Abstract
Efficient loading of major histocompatibility complex class II molecules with peptides requires the invariant chain (Ii) and the class II–like molecule H-2M. Recent in vitro biochemical studies suggest that H2-M may function as a chaperone to rescue empty class II dimers. To test this hypothesis in vivo, we generated mice lacking both Ii and H-2M (Ii−/−M−/−). Antigen presenting cells (APCs) from Ii−/−M−/− mice, as compared with APCs from Ii−/− mice, exhibit a significant reduction in their ability to present self-peptides to a panel of class II I-Ab–restricted T cells. As a consequence of this defect in the loading of self peptides, CD4+ thymocyte development is profoundly impaired in Ii−/−M−/− mice, resulting in a peripheral CD4+ T cell population with low levels of T cell receptor expression. These findings are consistent with the idea that H-2M functions as a chaperone in the peptide loading of class II molecules in vivo.
Newly synthesized MHC class II α/β dimers are assembled in the endoplasmic reticulum by association with invariant chain (Ii)1 and then transported into endocytic vesicles (MHC class II peptide–loading compartment MIIC or CIIV) with lysosomal and endosomal characteristics, where Ii is proteolytically degraded (for review see reference 1). The final products of Ii processing are short fragments originating from the 81–104 region of Ii, termed CLIP (class II–associated invariant chain peptide), which remain bound to the groove of class II molecules until they are exchanged for self or foreign peptides (2). Mice lacking Ii (Ii−/−) have significantly reduced expression of cell surface class II molecules, and have only small numbers of CD4+ T cells in the thymus and periphery (3, 4). Ii−/− splenocytes are severely deficient in presentation of the majority of exogenous protein antigens yet are more amenable to loading of cell surface class II molecules with exogenously supplied peptides. Biochemical experiments have shown that in Ii−/− cells, class II molecules are associated with 20–50-kD polypeptides (5). However, endogenous peptide–class II complexes, discernible by T cells, have been detected in Ii-negative fibroblasts (6) or in Ii−/− mice carrying a known epitope encoded by a transgene (7).
The class II–like molecule, H-2M (or HLA-DM in humans) uniquely resides in MIIC or CIIV vesicles (8), and binds to class II molecules at the low pH of these acidic organelles (9, 10). Experiments with purified class II and HLA-DM molecules have provided clues to potential functions of HLA-DM in vivo (for review see reference 11). In these experiments, HLA-DM was shown to catalyze antigenic peptide loading by accelerating the dissociation of CLIP, to act as a peptide editor by removing those peptides with high dissociation rates, and to prevent isolated empty class II α/β dimers from unfolding or aggregating at low pH (10–15). The consequences of H-2M or HLA-DM deficiency include the predominant expression of CLIP–class II complexes and profound defects in presentation of exogenous antigens (16–19). However, this defect is not absolute in that some peptides derived from endogenous or exogenous antigens have been shown to be displayed by class II molecules in the absence of HLA-DM or H-2M (20– 22). Although the abundance of CLIP–class II complexes does not alter the level of surface class II expression, mice lacking H-2M (M−/−) do have moderately reduced numbers of CD4+ T cells in the thymus and periphery, reflecting the altered peptide repertoire displayed by class II molecules.
To investigate the in vivo functions of H-2M other than facilitating the removal of CLIP, we analyzed mutant mice lacking both Ii and H-2M (Ii−/−M−/−). A recent publication also describing these mice reported that maturation, transport, and surface expression of class II molecules was comparable in Ii−/− and Ii−/−M−/− splenocytes (23). Surprisingly enough in view of these data, CD4+ T cell development in Ii−/−M−/− mice was impaired profoundly compared to mice lacking either Ii or H-2M alone, a finding not explained by the published analyses of the mice (23). We hypothesized that the limited repertoire of class II– bound self peptides displayed by Ii−/− cells, accounting for CD4+ T cell development in Ii−/− mice, is dramatically reduced in Ii−/−M−/− mice because an Ii-independent H-2M function is required for the presentation of the majority of endogenous peptides. To assess the effect of H-2M on the loading of high-affinity endogenous self peptides, we generated a novel panel of T cells specific for self peptide–I-Ab complexes. We found that the presentation of the majority of these self epitopes was significantly reduced in the doubly mutant Ii−/−M−/− APCs, relative to Ii−/− APCs. These studies demonstrate that even in the absence of Ii, H-2M can greatly enhance self peptide–class II complex formation, suggesting that it functions as a general chaperone in the peptide loading process. In addition, our data suggest that in the absence of Ii, surface class II molecules that internalize into or traffic through the endocytic pathway have access to the variety of antigenic peptides that are present in H-2M–containing early and late endocytic compartments.
Materials and Methods
Animals.
Each of the mutant mice used in this study are of a mixed B6x129 (H-2b) background, which expresses the MHC class II molecule I-Ab. Thus, the phenotypes of Ii−/− (4), M−/− (19), or Ii−/−M−/− mice were compared to that of wild-type B6x129 mice. Ii−/− and M−/− mice were bred to yield mice lacking both Ii and H-2M. All wild-type and mutant mouse strains were maintained under specific pathogen-free conditions at Vanderbilt University or University of Washington. Ii−/− mice were provided by Dr. Elizabeth Bikoff (Harvard University, Cambridge, MA). MHC-II−/− mice were purchased from Taconic Farms Inc. (Germantown, NY). Mice were used at 6–8 wk of age.
FACS® Analyses.
