Abstract
Peripheral lymph nodes (PLN) are critical for immunologic memory formation in response to antigens that penetrate the skin. Blood-borne lymphocytes first encounter such antigens after they home to PLN through a multi-step adhesion process that is normally initiated by L-selectin (CD62L) in high endothelial venules (HEV). Since naive T cells can not enter PLN normally in L-selectin–deficient mice, a delayed type hypersensitivity response to cutaneously applied antigen cannot be mounted. In this study, we report that the administration of activated platelets into the systemic circulation of L-selectin knockout mice restores lymphocyte trafficking to PLN, and reconstitutes T cell–mediated immunity in response to a cutaneous antigen. These effects required platelet-expressed P-selectin that allows activated platelets to transiently form a bridge between lymphocytes and HEV, thereby enabling lymphocytes to undergo subsequent β2 integrin-dependent firm adhesion. These profound effects of platelet-mediated cell–cell interactions on lymphocyte trafficking and formation of immunologic memory may impact on a variety of autoimmune and inflammatory conditions.
Immunocompetent lymphocytes continuously patrol the body in search of foreign antigen (1). To effectively do this, a sequence of critical adhesion events that allows these cells to migrate from the circulation into tissues must occur. In peripheral lymph nodes (PLN), the interaction of the lymph node homing receptor, L-selectin (CD62L), with its counterreceptor expressed on high endothelial venules (HEV), the peripheral node addressin (PNAd), is the first essential step in the extravasation of immunologically naive lymphocytes (2–4). L-selectin–PNAd interaction mediates the tethering and rolling of most lymphocytes in HEV, but is not sufficient by itself for lymphocyte emigration (5). The rolling cells must first encounter an activating stimulus that transmits a G protein–dependent transmembrane signal and triggers stationary adhesion through functional upregulation of the β2 integrin LFA-1 (6). The importance of L-selectin as the physiologic initiator of this adhesion cascade is best exemplified by the inability of naive T cells to home to PLN in L-selectin–deficient mice (7, 8). In fact, the lack of T cell trafficking through PLN in these animals was shown to be responsible for the inability to elicit a contact hypersensitivity (CHS) immune response by cutaneous exposure to a hapten antigen (7, 9).
In addition to L-selectin, circulating activated platelets can provide a second mechanism for initiating lymphocyte interactions with the HEV of PLN (10). P-selectin (CD62P) expressed on activated platelets can mediate adhesion to both lymphocytes and HEV via interactions with P-selectin glycoprotein ligand 1 (PSGL-1) and PNAd, respectively, resulting in sustained rolling of lymphocyte–platelet aggregates even in the absence of functional L-selectin. In this study, we have set out to examine the functional significance of this platelet-mediated lymphocyte delivery to HEV. We tested whether rolling promoted by this alternative adhesion pathway is sufficient to reestablish lymphocyte trafficking in L-selectin–deficient mice and whether lymphocytes delivered in this manner to PLN were capable of participating in a CHS immune reaction. We found that lymphocytes delivered by platelets could indeed accumulate and extravasate in PLN–HEV. L-selectin–deficient lymphocytes homed to PLN via a multi-step adhesive process that was initiated by platelet P-selectin and was followed by activation of rolling lymphocytes that arrested via engagement of LFA-1. Importantly, a transfusion of activated platelets 1 d after cutaneous sensitization of L-selectin–deficient mice normalized the CHS response in these animals, suggesting that platelet-delivered T cells were immunocompetent and could efficiently detect and respond to antigen presented in PLN.
Materials and Methods
Antibodies.
Function blocking anti–murine L-selectin mAb Mel-14, anti–murine LFA-1 (CD11a) mAb TIB 213, and anti– human P-selectin mAb WAPS 12.2 were provided by Dr. E.C. Butcher (Stanford University, Stanford, CA). Anti–human CD41 mAb 7E3 was a gift of Dr. B. Coller (Mt. Sinai Medical Center, New York). FITC-conjugated mAbs to murine TCRα/β, Mac-1 (CD11b), and anti-B220, and PE-conjugated nonblocking anti– human P-selectin (mAb S12) were purchased from PharMingen (San Diego, CA) and Becton-Dickinson (San Jose, CA), respectively. Anti–human CD31 mAb Hec 7 and unlabeled anti–human P-selectin mAb S12 were gifts of Drs. W.A. Muller (The Rockefeller University, New York) and R.P. McEver (University of Oklahoma Health Sciences Center, Oklahoma City, OK), respectively. Nonbinding isotype-matched antibodies were used as negative controls.
Intravital Microscopy.
Mice were anesthetized and catheterized, and the left subiliac LN was microsurgically prepared as previously described (5). Human platelets were isolated from blood of healthy donors following established procedures (11). For microscopic visualization of endogenous white blood cell (WBC) interactions with vascular endothelium, a bolus injection of saline (10 ml/kg body weight) containing 1 mg/ml of the nuclear dye Rhodamine 6G (Molecular Probes, Inc., Eugene, OR) was given intravenously. Interacting and freely flowing blood-borne WBCs were videotaped during their passage through LN HEV under fluorescent stroboscopic epi-illumination and observation through a ×40 Zeiss objective (Achroplan, numerical aperture 0.75 ∞•, water; Carl Zeiss, Inc., Thornwood, NY). The rolling fraction, defined as the percentage of interacting WBCs in the total number of fluorescent cells passing through a venule during 3 min, was determined by off-line analysis of video recordings. The sticking fraction, defined as the percentage of rolling WBCs that became stationary for a minimum of 30 s, was determined during the same period. After an initial observation period to establish baseline interactions, some animals were injected with 3 boluses (200 μl each at 2 min intervals) of resting or TRAP (thrombin receptor activating peptide)-activated human platelets (2 × 108/ml), and WBC behavior was recorded in the same vascular bed. Some aliquots of activated platelets were pretreated with mAb WAPS12.2 (20 μg/ml) before injection. In some experiments, platelets were fluorescently labeled with 2′7′,-bis-(2-carboxyethyl)-5(and 6) carboxyfluorescein (Molecular Probes, Inc.) to distinguish WBCs and injected platelets as previously described (10). To test the role of L-selectin and LFA-1 in HEV, some animals were treated with a 300 μl bolus of saline containing 100 μg of mAb Mel-14 or mAb TIB 213, and WBC adhesion before and after injection of activated platelets was analyzed.
Flow Cytometry and Platelet-PBMC Aggregation Assay.
Murine PBMCs were isolated by density gradient centrifugation using lympholyte-M (Accurate Chemical and Science Corp., Westbury, New York), washed in PBS with 1% heat-inactivated human serum albumin (HSA) and 5 mM EDTA, pH 7.4 (PBE) and subsequently resuspended in HBSS containing 10 mM Hepes, 2 mM CaCl2, and 0.2% HSA, pH 7.4 (assay media).
