Abstract
A large, transient population of natural killer (NK) cells appears in the murine uterine mesometrial triangle during pregnancy. Depletion of uterine (u) NK cells, recently achieved using gene-ablated and transgenic mice, results in pathology. Pregnancies from matings of homozygous NK and T cell–deficient tgε26 mice have <1% of normal uNK cell frequency, no development of an implantation site–associated metrial gland, and an edematous decidua with vascular pathology that includes abnormally high vessel walls/lumens ratios. Fetal loss of 64% occurs midgestation and placentae are small. None of these features are seen in pregnant T cell–deficient mice. To confirm the role of the NK cell deficiency in these reproductive deficits, transplantation of tgε26 females was undertaken using bone marrow from B and T cell–deficient scid/scid donors. Engrafted pregnant females have restoration of the uNK cell population, induced metrial gland differentiation, reduced anomalies in the decidua and decidual blood vessels, increased placental sizes, and restoration of fetal viability at all gestational days studied (days 10, 12, and 14). Thus, uNK cells appear to have critical functions in pregnancy that promote decidual health, the appropriate vascularization of implantation sites, and placental size.
Large, heavily granulated lymphocytes (LGLs)1 are found in the pregnant uteri of many species, including mice, rats, pigs, and humans (1). Due to their dependency on estrogen and progesterone and their absence from the uteri of virgin and postpartum animals, these lymphocytes are expected, but have not yet been proven, to have important, pregnancy-associated functions. By midgestation in rodents (days 10–14 of gestation in mice), up to 35% of the cells in a specialized, pregnancy-induced region within the uterine musculature called the metrial gland are granulated cells commonly called granulated metrial gland cells (2). In pregnant women, uterine LGLs are more widely dispersed throughout decidual tissue and represent 70% of the leukocytes in decidual cell suspensions (3).
Immunophenotyping of the surface and the cytoplasmic granules of rodent and human pregnancy-associated LGLs has led to the hypothesis that uterine LGLs are lymphocytes of the NK cell lineage (4, 5). Studies using immune-deficient mice support this conclusion, since LGLs typical in morphology, location, and frequency have been reported in T cell–deficient mice (6, 7), T and B cell–deficient mice (7), and NK1+ T cell–deficient mice (8). However, LGLs and a metrial gland structure were absent from the uteri of pregnant IL-2Rγ null, p56lck null × IL-2Rβ null, and tgε26 mice (8, 9). Because an NK cell deficiency accompanies the T cell deficiency in the three latter strains, uterine LGLs are now regarded as members of the NK cell lineage and designated uterine (u)NK cells (8, 9).
Depletion of uNK cells during pregnancy has only been achieved genetically. A transgenic strain, tgε26, carrying high copy numbers (30–35) of the full human (hu) CD3ε gene exhibits NK and T cell deficiencies from fetal life (10, 11). In addition to their splenic NK cell deficiency (10), uNK cells are deficient in pregnant tgε26 (range from 0 to 3% of the frequency found in immune-competent, random-bred H-2b CD1 mice and inbred H-2k CBA/J mice [8]). Metrial glands also fail to differentiate in tgε26 implantation sites (8).
In immune-competent mice, the metrial gland is associated with the maternal portion of the placenta that consists of transformed stromal cells known as the decidua. The fetal portion of the placenta has three layers: giant cell trophoblast, spongiotrophoblast, and labyrinthine trophoblast. Implantation sites in tgε26 mice appear normal, both grossly and histologically, until day 9 of gestation (counting from the copulation plug as day 0). By day 10 of gestation, the decidua lacks normal cellularity and the large ablumenal decidual blood vessels have thickened walls. There are progressive degenerative changes to both the media and endothelium over the next four d of gestation. Vascular anomalies are not found in other organs of pregnant or nonpregnant tgε26 mice (8).
Death of >50% of the tgε26 fetuses occurs in each litter between days 10 and 14 of gestation, a time correlating with the decidual pathologies. Surviving tgε26 fetuses have very small placentae (∼50% of normal) and from birth to adulthood tgε26 mice are smaller than immune-competent, age-matched controls (36% smaller at 24 h after birth and 27% smaller at 7 wk of age) (8). These data suggested that uNK cells have major functional roles in optimizing pregnancy success.
To understand the role of uNK cells in the reproductive deficits of tgε26 females, three further studies were undertaken. First, ectoplacental cones from tgε26 mice were examined for expression of the huCD3ε transgene. No expression was detected. Second, morphometry was applied to the maternal decidual arterial sinuses, which are murine equivalents to human spiral arteries (12). Elevated wall to lumen ratios were found, suggestive of hypertension. Third, bone marrow was transplanted from scid/scid mice into tgε26 females. These donor cells are known to establish peripheral NK cells but do not generate mature T cells (13). The uNK cell lineage was established and the quantified reproductive deficiencies of tgε26 mice were reversed or significantly reduced in engrafted, pregnant tgε26 mice.