Analyses of T cell surface markers and MHC class II were performed as previously described (20). Experiments to assess cell surface binding of exogenous peptides using synthetic Eα52-68 peptide and mAb YAe were done as previously described (24).
T Cell Assays.
T cell hybrids specific for I-Ab bound to β2m 48–58 (4.1) and IgM 377–392 (77.1) peptides have been previously described (20). T cell hybrids specific for I-Ab bound to naturally processed epitopes of actin (15.10), Clp36 (3.5), amino aspartate aminotransferase (AAT) (15.15), and low density lipoprotein (LDL) receptor (41.2) were generated after peptide immunization of H-2M–deficient mice (Dongre, A., S. Kovats, P. deRoos, and A.Y. Rudensky, manuscript submitted). Individual T cell hybrids subcloned by limiting dilution were tested for MHC restriction and peptide specificity using M−/− APCs. T cell hybrids (2–10 × 104/well) were incubated with variable numbers of ex vivo splenocytes (depleted of red blood cells) of the indicated genotype (102– 106/well) for 20 h. Peptide specificity of the T cell hybrids was shown by addition of either cognate peptides or irrelevant peptides (10 μg/ml) to cultures of M−/− splenocytes (106) and T cells.
Synthetic Peptides and Peptide Binding Studies.
Peptides were synthesized on an automated peptide synthesizer (Synergy 432; Applied Biosystems, Foster City, CA) using F-moc chemistry. The purity of peptides used was >90%. For binding assays, affinity purified I-Ab (500 pM) was incubated with titrated amounts of inhibitor peptides (300 nM–700 μM) and biotinylated Eα52–68 peptide (48 μM) for 16 h at 37°C in 0.1 M citrate-phosphate buffer, pH 5.0, containing 1% octylglucoside. After neutralizing pH, reaction mixtures were transferred into mAb Y3P-coated plates and incubated for 4 h at 4°C. After washing, plates were incubated for 2 h with Eu3+-streptavidin (O.Y. Wallac, Turku, Finland), then washed and read on a fluorometer (1234 DELFIA; O.Y. Wallac) after adding fluorescence enhance solution (O.Y. Wallac).
Results
The Absence of Ii Chain and H-2M Results in a Synergistic Reduction in CD4+ T Cell Development.
Analyses of mice lacking Ii have revealed a significant reduction in CD4+ thymocytes, 20–30% of wild-type numbers, suggesting that efficient CD4+ thymic selection requires high levels of surface MHC class II molecules and/or the diverse array of endogenous peptides that are displayed by thymic epithelial cells in the presence of Ii. In contrast, in H-2M–deficient mice, which display wild-type levels of class II molecules predominantly occupied by a single peptide, CLIP, the number of CD4+ thymocytes is significantly higher than in Ii−/− mice, ∼30–50% of the number in wild-type mice.
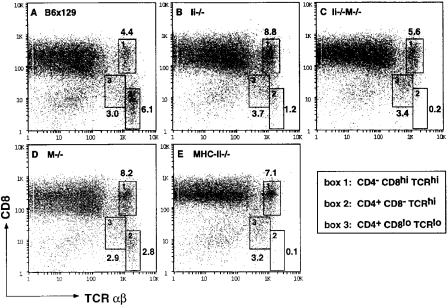
To assess CD4+ T cell development in Ii−/−M−/− mice, thymocytes and splenic T cells were assessed by flow cytometry for expression of CD4, CD8, and α/β TCR. The total number of thymocytes did not vary significantly in the mice of various genotypes. Three electronic gates were used to analyze the presence of CD4−CD8hiTCRhi (Fig. 1, box 1), CD4+CD8−TCRhi (Fig. 1, box 2), or CD4+CD8loTCRlo (Fig. 1, box 3) thymocyte populations isolated from mice of five distinct genotypes (B6x129, Ii−/−, M−/−, Ii−/−M−/−, and MHC-II−/−), shown as two parameter dot plots of CD8 versus TCR-α/β in Fig. 1. The CD4+CD8loTCRlo cells (box 3) represent a minor population in a wild-type thymus (Fig. 1 A), which is more prominent in an Ii−/− thymus, concomitant with a marked decrease in CD4+ TCRhi SP thymocytes (Fig. 1 B, box 2). In contrast, the M−/− thymus shows a 50% reduction in CD4+TCRhi single positive thymocytes (box 2) but no change in the intermediate (box 3) population (Fig. 1 D). Notably, the Ii−/−M−/− thymus shows a dramatic reduction (approximately sixfold relative to the Ii−/− thymus) in the number of CD4+TCRhi cells (Fig. 1 C), similar to that observed in mice lacking class II molecules (MHC-II−/−; Fig. 1 E). In both Ii−/−M−/− and MHC-II−/− mice, virtually all of the CD4+ thymocytes display low levels of TCR and have incomplete downregulation of CD8 (box 3). Thus, the partial block in positive selection of CD4 SP thymocytes observed in Ii−/− thymi is exacerbated by the additional loss of H-2M function in doubly mutant Ii−/−M−/− thymi.
Figure 1.