Purified PBMCs (2 × 106/ml) in PBE were preincubated at 4°C for 30 min with FITC-conjugated antibodies that recognize murine Mac-1, TCR-α/β, or B220. TRAP-activated human platelets (108/ml) were labeled with nonblocking PE-conjugated mAb S12 to human P-selectin. Stained cells were washed twice and resuspended in assay media. 50 μl each of platelets and PBMCs were subsequently coincubated in microtiter wells at 4°C for 1 h. In some experiments, labeled platelets were pretreated for 30 min with 20 μg/ml P-selectin mAb WAPS 12.2 (which blocks the lectin domain) or nonblocking control mAb S12 (which recognizes the SCR domain) and subsequently mixed with the PBMC suspension without washing. The two mAbs do not interfere with each other's binding to P-selectin (Diacovo, T.Y., and U.H. von Andrian, unpublished data). Platelet adhesion to PBMCs was quantitated by measuring red and green fluorescence on a FACScan® flow cytometer (Becton Dickinson, San Jose, CA). Gating on events identified by light scatter as lymphocytes or monocytes was performed to exclude single platelets. For each experiment, a sample containing either labeled platelets mixed with unlabeled PBMCs or labeled PBMCs alone was used to set threshold markers on the red and green fluorescence scale, respectively. MAb- labeled PBMCs showing the platelet marker were interpreted as cells that bound platelets. Results are presented as percentage of platelet-bound PBMCs expressing Mac-1, TCR-α/β, or B220 in the total number of cells in the same subset.
Elicitation of CHS Response.
A cutaneous delayed type hypersensitivity immune response was induced by application of 0.5% 2,4-dinitrofluorobenzene (DNFB) in acetone/olive oil (4:1) on the shaved abdomina of mice as previously described (7). 18 h after sensitization, mice were anesthetized, the right external jugular vein was cannulated, and a total volume of 3 ml of TRAP-activated human platelets (108/ml) were administered intravenously over 6 h as repeated small boluses. Activation status of platelets was confirmed by flow cytometry of P-selectin expression. For antibody inhibition studies, platelets and mice were pretreated with function blocking antibodies (50 μg/ml and 100 μg/mouse, respectively) to human P-selectin (mAb WAPS 12.2) or human CD31 (mAb Hec 7). Mice were rechallenged with 0.25% of DNFB on the left pinna (10 μl) on day 5, and ear swelling responses were measured 24 h after elicitation. Subsequently, the animals were killed and lymphocytes pooled from cervical, subiliac, and axillary nodes were counted on a hemocytometer.
In Vitro Lymphocyte Proliferation Assay.
For analysis of antigen-specific T cells in the PLN of mice, nodes were harvested 5 d after DNFB sensitization of mice with and without infusion of platelets and antibodies as described above. Isolated lymphocytes (2 × 104–1.6 × 105 cells) were cultured for 36 h in triplicate in the presence or absence of DNBS, the water soluble form of DNFB (50 μg/ml), and then pulsed with 1 μCi/well of [3H]thymidine for 18 h before harvesting cells as previously described (7).
Statistical Methods.
Where appropriate, data are presented as the arithmetic mean ± 1 SD. A statistical analysis of the ear swelling response was performed using the Kruskal-Wallis test for one-way analysis of variance followed by multiple comparisons on ranks using the Bonferroni correction of P. Results were considered statistically significant when P <0.05.
Results
Activated Platelets Promote Lymphocyte Rolling in PLN– HEV of L-selectin–deficient Mice.
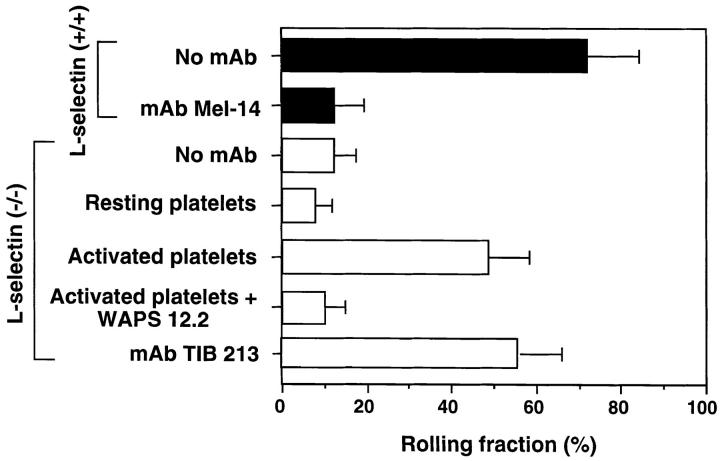
The behavior of rhodamine 6G–labeled circulating WBCs in PLN–HEV of L-selectin– deficient mice was evaluated using intravital microscopy (5). In agreement with previous studies using a neutralizing antibody to L-selectin (10), the rolling fraction of WBCs in mice lacking this homing receptor (12.1 ± 5.3%, mean ± SD) was considerably reduced in comparison to that in wild-type littermates (72.1 ± 12.1%; Figs. 1 and 2 a). Injection of activated, but not resting, human platelets enhanced leukocyte rolling in the HEV of mutant mice (rolling fraction of 48.9 ± 9.6%), approaching a level similar to that seen in wild-type mice. The pivotal role of platelet P-selectin was confirmed by the ability of mAb WAPS 12.2, directed against human, but not murine, P-selectin, to abolish this effect.
Figure 1.
Effect of circulating, activated platelets on WBCs rolling in PLN–HEV of mice deficient in L-selectin. WBCs (mononuclear cells and granulocytes) were fluorescently labeled in situ with rhodamine 6G, visualized in HEVs of PLN of wild-type (closed bars) and L-selectin–deficient (open bars) mice by fluorescence intravital microscopy, and rolling fractions quantitated as previously described (10). The effect of mAbs and platelet injections on the WBC rolling fraction was determined in identical fields of view. The L-selectin blocking mAb Mel-14 was tested in 10 venules of 4 wild-type animals. The ability of platelets to reconstitute rolling in mutant animals was examined by injecting TRAP-activated or resting, human platelets (12 venules in 5 animals). The ability of the human P-selectin mAb WAPS 12.2 and murine LFA-1 mAb TIB 213 to inhibit WBC rolling was also evaluated (four venules in three animals each). Data are shown as mean ± SD.
