Abstract
Interleukin (IL)-4–deficient mice were used to assess susceptibility to systemic or gastrointestinal Candida albicans infections, as well as parameters of innate and elicited T helper immunity. In the early stage of systemic infection with virulent C. albicans, an unopposed interferon (IFN)-γ response renders IL-4–deficient mice more resistant than wild-type mice to infection. Yet, IL-4–deficient mice failed to efficiently control infection in the late stage and succumbed to it. Defective IFN-γ and IL-12 production, but not IL-12 responsiveness, was observed in IL-4–deficient mice that failed to mount protective T helper type 1 cell (Th1)-mediated acquired immunity in response to a live vaccine strain of the yeast or upon mucosal immunization in vivo. In vitro, IL-4 primed neutrophils for cytokine release, including IL-12. However, late treatment with exogenous IL-4, while improving the outcome of infection, potentiated CD4+ Th1 responses even in the absence of neutrophils. These findings indicate that endogenous IL-4 is required for the induction of CD4+ Th1 protective antifungal responses, possibly through the combined activity on cells of the innate and adaptive immune systems.
Thelper cells play a central role in regulating immune responses to the fungus Candida albicans by secreting cytokines that modulate the development and activity of immune effectors. The dominance of either one of the two Th subsets (Th1 and Th2) directly correlates with the outcome and severity of infection (1, 2). In experimental models of candidiasis, protection correlates with the occurrence of Th1 responses (3–5), whereas Th2 responses are associated with disease exacerbation and pathology (6–8). A variety of factors control CD4+ Th subset development and regulation in murine candidiasis (1, 2). Cytokines are among these, acting not only as modulators of antifungal effector functions but also as key regulators in the development of the different Th subsets from precursor Ths. Early in infection, neutralization of Th1 cytokines (IFN-γ and IL-12; references 6, 9–11) and administration of Th2 cytokines (IL-4 or IL-10; reference 12) lead to the onset of Th2 rather than Th1 responses, whereas Th2 cytokine neutralization allows the development of Th1 rather than Th2 responses (4, 5, 8).
The finding that IL-4 fails to convert an already established Th1 response into a Th2 response (12) and that it is indeed required for the expression of protective immunity in mice with candidiasis (13), suggests complex levels of cytokine-mediated regulation of Th development and effector function, which were previously unappreciated. In particular, recent studies in IL-4–deficient mice have revealed important novel roles for IL-4, which would not have been predicted from data on IL-4 neutralization in vivo (14–16). Thus, in genetically susceptible IL-4–deficient mice with toxoplasmosis, IL-4 appears to have different roles, depending on the phase of infection (16). The infection is also exacerbated in genetically resistant IL-4–deficient mice, IL-4 being required for protection in the late stage of infection (15). The protective effect of IL-4 appears to be due to its ability to sustain IFN-γ production by activated CD4+ T cells (15), a finding that, although previously described (17, 18), is not easy to accommodate in the paradigm of CD4+ Th regulation by IL-4 (19). However, recent lineage ablation studies by Flavell et al. provide convincing evidence on the requirement of IL-4 in the differentiation of both Th2 and Th1 effector cells from precursor CD4+ Ths (20–22).
In this study, we assessed susceptibility and parameters of innate and adaptive Th immunity in IL-4–deficient mice infected with C. albicans. The results show the ability of these mice to successfully control the infection in the early stage but not the late, IL-4 being required for induction of protective CD4+ Th1 anticandidal responses.
Materials and Methods
Mice.
Breeding pairs of homozygous IL-4–deficient (IL-4−/−) and control (IL-4+/+) BALB/c mice (23) were bred under specific, pathogen-free conditions. Mice of both sexes, 8–10 wk old, were used. Procedures involving animals and their care were conducted in conformity with national and international laws and policies.
Yeasts, Infections, In Vivo Analyses, and Treatments.
The origin and characteristics of the C. albicans highly virulent CA-6 strain and the live vaccine strain PCA-2 used in this study have already been described in detail (4–13). For infection, cells were washed twice in saline, and diluted to the desired density to be injected intravenously via the lateral tail vein in a volume of 0.5 ml/mouse or intragastrically via an 18-gauge 4-cm-long plastic catheter, as described (24). The viability of the cells was >95% by trypan blue exclusion and quantitative cultures. Quantification of yeast cells in organs of infected mice (6–8/group) was performed by a plate-dilution method, using Sabouraud dextrose agar, and results (mean ± SEM) were expressed as CFU per organ. Resistance to reinfection was assessed by injecting mice with 106 CA-6 cells intravenously, 14 d after primary infection. Mice succumbing to yeast challenge were routinely necropsied for histopathological confirmation of disseminated candidiasis. Recombinant murine IL-4 (provided by Dr. Robert Coffman, DNAX Research Institute, Palo Alto, CA) was given intraperitoneally together with the anti–IL-4 mAb 11B11 hybridoma (American Type Culture Collection, Rockville, MD; 3 μg IL-4 + 30 μg mAb, each injection) as described (12, 13). Anti–IL-4 mAb was injected intraperitoneally at the dose of 0.5 mg affinity-purified mAb/injection, as described (4, 13). Control groups were injected with saline or isotype-matched Ab (Zymed Laboratories, Inc., South San Francisco, CA). Both treatments were performed on days 6, 8, 10, and 12 after C. albicans infection. Long-lasting neutrophil depletion was obtained as described (25), by administering RB6-8C5 (anti-Ly6G) mAb intraperitoneally at the dose of 100 μg/mouse 2 d before reinfection. Endotoxin was removed from all solutions with Detoxi-gel (Pierce Chemical Co., Rockford, IL).
Purification and Culture of Cells.
CD4+ T lymphocytes were positively selected from pools of spleen cells by means of a panning procedure using purified anti–murine CD4 mAb (GK1.5 hybridoma from American Type Culture Collection), which resulted in a >90% pure population on flow cytometric analysis (3–5). CD4+ cells (5 × 106) were cultured in the presence of 5 × 105 accessory macrophages and 5 × 105 heat-inactivated yeast cells. Unfractionated splenocytes (5 × 106/ml) were cultured in the presence of 10 μg/ml of Concanavalin A (Sigma Chemical Co., St. Louis, MO). Cultures of purified peritoneal neutrophils and macrophages, collected 18 or 72 h, respectively, after intraperitoneal inoculation of aged, endotoxin-free 10% thioglycollate solution (Difco, Detroit, MI), were done as described (25, 26) by incubating 5 × 105 cells in the presence of IFN-γ (400 U/ml) and LPS (40 ng/ml, Sigma Chemical Co.) or 5 × 104 C. albicans, PCA-2. In some experiments, cells were exposed in vitro to rIL-4 (250 U/ml) for 8 h before addition of stimuli. Cytokine measurement was performed in supernatants collected after 48 h (for lymphocytes) and 24 h (for neutrophils and macrophages).
Cytokine Assays.
The levels of IFN-γ, IL-2, IL-4, IL-6, and IL-10 were determined by means of cytokine-specific ELISA, using pairs of anticytokine mAbs, as described (3–13, 25, 26). The Ab pairs used were as follows, listed by capture/biotinylated detection: IFN-γ, R4-6A2/XMG1.2; IL-2, JES6-1A12/JE5H4; IL-4, BVD4-1D11/BVD6-24G2; IL-6, MP5-20F3/MP5-32c11; IL-10, JES5-2A5/SXC-1 (PharMingen, San Diego, CA). For IL-12p70 measurement, a modified Ab-capture bioassay was used, as described (7). Cytokine titers were calculated by reference to standard curves constructed with known amounts of recombinant cytokines (from PharMingen, except IL-12, from Genetics Institute, Boston, MA).