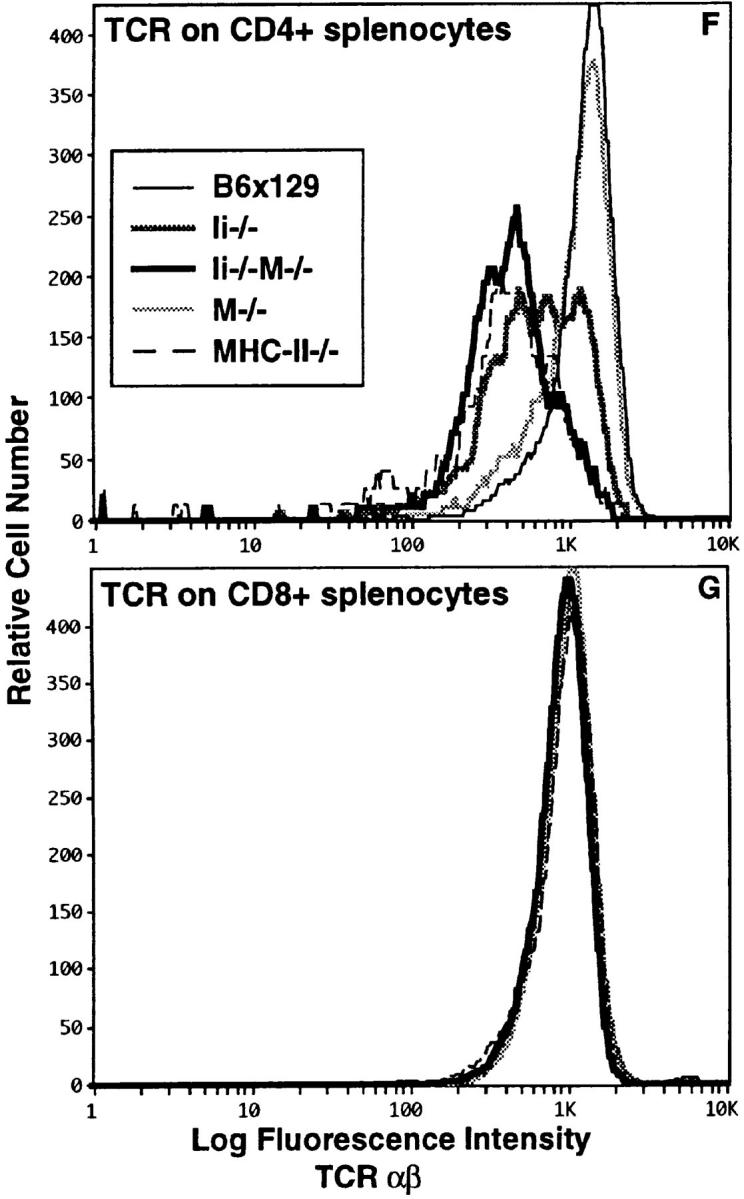
The absence of Ii chain and H-2M results in synergistic reduction of CD4+ T cell development. Thymocytes from (A) wild-type B6x129 and mutant (B) Ii−/−, (C) Ii−/−M−/−, (D) M−/−, and (E) MHC-II−/− mice were analyzed for expression of CD4, CD8, and TCR-α/β using flow cytometry. Shown are two parameter dot plots of CD8 versus TCR-α/β, in which three electronic gates are indicated, delineating the following populations: CD4−CD8hiTCRhi (box 1), CD4+CD8−TCRhi (box 2), and CD4+CD8loTCRlo (box 3). Also shown are the percentages of cells that fall within each box. Cells in boxes 2 and 3 are >97% CD4+. Splenocytes from wild-type and mutant mice were analyzed for expression of CD4, CD8, and TCR-α/β using flow cytometry. Shown are comparisons of TCR expression on (F) CD4+ T cells and (G) CD8+ T cells in the mice of the five genotypes, as indicated. Data are representative of analyses of four mice of each genotype.
Analyses of peripheral T cells showed that the number of splenic CD4+ T cells in Ii−/−M−/− mice were reduced relative to the number in Ii−/− or M−/− mice (data not shown). The level of TCR-α/β displayed on the surface of peripheral CD4+ T cells in M−/− mice was identical to that of wild-type mice (Fig. 1 F). In contrast, Ii−/− CD4+ T cells existed as two populations which differed in levels of TCR expression, one of which overlapped with wild-type T cells. Remarkably, CD4+ T cells with normal TCR expression were not present in spleens of doubly mutant Ii−/− M−/− mice; the low level of TCR exhibited by all these CD4+ T cells coincided with that of the CD4+TCRlo population in Ii−/− mice (Fig. 1 F). This low level of TCR expression was also observed on all CD4+ T cells present in MHC-II−/− mice. The level of TCR-α/β displayed on splenic CD8+ T cells from each of the mutant mice was not different from that of wild-type T cells (Fig. 1 G). Thus, Ii−/−M−/− mice have a nearly complete block in normal CD4+ T cell development, akin to that observed in mice lacking MHC class II molecules. These data led us to suggest that a diverse repertoire of endogenous peptides is achieved via an Ii-independent H-2M function and must be displayed by class II molecules in the thymus in order to achieve efficient CD4+ T cell development. To test this hypothesis, we next studied endogenous peptide:class II complex formation in the double mutant mice.
Occupancy of Surface Class II Molecules with High-Affinity Endogenous Peptides Was Diminished in Ii− /−M− /− Cells.
