Abstract
A growing number of human tumor antigens have been described that can be recognized by cytotoxic T lymphocytes (CTLs) in a major histocompatibility complex (MHC) class I–restricted fashion. Serological screening of cDNA expression libraries, SEREX, has recently been shown to provide another route for defining immunogenic human tumor antigens. The detection of antibody responses against known CTL-defined tumor antigens, e.g., MAGE-1 and tyrosinase, raised the question whether antibody and CTL responses against a defined tumor antigen can occur simultaneously in a single patient. In this paper, we report on a melanoma patient with a high-titer antibody response against the “cancer–testis” antigen NY-ESO-1. Concurrently, a strong MHC class I–restricted CTL reactivity against the autologous NY-ESO-1–positive tumor cell line was found. A stable CTL line (NW38-IVS-1) was established from this patient that reacted with autologous melanoma cells and with allogeneic human histocompatibility leukocyte antigen (HLA)-A2−, NY-ESO-1–positive, but not NY-ESO-1–negative, melanoma cells. Screening of NY-ESO-1 transfectants with NW38-IVS-1 revealed NY-ESO-1 as the relevant CTL target presented by HLA-A2. Computer calculation identified 26 peptides with HLA-A2–binding motifs encoded by NY-ESO-1. Of these, three peptides were efficiently recognized by NW38-IVS-1. Thus, we show that antigen-specific humoral and cellular immune responses against human tumor antigens may occur simultaneously. In addition, our analysis provides a general strategy for identifying the CTL-recognizing peptides of tumor antigens initially defined by autologous antibody.
There is growing evidence for humoral and cellular immune recognition of cancer by the autologous human host (1–6). Based on CTL-dependent lysis of cultured melanoma cell lines, several categories of autoimmunogenic tumor antigens have been characterized, including differentiation antigens of specific cell lineages (7–9), individual antigens caused by point mutations (10, 11), and tumor antigens, such as MAGE, which are expressed in a variable proportion of different tumor types, but are silent in most normal tissues except the testis (12). CTL responses against melanoma antigens induced by peptide vaccines in vivo have been associated with a favorable development of advanced melanoma in some patients (6, 13). As immunoselection of antigen-negative tumor cell variants has been observed during peptide vaccination (14), the molecular characterization of additional CTL-defined tumor antigens is needed to develop polyvalent vaccines with broader immunotherapeutic effects.
Sahin et al. have recently introduced a powerful new methodology for identifying human tumor antigens eliciting humoral immune response (5). The method has been called SEREX, for serological expression cloning of recombinant cDNA libraries of human tumors. Novel and previously defined tumor antigens have been identified by the SEREX method, including MAGE-1 and tyrosinase, both originally identified by cloning the epitopes recognized by CTLs. Thus, antibody screening of cDNA libraries prepared from human tumors can be used to identify antigens eliciting a cellular immune response, including CTLs, circumventing the need for established cultured autologous cell lines and stable CTL lines.
We have recently identified a novel human tumor antigen by SEREX analysis of a human esophageal cancer (15). The antigen, NY-ESO-1, belongs to a growing number of human tumor antigens we have called “cancer–testis” antigens that include MAGE, GAGE, BAGE (1), and SSX2 (HOM-MEL-40) (5, 16). These antigens have the following characteristics: (a) they are expressed in a variable portion of a wide range of cancers, (b) their normal tissue expression is generally restricted to the testis, and (c) they are generally coded for by genes on the X chromosome. In a recent survey of sera from normal individuals and cancer patients, antibodies against NY-ESO-1 were found in ∼10% of patients with melanoma, ovarian cancer, and other cancers, but not in normal individuals (Stockert, E., manuscript in preparation). One patient with a high NY-ESO-1 antibody response was found to have specific CTL reactivity against cultured autologous melanoma cells. In the present study, we report that NY-ESO-1 encodes the CTL target in this patient and identify the NY-ESO-1 peptides that are recognized.
Materials and Methods
Patient.
The 81-yr-old female patient NW38 was diagnosed with malignant melanoma involving her right thigh in 1993. After resection of the primary tumor, she developed metastatic disease in inguinal, parailiacal, and paraaortic lymph nodes. Disease progression was observed in the right inguinal region, leading to a necrotising tumor metastases of >9 cm in diameter. A transcutaneous needle biopsy was obtained from this area in 1995 to confirm the diagnosis of melanoma and to establish the tumor cell line NW-MEL-38. The patient typed HLA-A1 and -A2 positive. PBLs were collected, and a 6-wk course of chemotherapy with vindesin was given. Serum was collected repeatedly before and during chemotherapy. The patient died of tumor progression 26 wk after the start of chemotherapy, without evidence of brain metastasis.