Candidacidal Assay and Nitrite Determination.
For the candidacidal assay, 5 × 105 splenic macrophages (obtained by 2-h plastic adherence, >95% pure on esterase staining) or elicited peritoneal neutrophils were plated (0.1 ml/well) in 96-well flat-bottomed microtiter plates (Costar, Cambridge, MA) and incubated with 105 PCA-2 cells for 4 or 1 h, respectively, as described (27). Triton X-100 (final concentration: 0.1%) was then added to the wells, and serial dilutions from each well were made in distilled water. Pour plates (four to six replicate samples) were made by spreading each sample on Sabouraud glucose agar. The number of CFU was determined after incubation at 37°C and the percentage of CFU inhibition (means ± SE) was determined as follows: percentage of colony formation inhibition = 100 − (CFU experimental group/CFU control cultures) × 100. Control cultures consisted of C. albicans cells incubated without effector cells. Nitrite concentration, a measure of nitric oxide (NO)1 synthesis, was assayed in culture supernatants by a standard Griess reaction adapted to microplates, as described (8). The Griess reagent was prepared by mixing equal volumes of sulfanilamide (1.5% in 1 N HCl) and naphthylethylene diamine dihydrochloride (0.15% in H2O) (Sigma Chemical Co.). A volume of 100 μl of reagent was mixed with 100 μl of supernatant and incubated for 30 min in the dark. Absorbance of the chromophore formed was measured at 540 nm in an automated microplate reader. The data represent the means ± SE of quadruplicate determinations and are expressed as μg NO2 −/ml.
RNA Preparation and Reverse Transcriptase PCR.
Splenic macrophages, peritoneal neutrophils, and CD4+ splenocytes were subjected to RNA extraction by the guanidium thiocyanate-phenol-chloroform procedure, as described (28). In brief, 3 μg of total RNA was incubated with 0.5 μg of oligo(dT) (Pharmacia Biotech AB, Uppsala, Sweden) for 3 min at 65°C and chilled on ice for 5 min. Each sample was then incubated for 2 h at 42°C after adding 20 U RNase inhibitors (Boehringer Mannheim, Milan, Italy), 1.5 mM deoxynucleoside triphosphates, 25 U avian myeloblastosis virus reverse transcriptase (RT) (Boehringer Mannheim), and RT buffer (50 mM Tris-HCl, pH 8.3, 8 mM MgCl2, 30 mM KCl, and 10 mM dithiothreitol, final concentrations) in a final volume of 20 μl. cDNA was diluted to a total volume of 500 μl with TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and frozen at −20°C until use. Amplification of synthesized cDNA from each sample was carried out as described previously (29). In brief, 5 μl of cDNA was added to a reaction mixture containing 50 mM KCl, 10 mM Tris-HCl, pH 8.3, 3.0 mM MgCl2, 0.01% gelatin, 0.2 mM deoxynucleoside triphosphates, 1 μM of each primer, and 2.5 U AmpliTaq polymerase (Perkin-Elmer Corp., Hayward, CA). Each 100-μl sample was overlaid with 75 μl mineral oil (Sigma Chemical Co.) and incubated in a DNA Thermal Cycler 480 (Perkin-Elmer Corp.) for a total of 30–35 cycles for each cytokine. For hypoxanthine-guanine phosphoribosyl transferase (HPRT) and IL-12, the primers, positive controls, cycles, and temperature were as described elsewhere (9, 10). For IL-12Rβ1, IL-12Rβ2, and IL-4R, the primers were synthesized using a 391 DNA synthesizer (PCR-MATE; Applied Biosystems, Foster City, CA). The sequences of 5′ sense primers and 3′ antisense primers for IL-12Rβ1 and IL-12Rβ2 were as follows: IL-12β1, 5′- GAA CCA CAC ACA CTG TAC CCT G, 3′- TTT AGT GGG TGG CAC GAG CC; IL-12β2, 5′- CAA GAC ATC GAC TAT GAC AGA C, 3′- CAG GTT GTG CTG TCG AGT CTC G. For IL-12Rβ1 and β2, each cycle consisted of 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. The positive controls were obtained through the courtesy of Dr. Giorgio Trinchieri (Wistar Institute, Philadelphia, PA). For IL-4R, the sequences of 5′ sense primers and 3′ antisense primers were as follows: 5′- TGT GAC CTA CAA GGA ACC CA, 3′- GCA AAA CAA CGG GAT GCA GA. For IL-4R, each cycle consisted of 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. The HPRT primers were used as a control for both reverse transcription and the PCR reaction itself, and also for comparing the amount of products from samples obtained with the same primer. The PCR fragments were analyzed by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining. PCR-assisted messenger RNA (mRNA) amplification was repeated at least twice for at least two separately prepared cDNA samples for each experiment. Data are representative of at least three different experiments.
Competitive RT-PCR.
The semiquantitative competitive PCR developed by Reiner et al. (30), was performed using the competitor construct containing sequences for multiple cytokines, the primers for HPRT and IFN-γ, and the PCR conditions described by the authors. In brief, aliquots of cDNA were assayed for levels of HPRT by placing serial dilutions from 1:1 to 1:40 of the experimental cDNA against a fixed concentration of the competitor construct and examining the ratio of competitor/wild-type band intensity after amplification with HPRT-specific primers. Adjustments were made in the amount of cDNAs needed to standardize the HPRT levels to comparable levels among all groups. Serial dilutions of these adjusted volumes of cDNA were then used to quantitate cytokine levels using a fixed concentration of competitor (3 and 1.5 pg/ml for HPRT and IFN-γ, respectively) in each reaction in the presence of cytokine-specific primers. The PCR products were separated by electrophoresis in 2% agarose gels containing ethidium bromide. The point of equivalence in intensity between the competitor (upper band) and the cDNA (lower band) indicates the relative concentration of mRNA.
Statistical Analysis.
Survival and organ clearance data from each group of wild-type mice were compared with those from IL-4–deficient mice using the Mann-Whitney U test; P < 0.05 was considered significant. Student's t test was used to determine statistical significance between cytokine production of the two groups. In vivo groups consisted of four to six animals. The data reported are pooled from three experiments.
Results
IL-4−/− Mice Successfully Control C. albicans Infection in the Early Stage, but Succumb to Infection in the Late Stage.
IL-4−/− and IL-4+/+ mice were injected intravenously with different doses (106, 5 × 105 and 2 × 105) of highly virulent CA-6, with 106 cells of the live vaccine strain PCA-2, or intragastrically with 108 CA-6. Mice were monitored for resistance to primary and secondary infections (Table 1) and for fungal growth in the organs (Fig. 1). The results show that, although the median survival time (MST) of mice injected with the highest inoculum of CA-6 did not differ between the two types of mice, IL-4–deficient mice were more resistant than IL-4+/+ to the lower inocula of the yeast, as observed by the increased survival. IL-4−/− mice were as resistant as wild-type mice to infection with PCA-2 or to intragastric infection with CA-6. However, on assaying the susceptibility of survivors to a subsequent lethal CA-6 challenge, IL-4+/+ mice either survived or showed a remarkable resistance to reinfection, whereas IL-4–deficient mice did not.
Table 1.