Materials and Methods
Mice.
8-wk-old random-bred CD1 (H-2b) mice (Charles River Laboratories, St. Constant, Québec, Canada), inbred CBA/J mice (H-2k; The Jackson Laboratory, Bar Harbor, ME), C.B-17 scid/scid (SCID) mice (bred at Guelph) and transgenic tgε26 (H-2k bred at Guelph) were used for experiments. Time-matched pregnant uteri from syngeneically mated CD1 and CBA/J mice were used as controls for syngeneically mated tgε26 females counting the copulation plug as day 0. After euthanasia (CO2 + cervical dislocation), pregnant uteri were examined and dissected. Fetal viability was grossly scored by implant size and color, and then the implantation sites were processed for histology.
Bone Marrow Transplantation.
Bone marrow transplantation was performed in four experiments, as previously described (13, 14). In brief, female recipients (8-wk-old tgε26 mice; 5/experiment) were infused with pooled intravenous bone marrow cells, untreated or treated with two rounds of anti–Thy-1 antibody (Ab) diluted 1:20 in 10 mM PBS + guinea pig complement diluted 1:3 in 10 mM PBS (Cedarlane Labs., Hornby, Ontario, Canada) from male or female C.B-17 SCID mice. Each tgε26 mouse was preconditioned by a single intraperitoneal injection of 5-fluorouracil (5-FU, 150 mg/kg; Hoffmann-La Roche Ltd., Mississauga, Ontario, Canada) 48 h before grafting of four donor animal long bone cell equivalents (ranging from 2.5 × 106 to 1.75 × 107 bone marrow cells). After 3 wk of recovery, the females were paired with tgε26 males for pregnancies at days 10, 12, or 14 of gestation. A similar protocol has been shown to establish splenic NK cells in tgε26 mice (13).
Histology.
Uteri were fixed in Bouin's fixative (Fisher Scientific, Whitby, Ontario, Canada) and processed into paraffin. All implantation sites were serially sectioned transversely at 6 μm and stained using standard protocols for hematoxylin and eosin, periodic acid–Schiff (PAS) reaction, and Masson's trichrome connective tissue stain. The PAS reaction stains the granules of uNK cells a bright magenta and makes it possible to distinguish them from other lymphocytes. Masson's trichrome was used for assessment of decidual blood vessels. All serial sections were examined.
Image Analysis.
Micrometer-based uNK cell enumeration has been previously described (9). All other histological measurements used Northern Exposure System 2.6a (Imagexperts Inc., Hollywood, CA) running in Microsoft Windows 3.1 (Microsoft Corp., Redmond, WA). Eight median independent tissue sections were analyzed, including the central section and sections on both sides that were at least 36 μm apart. Cross-sectional area measurements of decidual blood vessel wall and vessel lumen and placental cross-sectional areas were measured from a minimum of two implantation sites per pregnancy. The limits used for the definition of placental cross-sectional area were trophoblast populations up to and including the trophoblast giant cell layer. The decidua and metrial gland were excluded.
Statistical Analysis.
Means, standard errors, standard deviations, P values, and paired t tests were performed using the computer software program Microsoft Excel 5.0 for Windows (Microsoft Corp.).
Results and Discussion
Tgε26 Trophoblast Does Not Express the huCD3ε Transgene.
One possible explanation for small placentae in tgε26 pregnancy is aberrant expression of the huCD3ε transgene in trophoblast cells. We first established that neither CD1 nor tgε26 trophoblast expressed immunoreactive murine CD3 (data not shown). Then, day 7 tgε26 ectoplacental cone tissue (the fetally derived structure from which all trophoblast arises) was examined for huCD3ε expression by Northern blotting and a more sensitive reverse transcriptase PCR approach. Day 7 was selected because it is the latest developmental time point at which pure trophoblast can be isolated. The positive control was RNA from thymocytes of heterozygous tgε600 mice (13), which carry a different copy number of the same transgene. Human CD3ε mRNA was not detected by either method in the trophoblast tissue but was present in the heterozygote thymocyte RNA (data not shown). Thus, it is unlikely that the reduced size of placental trophoblast in tgε26 is due to transgene expression within the trophoblastic lineages.
Tgε26 Decidua and Quantification of Decidual Vessel Histopathology.
The mesometrial decidua in tgε26 is very unusual, being relatively acellular and containing vessels with thickened walls. Masson's trichrome staining did not detect matrix deposition in the cell-deficient regions (data not shown). Since neutral red staining of cryostat sections has also eliminated fat deposition as an explanation for this relative acellularity, we interpret the decidual region as edematous (9).