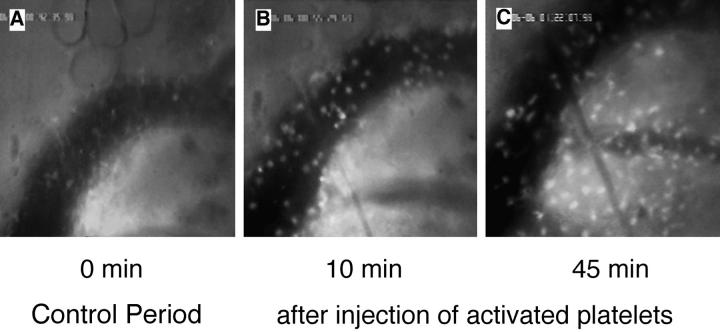
Figure 2.
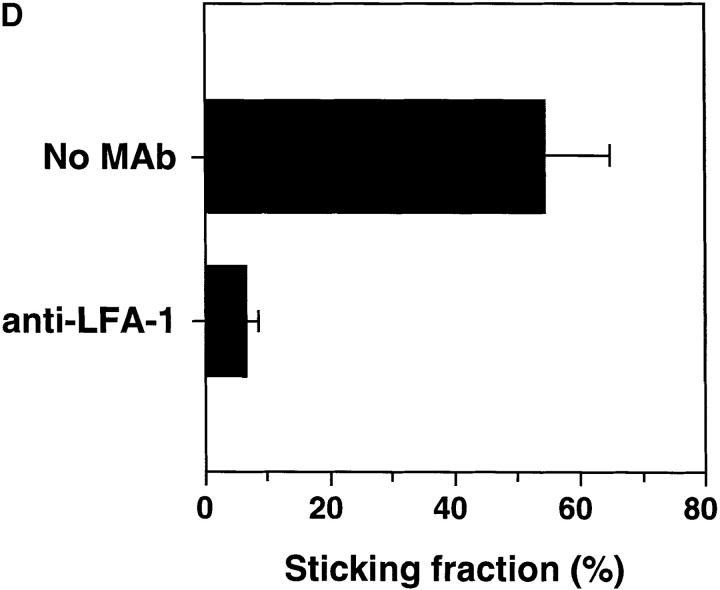
Activated human platelets support WBCs homing to subiliac PLN in L-selectin–deficient mice. WBCs were fluorescently labeled in situ using rhodamine 6G and recorded during their passage through single HEV. (A) Digitized intravital fluorescence micrograph of a typical HEV in an L-selectin–deficient mouse. Relatively few blood-borne cells (arrows) interacted with the vascular wall over a 45-min control period (0 min). (B) Intraarterial injection of TRAP-stimulated human platelets over a 10-min period resulted in rolling and accumulation of sticking endogenous WBCs (10 min). (C) A significant number of stuck cells subsequently transmigrated into the node (45 min). (D) LFA-1 (CD11a) receptor blockade abrogates firm adhesion of L-selectin–deficient WBCs delivered to HEV by platelets. The percentage of rolling cells that became firmly adherent for a mininum of 30 s (sticking fraction) was determined in the presence or absence of LFA-1 mAb TIB 213. The data shown represent the mean ± SD of seven venules in three animals.
Lymphocytes Delivered by Platelets Firmly Adhere to HEV via LFA-1.
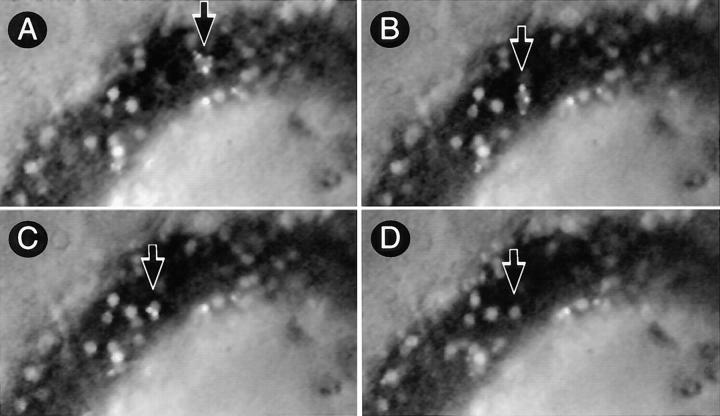
Although selectin binding to PNAd is highly efficient at initiating rolling interactions in flow, in vitro studies indicate that engagement of additional adhesion receptors is required for lymphocyte arrest in HEV (12). Indeed, recent in vivo experiments have shown that L-selectin–dependent lymphocyte rolling is followed by an activation–dependent arrest mediated by LFA-1 (6). We hypothesized that in order for L-selectin–deficient lymphocytes to accumulate in tissues where PNAd is expressed, a similar sequence of events must occur to stabilize dynamic interactions initiated by platelet P-selectin. Infusion of activated platelets in L-selectin–deficient mice not only restored rolling, but >50% of interacting WBCs became stably adherent to HEV, and a large fraction of stuck cells eventually transmigrated (Fig. 2, b and c). Since both WBCs and platelets express several integrin receptors that are capable of strengthening adhesive interactions with vascular endothelial cells, mutant mice or platelets were treated with function-blocking mAb to LFA-1 (TIB 213) or to CD41 (7E3) before platelet injection. MAb TIB 213, but not mAb 7E3, reduced leukocyte sticking by ∼90%; neither had any effect on rolling (Figs. 1 and 2 d and data not shown). Since human platelets do not express β2-integrins and TIB 213 binds only murine, but not human LFA-1 (reference 13 and our unpublished data), these results demonstrate that the sticking of WBC–platelet aggregates was mediated by LFA-1 on WBCs. Therefore, the cellular bridge between lymphocytes and HEV that was initially created by platelets allowed for a critical period of exposure of lymphocytes to chemoattractant signals on HEV that was sufficient to activate and engage LFA-1. Moreover, in intravital microscopy experiments where WBC and platelets were labeled with different fluorescent dyes, we observed that platelets frequently returned to the circulation after the WBCs they had delivered became firmly adherent on the surface of an HEV (Fig. 3). This suggests that platelets may be able to participate repeatedly in the recruitment of leukocytes.
Figure 3.
Lymphocyte delivery to PLN of L-selectin–deficient mice does not lead to platelet accumulation in HEV. The micrographs show a segment of the same HEV as in Fig. 2 (blood flow from right to left) 10 min after injection of activated human platelets. (A) A rolling aggregate of bright 2′7′-bis-(2-carboxyethyl)-5(and 6) carboxyfluorescein– labeled platelets with a fainter rhodamine 6G labeled WBC in their midst can be seen (arrow). (B) 2 s later, the platelet–WBC aggregate has arrested firmly. Labeled platelets are initially distributed randomly on the surface of the stuck cell. (C) Within 5 s after the WBC had become stuck, platelets roll slowly downstream across the body of the adherent cell and eventually detach. (D) 30 s later, all platelets have detached and returned to the blood stream, whereas the WBC remains stuck at the HEV surface.