Susceptibility of IL-4−/− and IL-4+ /+ Mice to C. albicans Infection
| Mice | Primary infection | Secondary infection* | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yeast | Dose | Route | MST | D/T | Yeast | Dose | Route | MST | D/T | |||||||||||
| IL-4−/− | CA-6 | 106 | i.v. | 4 | 8/8 | – | – | – | – | – | ||||||||||
| CA-6 | 5 × 105 | i.v. | 16† | 8/8 | – | – | – | – | – | |||||||||||
| CA-6 | 2 × 105 | i.v. | >60† | 0/8 | CA-6 | 106 | i.v. | 5.5 | 4/4 | |||||||||||
| CA-6 | 108 | i.g. | >60 | 0/8 | CA-6 | 5 × 105 | i.v. | 10 | 4/4 | |||||||||||
| PCA-2 | 106 | i.v. | >60 | 0/12 | CA-6 | 106 | i.v. | 6 | 6/6 | |||||||||||
| IL-4+/+ | CA-6 | 106 | i.v. | 5 | 12/12 | – | – | – | – | – | ||||||||||
| CA-6 | 5 × 105 | i.v. | 7 | 8/8 | – | – | – | – | – | |||||||||||
| CA-6 | 2 × 105 | i.v. | 15 | 8/8 | – | – | – | – | – | |||||||||||
| CA-6 | 108 | i.g. | >60 | 0/12 | CA-6 | 5 × 105 | i.v. | 21 | 6/6 | |||||||||||
| PCA-2 | 106 | i.v. | >60 | 0/12 | CA-6 | 106 | i.v. | >60 | 0/6 | |||||||||||
i.g., intragastric; MST, median survival time (d); D/T, dead animals over total mice infected.
Surviving mice were reinfected 14 d after the primary challenge.
P < 0.05, Mann Whitney U test (IL-4−/− versus IL-4+/+).
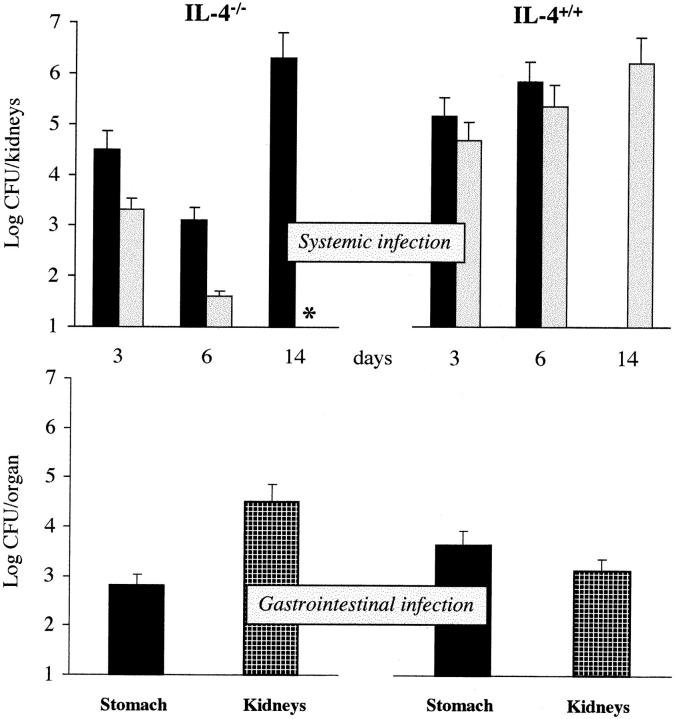
Figure 1.
Fungal growth in the organs of IL-4−/− and IL-4+/+ mice injected with 5 × 105 (solid colums) or 2 × 105 (dotted columns) CA-6 cells (Systemic infection) or 108 CA-6 cells (Gastrointestinal infection). Enumeration of yeast cells recovered from the kidneys of mice with systemic infection or from the stomach and kidneys of mice with gastrointestinal infection was performed at different days after infection. In the gastrointestinal infection, yeast cell counts in the kidneys were performed 4 d after intravenous reinfection with 5 × 106 CA-6. Cumulative data from two experiments (mean ± SE, four to six animals per group). Vertical bars, upper limit of the standard error; *, no viable yeast units found. For each CFU value, P < 0.05 (Student's t test, IL-4−/− versus IL-4+/+ mice).
Quantification of yeast cells recovered from infected mice revealed that the number of C. albicans cells decreased in the kidneys of IL-4−/− mice throughout the first week of infection with either 5 × 105 or 2 × 105 CA-6 cells. However, at 14 d after infection, when no yeast cells were recovered in mice surviving the lowest inoculum, an extensive fungal growth was observed in the kidneys of mice succumbing to infection. In contrast, the fungal load in the kidneys of wild-type mice was always higher and increased continuously until death. In mice with gastrointestinal infection, the number of yeast cells recovered from the stomach was significantly lower in IL-4−/− than in IL-4+/+ mice, even though both types of mice eventually survived the infection. Resistance to CA-6 intravenous reinfection was lower in IL-4−/− than IL-4+/+ mice, as revealed by the high number of yeast cells recovered from the kidneys of IL-4−/− reinfected mice. Histopathological examination of the kidneys of systemically infected IL-4–deficient mice revealed patterns of lesions similar to those observed in resistant or susceptible strains of mice infected intravenously (3– 5), with signs of extensive fungal growth and numerous foci of inflammatory reaction (mainly consisting of polymorphonuclear cells) throughout the kidney parenchyma of mice succumbing to infection and absence of pathological lesions and fungal growth in kidneys of mice resistant to infection (data not shown). Therefore, these data clearly show a two-stage control of C. albicans infection in mice. IL-4–deficient mice were highly resistant in the early stage of infection, but highly susceptible in the late stage.
Early Control of C. albicans Infection in IL-4−/− Mice Is Associated with Unimpaired Antifungal Activity of Phagocytic Cells.
To evaluate the contribution of cells of the innate immune system on the ability of IL-4–deficient mice to efficiently oppose infectivity in the early stage of infection, the antifungal effector functions of macrophages and neutrophils were assessed. Splenic macrophages and peritoneal neutrophils were obtained from IL-4−/− and +/+ mice at 3 d after intravenous infection with 5 × 105 CA-6 cells and assessed for candidacidal activity and production of NO. The results (Table 2) showed that the candidacidal activity of both types of cells was severely depressed in IL-4+/+ mice upon infection, as opposed to the unimpaired activity of those from IL-4−/− mice. Interestingly, the candidacidal activity of macrophages, and lesser that of neutrophils, appeared to be higher in uninfected IL-4−/− mice as opposed to wild-type mice. Likewise, production of NO occurred successfully in IL-4–deficient mice, and to a lesser extent in wild-type mice. Moreover, the number of peripheral white blood cells did not differ between IL-4−/− and IL-4+/+ uninfected mice and increased comparably upon infection (data not shown). Therefore, these data suggest that a successful innate antifungal immune response occurs in IL-4−/− mice upon infection.
Table 2.
Candidacidal Activity and NO2 − Production by Macrophages and Neutrophils from IL-4−/− and IL-4+ /+ Mice Infected with C. albicans
| Cells* | Infection | Candidacidal activity† | NO2 −§ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-4−/− | IL-4+/+ | IL-4−/− | IL-4+/+ | |||||||
| % | % | μg/ml | μg/ml | |||||||
| Macrophages | None | 51 ± 4 | 33 ± 4 | 1.4 ± 0.5 | 2.6 ± 1.1 | |||||
| CA-6 | 53 ± 5 | 20 ± 3 | 7.1 ± 1.1 | 3.8 ± 2.0 | ||||||
| Neutrophils | None | 68 ± 6 | 60 ± 5 | 10.2 ± 3.1 | 9.0 ± 3.1 | |||||
| CA-6 | 68 ± 5 | 14 ± 2 | 10.6 ± 2.5 | 11.2 ± 3.2 | ||||||
Macrophages were from the spleens and neutrophils from the peritoneal cavity of mice uninfected or infected with 5 × 105 CA-6 cells 3 d before.
Macrophages and neutrophils were incubated with C. albicans at a cell/cell ratio of 5:1 for 4 and 1 h, respectively, before evaluation of Candida growth inhibition activity.