Normal pregnancy is associated with progressive dilation of uterine blood vessels and their derivative branches. Since this was apparently not occurring in tgε26 pregnancies, the anomalous vessels were studied morphometrically. Table 1 summarizes the ratios of tgε26 vessel wall to lumen cross-sectional area measurements and compares them to measurements of the similar vessels in the ablumenal decidua of CBA/J. In immune-competent control animals, a progressive increase in the ratio of the lumen to wall was found. This is consistent with the hypotensive environment promoted in mammalian uterus during mid-gestation (15–17). At days 10, 12, and 14 of gestation, tgε26 vessel walls showed a thickened media. This was measured at 2.7, 2.0, and 4.7 times greater than the media thickness in control, gestational day–matched CBA/J. The decidual vessels in tgε26 had relatively less lumen, suggesting vasoconstriction or failure to undergo arterial vessel modification that would facilitate vasodilation. Either mechanism would be expected to promote thickened decidual vessel walls and a more hypertensive environment in the tgε26 uterus at midpregnancy. Masson's trichrome intensely stained the muscular component of the vessel walls (red). There was little evidence of increased connective tissue reactivity (blue) on days 10, 12, and 14 of gestation. This demonstrates that the media was the thickened component in tgε26 decidual vessel walls.
Table 1.
Summary of Ratios of Mean Decidual Vessel Wall to Lumen Cross-sectional Area Measured at Days 10, 12, and 14 of Gestation in tgε26 and CBA/J Mice
| Mouse strain | Day 10 of gestation (mean W/L* ± SEM) | Day 12 of gestation (mean W/L ± SEM) | Day 14 of gestation (mean W/L ± SEM) | |||
|---|---|---|---|---|---|---|
| tgε26 | 2.77 ± 0.55‡ | 1.32 ± 0.51§ | 2.48 ± 0.56‡ | |||
| CBA/J | 1.03 ± 0.17 | 0.67 ± 0.42 | 0.53 ± 0.10 |
W/L = vessel wall cross-sectional area/lumen cross-sectional area ratio measured at ×200.
P <0.01 as compared to CBA/J.
P <0.1 as compared to CBA/J.
NK Cell Reconstitution in tgε26 Females Infused with SCID Bone Marrow.
Six features of pregnancies in tgε26 females have been identified as different from pregnancies in immune-competent or in T cell–deficient mice. These features are as follows: loss of uNK cells to <1% of normal; absence of metrial gland development; acellularity and edema in the decidua; increased wall/lumen ratios in the major ablumenal mesometrial vessels of the decidua; small placental size; and midgestational fetal death (8). To determine whether NK cell deficiency makes a major contribution to any or all of these reproductive deficits, females were grafted with SCID (NK+ T− B−) bone marrow, which cannot establish the T cell lineage. Half of the recipients received bone marrow that had been pretreated with α-Thy-1 Ab + complement before injection while the other half received untreated bone marrow. Of the 20 recipients, only 10 mated successfully and these were from both of the marrow pretreatment protocols (Table 2). Of these 10, 7 were considered to have splenic NK cell reconstituted as assessed by standard chromium release assays (mean percentage of specific lysis of 36.2% ± 2.1 at E:T = 100:1 compared to mean percentage of specific lysis of 42.0% ± 5.0 for CD1 at the same E:T ratio). Three females who had relatively nonlytic spleen cells (mean percentage of specific lysis of 9.1% ± 2.0 at E:T = 100:1 compared to mean percentage of specific lysis of 4.8% ± 3.7 for unmanipulated tgε26 at the same E:T) were considered to be non-reconstituted, and were analyzed separately from the first seven females.
Table 2.
Data Summary for SCID Bone Marrow–inoculated tgε26 Females at Days 10, 12, and 14 of Gestation
| Day of gestation | No. of mice | Thy-1 tx* | No. of uNK cells‡ (mean ± SEM) | No. of viable/ total No. of implants | Percent viability in utero | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NK reconstituted § | ||||||||||
| 10 | 1 | no | 54.2 ± 10.9‖ | 8/9 | 88.8 | |||||
| 12 | 1 | yes | 90.6 ± 14.2‖ | 5/6 | 83.3 | |||||
| 14 | 2 | no | 108.9 ± 20.3‖ | 13/14 | 92.9 | |||||
| 14 | 3 | yes | 124.3 ± 26.3‖ | 24/27 | 88.8 | |||||
| NK nonreconstituted § | ||||||||||
| 10 | 1 | no | 4.1 ± 1.9‖ | 3/4 | 75.0 | |||||
| 12 | 1 | yes | 5.0 ± 5.8‖ | 7/10 | 70.0 | |||||
| 14 | 1 | no | 1.45 ± 1.2‖ | 8/9 | 88.8 | |||||
| tgε26 | ||||||||||
| 10 | 6 | no | 1.3 ± 1.2‖ | 47/59 | 79.7 | |||||
| 12 | 4 | no | 0.2 ± 0.3‖ | 25/41 | 61.0 | |||||
| 14 | 4 | no | 1.7 ± 1.2‖ | 9/25 | 36.0 | |||||
| CD1/CBA/J | ||||||||||
| 10 | 11 | no | 134.8 ± 12.4 | 110/121 | 90.9 | |||||
| 12 | 30 | no | 161.4 ± 16.8 | 358/371 | 96.5 | |||||
| 14 | 13 | no | 229.2 ± 12.2 | 127/138 | 92.0 |
Some SCID bone marrow was pretreated with α-Thy-1 Ab before inoculation of tgε26 females.