Cell Cultures.
Melanoma cell line NW-MEL-38 was established in our laboratory (Krankenhaus Nordwest, Frankfurt, Germany) from a lymph node metastasis of patient NW38. Cell lines designated MZ have been established in our former laboratory (Johannes Gutenberg Universität, Mainz, Germany) and cell lines designated SK were from the tumor cell bank at the Ludwig Institute for Cancer Research, (New York). Cells were cultured in DME (GIBCO, BRL, Gaithersburg, MD) containing 10 mM Hepes buffer, l-arginine (84 mg/liter), l-glutamine (584 mg/liter), penicillin (10 IU/ml), streptomycin (100 μg/ml), and 10% FCS. EBV-transformed B lymphocytes, MZ1257-EBV, used as feeder cells in the mixed lymphocyte tumor cell culture, and the mutant cell line CEM × 721.174.T2 (T2) were maintained in RPMI 1640 medium supplemented with 10 mM Hepes, l-arginine (242 mg/liter), l-asparagine (50 mg/liter), l-glutamine (300 mg/liter), penicillin (10 IU/ml), streptomycin (100 μg/ml), 1% non-essential amino acids, and 10% FCS.
Mixed lymphocyte tumor cell cultures of PBLs and the autologous tumor cell line from patient NW38 were performed as previously described (17). The stable CTL line NW38-IVS-1 was maintained by weekly restimulation of 2–3 × 105 CTLs with 5 × 104 autologous melanoma cells and 2 × 105 MZ1257-EBV cells as feeders.
Reverse Transcriptase PCR Analysis of NY-ESO-1 Expression.
Total RNA was isolated from tumor samples frozen in liquid nitrogen and from melanoma cell lines using the RNeasy kit (Qiagen, Chatsworth, CA), according to the instructions of the manufacturer. 2 μg of each sample were subjected to cDNA synthesis using the Ready-To-Go first strand synthesis kit (Pharmacia Biotech, Piscataway, NJ). PCR was subsequently performed to analyze the expression of NY-ESO-1. Primers were: ESO1A, 5′-CACACAGGATCCATGGATGCTGCAGATGCGG-3′; and ESO1B, 5′-CACACAAAGCTTGGCTTAGCGCCTCTGCCCTG-3′ (Operon Technologies, Alameda). Amplification was performed using 35 cycles at an annealing temperature of 60°C. PCR products were visualized by ethidium bromide staining after separation over a 1.5% agarose gel.
Prokaryotic Expression Cloning of NY-ESO-1.
To produce full-length NY-ESO-1 recombinant protein, the coding sequence for the protein was PCR amplified from a corresponding cDNA clone in pBK-CMV phagemid, and cloned into pQE9, a plasmid vector containing histidine tags (Qiagen). PCR primers for NY-ESO-1 amplification were ESO1A(FL), 5′-CACACAGGATCCATGCAGGCCGAAGGCCGG-3′; and ESO1B (described above). After transformation into Escherichia coli strain XL1-Blue, positive transformants were confirmed to contain the appropriate insert by restriction mapping and DNA sequencing. Recombinant NY-ESO-1 protein was then produced by isopropyl β-d-thiogalactoside (IPTG) induction and purified by Ni2+ affinity chromatography, following procedures recommended by the manufacturer (Qiagen). Concentration of the purified protein was determined by colorimetric protein quantification assay (BioRad Labs., Hercules, CA).
Immunoblot Analysis.
Serum antibody responses against the full-length recombinant NY-ESO-1 protein, a lysate of NY-ESO-1–transfected COS-7 cells, and a lysate of the autologous tumor cell line NW-MEL-38 were tested by standard Western blot analysis (18). In brief, 1 μg of NY-ESO-1 protein or lysates of 2 × 104 NY-ESO-1–transfected COS-7 cells (containing 7.7 μg of protein) or of 5 × 103 tumor cells were diluted in SDS, and electrophoresed on a 15% SDS gel. After overnight blotting on a nitrocellulose filter (0.45 μm; Sartorius, Göttingen, Germany) and blocking with 3% BSA, blots were incubated with serum of patient NW38 at 1:1,000, 1:10,000, and 1:100,000 dilution, or with a mouse monoclonal antibody against NY-ESO-1 (Stockert, E., manuscript in preparation) as a positive control. Serum antibodies binding to NY-ESO-1 were detected by incubation with goat anti–human IgG (Fc-specific; Sigma Chemical Co., St. Louis, MO) at a ratio of 1:10,000, and visualized with NBT/X-phosphate (Sigma Chemical Co.).