Nitrate determination in supernatants from cells cultured as in
† was assessed by a standard Griess reaction, as detailed in Materials and Methods.
Susceptibility of IL-4−/− Mice to Candidiasis Is Associated with Impaired Development of Antifungal CD4+ Th1 Responses.
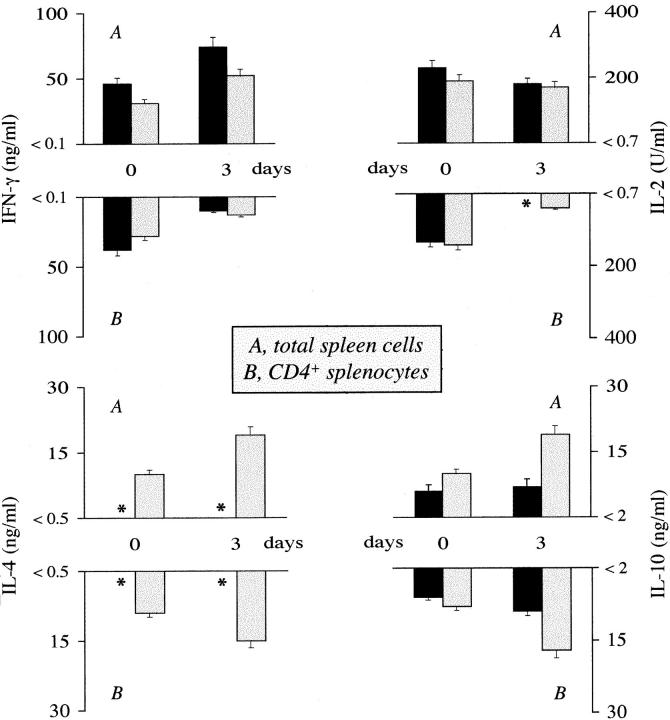
IFN-γ is a potent activator of antifungal effector functions of phagocytic cells (31, 32) and is produced by a variety of cells, including NK and CD4+ Th1s (33). Therefore, we assessed the level of IFN-γ production in cultures of unfractionated or purified CD4+ T splenocytes obtained from mice infected with 5 × 105 CA-6 cells, soon after infection. We also extended the analysis to IL-2, IL-4, and IL-10 production, as resistance and susceptibility to C. albicans is associated with preferential expansion of cells producing Th1 and Th2 cytokines, respectively (1, 2). We found that production of IFN-γ by mitogen-stimulated splenocytes was higher in IL-4−/− than +/+ mice, either uninfected or at 3 d after infection, at a time when no IL-4 or minimal IL-10 were detected in the former mice compared to wild-type mice (Fig. 2). On assaying cytokine levels produced by antigen-stimulated CD4+ splenocytes, a similar pattern of Th1 cytokine production was observed in IL-4−/− and +/+ mice, in that minimal or no IFN-γ and IL-2 were produced. As expected, IL-4 and IL-10 productions were increased in infected wild-type mice, as opposed to no (IL-4) or minimal (IL-10) detection in mutant mice (Fig. 2). Therefore, these data indicated that the early sustained production of IFN-γ in IL-4−/− mice was derived from cells other than CD4+ Th1 lymphocytes.
Figure 2.
Cytokine production in IL-4−/− (solid columns) and IL-4+/+ (dotted columns) mice infected with C. albicans. Total spleen cells (A) and purified CD4+ T splenocytes (B), obtained from uninfected mice (0) or 3 d after intravenous challenge with 5 × 105 CA-6 cells, were mitogen or antigen stimulated in vitro, respectively, for 48 h. Cytokine levels were determined by means of cytokine-specific ELISA. *, Cytokine levels below the detection limit of the assay, as indicated on the y-axis.
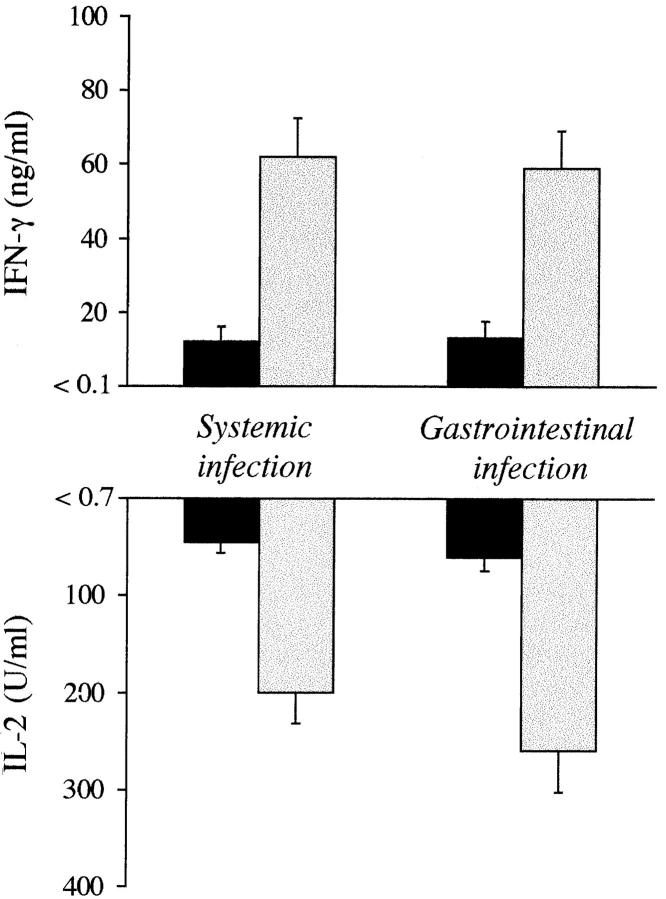
To investigate whether CD4+ Th1 development may occur in IL-4–deficient mice, we measured cytokine production in culture supernatants of purified CD4+ splenocytes from IL-4–deficient mice infected under conditions that would otherwise result in the activation of protective CD4+ Th1s. For this purpose, cytokine production by splenic CD4+ T cells was assessed in mice reinfected with virulent CA-6, 14 d after the primary intravenous challenge with PCA-2 or after the primary intragastic challenge with CA-6. The results showed (Fig. 3) that production of IFN-γ and IL-2 were elevated, as expected, in IL-4+/+ mice either surviving reinfection or showing increased resistance to it (Table 1). In contrast, minimal production of both cytokines was observed in IL-4 mutant mice, succumbing to reinfection. We also assessed levels of IFN-γ gene expression in CD4+ T cells from both types of mice by competitive PCR. IL-4−/− and IL-4+/+ mice were injected with PCA-2 cells and assessed for IFN-γ gene expression 3 d after reinfection with CA-6 cells. The results showed a three- to fourfold increase in the IFN-γ message in CD4+ cells from wild-type as compared to mutant mice (Fig. 4). Interestingly, treatment with exogenous IL-4 restored IFN-γ gene expression to a level comparable to that observed in wild-type–resistant mice.
Figure 3.
Production of IFN-γ and IL-2 in IL-4−/− (solid columns) and IL-4+/+ (dotted columns) mice infected with 106 PCA-2 cells intravenously (Systemic infection) or 108 CA-6 intragastrically (Gastrointestinal infection). Purified CD4+ T splenocytes were obtained at 4 d after reinfection with 5 × 105 CA-6 cells, 14 d after primary infection. Cells were cultured in the presence of Candida antigen and macrophages as accessory cells, as described in Materials and Methods. Cytokine levels in unstimulated cultures were below the detection limit of the assay, as indicated on the y-axis.
Figure 4.