The majority of I-Ab molecules that reach the cell surface in the absence of Ii are associated with kinetically unstable peptides or polypeptides that can be easily displaced by exogenous high-affinity peptides; in contrast, class II–bound high-affinity peptides in wild-type cells cannot be displaced (4). The surface expression of class II molecules on Ii−/− and Ii−/−M−/− splenocytes was reduced comparably about fivefold relative to wild-type levels, as assessed by the I-Ab– specific mAb Y3P (Fig. 2 A). To demonstrate endogenous peptide occupancy of these surface class II molecules, wild-type and mutant cells were loaded with 30 μM of the Eα52–68 peptide and expression of the resulting Eα52–68– I-Ab complexes was assessed with the complex-specific mAb YAe (reference 24; Fig. 2, B and C). M−/− cells did not bind mAb YAe after incubation with Eα peptide. Relative to wild-type cells, Ii−/− cells showed a threefold increase in binding of mAb YAe. Significantly, the doubly mutant Ii−/−M−/− splenocytes showed a further modest increase in mAb YAe binding. Since the fraction of class II molecules containing high-affinity peptides in Ii−/− splenocytes is already small, resulting in very efficient loading of surface class II molecules with exogenous Eα52–68, it may be difficult to observe a further large effect of H-2M deficiency on exogenous peptide loading. Nonetheless, these data suggest that in the absence of both Ii and H-2M, even fewer self-peptides are stably loaded onto class II molecules.
Figure 2.
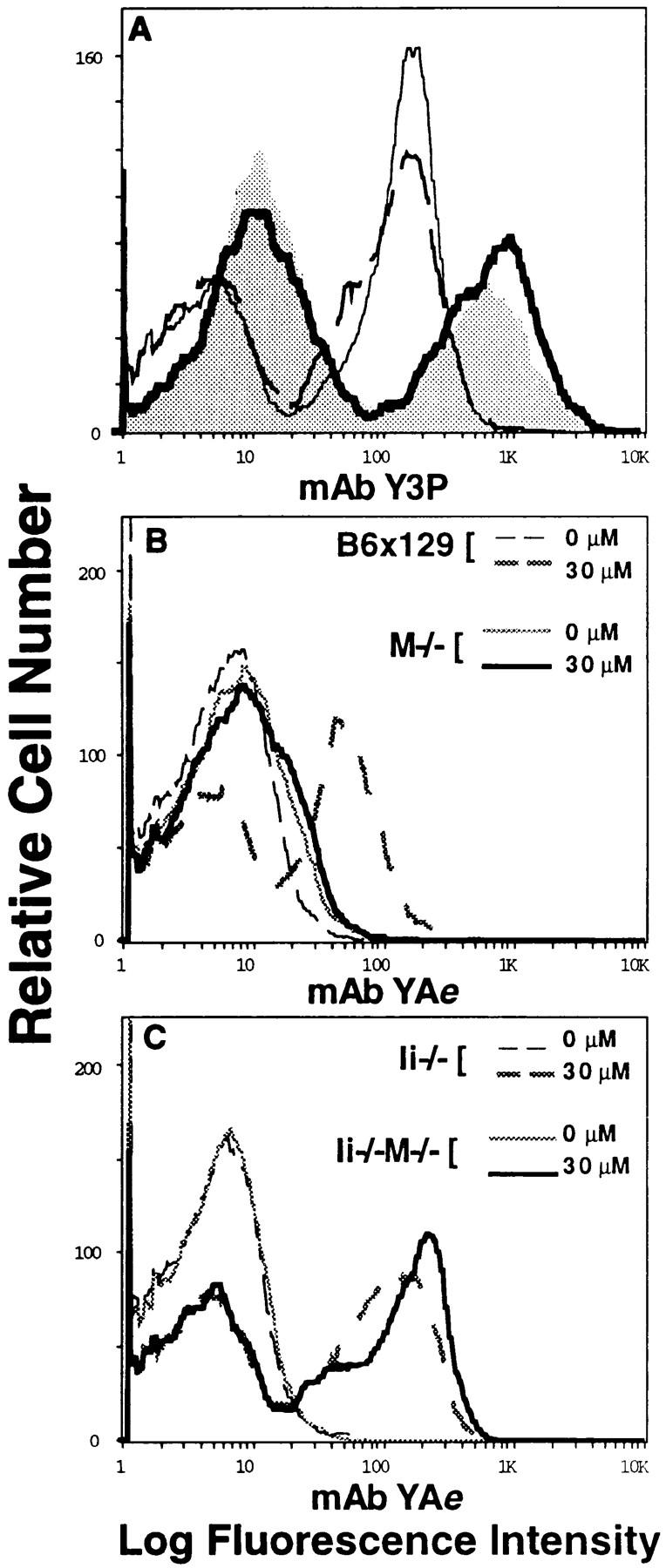
Occupancy of surface class II molecules with high affinity endogenous peptides is diminished in Ii−/−M−/− splenocytes. (A) Cell surface class II expression on splenocytes from wild-type and mutant mice was assessed by the binding of biotinylated mAb Y3P and streptavidin-PE, followed by flow cytometry. Shown are profiles from wild-type B6x129 (shaded histogram), M−/− (thick solid line), Ii−/− (thin dotted line), and Ii−/− M−/− (thin solid line) mice. For assessment of peptide occupancy, splenocytes were incubated in the absence (0 μM) or presence (30 μM) of synthetic Eα52–68 peptide (as indicated) for 3 h at 37°C, before assessment of the resulting Eα52–68–I-Ab complex formation using biotinylated mAb YAe and streptavidin-PE in flow cytometric analyses. Shown is binding of mAb YAe to (B) wild-type B6x129 and M−/− cells and (C) Ii−/− and Ii−/− M−/− cells. Data are representative of four independent experiments.