Prediction of HLA-A2–binding Peptides Encoded by NY-ESO-1 and Peptide Synthesis.
NY-ESO-1 peptides with HLA-A2–binding motifs were predicted by computer analysis as described (19). Peptides were synthesized using a multiple peptide synthesizer (Abimed 422; Abimed, Langenfeld, Germany). Peptides were isolated and purified by repeated ether precipitations. The purity was determined by analytical reversed phase HPLC and proved to be at least 80% pure. Integrity of the peptides was determined by laser desorption time-of-flight mass spectrometry on a Lasermat mass spectrometer (Finnigan MAT, UK).
Cytotoxicity Assays.
Lytic activity of CTLs was tested in a 4-h chromium release assay as previously described (3). Peptide-specific CTL reactivity was determined on T2 cells labeled with 100 μCi of Na(51Cr)O4, 10 μg/ml of peptide, and 2.5 μg/ml of β2-microglobulin. CTLs were added to the peptide-pulsed targets at effector/target ratios of 90:1, 30:1, 10:1, and 1:1 for 4 h in V-bottomed microwells. Radioactivity was measured in supernatants (100 μl) in a gamma counter.
Eukaryotic Expression Cloning of NY-ESO-1.
For transfectional studies, the full-length NY-ESO-1 sequence was isolated from the corresponding pQE9 clone by restriction enzyme digestion, and cloned into BamHI–HindIII sites of the pcDNA3.1(−) vector (Invitrogen, Carlsbad, CA). Transfection of COS-7 cells was carried out as described (20). In brief, 2 × 104 COS-7 cells were transfected with 150 ng of plasmid pcDNA3.1(−) containing NY-ESO-1 cDNA, and 150 ng of plasmid pcDNA1Amp containing HLA-A2.1 or HLA-A1 cDNA using the DEAE-dextran-chloroquine method. The transfectants were incubated at 37°C for 48 h, and tested in a CTLs stimulation assay after 24 h.
Immunoscreening of NY-ESO-1 Transfectants.
Transfectants were tested for their ability to stimulate the production of TNF-α by NW38-IVS-1, as described (20). In brief, 2,500 CTLs in 100 μl RPMI supplemented with 10% human serum and 25 U/ml recombinant human IL-2 were added to microwells containing COS-7 transfectants. After 24 h, the content of TNF-α/50 μl of supernatant was determined by testing the cytotoxicity against WEHI 164 clone 13 cells in a 3-(4,5dimethylthiazol-2-yl)- 2,5diphenyltetrazolium bromide (MTT) colorimetric assay.
Results
High-titer Antibody Reactivity against NY-ESO-1.
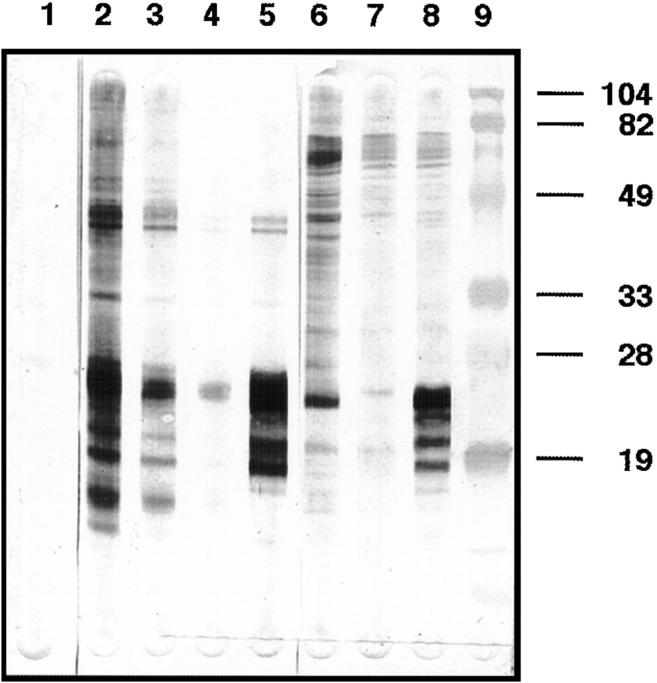
Melanoma patient NW38 presented with extensive metastases to inguinal lymph nodes having large areas of necrosis. Reverse transcriptase PCR of tumor RNA showed that this tumor expressed NY-ESO-1. Based on the hypothesis that exposure of the immune system to large amounts of intracellular tumor proteins released from the necrotic tumor might elicit a strong humoral immune response, the serum of patient NW38 was tested for specific reactivity against recombinant NY-ESO-1 protein. Fig. 1 shows the reactivity of NW38 serum with the recombinant NY-ESO-1 protein, with a lysate of NY-ESO-1–transfected COS-7 cells, and with a lysate of the autologous NY-ESO-1 messenger RNA–positive tumor cell line NW-MEL-38. A 22-kD protein species was identified in both cell lysates, and comigrated with the purified recombinant NY-ESO-1 protein. The identity of this protein species as NY-ESO-1 was further confirmed by using an anti–NY-ESO-1 mouse monoclonal antibody. Reactivity against recombinant NY-ESO-1 protein was still detectable at a serum dilution of 1:100,000. No reactivity was detected against a lysate of untransfected COS-7 cells.