The frequency of uNK cells, as detected by periodic acid-Schiff–reactive granules was counted using a 10 mm2 grid, at ×250 for all implantation sites, using 10 median sections per implantation site, a minimum of 2 implantation sites per pregnancy.
As assessed by splenic effector lytic cell assay against 51Cr-labeled YAC-1 targets.
P <0.01 as compared to gestational day–matched pooled CBA/J + CD1 values.
Uterine Changes in Pregnant Reconstituted tgε26.
uNK cells were present in considerable numbers in the seven NK cell reconstituted females but not in the remaining three females. Implantation sites from two immune-competent strains of mice (CD1 and CBA/J) were used to establish the parameters for normal number, size, and granularity of uNK cells on each of days 10, 12, and 14 of gestation. The frequency of uNK cells was not significantly different between CD1 and CBA/J strains at any gestational day studied. Therefore, the values from these two strains were pooled and are referred to as “control” in Table 2. In nonmanipulated tgε26, the number of uNK cells were statistically <1% of control at days 10, 12, and 14 of gestation (reference 8 and Table 2). When compared to control immune-competent females, day 10 NK cell reconstituted females had 40.2%, day 12 females had 56.1% and day 14 females had 49.8% of normal frequencies of uNK cells (P <0.01; Table 2 and Fig. 1). Many uNK cells in the reconstituted females were associated with the main decidual blood vessels (Fig. 1). In contrast, low numbers of uNK cells (1–3.1% of immune-competent females, which is also equivalent to the number of uNK cells found in untreated tgε26 females) were present in the three bone marrow infused, nonreconstituted tgε26 females (Table 2).
Figure 1.
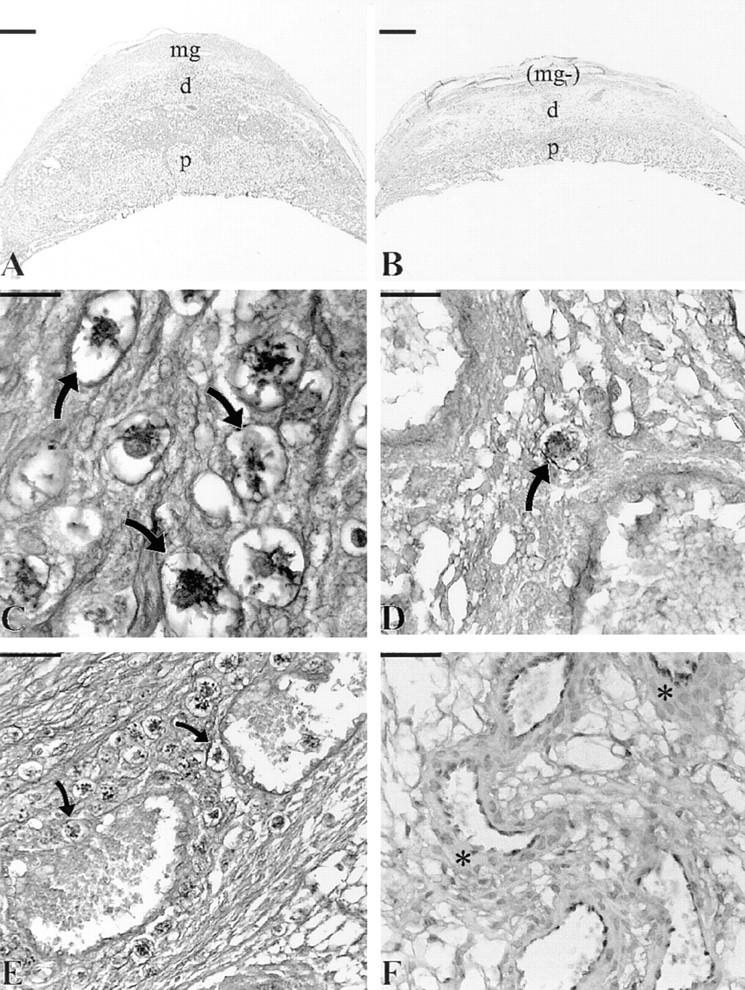
Comparison of day 12 placental morphology in NK cell– reconstituted tgε26 (A, C, and E) and tgε26 (B, D, and F) females. Placental (p) cross-sectional area measurements of NK cell–reconstituted tgε26 mice (NK+; A) were larger by 27–34% than gestational age-matched homozygous tgε26 mice (B). The NK+ females had well-developed metrial glands (mg) and higher cellularity in the decidua (d), whereas metrial gland development was absent (mg−) and the decidua was edematous in tgε26 females. When compared to control immune-competent females, NK+ females had 40–56% normal frequencies of uNK cells (arrows; C) whereas tgε26 females had 0–3% normal uNK cell frequencies (arrow; D). In the NK+ group of females, uNK cells (arrows) were frequently found surrounding decidual blood vessels and occasionally found within the vessel lumens (E). (F) demonstrates the decidual vessel anomalies found in tgε26 mice including thickened vessel walls (asterisks); A and B, bar = 1,000 μm; C and D, bar = 40 μm, E and F, bar = 100 μm; (A, B, and F) stained with hematoxylin and eosin; (C–E) stained with periodic acid– Schiff.