Activated Platelets Bind to Murine PBMC In Vitro.
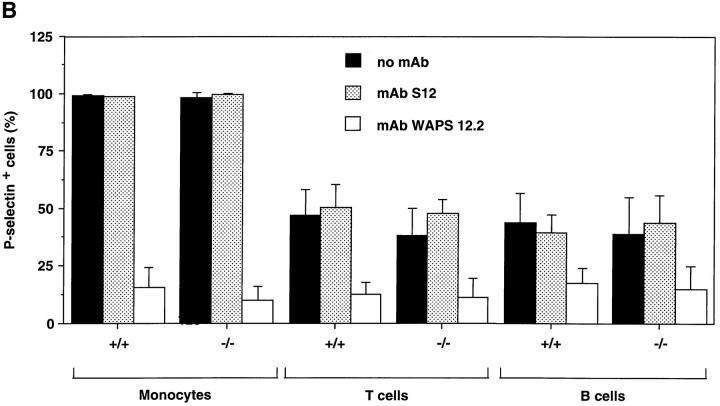
Although in situ labeling of circulating WBCs with rhodamine 6G allows adequate visualization of nucleated cells in HEV, it does not enable us to differentiate between the various leukocyte populations that may participate in antigen-specific immune responses. To identify the specific subsets of murine lymphocytes capable of interacting with human platelets, PBMCs from wild-type or L-selectin–deficient mice were preincubated with fluoresceinated mAbs detecting specific antigens for either α/β T cells or B cells. Cells were then incubated with activated human platelets prelabeled with a nonblocking PE-conjugated P-selectin antibody, and platelet adhesion was quantified using two-color flow cytometry (Fig. 4 a). For reference, platelet binding to monocytes was also analyzed. Approximately half of all B and T cells from both wild-type and L-selectin–deficient mice formed aggregates with activated platelets (Fig. 4 b). Over 98% of monocytes from either genotype also interacted with platelets. The critical role of P-selectin in mediating these interactions was determined by the ability of mAb WAPS 12.2, but not by the nonblocking P-selectin mAb S12, to significantly reduce the percentage of all PBMC subsets bound by platelets. These data suggest that a substantial fraction of circulating B and T lymphocytes can associate with activated platelets in the blood stream.
Figure 4.
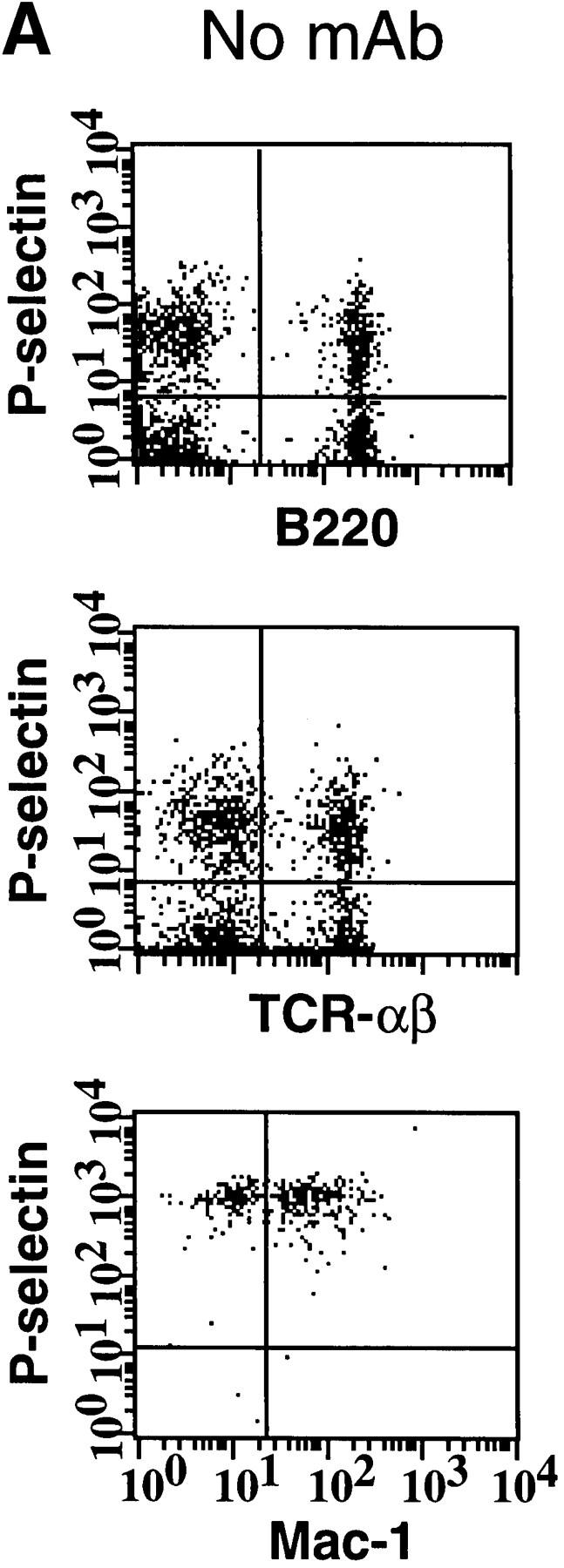
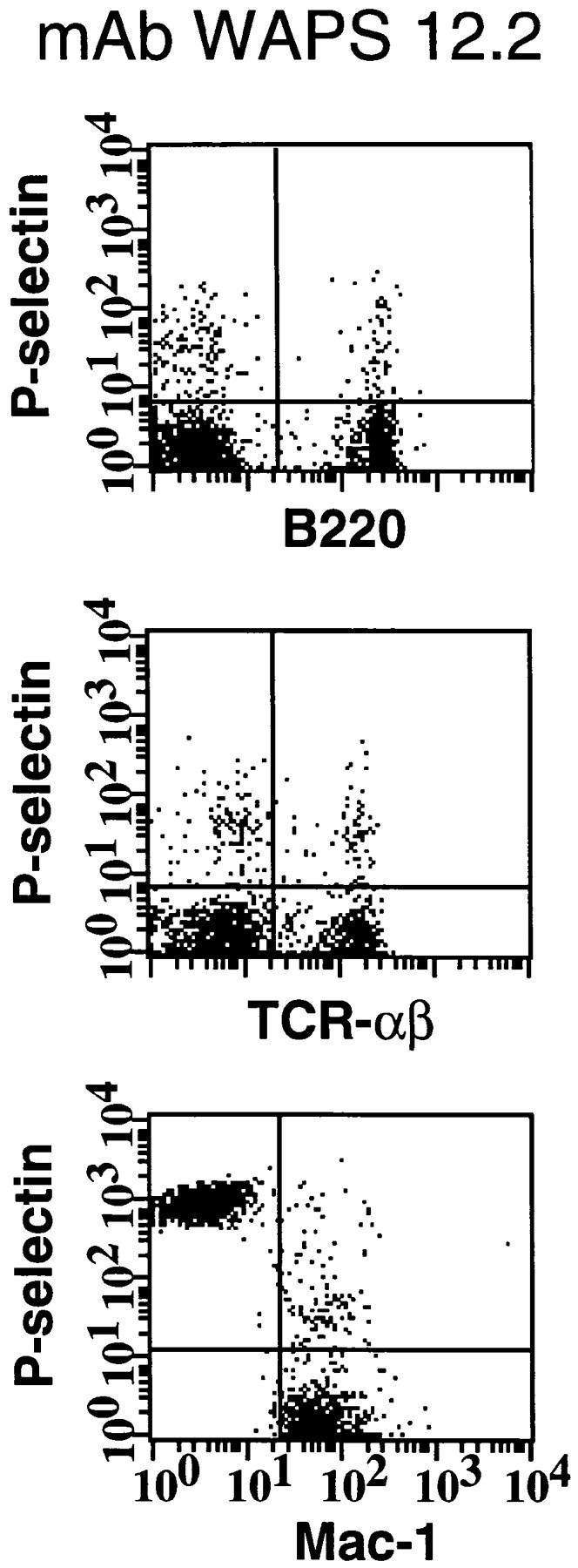
Activated human platelets bind to murine lymphocytes in suspension. Freshly isolated murine PBMCs were stained with FITC-conjugated antibodies to TCR-α/β, B220, and Mac-1 to identify T cells, B cells, and monocytes, respectively. Activated human platelets were stained with PE-conjugated nonblocking antibody to P-selectin and then mixed with labeled PBMCs. (A) Identification of PBMC subsets from L-selectin–deficient mice capable of aggregating with platelets as determined by two-color flow cytometry. The quadrant markers were drawn based on appropriate negative controls after gating separately for lymphocytes and monocytes. Double positive cells (upper right quadrant) represent the members of each subset capable of platelet binding. Events in the monocyte gate that show positive staining for P-selectin, but not Mac-1, are platelet aggregates (upper left quadrant). P-selectin–dependent adhesion was evaluated by treating platelets with mAb WAPS 12.2 before and during incubation with PBMCs. Similar results were obtained in wild-type mice. (B) Percentage of monocytes, T cells, and B cells of wild-type (+/+) and L-selectin-deficient (−/−) donor mice that interacted with activated human platelets. The effect of P-selectin blocking mAb WAPS 12.2 (open bar) and nonblocking mAb S12 (dotted bars) was evaluated as described in Materials and Methods. Bars reflect mean ± SD of four independent experiments.