Figure 1.
Western blot analysis of NW38 serum antibody reactivity. Lanes 1–5 contained 1 μg of recombinant NY-ESO-1 protein, tested against serum from a healthy donor (lane 1), NW38 serum (lanes 2–4, serum dilutions 1:1,000, 1:10,000, and 1:100,000), or an anti–NY-ESO-1 mouse monoclonal antibody (lane 5, hybridoma supernatant 1:50 dilution). NW38 serum was also tested, at 1:1,000 dilution, against lysates of 5 × 103 autologous NW-MEL-38 tumor cells (lane 6), of 2 × 104 untransfected COS-7 cells (lane 7), and of 2 × 104 NY-ESO-1–transfected COS-7 cells (lane 8).
CTL Reactivity against Autologous and Allogeneic ESO-1+ Tumor Cell Lines.
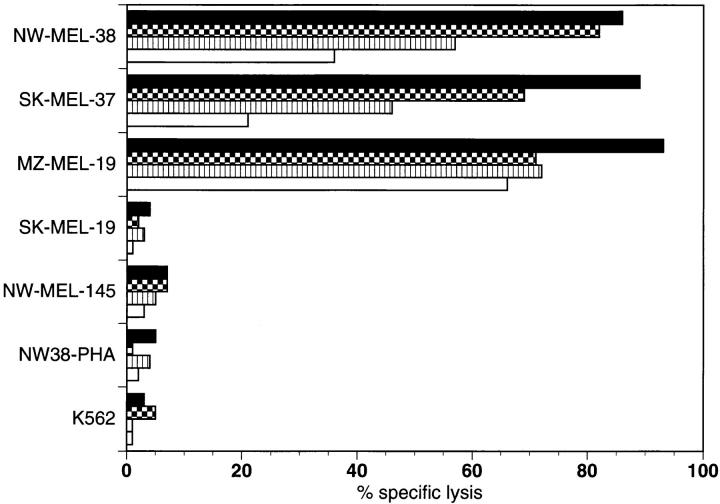
A tumor cell line was established from tumor tissue obtained from an inguinal lymph node metastasis by needle biopsy. Stimulation of autologous PBLs by the tumor cell line, NW38-MEL-38 resulted in significant lysis of the tumor cell targets, and a stable CTL cell line, designated NW38-IVS-1, maintaining this reactivity was established. The natural killer cell target K562 and autologous PHA blasts NW38-PHA were not lysed by NW38-IVS-1. The observation of high-titer seroreactivity against NY-ESO-1 indicated recognition of NY-ESO-1 by helper T cells and suggested NY-ESO-1 as a possible antigenic target for NW38-IVS-1. To evaluate this, several NY-ESO-1 messenger RNA-positive and -negative allogeneic melanoma lines were tested (Fig. 2). The allogeneic HLA-A2+, NY-ESO-1+ melanoma cell lines SK-MEL-37 and MZ-MEL-19 were lysed by NW38-IVS-1. The NY-ESO-1+ melanoma cell line SK-MEL-19 lacking MHC class I expression was not lysed, nor was the HLA-A2+, NY-ESO-1− melanoma cell line NW-MEL-145.
Figure 2.