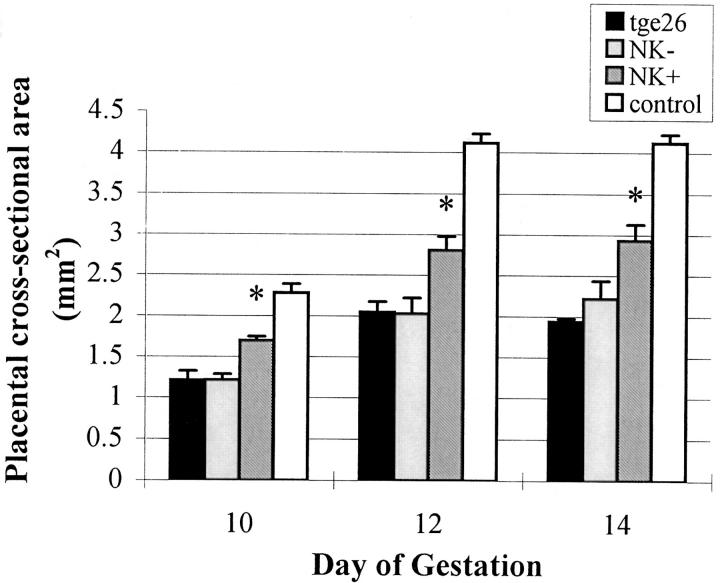
Histologically, the placentae from the NK cell reconstituted females appeared larger than those in tgε26 females and the implantation sites resembled those in immune-competent females, because they included a well developed metrial gland (Fig. 1). Reference standards for placental cross-sectional areas were obtained by measurements of CD1 and CBA/J implantation sites. Since these were not statistically different from each other at the gestational days used (10, 12, and 14), the data were pooled to provide a larger immune competent control data set than that used for control reference values in our earlier publication (8). The mean cross-sectional areas of unmanipulated tgε26 placentae were 53% of the mean at day 10 (P <0.01, n = 6 mice), 50% of the mean at day 12 (P <0.01, n = 4 mice) and 47% of the mean at day 14 (P <0.01, n = 4 mice) control placentae (Fig. 2).
Figure 2.
Placental cross-sectional area measurements of tgε26 recipients of SCID bone marrow, homozygous tgε26 and control mice. Placental cross-sectional area measurements of NK cell–reconstituted tgε26 mice (NK +) were larger by 28% at day 10, 27% at day 12, and 34% at day 14 of gestation as compared to gestational age-matched homozygous tgε26 mice (*P <0.01). Day 10, day 12, and day 14 placental cross-sectional area measurements from bone marrow–infused, nonreconstituted mice (NK−) were not statistically different than homozygous tgε26 placentae. Day 10 and day 12 placentae were measured at ×160 magnification, whereas day 14 placentae were measured at ×140 magnification.
Placental cross-sectional areas for the NK cell–reconstituted females were significantly larger than cross-sectional areas measured for time-matched homozygous tgε26 females or for SCID bone marrow–infused females who failed to show evidence for engraftment (Fig. 2). Placentae from all NK cell reconstituted tgε26 mothers (independent of the marrow graft preparation protocol) were 28% (day 10), 27% (day 12), and 34% (day 14) larger than placentae from homozygous tgε26 mice (P <0.01) but were 26% (day 10), 32% (day 12), and 29% (day 14) smaller than placentae from the immune-competent control mice (P <0.01). Days 10, 12, and 14 placental cross-sectional areas from bone marrow–infused, nonreconstituted mice were not statistically different than those from homozygous tgε26 placentae (Fig. 2). Since normal levels of uNK cells were not established in the engrafted mice, it is unclear whether or not the placental sizes achieved represent the maximum placental growth effects that can be promoted by uNK cells.