Circulating Activated Platelets Reconstitute the CHS Response in L-selectin–deficient Mice.
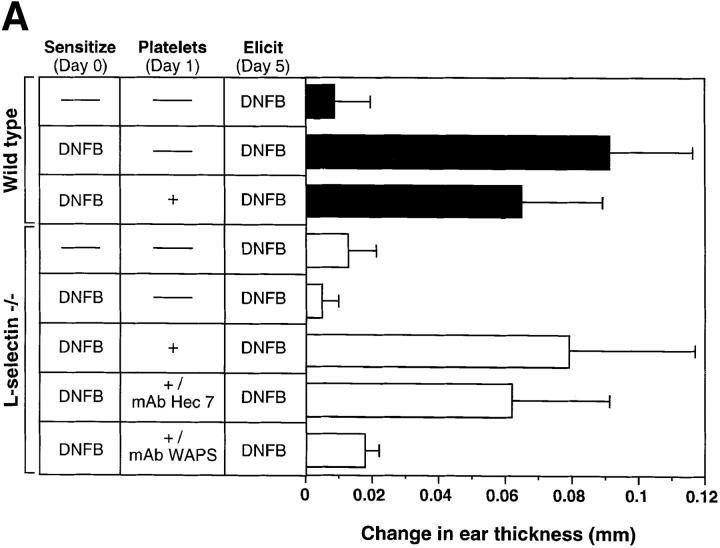
Our results indicate that lymphocytes can use the platelet delivery mechanism to enter the LN interstitium where they may encounter their cognate antigen. Since this intravascular adhesion cascade does not mandate a contribution by L-selectin, we tested whether activated platelets in the circulation of L-selectin–deficient mice can reconstitute the defect in CHS that has been previously described in these animals (7, 9). To address this issue, the abdominal skin of mutant mice was exposed to DNFB. Topically applied antigens are known to be taken up primarily by dermal Langerhans' cells which then migrate to draining PLN where they present antigen to homed lymphocytes. Our analysis has indicated that a peak in the number of Langerhans' cells is achieved 18–24 h after sensitization (Catalina, M.D., and M.H. Siegelman, unpublished data). To ensure optimal lymphocyte delivery to PLN, mutant mice were repeatedly injected during this 6 h time interval with a total number of 3 × 108 activated platelets.
When ears of sham-treated mutant mice were rechallenged with DNFB 5 d after sensitization, there was no detectable CHS response (Fig. 5 a). Moreover, activated platelet treatment of sensitized wild-type mice did not enhance the CHS response, which, in fact, was slightly lower in this group (P <0.05 versus sensitized wild-type without platelets). In contrast, a dramatic CHS immune reaction was elicited in sensitized mutant animals that had received activated platelets. The extent of ear swelling elicited with DNFB in platelet-treated L-selectin–deficient mice was not different from that elicited in sensitized wild-type mice irrespective of whether or not the latter had received activated platelets. Pretreatment with P-selectin function-blocking mAb WAPS 12.2, but not control mAb Hec 7 to the human platelet antigen CD31, abolished the CHS response in L-selectin–deficient mice. Since WAPS 12.2 neutralizes human, but not murine, P-selectin, its inhibitory effect was most likely due to blocking of platelet-mediated lymphocyte delivery to PLN during the sensitization phase, and not a result of the inhibition of endothelial P-selectin– mediated trafficking of effector cells during the elicitation phase.
Figure 5.