Cytotoxicity of NW38-IVS-1 against NY-ESO-1+ and NY-ESO-1− cell lines. Specific lysis of the autologous NY-ESO-1+ melanoma cell line NW-MEL-38, and the NY-ESO-1+/HLA-A2+ allogeneic melanoma cell lines SK-MEL-37 and MZ-MEL-19 was observed. The NY-ESO-1+ MHC class I- melanoma cell line SK-MEL-19, the NY-ESO-1− HLA-A2+ melanoma cell line NW-MEL-145, K562, and NW38 PHA blasts used as controls were not lysed. Values represent the specific lysis of 51Cr-labeled target cells (E/T ratios: 90 [solid], 30 [checkered], 10 [striped], and 1:1 [open], as assessed by a standard 51Cr-release assay.
HLA-A2–restricted CTL Reactivity against NY-ESO-1 Transfectants.
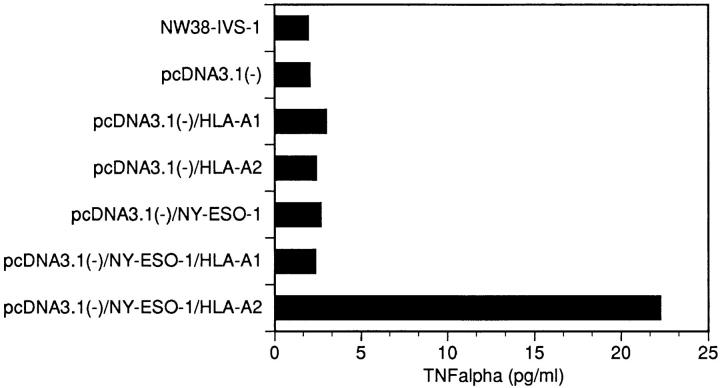
The correlation between NY-ESO-1 expression and NW38-IVS-1 reactivity suggested NY-ESO-1 as the antigenic target. To prove this, COS-7 cells were transfected with NY-ESO-1 cDNA and different MHC class I molecules and used as targets for NW38-IVS-1. Reactivity was measured in a standard TNF-α release assay. TNF release was found after stimulation of NW38-IVS-1 with COS-7 cells cotransfected with HLA-A2− and NY-ESO-1 cDNA. No reactivity was detected after stimulation with cotransfectants of pcDNA3.1(−)-NY-ESO-1− and pcDNA1Amp-HLA-A1 cDNA, COS-7 cells transfected with pcDNA3.1(−), or untransfected COS-7 cells (Fig. 3).
Figure 3.
TNF-α–release assays after stimulation of the CTL line NW38-IVS-1 by COS-7 cell transfectants. TNF release was detected against COS-7 cells cotransfected with the expression vector pcDNA3.1 (−) containing NY-ESO-1 cDNA, and pcDNA1Amp containing HLA-A2 cDNA. No TNF release was detected against COS-7 transfected with vector alone, vector/HLA-A1, vector/HLA-A2, vector/NY-ESO-1, and vector/NY-ESO-1/HLA-A1.
Peptide-specific CTLs.
26 different peptides encoded by NY-ESO-1 with theoretical binding motifs to the HLA-A2.1 molecule were tested for specific recognition by NW38-IVS-1. The target cells were peptide-pulsed T2 cells. Of these 26 peptides, three were recognized by NW38-IVS-1 as determined by a standard 51Cr–release assay (Table 1). The peptide sequences SLLMWITQCFL, SLLMWITQC, and QLSLLMWIT are located between positions 155 and 167 of the NY-ESO-1 protein (15), and show overlapping sequences. The 11-mer SLLMWITQCFL (2 in Table 1) and the 9-mer SLLMWITQC (12 in Table 1) consist of identical amino acids at positions 1–9.
Table 1.