The vascular anomalies found in unmanipulated pregnant tgε26 mice may or may not underlie the limited placental growth seen in these pregnancies. In the NK cell– reconstituted tgε26 females, no major anomalies of the decidua or placental vasculature were found (Fig. 1). The lumen size in decidual vessels of the NK cell–reconstituted females was larger than that in nonreconstituted tgε26 mice and appeared to increase with gestational length. The wall/ lumen ratios were similar to those in the immune-competent controls (Tables 1 and 3), suggesting that engraftment had restored normal dilation of the decidual arterioles. This occurred in spite of the observation that uNK cell numbers were lower than in immune-competent mice. Minor variations from the immune-competent controls were found in implantation sites from only two of the seven NK cell– reconstituted females, the two females with the lowest uNK cell numbers (38.7 and 42.9% of immune-competent controls, respectively), and this suggests a redundancy in the uNK cell numbers regarding this trait. In these two females, the decidual region appeared fluid filled, the width of the myometrial smooth muscle was increased on the mesometrial side with uNK cells scattered within these smooth muscle fibers, and a small developing metrial gland was present. The trophoblast giant cell layer appeared mildly disorganized, having produced isolated islands of trophoblast giant cells, and trophoblast giant cells were found in apposition to decidual vessels. This is the location that many uNK cells occupy in mice. Blood vessels and placentae from bone marrow–infused but NK cell–nonreconstituted tgε26 females resembled those from homozygously mated, nonmanipulated tgε26 females (Tables 1 and 3).
Table 3.
Summary of Ratios of Mean Decidual Vessel Wall to Lumen Cross-sectional Area Measured at Days 10, 12, and 14 of Gestation
| Mouse strain | Day 10 of gestation (mean W/L* ± SEM) | Day 12 of gestation (mean W/L ± SEM) | Day 14 of gestation (mean W/L ± SEM) | |||
|---|---|---|---|---|---|---|
| NK+‡ | 1.27 ± 0.32§ | 0.44 ± 0.27§ | 0.68 ± 0.12§ | |||
| NK−‡ | 2.51 ± 0.41‖ | 1.21 ± 0.39¶ | 2.13 ± 0.37‖ |
W/L = vessel wall cross-sectional area/lumen cross-sectional area ratio measured at ×200 magnification.
NK− = three NK cell nonreconstituted tgε26 females; NK+ = seven NK cell reconstituted tgε26 females.
Not statistically different than CBA/J.
P <0.01 as compared to CBA/J.
P <0.1 as compared to CBA/J.
Viability of the fetuses from the NK cell–reconstituted tgε26 females was equivalent to fetal viability in immune-competent mice at all gestational days studied (Table 2). Viability of the fetuses from the single time-matched pregnancies available in bone marrow–infused, nonreconstituted females was below control levels at days 10 and 12 of gestation but, unexpectedly, equivalent to control in the one day 14 female, for which we have no explanation. Thus, reestablishment of 40–56% of uNK cells restored vasodilation in decidual arteries, promoted placental size, and promoted fetal viability.
One interpretation of the tgε26 experiments is that uNK cells downregulate vascular smooth muscle cell (VSMC) numbers during pregnancy or promote VSMC relaxation. IFN-γ inhibits proliferation of rat VSMC through the induction of nitric oxide synthase (NOS) activity which in turn generates NO (18). NO is a major vasodilator that acts on vascular smooth muscle in many sites, including the uterus (19, 20). Infusion of an inhibitor of NOSs during pregnancy in rats caused hypertension and fetal growth retardation, suggesting that a reduction in the synthesis of NO may contribute to the pathogenesis of pregnancy-induced hypertension (21). IFN-γ and iNOS are both products of mouse uNK cells (7, 22), as is TNF-α (23), another hypotensive molecule (24, 25). A number of histologists have noted a strong association of rodent uNK cells with blood vessels, and this association was reestablished in bone marrow–reconstituted tgε26 females (Fig. 1 F). It has been previously suggested that uNK cells are involved in blood pressure regulation because hypertensive rats in which an artificial deciduomata had been induced experienced a fall of blood pressure at the time uNK cells appeared and an elevation of blood pressure when uNK cells disappeared despite persistence of the deciduomata (26). However, preliminary experiments involving serial blood pressure recordings by tail cuff in four conscious pregnant tgε26 females failed to demonstrate statistically significant systemic hypertension over the first 16 d of gestation in comparison to four nonpregnant tgε26 females (Luross, J.A., and B.A. Croy, unpublished data).