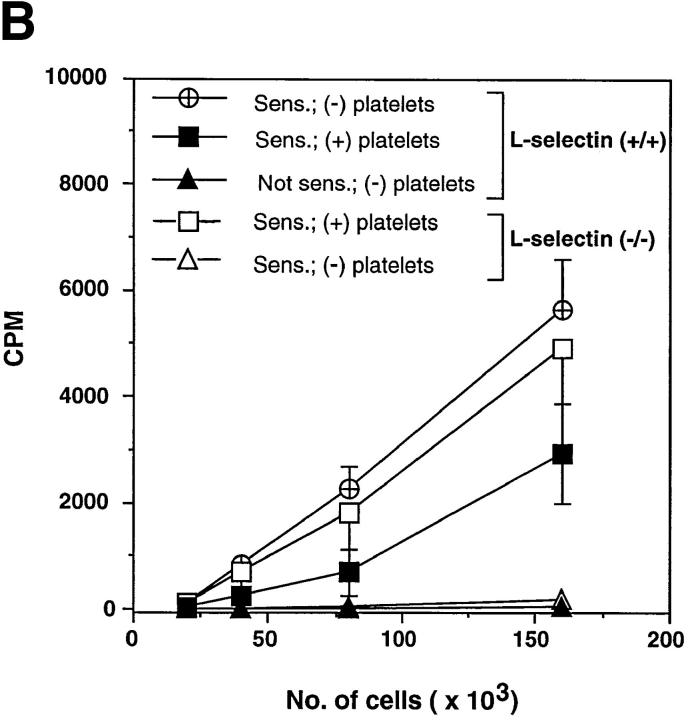
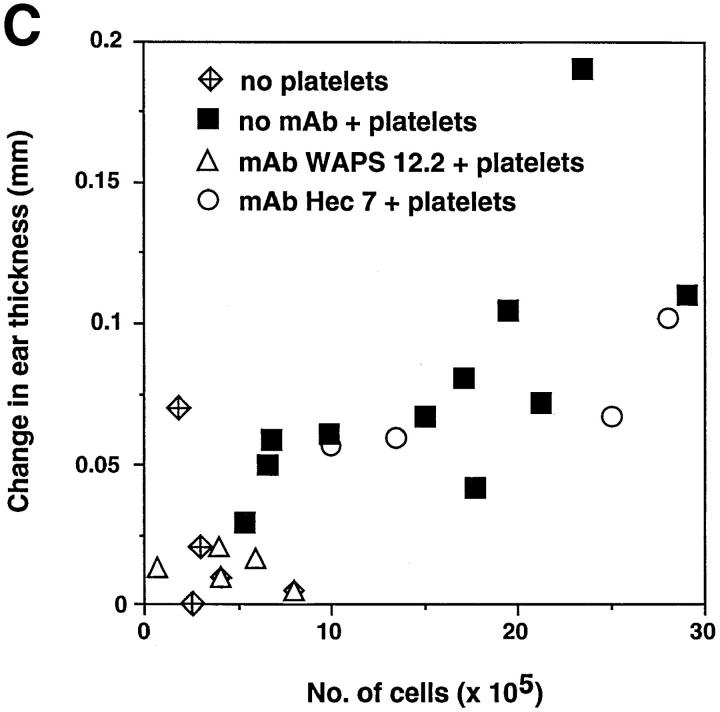
Circulating, activated platelets reconstitute CHS response in L-selectin–deficient mice. (A) Mice were sensitized to DNFB on day 0. CHS was elicited at day 5, and the subsequent ear swelling response was measured 24 h later. 18 h after sensitization, some animals were injected intravenously with TRAP-activated human platelets (3 × 108 in 3 ml over 6 h). Some L-selectin−/− animals were also treated with either P-selectin mAb WAPS 12.2 or CD31 mAb Hec 7. There was no significant difference between the response of L-selectin–deficient mice and wild-type mice which were elicited without earlier sensitization (P >0.05). Ear swelling in sensitized wild-type animals with or without platelet infusion was highly significant (P <0.001 versus elicit only). In sensitized L-selectin−/− mice, elicitation alone had no effect, whereas mutant mice that also received activated platelets displayed a dramatic swelling response (P <0.001) that was not different from that in sensitized wild-type animals with or without platelet treatment (P >0.05 versus both). MAb Hec 7 had no significant effect (P >0.05 versus sensitized L-selectin−/− receiving activated platelets only), whereas coinjection of platelets and MAb WAPS12.2 abolished the delayed type hypersensitivity effect (P >0.05 versus both L-selectin−/− groups without platelet infusion; P <0.001 versus sensitized, platelet-treated L-selectin−/− animals; P <0.01 versus sensitized, platelet plus MAb Hec 7–treated L-selectin−/− animals). Data shown are mean ± SD from 4 to 11 mice in each group (5 measurements per ear before and after elicitation). (B) Antigen–specific T cells are present in PLN of L-selectin–deficient mice only after treatment with activated platelets. Cells from mice sensitized 5 d earlier with DNFB were cultured for 36 h in the presence of DNBS after which the cultures were pulsed with [3H]thymidine for 18 h. Each point represents the mean ± SD of three separate mouse groups, with each group consisting of pooled PLN from two mice. Each group was done in triplicate. Background proliferation was subtracted as previously described (7). (C) Magnitude of CHS response in L-selectin–deficient mice receiving activated platelets correlates with the number of resident lymphocytes in PLN. After eliciting a CHS response, six PLN per mouse (subiliac, axillary, and cervical) were harvested and the number of resident cells was counted and correlated to the extent of the ear swelling. PLN were obtained from mice which had received no platelets (n = 5), activated platelets (n = 11), activated platelets pretreated with control mAb Hec 7 (n = 4), or activated platelets pretreated with mAb WAPS 12.2 (n = 5).
Antigen-specific T Cells Reside in the PLN of Platelet-treated L-selectin–deficient Mice.
To address whether the effect of activated platelets on the CHS response was in the afferent phase of the response, the presence of antigen-specific T cells within draining PLN of sensitized mice was determined by the ability of DNBS to induce in vitro lymphocyte proliferation (Fig. 5 b). LN T cells isolated from sensitized platelet-treated mutant mice proliferated in response to the antigen comparably to those of wild-type animals, whereas T cells from sensitized untreated L-selectin–deficient animals did not proliferate at all. Furthermore, DNFB-reactive T cells resided overwhelmingly in the PLN of both L-selectin–deficient and wild-type mice after contact sensitization, as no detectable proliferation was found in either mesenteric LNs or spleens of these animals (reference 7 and data not shown). The magnitude of the CHS response in platelet-treated mutant animals directly correlated with the number of resident lymphocytes in PLN, suggesting that the number of antigen-specific T cell clones generated during the sensitization phase determined the extent of the cutaneous reaction (Fig. 5 c).