Relative levels of IFN-γ mRNA in CD4+ T cells from naive or C. albicans–infected IL-4+/+ and IL-4−/− mice. Mice were infected with 106 PCA-2 intravenously and reinfected with 106 virulent CA-6 14 d later. Cells were purified from spleens at 3 d after reinfection. *, Mice were infected as above and treated with rIL-4 (3 μg/each injection) on days 6, 8, 10, and 12 after PCA-2 infection. Semiquantitative competitive PCR was performed as described by Reiner et al. (30). Photographs are shown of ethidium bromide–stained gels of representative PCR reactions using HPRT– and IFN-γ–specific primers in the presence of serial fivefold dilutions of the experimental sample and a fixed concentration of the competitor, as described in Materials and Methods. The upper band was due to amplification of the competitor construct and the lower band was due to amplification of cDNA. The point of equivalence in intensity between the competitor and cDNA indicates the relative concentration of mRNA. The relative concentration of HPRT was used to control differences in mRNA concentration and efficiency of cDNA synthesis.
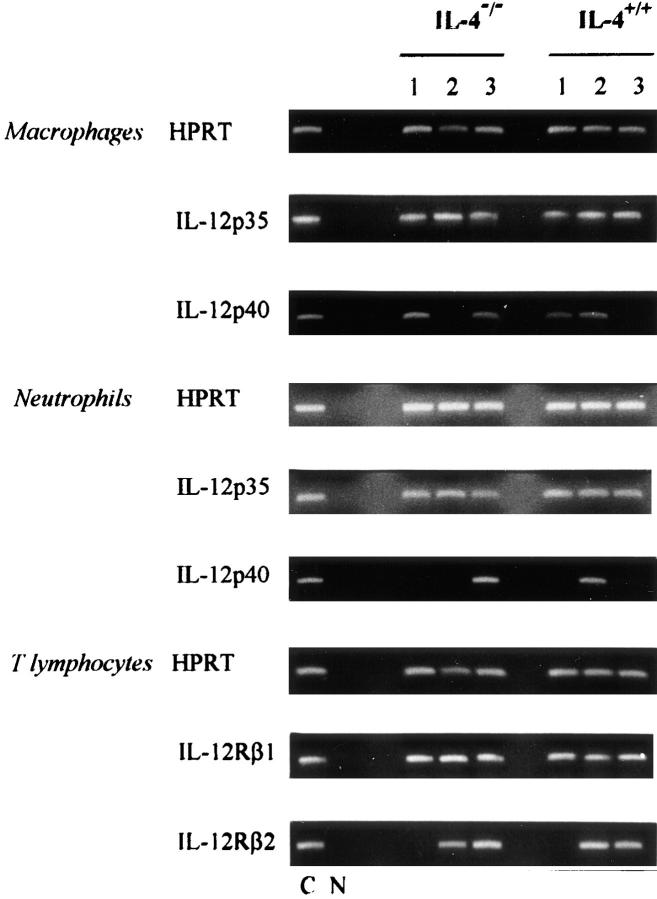
IL-12 Production, but Not IL-12 Responsiveness, Is Impaired in IL-4–deficient Mice Infected with C. albicans.
Because IL-12 is both required and prognostic for Th1 development in mice with candidiasis (9, 10), we evaluated whether the failure of IL-4–deficient mice to default to the Th1 pathway was associated with a defective production of IL-12. We have recently shown that neutrophils, more than macrophages, are important sources of IL-12 in vivo in C. albicans infection (25, 26). Therefore, we looked for IL-12 gene expression in both neutrophils and macrophages from either type of mouse after PCA-2 infection. We found that IL-12p40 message, while present in both neutrophils and macrophages from either type of mouse soon after infection (data not shown), could not be detected in IL-4–deficient mice later in infection unless mice were treated with IL-4 (Fig. 5). The message also disappeared in IL-4+/+ mice upon IL-4 neutralization, a finding in line with previous data (9). We also measured IL-12 responsiveness in both types of mice, by assessing levels of IL-12Rβ1 and IL-12Rβ2 expression, as it has recently been demonstrated that loss of IL-12 responsiveness, due to a selective loss of IL-12Rβ2 subunit expression, represents an early step in the commitment of T cells to the Th2 pathway (34, 35). We found that expression of the IL-12Rβ1 subunit gene was similar in either type of uninfected mice and was not modified upon C. albicans infection. For the IL-12Rβ2 subunit, expression was induced by infection and was continuously present in either type of mouse (Fig. 5). Therefore, these data suggest that IL-12 production, but not IL-12 responsiveness, was impaired in IL-4–deficient mice infected with C. albicans. To verify whether IL-12 deficiency could be responsible for the inability of IL-4–deficient mice to mount a protective CD4+ Th1 response, an obvious approach would have been to supply mice with exogenous IL-12. However, due to an adverse effect on neutrophils (11), administration of exogenous IL-12 to mice with candidiasis does not have a beneficial effect (9, 11). Nevertheless, the finding that in vitro exposure to IL-12 greatly increased production of IFN-γ by CD4+ T cells from IL-4−/− mice cultured with Candida Ag and accessory cells (data not shown), indicated that the defective production of IL-12 may be a limiting factor in the induction of CD4+ Th1 responses in IL-4−/− deficient mice.
Figure 5.
Expression of IL-12 and IL-12R genes in IL-4−/− and IL-4+/+ mice infected with C. albicans. Purified splenic macrophages, peritoneal neutrophils, and CD4+ splenocytes were obtained from uninfected mice (lane 1) or from PCA-2–infected mice 3 d after reinfection with 106 CA-6 cells (lane 2). PCA-2–infected mice were treated with rIL-4 (IL-4−/− mice) or with anti-IL–4 mAb (IL-4+/+) before reinfection (lane 3), as described in Table 3. Cytokine gene expression was assessed by RT-PCR. N, no DNA added to the amplification mix during PCR; C, HPRT- or cytokine-specific controls.
IL-4 Induces IL-12 Production in Response to C. albicans.
To assess whether IL-4 would directly induce production of IL-12 in mice with candidiasis, we evaluated the effect of IL-4 priming on the ability of neutrophils and macrophages to release immunomodulating cytokines, including IL-12. Elicited peritoneal neutrophils and macrophages from IL-4–deficient mice were exposed in vitro to IL-4 before assessing cytokine levels produced in response to yeast cells. The results showed that priming with IL-4 greatly increased the ability of neutrophils, but not of macrophages, to release IL-12, with the levels of production being similar to those observed in IL-4+/+ mice (Fig. 6 A). Interestingly, priming with IL-4 increased IL-6 production from both types of cells. The potent stimulatory effect on neutrophils appeared to be due to a direct effect of IL-4 on these cells. In fact, expression of IL-4R in response to Candida cells was induced by priming with IL-4 as opposed to what observed in macrophages, whose IL-4R expression appeared to be decreased upon IL-4 priming (Fig. 6 B). The same potentiating effect was observed by adding IL-4 and yeast cells simultaneously (data not shown). These data suggest that IL-4 may be required for an optimal production of IL-12 by neutrophils in mice with candidiasis.
Figure 6.
Effect of IL-4 on cytokine production (A) and IL-4R expression (B) on neutrophils and macrophages from IL-4−/− (solid columns) or IL-4+/+ (dotted columns) mice. Elicited peritoneal neutrophils or macrophages were cultured in vitro with IFN-γ + LPS or C. albicans cells. Priming with IL-4 was done by adding 250 U/ml of rIL-4 8 h before C. albicans (striped columns, A; and lane 2, B). After 24 h incubation, supernatants were assayed for cytokine contents and cells for IL-4R expression by RT-PCR. *, Cytokine levels below the detection limit of the assay, as indicated on the y-axis. Lane 1, C. albicans alone. N, No DNA added to the amplification mix during PCR; C, HPRT- or cytokine-specific controls.