NY-ESO-1–derived Peptides with Binding Motifs to HLA-A2
| No. | Peptide sequence | Position | Specific lysis | |||
|---|---|---|---|---|---|---|
| 1 | SLAQDAPPLPV | 108–118 | 0 | |||
| 2 | SLLMWITQCFL | 157–167 | 55 | |||
| 3 | QLSISSCLQQL | 146–156 | 1 | |||
| 4 | QLQLSISSCL | 144–153 | 1 | |||
| 5 | LLMWITQCFL | 158–167 | 15 | |||
| 6 | RLTAADHRQL | 136–145 | 5 | |||
| 7 | FTVSGNILTI | 126–135 | 1 | |||
| 8 | ITQCFLPVFL | 162–171 | 1 | |||
| 9 | SLAQDAPPL | 108–116 | 3 | |||
| 10 | PLPVPGVLL | 115–123 | 1 | |||
| 11 | WITQCFLPV | 161–169 | 5 | |||
| 12 | SLLMWITQC | 157–165 | 78 | |||
| 13 | RLLEFYLAM | 86–94 | 7 | |||
| 14 | SISSCLQQL | 148–156 | 6 | |||
| 15 | LMWITQCFL | 159–167 | 4 | |||
| 16 | QLQLSISSC | 144–152 | 1 | |||
| 17 | CLQQLSLLM | 152–160 | 1 | |||
| 18 | QLSLLMWIT | 155–163 | 84 | |||
| 19 | NILTIRLTA | 131–139 | 2 | |||
| 20 | GVLLKEFTV | 120–128 | 3 | |||
| 21 | ILTIRLTAA | 132–140 | 12 | |||
| 22 | TVSGNILTI | 127–135 | 4 | |||
| 23 | GTGGSTGDA | 7–15 | 9 | |||
| 24 | ATPMEAELA | 97–105 | 1 | |||
| 25 | FTVSGNILT | 126–134 | 1 | |||
| 26 | LTAADHRQL | 137–145 | 9 |
NY-ESO-1 peptides with binding motifs to HLA-A2, as analyzed by computer calculation, and specific lysis of T2 cells pulsed with peptide at 10 μg/ml (E/T = 90:1) as assessed by a standard 51Cr–release assay.
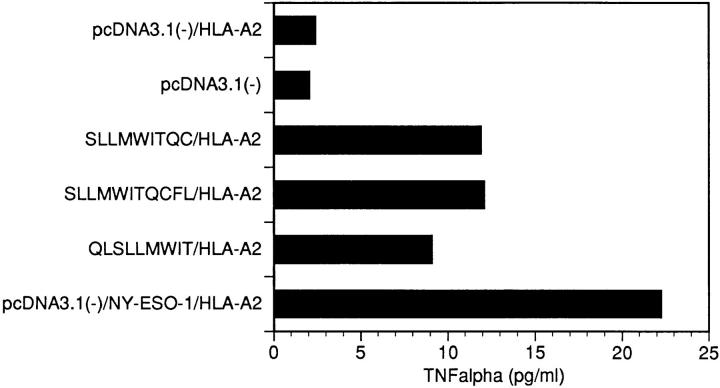
To provide additional confirmation of the peptide specificity, the 26 synthetic peptides were individually incubated with HLA-A2–transfected COS-7 cells and tested in the TNF release assay. Consistent with the results of 51Cr–release assay, specific TNF-α release was detected in tests with peptides SLLMWITQCFL, SLLMWITQC, and QLSLLMWIT. NY-ESO-1/HLA-A2 transfectants were used as a positive control in these assays (Fig. 4).
Figure 4.
TNF-α–release assays after stimulation of CTL line NW38-IVS-1 by HLA-A2–transfected COS-7 cells pulsed with HLA-A2–binding peptides encoded by NY-ESO-1-at 10 μg/ml. Cotransfectants of NY-ESO-1 and HLA-A2 were used as a positive control.
Discussion
The search for tumor antigens that induce specific immune responses in cancer patients is the ongoing challenge in tumor immunology. Evidence for a specific humoral response to human cancer came from serological analysis of cell surface reactivity of sera from cancer patients for autologous cancer cells, an approach called autologous typing (4). However, with only a few exceptions, this approach did not allow for the structural definition of the antigenic target. An autologous typing system also provided the first evidence for the development of CTLs with specificity for human melanoma cells (3, 17, 21–24). Using specific antitumor CTLs as probes, a number of CTL targets have been cloned on the basis of MHC class I–restricted recognition (1, 6). However, this approach involves cultured cancer cell lines and stable CTL lines from the same patient, two requirements that cannot easily be met with many tumor types. With the demonstration that genes coding for CTL-recognized tumor antigens elicit humoral immunity and can be cloned by SEREX methodology, a technically less demanding approach defining immunogenic tumor antigens is now available, one that extends the range of analysis to tumor types that are not easily adaptable to in vitro growth and are not sensitive targets for CTLs. A number of novel tumor antigens have been defined by SEREX, including two new members of the cancer–testis antigenic family, SSX2 (HOM-MEL-40) (5, 16), and NY-ESO-1 (15).