Discussion
The observation that circulating, activated platelets can promote not only adhesion of lymphocytes to HEV but also reconstitute cell-mediated immunity in L-selectin– deficient mice is important in several ways. It emphasizes the mandatory requirement for lymphocyte localization to PLN in a T cell–dependent immune response to a cutaneous antigen challenge. Moreover, it shows that L-selectin per se is not the exclusive mechanism for lymphocyte homing to PLN, at least in situations that lead to platelet activation in the blood stream. Platelets, through surface P-selectin, can provide a significant compensatory mechanism for initiating lymphocyte–HEV interactions without perturbing subsequent adhesion steps or later events such as antigen recognition or lymphocyte migration and differentiation, which are requisite for activation and clonal expansion of immunocompetent T cells.
Our intravital microscopy observations of PLN–HEV in L-selectin–deficient mice also suggest a second constitutive adhesion pathway that can mediate rolling and perhaps lymphocyte recruitment to HEV. Although impaired in their ability to interact with HEV, a minority of circulating L-selectin–deficient WBCs (∼12%) were still capable of rolling, even when no activated platelets were injected. This low level interaction was also observed in wild-type mice treated with an L-selectin function-blocking antibody (10). Interestingly, treatment of L-selectin–deficient animals with a P-selectin function-blocking antibody reduced these residual lymphocyte–HEV interactions by ∼75% (Diacovo, T.G., and U.H. von Andrian, unpublished data). However, it remains to be determined whether this L-selectin–independent component of rolling is mediated by endogenous P-selectin expressed on platelets, on endothelial cells, or on both.
Recent intravital microscopy studies of PLN–HEV in wild-type mice have shown that stationary adhesion of lymphocytes requires that L-selectin–mediated rolling is followed by a G protein–coupled transmembrane signal that results in the upregulation and subsequent engagement of LFA-1 (6). Consistent with these observations, our present results indicate that lymphocytes that become tethered to HEV by platelets must also activate LFA-1 before they can arrest. Although the precise mechanism(s) that control(s) the physiologic modulation of LFA-1 activity remain(s) to be defined, previous in vitro studies have implicated a role for L-selectin (14–17). In fact, cross-linking of L-selectin on naive, but not memory, T cells with either antibodies or the HEV-derived L-selectin ligand GlyCAM-1, has been reported to promote binding of LFA-1 to one of its counterreceptors, ICAM (intercellular adhesion molecule)-1 (17). Signal transduction through PSGL-1 has been suggested to mediate similar interactions in nonlymphoid leukocytes (18–20). However, platelet-mediated cross-linking of PSGL-1 on T cells did not appear to directly induce integrin activation; platelets bound to purified human peripheral blood T cells failed to convert rolling interactions to firm adherence on PNAd coimmobilized with ICAM-1 in flow assays (Diacovo, T.G., and U.H. von Andrian, unpublished data). Similarly, surface-adherent platelet monolayers supported P-selectin–dependent T cell rolling, but not firm adhesion, which has been shown to occur during platelet interactions with neutrophils (21, 22). Thus, the predominant contribution of platelets to lymphocyte trafficking appears to be the facilitation of lymphocyte margination. Although our data do not exclude more subtle platelet-mediated effects that could potentially affect integrin function on lymphocytes, it appears likely that additional direct communication events between the passively tethered lymphocyte and the vascular wall must occur to initiate the necessary downstream events that are required for lymphocyte accumulation in lymphoid or chronically inflamed tissues.
Our results demonstrate that activated platelets can have a profound effect on the formation of immunologic memory because they can significantly impact on the trafficking pattern of lymphocytes. In particular, activated platelets are likely to affect the migratory behavior of lymphocyte subsets that express little or no L-selectin. This would include populations such as activated and/or memory T cells, which play a vital role in the initiation and maintenance of inflammatory conditions. It is interesting in this context that Th1, but not Th2, cells were recently found to acquire the ability to interact with P-selectin, and this differential ability was shown to be responsible for the preferential recruitment of Th1 cells into inflamed skin and joints (23). However, it appears that P-selectin binding activity is probably not restricted to this specific subset of T cells, as our studies indicate that a much larger proportion (40–50%) of both T and B cells can bind activated platelets, at least in the murine system.
In humans, platelets may not only participate in hemostasis but may similarly modulate immune function by virtue of their ability to interact with both vascular endothelium and circulating leukocytes. This dual function of platelets may contribute to vascular pathology associated with chronic inflammation. For instance, it is conceivable that platelets may play a role in exacerbations of inflammatory bowel disease, a pathological condition associated with an increase in activated platelets in the systemic circulation (24), and endothelial cell expression of PNAd in inflamed tissues (25). Circulating, activated platelets may not only occlude the intestinal microvasculature resulting in tissue infarction, but also alter the homing pattern of T cells that play a pivotal role in the pathogenesis of this and other chronic inflammatory diseases (26–28).
Clinical studies suggest that intravascular platelet activation may be a frequent occurrence in other settings as well. For instance, elevated numbers of P-selectin–expressing platelets have been detected in patients with diabetes mellitus, and in healthy subjects at increased risk for developing insulin-dependent diabetes mellitus (29, 30). In these settings as well as in inflammatory bowel disease, activated platelets may be encountered in the blood stream for extended periods of time. However, our experiments indicate that even a single episode during which large numbers of activated platelets are present in the systemic circulation can affect lymphocyte trafficking. The total number of platelets that were infused into L-selectin–deficient mice during a 6-h period corresponded to ∼40–50% of the circulating platelet pool in each animal. This dose of activated platelets may be clinically relevant since similar levels may be achieved as a result of platelet transfusions to individuals with thrombocytopenia. At the time of transfusion, 15–50% of platelets stored under blood bank conditions have been reported to express P-selectin, depending on the method of isolation and concentration in the transfusate (31, 32). Acute activation of circulating platelets can also occur as a consequence of surgical procedures requiring cardiopulmonary bypass or hemodialysis with bioincompatible filter membranes (33, 34). Thus, our studies simulate an extremely common clinical condition that has not been examined so far with regards to its consequences for a patient's immune system.
Acknowledgments
We wish to thank Drs. E.C. Butcher, B. Coller, R.P. McEver, and W. Muller for supplying antibodies.
This work was supported by National Institutes of Health grants K08 HL-03361 (to T.G. Diacovo); HL-54936, HL-48675, and HL-56949 (to U.H. von Andrian); and CA-56746 and HL-56746 (to M.H. Siegelman); and a grant from the Arthritis Foundation (to M.H. Siegelman). M.H. Siegelman is an Established Investigator of the American Heart Association.
Footnotes
Address corrrespondence to Dr. Ulrich H. von Andrian, The Center for Blood Research, 200 Longwood Ave., Boston, MA 02115. Phone: 617-278-3130; Fax: 617-278-3190; E-mail: uva@cbr.med.harvard.edu
M.H. Siegelman and U.H. von Andrian contributed equally to this work.