Late Treatment with Exogenous IL-4 Increased Th1-mediated Anticandidal Resistance in IL-4–deficient Mice.
We have already shown that IL-4 production late in infection is associated with the detection of protective CD4+ Th1s and is positively regulated by IL-12 (13). Neutralization, but not administration, of IL-4 late in the course of infection alters an already established CD4+ Th1 response to C. albicans (13). Here we show that treatment with IL-4 early in the course of infection greatly exacerbated the infection in both IL-4−/− and +/+ mice (data not shown), as expected (12). Late treatment with IL-4 greatly increased resistance of PCA-2–infected mutant mice to reinfection, as revealed by increased survival, decreased fungal growth in the kidneys, and increased production of IFN-γ and IL-2 by CD4+ T cells. In contrast, IL-4 neutralization reduced resistance of wild-type mice (Table 3). To assess whether mechanisms other than regulation of IL-12 produced by neutrophils may contribute to the ability of IL-4 to sustain Th1-dependent anticandidal immunity, IL-4 was administered to IL-4−/− and IL-4+/+ mice depleted of neutrophils. To this purpose, groups of mice with primary infection were injected with the anti-Ly6G mAb 2 d before reinfection and treated with exogenous IL-4. Depletion of neutrophils decreased resistance to reinfection in both types of mice, as expected (25). However, exogenous IL-4 partially restored resistance of neutrophil-depleted mice, as indicated by the increased survival, decreased fungal growth, and increased production of IFN-γ and IL-2. Interestingly, the effects of IL-4 administration were similar to those observed in mice with primary infection and treated with IL-12–neutralizing Ab before reinfection (data not shown). All together these data indicate that exogenous IL-4 can increase Th1-mediated anticandidal resistance in IL-4–deficient mice, and that mechanisms other than those regulating IL-12 production by neutrophils may significantly contribute to this effect.
Table 3.
Late IL-4 Administration Restores Resistance and CD4+ Th1 Cytokine Production in C. albicans–infected IL-4−/− Mice
| Mice* | Treatment† | MST | CFU × 103§ | Cytokine production‖ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rIL-4 | mAb Anti-Ly6G | mAb Anti–IL-4 | IFN-γ (ng/ml) | IL-2 (U/ml) | ||||||||||
| IL-4−/− | − | − | − | 7 | 1,220 ± 89 | <0.1 | 64 ± 5 | |||||||
| IL-4−/− | + | − | − | 27¶ | 360 ± 65¶ | 78 ± 10 | 156 ± 14 | |||||||
| IL-4−/− | − | + | − | 4 | 3,530 ± 148 | 19 ± 3 | 50 ± 8 | |||||||
| IL-4−/− | + | + | − | 11¶ | 1,047 ± 111¶ | 48 ± 8 | 144 ± 28 | |||||||
| IL-4+/+ | − | − | − | >60 | 225 ± 81 | 81 ± 13 | 187 ± 17 | |||||||
| IL-4+/+ | + | − | − | >60 | 304 ± 102 | 119 ± 12 | 148 ± 24 | |||||||
| IL-4+/+ | − | − | + | 21** | 581 ± 94 | 24 ± 3 | 89 ± 9 | |||||||
| IL-4+/+ | − | + | − | 5 | 2,824 ± 177 | 29 ± 4 | 66 ± 10 | |||||||
| IL-4+/+ | + | + | − | 14¶ | 811 ± 79¶ | 54 ± 2 | 104 ± 15 | |||||||
Mice were infected intravenously with 106 PCA-2 and reinfected 14 d later with 106 CA-6.
Treatment with rIL-4 (12 μg total dose, given together with the 11B11 anti–IL-4 mAb) or with the 11B11 mAb (2 mg, total dose) was done every other day from 6 to 12 d after primary PCA-2 infection. The neutrophil-depleting mAb (anti-Ly6G) was given intraperitoneally (100 μg/mouse) 2 d before reinfection. Neutrophil-depleted mice were treated twice with rIL-4 (as above) 2 and 1 d before reinfection.
Colony forming units in the kidneys at 3 d after reinfection.
Cytokine contents of supernatants of CD4+ T cells from spleens of 3-d reinfected mice cultured in vitro with C. albicans antigen and accessory macrophages.
P <0.05 Student's t test (rIL-4–treated versus untreated mice).
P <0.05 Student's t test (anti–IL-4 mAb-treated versus untreated IL-4+/+ mice).
Discussion
The results of the present study reveal a novel, previously unappreciated, role for IL-4 in vivo in mice with C. albicans infection, in which endogenous IL-4 is required for the induction of protective antifungal CD4+ Th1 responses. Previous studies demonstrating the beneficial (4, 5) or detrimental (12) effect of early IL-4 neutralization or administration, respectively, in mice with candidiasis led us to conclude that the occurrence of Th1 or Th2 responses positively correlated with the presence or the absence of IL-4. However, the subsequent finding that peak production of IL-4 occurred in mice with candidiasis concomitantly with the expression of protective, Th1-mediated resistance, and that depletion but not administration of IL-4 late in infection decreased resistance and production of IFN-γ and IL-12, suggested that IL-4 may have a positive effect in C. albicans infection, at least in the late stage (13). In the present study, we found that IL-4–deficient mice, while having an impaired Th2 response, did not default to the Th1 pathway, thus becoming highly susceptible in the late stage of C. albicans infection. Susceptibility to infection was not associated with signs of Th2 activation, such as production of IL-10 and high levels of circulating specific IgE (data not shown). Also, the message of IL-13 was only transiently induced in IL-4−/− and IL-4+/+ mice upon infection (data not shown), thus ruling out the possibility that IL-13, which signals through the IL-4Rα chain (36), may compensate for IL-4 deficiency. Therefore, IL-4 is the most important cytokine in the induction of CD4+ Th2 responses in candidiasis. A similar finding was observed in IL-4– deficient mice infected with a variety of pathogens, which have impaired Th2 responses but enhanced (23, 37–41) or not (14, 42) Th1 responses. We found that production of IFN-γ by CD4+ Th splenocytes was reduced in IL-4−/− mice compared to wild-type mice in response to virulent C. albicans, but also in experimental conditions of infection that otherwise result in the induction of CD4+ Th1. Defective activation of CD4+ Th1 was not associated with defective IL-12 responsiveness, as the expression of IL-12Rβ2s on these cells was not different from that observed in wild-type mice mounting a CD4+ Th1 response. Therefore, these data indicate that the unimpaired expression of IL-12β2 on CD4+ cells, due to the lack of inhibitory IL-4 (34, 35), may not correlate with the functional activation of CD4+ Th1s in murine candidiasis.
The results obtained in the present study clearly evidence a two-stage control of infection in mice with C. albicans infection. In the early stage, an innate immune response, if unopposed, may successfully control infectivity in the absence of a supportive CD4+ Th1 response, as observed in IL-4–deficient mice exposed to low or moderate yeast inocula or with gastrointestinal infection. In this condition, IFN-γ derived from a non–T cell compartment, presumably NK cells (33), represents one possible activator of antifungal effector cells (31, 32). However, in the late stage of infection, IL-12 and CD4+ Th1s producing IFN-γ are required to cope successfully with the pathogen. Indeed, IL-4−/− mice failed to develop protective CD4+ Th1 responses, as observed upon intravenous or mucosal immunization, thus becoming susceptible to infection at the late stage. Although IL-4–deficient mice appeared to be resistant to mucosal infection, the data of the present paper do not seem to suggest a possible late exacerbation of the infection, a finding compatible with the notion that IL-4 may mediate both protection (43) and pathology (44) at the mucosal level.