Three explanations have been considered for incomplete uNK cell reconstitution in tgε26 females. First, the number of bone marrow cells or the proportion NK cell progenitors within the bone marrow suspension used for treatment in this study may not have been sufficient for full reconstitution of the uNK cell population. Since bone marrow was pooled from four donors for each recipient, this is not a strong argument. Second, the 3-wk time interval between the time of bone marrow treatment and the time of mating, in addition to the 10–14 d of pregnancy may have been too long, and thus bypassed the optimal interval (3 wk) when splenic NK cell numbers peak in tgε26 mice reconstituted with recombination activating gene 2 (RAG-2) null bone marrow (13). Third, the 5-FU pretreatment may not have displaced the recipient marrow cells sufficiently to provide an adequate niche for optimal donor marrow engraftment.
The decidual blood vessel structure and placental morphology were improved or fully restored in the seven reconstituted tgε26 mice despite their lower than normal frequency of uNK cells. Therefore, the placental size increments achieved in the NK cell–reconstituted tgε26 versus nonmanipulated tgε26 may represent the maximal growth promotion by uNK cells with other mechanisms, accounting for the deficit in size between reconstituted tgε26 and immune-competent controls. One further requirement to promote full placental growth may be immune-competence in the fetal compartment. We have reported elsewhere that embryo transfers demonstrate that unilateral fetal or maternal NK cell deficiency can reduce placental size (8).
These in vivo studies have better defined the functional role of the immune system and the role of uNK cells in the pregnant uterus in particular. They exclude T cells and NK1 T cells as essential participants in pregnancy success, supporting earlier studies of nu/nu and IL-2 null × β2m null mice (6–8). These studies identify the NK cell lineage as important for normal development of implantation sites and highlight the mesometrial decidua and vasculature as important sites for uNK cell function. These studies do not support the previously postulated function of uNK cells to limiting trophoblast cell growth and invasion. On the contrary, they show that maternal uNK cells promote placental growth and thereby the growth of the developing fetus. It remains unclear whether promotion of trophoblast growth is a direct or indirect effect.
Acknowledgments
We thank Dr. Cox Terhorst (Harvard Medical School, Boston, MA) and Dr. R.A. Phillips (Hospital for Sick Children, Toronto, Ontario, CA) for providing tgε26 and scid/scid breeding pairs, and Andrew Moore (Ontario Ministry of Agriculture, Food and Rural Affairs, Guelph, Ontario, Canada) for support of image analysis.
Footnotes
This work was financed by awards from the Natural Sciences and Engineering Research Council of Canada and the Ontario Ministry of Agriculture, Food, and Rural Affairs.
Abbreviations used in this paper: hu, human; LGL, large granular lymphocytes; NO, nitric oxide; NOS, NO synthase; u, uterine.
References
- 1.Whitelaw PF, Croy BA. Granulated lymphocytes of pregnancy. Placenta. 1996;17:533–543. doi: 10.1016/s0143-4004(96)80070-1. [DOI] [PubMed] [Google Scholar]
- 2.Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- 3.King A, Loke YW. On the nature and function of human uterine granulated lymphocytes. Immunol Today. 1991;12:432–435. doi: 10.1016/0167-5699(91)90014-K. [DOI] [PubMed] [Google Scholar]
- 4.Zheng LM, Joag SV, Parr MB, Parr EL, Young JD-E. Perforin-expressing granulated metrial gland cells in murine deciduoma. J Exp Med. 1991;174:1221–1226. doi: 10.1084/jem.174.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croy BA, Kiso Y. Granulated metrial gland cells: a natural killer cell subset of the pregnant murine uterus. Microsc Res Tech. 1993;25:189–200. doi: 10.1002/jemt.1070250302. [DOI] [PubMed] [Google Scholar]
- 6.Stewart IJ, Peel S. Granulated metrial gland cells in pregnant athymic mice. J Anat. 1980;131:217. (Abstr.) [Google Scholar]
- 7.Croy, B.A., C. Chapeau, N. Reed, I.J. Stewart, and S. Peel. 1991. Is there an essential requirement for bone marrow– derived cells at the fetomaternal interface during successful pregnancy? A study of pregnancies in immunodeficient mice. In Molecular and Cellular Immunobiology of the Maternal Fetal Interface. T.G. Wegmann, T.J. Gill III, and E. Nisbet-Brown, editors. Oxford University Press, New York. 168–188.