Presentation of Some Endogenous Self-peptides Requires H-2M Even in the Absence of Ii-derived CLIP
To investigate whether H-2M acts to facilitate self-peptide binding in the absence of CLIP, we assessed the ability of Ii−/−M−/− splenocytes to form particular endogenous self-peptide–I-Ab complexes. Sequences of naturally processed self-peptides bound to I-Ab molecules in B cells or macrophages, corresponding to portions of both membrane-bound and cytosolic proteins, were recently identified in our laboratory using tandem mass spectrometry (Dongre, A., S. Kovats, P. deRoos, and A.Y. Rudensky, manuscript in preparation). The peptide epitopes studied in this work include those derived from membrane-bound proteins IgM (377–392), β2m (48–58), LDLr (486–501), and cytosolic proteins actin (163–177), Clp36 (138–153), and AAT (394–410). Synthetic peptides of identical amino acid sequences to those eluted from class II molecules were found to have a high affinity for purified I-Ab in in vitro binding assays (Fig. 3), comparable to that of the Eα52–68 peptide, a known high-affinity binder (24). These peptides were used to generate antigen-specific T cell hybrids upon immunization of H-2M−/− mice (LDLr, actin, AAT, Clp36) P. Dongre, S. Kovats, P. deRoos, and A.Y. Rudensky, manuscript in preparation), or mice lacking the specific self-protein, e.g., IgM−/− or β2m−/− (20). These T cell hybrids were specific for the immunizing peptide bound to I-Ab, as demonstrated by experiments in which exogenous cognate peptide was added to M−/− splenocytes (Fig. 4, bar graphs). Irrelevant peptides added to M−/− APCs did not stimulate the T cell hybrids (data not shown). The T cell hybrids also recognized endogenously formed peptide–I-Ab complexes on wild-type splenocytes (Fig. 4, line graphs).
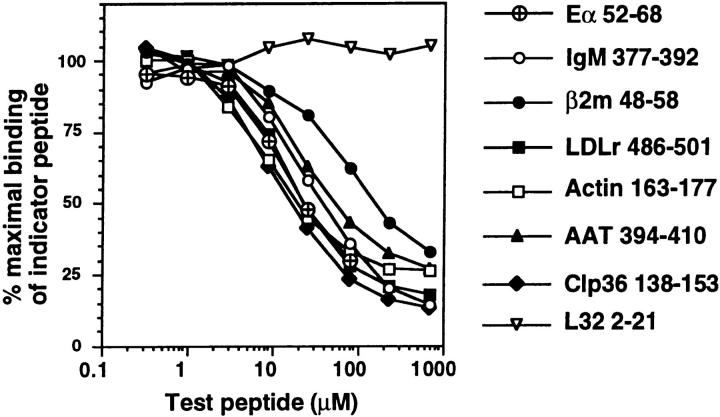
Figure 3.
Endogenous peptides bind with high affinity to purified I-Ab molecules in vitro. Purified I-Ab molecules were incubated with titrated amounts of unlabeled test peptides and a biotinylated indicator peptide Eα52–68. Subsequently, I-Ab molecules were captured on plates, and the amount of biotinylated Eα peptide bound was quantified using Eu3+-streptavidin and fluorometry. Data were normalized to the maximal binding of biotinylated Eα52–68 (65,000–150,000 fluorescence units) in the absence of inhibitor, and are reported as percentage maximal binding of this indicator peptide. Shown are binding curves in the presence of unlabeled Eα52–68 and a peptide that does not bind to I-Ab, L32 2–21. Background signal in the assay was 2,000–5,000 fluorescence units.
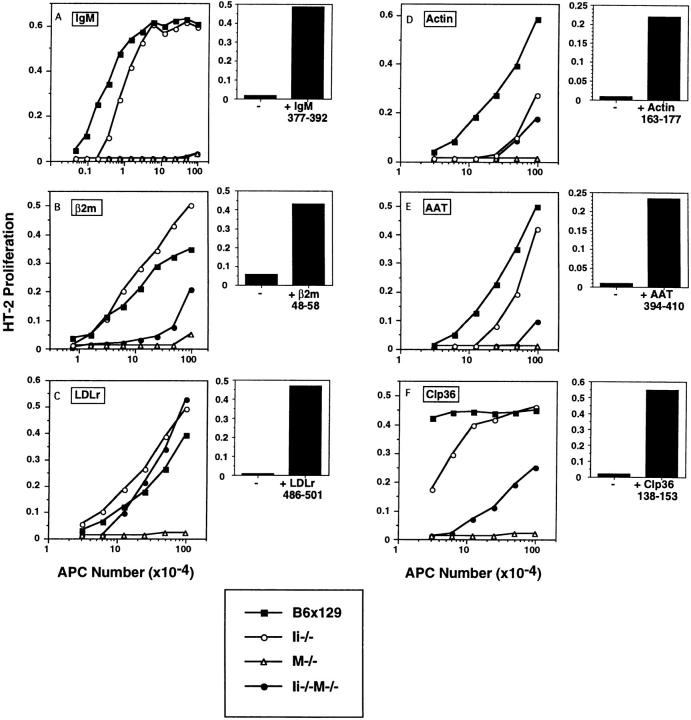
Figure 4.