IL-4 appears to be required for the optimal occurrence of both innate and adaptive immune responses. Fungal elimination in IL-4−/− BALB/c mice was not as efficient as in genetically resistant similarly infected mice (our unpublished observation), thus suggesting that IL-4 may exert a positive effect on the antifungal effector function of phagocytic cells. In this regard, IL-4 has been reported to enhance murine macrophage mannose receptor activity (45) and to stimulate phagocytosis and killing of yeast cells by macrophages (46) and neutrophils (47). Both types of cells express surface receptors for IL-4 (48, 49). We have previously reported that IL-4 inhibits candidacidal activity and NO release by macrophages (27), and in the present study we found that both activities were unimpaired in IL-4– deficient mice. It is likely that IL-4 may both positively and negatively affect the antifungal effector functions of phagocytic cells, the net result being dependent on the dose and time of infection, as observed in leishmaniasis (50).
Whatever the effect of IL-4 on the antifungal effector functions of phagocytic cells, in this study we found that IL-4 efficiently primed neutrophils for IL-12 production in response to the fungus. The effect was associated with the induction of IL-4R on these cells. That IL-4 can prime for IL-12 production has already been observed (51, 52). In human mononuclear cells (51) the positive effect of IL-4 priming on IL-12 production appeared to be due to IL-10 inhibition. Because neutrophils produce IL-12 and IL-10 in response to C. albicans (25, 26), this mechanism could be at work in our system. Interestingly, priming with IL-4 also resulted in the release of high levels of IL-6, which is known to regulate IL-4 receptors on murine myeloid progenitor cells (53). Therefore, it appears that a positive amplification loop exists between IL-6 and IL-4 at the level of neutrophil response, which may be one possible mechanism underlying the beneficial effect of IL-6 in mice with candidiasis (54).
The defective production of IL-12 may likely contribute to the impairment of CD4+ Th1 development in IL-4–deficient mice with C. albicans infection, as it has been shown that exposure to IL-12 restores IFN-γ production in CD4+ T cells from IL-4−/− mice (22). However, the ability of IL-4 to increase IFN-γ and IL-2 production in the relative absence of neutrophils also suggests a possible direct effect of IL-4 on effector Th1s. Elegant studies by Flavell et al. have recently shown that developing Th1 lineage cells produce low levels of IL-4 as they differentiate into Th1 effectors (20, 21), thus implying that endogenous IL-4 could play an as yet not completely defined physiologic role in modulating Th1 effectors (20–22). Further studies will elucidate the important role of endogenous IL-4 in CD4+ Th1 differentiation and maintenance in C. albicans infection.
One interesting and still partially unresolved issue raised by our studies is where does IL-4 come from and which yeast/host factors regulate its production in mice with C. albicans infection. Evidence indicates that TCR-α/β+ cells, with an activated phenotype and a biased Vβ receptor expression, are the early producers of IL-4 in infected mice (55). The interaction between Candida cells and/or fungal products with cells producing IL-4 appears to occur through a superantigen-like mediated mechanism (55, 56). Whether the late peak production of IL-4 occurring in mice with candidiasis also occurs in recognition of superantigen-like molecules produced by fungal processing or fungal growth polymorphism remains an interesting possibility. Late peak production of IL-4 has also been observed in resistant mice infected with Leishmania major, and appears to be an essential component of the immune response to this parasite (57, 58).
In conclusion, this study provides several novel features of the immune response to C. albicans, including a physiologic role for IL-4 in the induction and maintenance of IL-12–dependent protective cell-mediated immunity.
Acknowledgments
The authors are grateful to Eileen Mahoney Zannetti for dedicated secretarial and editorial assistance.
This study was supported by AIDS Project 50A.0.28, Italy. The Basel Institute of Immunology has been founded and is supported by Hoffman LaRoche.
Footnotes
Abbreviations used in this paper: HPRT, hypoxanthine-guanine phosphoribosyl transferase; mRNA, messenger RNA: MST, median survival time; NO, nitric oxide; RT, reverse transcriptase.
References
- 1.Romani L, Puccetti P, Bistoni F. Biological role of Th cell subsets in candidiasis. Chem Immunol. 1996;63:115–137. [PubMed] [Google Scholar]
- 2.Puccetti P, Romani L, Bistoni F. A TH1-TH2-like switch in candidiasis: new perspectives for therapy. Trends Microbiol. 1995;3:237–240. doi: 10.1016/s0966-842x(00)88931-3. [DOI] [PubMed] [Google Scholar]
- 3.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993;150:925–931. [PubMed] [Google Scholar]
- 4.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1–associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Enssle K-H, Romani L, Bistoni F. Cure of murine candidiasis by recombinant soluble interleukin-4 receptor. J Infect Dis. 1994;169:1325–1331. doi: 10.1093/infdis/169.6.1325. [DOI] [PubMed] [Google Scholar]
- 6.Romani L, Cenci E, Mencacci A, Spaccapelo R, Grohmann U, Puccetti P, Bistoni F. Gamma interferon modifies CD4+subset expression in murine candidiasis. Infect Immun. 1992;60:4950–4952. doi: 10.1128/iai.60.11.4950-4952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaccapelo R, Romani L, Tonnetti L, Cenci E, Mencacci A, Tognellini R, Reed SG, Puccetti P, Bistoni F. TGF-β is important in determining the in vivo of susceptibility or resistance in mice infected with Candida albicans. . J Immunol. 1995;155:1349–1360. [PubMed] [Google Scholar]
- 8.Romani L, Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Tonnetti L, Grohmann U, Bistoni F. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. . J Immunol. 1994;152:3514–3521. [PubMed] [Google Scholar]
- 9.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf SF, Bistoni F. Interleukin-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J Immunol. 1994;153:5167–5175. [PubMed] [Google Scholar]
- 10.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Wolf S, Puccetti P, Bistoni F. Interleukin-12 but not interferon-γ production correlates with induction of T helper type-1 phenotype in murine candidiasis. Eur J Immunol. 1994;22:909–915. doi: 10.1002/eji.1830240419. [DOI] [PubMed] [Google Scholar]
- 11.Romani L, Bistoni F, Mencacci A, Cenci E, Spaccapelo R, Puccetti P. IL12 in Candida albicansinfections. Res Immunol. 1996;146:532–538. doi: 10.1016/0923-2494(96)83028-8. [DOI] [PubMed] [Google Scholar]
- 12.Tonnetti L, Spaccapelo R, Cenci E, Mencacci A, Puccetti P, Coffman RL, Bistoni F, Romani L. Interleukin-4 and -10 exacerbate candidiasis in mice. Eur J Immunol. 1995;25:1559–1565. doi: 10.1002/eji.1830250614. [DOI] [PubMed] [Google Scholar]
- 13.Mencacci A, Spaccapelo R, Del Sero G, Enssle K-H, Cassone A, Bistoni F, Romani L. CD4+ T-helper-cell responses in mice with low-level Candida albicansinfection. Infect Immun. 1996;64:4907–4914. doi: 10.1128/iai.64.12.4907-4914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann K-H, Lefrang K, Humborg C, Lederman B, Solbach W. IL-4–deficient BALB/c mice resist infection with Leishmania major. . J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki Y, Yang Q, Yang S, Nguyen N, Lim S, Liesenfeld O, Kojima T, Remington JS. IL-4 is protective against development of toxoplasmic encephalitis. J Immunol. 1996;157:2564–2569. [PubMed] [Google Scholar]
- 16.Roberts CW, Ferguson DJP, Jebbari H, Satoskar A, Bluethmann H, Alexander J. Differential roles for interleukin-4 during the course of Toxoplasma gondiiinfection. Infect Immun. 1996;64:897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noble A, Kemeny DM. Interleukin-4 enhances interferon-γ synthesis but inhibits development of interferon-γ-producing cells. Immunology. 1995;85:357–363. [PMC free article] [PubMed] [Google Scholar]
- 18.Platzer C, Ricther G, Muller W, Blocker H, Diamantstein T, Blackenstein T. Analysis of cytokine mRNA levels in interleukin-4-transgenic mice by quantitative polymerase chain reaction. Eur J Immunol. 1992;22:1179–1184. doi: 10.1002/eji.1830220511. [DOI] [PubMed] [Google Scholar]
- 19.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 20.Kamogawa Y, Minasi LE, Carding SR, Bottomly K, Flavell RA. The relationship of IL-4– and IFN-γ-producing T cells studied by lineage ablation of IL-4–producing cells. Cell. 1993;75:985–995. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4– and IFN-γ–producing CD4+ T cells following activation of naive CD4+T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- 22.Nakamura T, Lee RK, Nam SY, Podack ER, Bottomly K, Flavell RA. Roles of IL-4 and IFN-γ in stabilizing the T helper cell type 1 and 2 phenotype. J Immunol. 1997;158:2648–2653. [PubMed] [Google Scholar]
- 23.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 24.Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle K-H, Puccetti P, Romani L, Bistoni F. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis. 1995;171:1279–1288. doi: 10.1093/infdis/171.5.1279. [DOI] [PubMed] [Google Scholar]
- 25.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 26.Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 27.Cenci E, Romani L, Mencacci A, Spaccapelo R, Schiaffella E, Puccetti P, Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. . Eur J Immunol. 1993;23:1034–1038. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 29.Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 30.Reiner SL, Zheng S, Corry DB, Locksley RM. Constructing polycompetitor cDNA for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 31.Djeu, J.Y. 1993. Modulators of immune responses to fungi. In Fungal Infections and Immune Responses. J.W. Murphy, H. Friedman, and M. Bendinelli, editors. Plenum Press, New York. 521–532.