Abbreviations used in this paper: CHS, contact hypersensitivity; DNBS, 2,4-dinitro-benzene sulfonic acid; DNFB, 2,4-dinitrofluorobenzene; HEV, high endothelial venules; PLN, peripheral lymph nodes; PNAd, peripheral node addressin; PSGL-1, P-selectin glycoprotein ligand 1; TRAP, thrombin receptor-activating peptide; WBC, white blood cell.
References
- 1.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 2.Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 3.Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai Y, Singer MS, Fennie C, Lasky LA, Rosen SD. Identification of a carbohydrate based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991;113:1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Andrian UH. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation. 1996;3:287–300. doi: 10.3109/10739689609148303. [DOI] [PubMed] [Google Scholar]
- 6.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalina MD, Carroll MC, Arizpe H, Takashima A, Estess P, Siegelman MH. The route of antigen entry determines the requirement for L-selectin during immune responses. J Exp Med. 1996;184:2341–2351. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbonès ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin–deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 9.Tedder TF, Steeber DA, Pizcueta P. L-selectin–deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- 11.Diacovo TG, de Fougerolles AR, Bainton DF, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule-2. J Clin Invest. 1994;94:1243–1251. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence MB, Berg EL, Butcher EC, Springer TA. Rolling of lymphocytes and neutrophils on peripheral node addressin and subsequent arrest on ICAM-1 in shear flow. Eur J Immunol. 1995;25:1025–1031. doi: 10.1002/eji.1830250425. [DOI] [PubMed] [Google Scholar]
- 13.McCaffery PJ, Berridge MV. Expression of the leukocyte functional molecule (LFA-1) on mouse platelets. Blood. 1986;67:1757–1764. [PubMed] [Google Scholar]
- 14.Laudanna C, Constantin G, Baron P, Scarpini E, Scarlato G, Cabrini G, Dechecchi C. Sulfatides trigger increase of cytosolic free calcium and enhanced expression of tumor necrosis factor-α and interleukin-8 mRNA in human neutrophils. J Biol Chem. 1994;269:4021–4026. [PubMed] [Google Scholar]
- 15.Waddell TK, Fialkow L, Chen CK, Kishimoto TK, Downey GP. Potentiation of the oxidative burst of human neutrophils: a signaling role for L-selectin. J Biol Chem. 1994;269:18485–18491. [PubMed] [Google Scholar]
- 16.Brenner B, Gulbins E, Schlottmann K, Koppenhoefer U, Busch GL, Walzog B, Steinhausen M, Coggeshall KM, Linderkamp O, Lang F. L-selectin activates the Ras pathway via the tyrosine kinase p56lck. Proc Natl Acad Sci USA. 1996;93:15376–15381. doi: 10.1073/pnas.93.26.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang ST, Singer MS, Giblin PA, Yednock TA, Bacon KB, Simon SI, Rosen SD. GlyCam-1, a physiologic ligand for L-selectin, activates β2 integrins on naive peripheral lymphocytes. J Exp Med. 1996;184:1343–1348. doi: 10.1084/jem.184.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata K, Tsuji T, Todoroki N, Katagiri Y, Tanoue K, Yamazaki H, Hanai N. Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62) J Immunol. 1993;151:3267–3273. [PubMed] [Google Scholar]
- 19.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, Furie B P-selectin induces the expression of tissue factor on monocytes Proc Natl Acad Sci USA. 1994;91:8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the β2-integrin CD11b/CD18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- 22.Yeo EL, Sheppard J, Feuerstein IA. Role for P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model) Blood. 1994;83:2498–2507. [PubMed] [Google Scholar]
- 23.Astrup FD, Vestweber D, Borges E, Lohnig M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 24.Collins CE, Cahill MR, Newland AC, Rampton DS. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106:840–845. doi: 10.1016/0016-5085(94)90741-2. [DOI] [PubMed] [Google Scholar]
- 25.Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ. The human peripheral lymph node vascular addressin: an inducible endothelial antigen involved in lymphocyte homing. Am J Pathol. 1993;143:1688–1698. [PMC free article] [PubMed] [Google Scholar]
- 26.Christopher SP, Andreas C, Turner JR, Saubermann LJ, Stevens AC, Bodinaku K, Elson CO, Balk SP, Blumberg RS. Persistent clonal expansion of peripheral blood CD4+lymphocytes in chronic inflammatory bowel disease. J Immunol. 1996;157:3183–3191. [PubMed] [Google Scholar]
- 27.Konttinen YT, Bergroth V, Nordstrom D, Segerberg-Konttinen M, Seppala K, Salaspuro M. Lymphocyte activation in vivo in the intestinal mucosa of patients with Crohn's disease. J Clin Lab Immunol. 1987;22:59–63. [PubMed] [Google Scholar]
- 28.Sauer D, Sauer H, Fiebig H. Circulating lymphocyte subpopulations in patients with Crohn's disease. Gastroenterology. 1991;51:27–32. [PubMed] [Google Scholar]
- 29.Tschoepe D, Roesen P, Esser J, Schwippert B, Nieuwenhuis HK, Kehrel B, Gries FA. Large platelets circulate in an activated state in diabetes mellitus. Semin Thromb Hemostasis. 1991;17:433–438. doi: 10.1055/s-2007-1002650. [DOI] [PubMed] [Google Scholar]
- 30.Tschoepe D, Driesch E, Schwippert B, Lampeter EF. Activated platelets in subjects at increased risk of IDDM. Diabetologia. 1997;40:573–577. doi: 10.1007/s001250050717. [DOI] [PubMed] [Google Scholar]
- 31.Fijhneer R, Modderman PW, Veldman H. Detection of platelet activation with monoclonal antibodies and flow cytometry: changes during platelet storage. Transfusion. 1990;30:20–25. doi: 10.1046/j.1537-2995.1990.30190117623.x. [DOI] [PubMed] [Google Scholar]
- 32.Rinder HM, Murphy M, Mitchell JG, Stocks J, Aly KA, Hillman RS. Progressive platelet activation with storage: evidence for shortened survival of activated platelets after transfusion. Transfusion. 1991;31:409–414. doi: 10.1046/j.1537-2995.1991.31591263195.x. [DOI] [PubMed] [Google Scholar]
- 33.Rinder CS, Bonan JL, Rinder HM, Mathew J, Hines R, Smith BR. Cardiopulmonary bypass induces leukocyte–platelet adhesion. Blood. 1992;79:1201–1205. [PubMed] [Google Scholar]
- 34.Gawaz MP, Mujais SK, Schmidt B, Gurland HJ. Platelet-leukocyte aggregation during hemodialysis. Kidney Int. 1994;46:489–495. doi: 10.1038/ki.1994.299. [DOI] [PubMed] [Google Scholar]