In this study, we identified a melanoma patient, NW38, with high-titered antibody against NY-ESO-1. This patient had a large and highly necrotic tumor, and the sustained release of intracellular antigens that are usually inaccessible to the immune system may account for the high NY-ESO-1 titer. The establishment of an autologous cell line that typed NY-ESO-1 positive provided target cells for assessing CTL reactivity in this patient. A CTL line was established from this patient that lysed the autologous melanoma cell line in an HLA-A2–restricted fashion. Using target cells transfected with NY-ESO-1 and HLA-A2, the specificity of CTL reactivity was found to be coded by NY-ESO-1. Computer analysis of the NY-ESO-1 sequence identified 26 peptides with HLA-A2–binding motifs. Screening of these peptides presented by T2 cells identified three sequences that were confirmed to be specifically recognized by NW38-IVS-1. This is the first conclusive demonstration of simultaneous antibody and CTL responses against a cancer–testis antigen in a single patient.
The strategy used in this study to generate and analyze CTL reactivity to a SEREX-defined antigen can be used as a model for investigating cellular immune responses to the growing list of other SEREX antigens. Identification of clones in SEREX requires high-titered IgG antibody, and the development of such antibodies requires the help of CD4+ T cells. In this sense, SEREX can be thought of as a method to define the CD4+ T cell repertoire to human tumor antigens. Also, the presence of both NY-ESO-1 antibody and CTLs in patient NW38 suggests that screening for an antibody response may be a simple and effective way to identify patients with concomitant CTL reactivity, and this possibility is now being tested in other patients with NY-ESO-1 antibody. In the absence of autologous tumor cell lines, CD8+ T cells can be stimulated with autologous antigen-presenting cells that have been transfected with the coding gene or fed purified protein antigens. A similar strategy can be used to identify peptide targets for CD4+ T cells.
A major objective in defining immunogenic human tumor targets is to explore their use in the development of cancer vaccines, and a number of clinical trials with various vaccine constructs are currently underway. Although tumor regression is the desired goal of a therapeutic vaccine, this end point cannot be expected to be an effective way to develop maximally immunogenic tumor vaccines. For this purpose, reliable immunological assays are needed to monitor the specificity and strength of specific immune reactions generated by the vaccine. With the exception of vaccines aimed at inducing a humoral immune response such as GM2 ganglioside vaccines, most vaccine trials are designed to stimulate cellular immunity, particularly the development of CTLs and CD4+ T cells. These have been difficult to detect in vaccine trials with MAGE peptides (25), and difficult to interpret in trials with vaccines containing melanocyte differentiation antigens, since CTLs against these antigens can be generated in vitro from nonvaccinated melanoma patients as well as normal individuals (26, 27). However, de novo induction and increase of preexisting CTL reactivity have been detected after vaccination with melanocyte differentiation antigens and observed to be associated with cancer regressions in a limited number of patients (13). The demonstration of a simultaneous antibody and CTL response to NY-ESO-1 in the same patient suggests that serological methods may be useful in monitoring vaccine trials with NY-ESO-1 and other tumor antigens eliciting a humoral immune response.
Footnotes
This work was partially funded by Krebs forschung Rhein Main and Ludwig Institute for Cancer Research.
References
- 1.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disis ML, Cheever MA. Oncogenic proteins as tumor antigens. Curr Opin Immunol. 1996;8:637–642. doi: 10.1016/s0952-7915(96)80079-3. [DOI] [PubMed] [Google Scholar]
- 3.Knuth A, Danowski B, Oettgen HF, Old LJ. T-cell–mediated cytotoxicity against autologous malignant melanoma: analysis with interleukin-2–dependent T-cell cultures. Proc Natl Acad Sci USA. 1984;81:3511–3515. doi: 10.1073/pnas.81.11.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Old LJ. Cancer immunology: the search for specificity. Cancer Res. 1981;41:361–375. [PubMed] [Google Scholar]
- 5.Sahin U, Türeci Ö, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA. Development of cancer immunotherapies based on identification of the genes encoding cancer regression antigens. J Natl Cancer Inst. 1996;88:1635–1644. doi: 10.1093/jnci/88.22.1635. [DOI] [PubMed] [Google Scholar]