Presentation of select endogenous self peptides requires H-2M even in the absence of Ii-derived CLIP. Activation of antigen-specific T cell hybrids by ex vivo splenic APCs isolated from wild-type and mutant mice. Specificities of the T cell hybrids are as follows: (A) IgM 377–392, (B) β2m 48– 58, (C) LDLr 486–501, (D) actin 163–177, (E) AAT 394–410, and (F) Clp36 138–153. Shown in the left panels (line graphs, A–F) are responses of T cell hybrids to variable numbers of splenocytes in the absence of exogenous peptide; splenocytes derived from wild-type B6x129 (squares), Ii−/− (open circles), M−/− (triangles), and Ii−/−M−/− (closed circles) mice are distinguished as indicated. Shown in the right panels (bar graphs, A–F) are responses of T cell hybrids to M−/− splenocytes in the absence or presence of exogenously supplied cognate peptide (10 μg/ml). IL-2 production by the T cell hybrids was assessed by proliferation of HT-2 cells using an Alamar blue colorimetric assay; results (average of duplicate wells) are expressed as arbitrary units of OD at 570 versus 600 nm. Data are representative of four independent experiments.
A comparison of the ability of wild-type and mutant APCs to form these endogenous peptide–I-Ab complexes was obtained by incubation of variable numbers of ex vivo isolated splenic APCs with a constant number of T cells in the absence of exogenously added antigen (Fig. 4, A–F). The comparison of Ii−/− and Ii−/−M−/− APCs allows direct assessment of the role of H-2M, since the surface class II expression is equivalent on splenocytes from these two types of mice (Fig. 2). Each of the six epitopes was efficiently presented by Ii−/− splenocytes (Fig. 4). The responses of T cells specific for IgM, actin, AAT, and Clp36 epitopes were reduced by approximately fivefold, whereas the responses of T cells specific for β2m and LDLr epitopes were enhanced about twofold, relative to wild-type APCs. These data indicate that these endogenous peptides can be efficiently loaded in vivo onto class II molecules that traffic through cellular compartments via an Ii-independent route.
In contrast, formation of the six distinct peptide–class II complexes by doubly mutant Ii−/−M−/− splenocytes was much more variable, demonstrating differential requirements for H-2M function in the absence of CLIP. Presentation of the IgM377–392 epitope was profoundly diminished in the absence of both Ii and H-2M, a striking result since the IgM377–392–I-Ab complex is expressed very well in Ii−/− APCs (Fig. 4 A). Presentation of the β2m48–58 epitope was also significantly diminished in Ii−/−M−/− APCs, (reduced ∼10-fold relative to Ii−/− APCs; Fig. 4 B). As previously described (20), these two epitopes were presented at undetectable or very low levels by M−/− splenocytes (Fig. 4, A and B). Presentation of peptides derived from the cytosolic antigens AAT and Clp36 by Ii−/−M−/− splenocytes was diminished 5–10-fold relative to Ii−/− APCs (Fig. 4, E and F), while the already low level of presentation of the actin epitope by Ii−/− deficient cells was not profoundly affected by the absence of H-2M (Fig. 4 D). Interestingly, formation of LDLr486-501–I-Ab complexes was essentially equivalent in Ii−/− and Ii−/− M−/− cells (Fig. 4 C), and slightly enhanced relative to wild-type cells, suggesting that this peptide preferentially binds to class II molecules via a mechanism independent of both Ii and H-2M. As expected, since the T cells specific for LDLr, actin, AAT, and Clp36 peptides were generated in H-2M–deficient mice, these peptides were not presented by M−/− splenocytes (Fig. 4, C–F).
Discussion
In this report, we demonstrate that H-2M functions in the in vivo loading of antigenic peptides onto class II molecules in a capacity that is distinguishable from its previously described role in CLIP removal. Using a panel of T cells specific for high-affinity endogenous self-peptides bound to the class II molecule I-Ab, we found that for the majority of epitopes normally expressed in the absence of Ii, additional loss of H-2M function significantly reduced the ability of APCs to assemble these self-peptide–class II complexes. Thus, even in the absence of Ii, class II molecules traffic through the endocytic vesicles in which H-2M facilitates the binding of antigenic peptides. As a consequence of this deficient loading of self-peptides, CD4+ thymocyte development is profoundly impaired in Ii−/− M−/− mice, resulting in a peripheral CD4+ T cell population with uniformly low levels of TCR. Thus, we hypothesize that the normal diversity of class II-bound endogenous peptides displayed on antigen presenting cells, made possible by the disparate chaperone functions of both Ii and H-2M, is required for development of a complete repertoire of CD4+ T cells.
Each of the endogenous epitopes we studied were presented well to T cells in the absence of Ii. With the exception of the response to the LDLr epitope, self-antigen– specific T cell responses to Ii−/−M−/− splenocytes were reduced significantly relative to Ii−/− APCs, suggesting that even in the absence of Ii, some endogenous peptide–class II complexes are formed in H-2M containing lysosomal compartments. There are two ways in which Ii-free class II molecules may enter the endocytic pathway and potentially benefit from the activities of H-2M. Cell surface class II molecules can internalize into early endocytic vesicles via signals in the cytoplasmic tails of class II α and β molecules, a pathway that is also functional in Ii-expressing cells (25, 26). Alternatively, a lysosomal targeting signal present at residues 80–82 of the class II β chain may facilitate entry of newly synthesized class II molecules into the endocytic pathway in the absence of Ii (27). In view of this, the self-epitopes analyzed here may be generated efficiently and bind to class II in early or late endocytic compartments accessed by class II molecules internalized from the cell surface or after diversion from the trans-Golgi network. Although some of the high affinity self-peptides studied here were presented in the absence of Ii and H-2M, the dramatic reduction of mature CD4+ T cells in the Ii−/−M−/− mice indicates that the function of H-2M is generally required in vivo to promote the binding of most self-peptides to class II molecules.