- 8.Guimond M-J, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in Tgε26 mice. Biol Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- 9.Croy BA, Ashkar AA, Foster RA, DiSanto JP, Magram J, Carson D, Gendler SJ, Grusby MJ, Wagner N, Müller W, Guimond M-J. Histological studies of gene-ablated mice support important functional roles for natural killer cells in the uterus during pregnancy. J Reprod Immunol. 1997;35:111–133. doi: 10.1016/s0165-0378(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Biron C, She J, Higgins K, Sunshine M-J, Lacy E, Lonberg N, Terhorst C. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3εgene. Proc Natl Acad Sci USA. 1994;91:9402–9406. doi: 10.1073/pnas.91.20.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Levelt C, Salio M, Zheng D, Sancho J, Liu C-P, She J, Huang M, Higgins K, Sunshine M-J, Eichmann K, et al. Over-expression of CD3εtransgenes blocks T lymphocyte development. Int Immunol. 1995;7:435–448. doi: 10.1093/intimm/7.3.435. [DOI] [PubMed] [Google Scholar]
- 12.Pijnenborg R, Robertson WB, Brosens I, Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2:71–91. doi: 10.1016/s0143-4004(81)80042-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Holländer GA, Nichogiannopoulou A, Simpson SJ, Orange JS, Gutierrez-Ramos J-C, Burakoff SJ, Biron CA, Terhorst C. Natural killer cell development is blocked in the context of aberrant T lymphocyte ontogeny. Int Immunol. 1996;8:939–949. doi: 10.1093/intimm/8.6.939. [DOI] [PubMed] [Google Scholar]
- 14.Holländer GA, Wang B, Nichogiannopoulou A, Platenburg PP, van Ewijk W, Burakoff SJ, Gutierrez-Ramos J-C, Terhorst C. Development control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373:350–353. doi: 10.1038/373350a0. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin MK, Keve TM. Pregnancy-induced changes in resistance blood vessels. Am J Obstet Gynecol. 1986;155:1296–1299. doi: 10.1016/0002-9378(86)90163-8. [DOI] [PubMed] [Google Scholar]
- 16.Meyers MC, Brayden JE, McLaughlin MK. Characteristics of vascular smooth muscle in the maternal resistance circulation during pregnancy in the rat. Am J Obstet Gynecol. 1993;169:1510–1516. doi: 10.1016/0002-9378(93)90427-k. [DOI] [PubMed] [Google Scholar]
- 17.Cipolla MJ, Binder ND, Osol G. Myoendometrial versus placental uterine arteries: structural, mechanical, and functional differences in late-pregnant rabbits. Am J Obstet Gynecol. 1997;177:215–221. doi: 10.1016/s0002-9378(97)70464-2. [DOI] [PubMed] [Google Scholar]
- 18.Nunokawa Y, Tanaka S. Interferon-γ inhibits proliferation of rat vascular smooth muscle cells by nitric oxide generation. Biochem Biophys Res Commun. 1992;188:409–415. doi: 10.1016/0006-291x(92)92400-r. [DOI] [PubMed] [Google Scholar]
- 19.Yallampalli C, Byam-Smith M, Nelson SO, Garfield RE. Steroid hormones modulate the production of nitric oxide and cGMP in the rat uterus. Endocrinology. 1994;134:1971–1974. doi: 10.1210/endo.134.4.8137765. [DOI] [PubMed] [Google Scholar]
- 20.Norman JE, Cameron IT. Nitric oxide in the human uterus. Reviews in Reproduction. 1996;1:61–68. doi: 10.1530/ror.0.0010061. [DOI] [PubMed] [Google Scholar]
- 21.Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol. 1993;169:1316–1320. doi: 10.1016/0002-9378(93)90299-x. [DOI] [PubMed] [Google Scholar]
- 22.Hunt JS, Miller L, Vassmer D, Croy BA. Expression of the inducible nitric oxide synthase gene in mouse uterine leukocytes and potential relationships with uterine function during pregnancy. Biol Reprod. 1997;57:827–836. doi: 10.1095/biolreprod57.4.827. [DOI] [PubMed] [Google Scholar]
- 23.Parr EL, Chen H-L, Parr MB, Hunt JS. Synthesis and granular localization of tumour necrosis factor-alpha in activated NK cells in the pregnant mouse uterus. J Reprod Immunol. 1995;28:31–40. doi: 10.1016/0165-0378(94)00905-m. [DOI] [PubMed] [Google Scholar]
- 24.Turner CR, Esser KM, Wheeldon EB, Slivjak M, Smith EF. Cardiovascular and pulmonary effects of human recombinant tumor necrosis factor in the conscious rat. Circ Shock. 1989;28:369–384. [PubMed] [Google Scholar]
- 25.Saks S, Rosenblum M. Recombinant human TNF-α: preclinical studies and results from early clinical trials. Immunol Ser. 1992;56:567–587. [PubMed] [Google Scholar]
- 26.Moore HC, Cserhati I, Wilson K. The duration of the fall of blood pressure following the induction of deciduomata and the administration of progesterone in steroid hypertensive rats. Acta Endocrinol. 1970;63:242–252. doi: 10.1530/acta.0.0630242. [DOI] [PubMed] [Google Scholar]