- 7.Brichard V, Van Pel A, Wölfel T, Wölfel C, DePlaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakker AB, Schreurs MWJ, deBoer AJ, Kawakami Y, Rosenberg SA, Adema GJ, Figdor CG. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulie PG, Brichard V, Van Pel A, Wölfel T, Schneider J, Traversari C, Mattei S, DePlaen E, Lurquin C, Szikora J-P, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulie PG, Lehmann F, Lethe B, Herman J, Lurquin C, Andrawiss M, Boon T. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc Natl Acad Sci USA. 1995;92:7976–7980. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wölfel T, Hauer M, Schneider J, Serrano M, Wölfel C, Klehmann-Hieb E, DePlaen E, Hankeln T, Meyer zum Büschenfelde K-H, Beach D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 12.Van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 13.Jäger E, Ringhoffer M, Dienes H-P, Arand M, Karbach J, Jäger D, Ilsemann C, Hagedorn M, Oesch F, Knuth A. Granulocyte macrophage colony-stimulating factor enhances immune responses to melanoma associated peptides in vivo. Int J Cancer. 1996;67:54–62. doi: 10.1002/(SICI)1097-0215(19960703)67:1<54::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Jäger E, Ringhoffer M, Altmannsberger M, Arand M, Karbach J, Jäger D, Oesch F, Knuth A. Immunoselection in vivo: independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int J Cancer. 1997;71:142–147. doi: 10.1002/(sici)1097-0215(19970410)71:2<142::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y-T, Scanlan MJ, Sahin U, Türeci Ö, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Türeci O, Sahin U, Schobert I, Koslowski M, Schmitt H, Schild HJ, Stenner F, Seitz G, Rammensee HG, Pfreundschuh M. The SSX-2 gene, which is involved in the t(X;18) translocation of synovial sarcomas, codes for the human tumor antigen HOM-MEL-40. Cancer Res. 1996;56:4766–4772. [PubMed] [Google Scholar]
- 17.Herin M, Lemoine C, Weynants P, Vessiere F, VanPel A, Knuth A, Devos R, Boon T. Production of stable cytolytic T cell clones directed against autologous human melanoma. Int J Cancer. 1987;39:390–396. doi: 10.1002/ijc.2910390320. [DOI] [PubMed] [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Amaro J, Houbiers JGA, Drijfhout JW, Brandt RMP, Schipper R, Bouwes JN, Bavinck, Melief CJM, Kast WM. A computer program for predicting possible cytotoxic T lymphocyte epitopes based on HLA class I peptide-binding motifs. Hum Immunol. 1995;43:13–18. doi: 10.1016/0198-8859(94)00153-h. [DOI] [PubMed] [Google Scholar]
- 20.Traversari C, van der Bruggen P, van den Eynde B, Hainaut P, Lemoine C, Ohta N, Old LJ, Boon T. Transfection and expression of gene coding for a human melanoma antigen recognized by autologous cytolytic T lymphocytes. Immunogenetics. 1992;35:145–148. doi: 10.1007/BF00185107. [DOI] [PubMed] [Google Scholar]
- 21.Knuth A, Wölfel T, Klehmann E, Boon T, Meyer zum Büschenfelde K-H. Cytolytic T-cell clones against an autologous human melanoma: specificity study and definition of three antigens by immunoselection. Proc Natl Acad Sci USA. 1989;86:2804–2808. doi: 10.1073/pnas.86.8.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knuth A, Wölfel T, Meyer zum Büschenfelde K-H. T cell responses to human malignant tumours. Cancer Surv. 1992;13:39–52. [PubMed] [Google Scholar]
- 23.Mukherji B, MacAlister TJ. Clonal analysis of cytotoxic T cell response against human melanoma. J Exp Med. 1983;158:240–244. doi: 10.1084/jem.158.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987;138:989–995. [PubMed] [Google Scholar]
- 25.Marchand M, Weymants P, Rankin E, Arienti F, Belli F, Parmiani G, Cascinelli N, Bourlond A, Vanwjick R, Humblet Y, et al. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. Int J Cancer. 1995;63:883–885. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 26.Jäger E, Arand M, Ringhoffer M, Karbach J, Jäger D, Ilsemann C, Hagedorn M, Oesch F, Knuth A. Cytolytic T cell reactivity against melanoma associated differentiation antigens in peripheral blood of melanoma patients and healthy individuals. Melanoma Res. 1996;6:419–425. doi: 10.1097/00008390-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Yee C, Gilbert MJ, Ridell SR, Brichard VG, Fefer A, Thompson JA, Boon T, Greenberg PD. Isolation of tyrosinase-specific CD8+ and CD4+ T cell clones from the peripheral blood of melanoma patients following in vitro stimulation with recombinant vaccinia virus. J Immunol. 1996;157:4079–4086. [PubMed] [Google Scholar]