H-2M may significantly increase the class II–bound repertoire of endogenous peptides in Ii−/− APCs by virtue of its ability to stabilize empty class II molecules or its capacity to mediate replacement of a limited set of unstable polypeptides with a larger repertoire of relatively stable peptides. Thus, it is perhaps unexpected that the absence of H-2M did not decrease the steady-state level of I-Ab expression in Ii−/−M−/− APCs. However, since the majority of class II molecules in Ii−/− cells are unstably bound to low-affinity polypeptides, the minor proportion of class II molecules stably bound to high-affinity peptides in the presence of H-2M may readily assemble with available polypeptides in the absence of H-2M, thus maintaining an apparently similar level of surface class II expression. Alternatively, the absence of this minor population in Ii−/−M−/− APCs would not significantly affect total surface class II expression as measured by flow cytometry.
Biochemical experiments have shown that empty class II α/β dimers are partially unfolded at pH 4.0–5.5 and tend to form aggregates (28). HLA-DM is able to protect these α/β dimers from denaturation through a stable interaction (10, 15). In vivo, such chaperone activity would maintain more class II molecules in a peptide-receptive state in endocytic vesicles until they contacted antigenic peptides, eventually increasing the number of stable peptide–class II complexes at the cell surface. For the high affinity self-peptides we studied, it is unlikely that the editing function of H-2M was required to select for their stable binding. These peptides are able to efficiently displace low stability or affinity peptides or polypeptides bound to surface class II molecules on Ii−/− cells at neutral pH without the aid of H-2M (Fig. 2 and data not shown). Rather, H-2M may be primarily enhancing their presentation by virtue of its ability to bind to and prolong the half-life of empty class II α/β dimers at acidic pH in vivo, as suggested by the in vitro studies.
The ability of a particular peptide to bind productively to class II molecules in the absence of H-2M may depend on its unique complement of amino acid side chains, or the location and stability of the antigenic fragment within the various endocytic vesicles. Thus, H-2M–independent peptide binding would occur if the on-rate of the binding reaction is very high, or if the half-life of an antigenic peptide is greater than the half-lives of empty class II molecules, a condition that might exist in the less proteolytic early endocytic compartments that contain little or no H-2M (29–31). In this regard, it is of interest to note that the LDL receptor protein contains a targeting signal for early endosomes, which may be the site where the H-2M– and Ii-independent epitope of LDLr is generated (32).
Observations with singly mutant Ii−/− or M−/− mice have indicated that efficient positive selection of CD4+ T cells requires high levels of surface class II molecules and/or a diverse array of class II–bound endogenous peptides (3, 4, 17–19). Since the majority of surface class II molecules in Ii−/− APCs are apparently associated with unstably bound peptides or larger polypeptides, the profound impairment in CD4+ T cell development in Ii−/− mice suggests that this majority of class II molecules does not mediate positive selection. Rather, CD4+ T cells in Ii−/− mice are likely to be selected on the small minority of stable endogenous peptide–class II complexes that do reach the cell surface, albeit with a contracted repertoire of high-affinity endogenous peptides. The absence of H-2M function in Ii-deficient cells is likely to further decrease the diversity of the class II–bound repertoire and/or the number of functional endogenous peptides, which may be replaced with unstably bound polypeptides. In both of these scenarios, steady state surface class II expression would be similar to that of Ii−/− cells. Although distinguishing between these two possibilities is key to understanding the requirements for positive selection in these mice, we currently lack the means to quantitate the proportion of class II molecules occupied with high affinity peptides in Ii−/− cells. Nevertheless, these reductions in the number or diversity of functional peptides apparently puts their expression below a threshold level required for CD4+ positive selection in Ii−/− M−/− mice. In view of our data, the impairment of CD4+ thymic selection in M−/− mice may be due to the dramatic reduction in diversity of endogenous peptides bound to wild-type levels of class II molecules, although this impairment may be less marked than in Ii−/− mice due to the high density of the single CLIP peptide–class II complex.
In summary, our findings are consistent with the idea that H-2M evolved as the primary chaperone assisting in the loading of antigenic peptides onto MHC class II molecules in the late endocytic compartment, presumably by promoting the stability of empty class II α/β dimers. The facilitated dissociation of CLIP, and of other peptides that bind to class II molecules with a high dissociation rate, may be more specific manifestations of the general chaperone function of H-2M.
Acknowledgments
We thank Deb Wilson for excellent animal care, and our colleagues for valuable discussions. We also thank E. Bikoff for Ii−/− mice.
This work was supported by National Institutes of Health grants to S.K. (NRSA AI-08903), C.E. Grubin (Predoctoral training grant), and A.Y. Rudensky (AI-34206). A.Y. Rudensky and L. Van Kaer are investigators of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used in this paper: AAT, aspartate aminotransferase; CLIP, Class II associated invariant chain peptide; Ii, invariant chain; LDLr, LDL receptor; M, H-2M.