- 32.Kullberg BJ, van't Want JW, Hoogstraten C, van Furth R. Recombinant interferon-γ enhances resistance to acute disseminated C. albicansinfection in mice. J Infect Dis. 1993;168:436–443. doi: 10.1093/infdis/168.2.436. [DOI] [PubMed] [Google Scholar]
- 33.Romani L, Mencacci A, Cenci E, Spaccapelo R, Schiaffella E, Tonnetti L, Puccetti P, Bistoni F. Natural killer cells do not play a dominant role in CD4+ subset differentiation in Candida albicans–infected mice. Infect Immun. 1993;61:3769–3774. doi: 10.1128/iai.61.9.3769-3774.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Wilson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci USA. 1996;81:7189–7193. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von-der-Weid T, Kopf M, Kohler G, Langhorne J. The immune response to Plasmodium chabaudimalaria in interleukin-4–deficient mice. Eur J Immunol. 1994;24:2285–2293. doi: 10.1002/eji.1830241004. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence RA, Allen JE, Gregory WF, Kopf M, Maizels RM. Infection of IL-4–deficient mice with the parasitic nematode Brugia malayidemonstrates that host resistance is not dependent on a T helper 2–dominated immune response. J Immunol. 1995;154:5995–6001. [PubMed] [Google Scholar]
- 39.Pearlman E, Lass JH, Bardenstein DS, Kopf M, Hazlett FE, Diaconu E, Kazura JW. IL-4 and Th2 cells are required for development of experimental onchocercal keratitis (river blindness) J Exp Med. 1995;182:931–940. doi: 10.1084/jem.182.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearce EJ, Cheever A, Leonard S, Covalesky M, Fernandez-Botran R, Kohler G, Kopf M. Schistosoma mansoniin IL-4–deficient mice. Int Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- 41.Kopf M, LeGros G, Coyle AJ, Kosco-Vilbois M, Brombacher F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 42.Metwali A, Elliot D, Blum AM, Li J, Sandor M, Lynch RR, Noben-Trauth N, Weinstock JV. The granulomatous response in murine Schistosomiasis mansonidoes not switch to Th1 in IL-4–deficient C57BL/6 mice. J Immunol. 1996;157:4546–4553. [PubMed] [Google Scholar]
- 43.Vajdy M, Kosko-Vilbois MH, Kopf M, Kohler G, Lycke N. Impaired mucosal immune responses in interleukin 4–targeted mice. J Exp Med. 1995;181:41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 44.Mowat AM, Widmer MB. A role for IL-4 in immunologically mediated enteropathy. Clin Exp Immunol. 1995;99:65–69. doi: 10.1111/j.1365-2249.1995.tb03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redmond, H., L. Schuchter, J. Shou, L.K. Hofmann, P. Leon, L. Marodi, R. Johnston, and J. Daly. 1990. Interleukin 4 (IL-4) enhances macrophage microbicidal function. J. Leukocyte Biol. 48(Suppl.):24.
- 47.Bobert LA, Waters TA, Pugliese-Sivo CC, Sullivan LM, Narula SK, Grace MJ. IL-4 induces neutrophilic maturation of HL-60 cells and activation of human peripheral blood neutrophils. Clin Exp Immunol. 1995;99:129–136. doi: 10.1111/j.1365-2249.1995.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldman GM, Finbloom DS. Induction and regulation of IL-4 receptor expression on murine macrophage cell lines and bone marrow–derived macrophages by IFN-γ. J Immunol. 1990;145:854–859. [PubMed] [Google Scholar]
- 49.Park L, Friend D, Sassenfeld H, Urdal D. Characterization of the human B cell stimulatory factor 1 receptor. J Exp Med. 1987;166:476–488. doi: 10.1084/jem.166.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lezama-Davila CM, Williams DM, Gallagher G, Alexander J. Cytokine control of Leishmaniainfection in the BALB/c mouse: enhancement and inhibition of parasite growth by local administration of IL-2 or IL-4 is species and time dependent. Parasite Immunol. 1992;14:37–48. doi: 10.1111/j.1365-3024.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 51.D'Andrea A, Ma X, Aste-Amegaza M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor α production. J Exp Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takenaka H, Maruo S, Yamamoto N, Wysocka M, Ono S, Kobayashi M, Yagita H, Okamura K, Hamaoka T, Trinchieri G, Fujiwara H. Regulation of T cell–dependent and independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J Leukocyte Biol. 1997;61:80–87. doi: 10.1002/jlb.61.1.80. [DOI] [PubMed] [Google Scholar]
- 53.Feldman GM, Ruhl S, Bickel M, Finbloom DS, Pluznik DH. Regulation of interleukin-4 receptors on murine myeloid progenitor cells by interleukin-6. Blood. 1991;78:1678–1684. [PubMed] [Google Scholar]
- 54.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell development in interleukin 6–deficient mice infected with Candida albicans. . J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romani L, Puccetti P, Mencacci A, Spaccapelo R, Cenci E, Tonnetti L, Bistoni F. Tolerance to staphylococcal enterotoxin B initiates Th1 cell differentiation in mice infected with Candida albicans. . Infect Immun. 1994;62:4047–4053. doi: 10.1128/iai.62.9.4047-4053.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romani L, Howard DH. Mechanisms of resistance to fungal infections. Curr Opin Immunol. 1995;7:517–523. doi: 10.1016/0952-7915(95)80097-2. [DOI] [PubMed] [Google Scholar]
- 57.Reiner SL, Zheng S, Wang Z-E, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin-12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Launois P, Ohteki T, Swihart K, MacDonald HR, Louis JA. In susceptible mice Leishmania major induces very rapid interleukin-4 production by CD4+ T cells which are NK1.1− . Eur J Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]