References
- 1.Harding CV. Intracellular organelles involved in antigen processing and the binding of peptides to class II MHC molecules. Semin Immunol. 1995;7:355–360. doi: 10.1006/smim.1995.0040. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–507. doi: 10.1016/s0092-8674(00)81025-9. [DOI] [PubMed] [Google Scholar]
- 3.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Mice lacking the MHC class II–associated invariant chain. Cell. 1993;72:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 4.Bikoff EK, Huang L-Y, Episkopou V, Meerwijk J, Germain RN, Robertson EJ. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+T cell selection in mice lacking invariant chain expression. J Exp Med. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busch R, Cloutier I, Sekaly R-P, Hammerling GJ. Invariant chain protects class II compatibility antigens from binding intact polypeptides in the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1996;15:418–428. [PMC free article] [PubMed] [Google Scholar]
- 6.Ceman S, Sant AJ. The function of invariant chain in class II–restricted antigen presentation. Semin Immunol. 1996;7:373–387. doi: 10.1006/smim.1995.0042. [DOI] [PubMed] [Google Scholar]
- 7.Bodmer H, Viville S, Benoist C, Mathis D. Diversity of endogenous epitopes bound to MHC class II molecules limited by invariant chain. Science. 1994;263:1284–1286. doi: 10.1126/science.7510069. [DOI] [PubMed] [Google Scholar]
- 8.Sanderson F, Kleijmeer MJ, Kelly A, Verwoerd D, Tulp A, Neefjes JJ, Geuze HJ, Trowsdale J. Accumulation of HLA-DM, a regulator of antigen presentation in MHC class II compartments. Science. 1994;266:1566–1569. doi: 10.1126/science.7985027. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson F, Thomas C, Neefjes J, Trowsdale J. Association between HLA-DM and HLA-DR in vivo. Immunity. 1996;4:87–96. doi: 10.1016/s1074-7613(00)80301-5. [DOI] [PubMed] [Google Scholar]
- 10.Denzin LK, Hammond C, Cresswell P. HLA-DM interactions with intermediates in HLA-DR maturation and a role for HLA-DM in stabilizing empty HLA-DR molecules. J Exp Med. 1996;184:2153–2165. doi: 10.1084/jem.184.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kropshofer H, Hammerling GJ, Vogt AB. How HLA-DM edits the MHC class II peptide repertoire: survival of the fittest? . Immunol Today. 1997;18:77–82. doi: 10.1016/s0167-5699(97)01006-2. [DOI] [PubMed] [Google Scholar]
- 12.Sloan VS, Camerson P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 13.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 14.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain–derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 15.Kropshofer H, Arndt SO, Moldenhauer G, Hammerling GJ, Vogt AB. HLA-DM acts as a molecular chaperone and rescues empty HLA-DR molecules at lysosomal pH. Immunity. 1997;6:293–302. doi: 10.1016/s1074-7613(00)80332-5. [DOI] [PubMed] [Google Scholar]
- 16.Mellins B, Smith L, Arp B, Cotner T, Celis E, Pious D. Defective processing and presentation of exogenous antigens in mutants with normal HLA class II genes. Nature. 1990;343:71–74. doi: 10.1038/343071a0. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki T, Wolf P, Tourne S, Waltzinger C, Dierich A, Barois N, Ploegh H, Benoist C, Mathis D. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide–loading pathway. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 18.Fung-Leung W-P, Surh CD, Liljedahl M, Pang J, Leturcq D, Peterson PA, Webb SR, Karlsson L. Antigen presentation and T cell development in H2-M–deficient mice. Science. 1996;271:1278–1281. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 19.Martin WD, Hicks GG, Leva HI, Ruley HE, Van Kaer L. H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 20.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 21.Pinet V, Malnati MS, Long EO. Two processing pathways for the MHC class II–restricted presentation of exogenous influenza virus antigen. J Immunol. 1994;152:4852–4860. [PubMed] [Google Scholar]
- 22.Avva RR, Cresswell P. In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain–derived peptides. Immunity. 1994;1:763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 23.Tourne S, Miyazaki T, Wolf P, Ploegh H, Benoist C, Mathis D. Functionality of major histocompatibility complex class II molecules in mice doubly deficient for invariant chain and H-2M complexes. Proc Natl Acad Sci USA. 1997;94:9255–9260. doi: 10.1073/pnas.94.17.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudensky AY, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr On the complexity of self. Nature. 1991;353:660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 25.Reid PA, Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990;346:655–657. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- 26.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recylcing of surface HLA-DR molecules. Nature. 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 27.Chervonsky AV, Gordon L, Sant AJ. A segment of the MHC class II β chain plays a critical role in targeting class II molecules to the endocytic pathway. Int Immunol. 1994;6:973–982. doi: 10.1093/intimm/6.7.973. [DOI] [PubMed] [Google Scholar]
- 28.Germain RN, Rinker AGJ. Peptide binding inhibits protein aggregation of invariant-chain free class II dimers and promotes surface expression of occupied molecules. Nature. 1993;363:725–728. doi: 10.1038/363725a0. [DOI] [PubMed] [Google Scholar]
- 29.Castellino F, Germain RN. Extensive trafficking of MHC class II–invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995;2:73–88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 30.Ma C, Blum JS. Receptor-mediated endocytosis of antigens overcomes the requirement for HLA-DM in class II–restricted antigen presentation. J Immunol. 1997;158:1–4. [PubMed] [Google Scholar]
- 31.Griffin JP, Chu R, Harding CV. Early endosomes and a late endocytic compartment generate different peptide–class II MHC complexes via distinct processing mechanisms. J Immunol. 1997;158:1523–1532. [PubMed] [Google Scholar]
- 32.Marks MS, Ohno H, Kirchhausen T, Bonifacino JS. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]