Abstract
We have demonstrated that intestinal epithelial cells produce interleukin 7 (IL-7), and IL-7 serves as a potent regulatory factor for proliferation of intestinal mucosal lymphocytes expressing functional IL-7 receptor. To clarify the mechanism by which locally produced IL-7 regulates the mucosal lymphocytes, we investigated IL-7 transgenic mice. Here we report that transgenic mice expressing murine IL-7 cDNA driver by the SRα promoter developed chronic colitis in concert with the expression of SRα/IL-7 transgene in the colonic mucosa. IL-7 transgenic but not littermate mice developed chronic colitis at 4–12 wk of age, with histopathological similarity to ulcerative colitis in humans. Southern blot hybridization and competitive PCR demonstrated that the expression of IL-7 messenger RNA was increased in the colonic mucosal lymphocytes but not in the colonic epithelial cells. IL-7 protein accumulation was decreased in the goblet cell–depleted colonic epithelium in the transgenic mice. Immunohistochemical and cytokine production analysis showed that lymphoid infiltrates in the lamina propria were dominated by T helper cell type 1 CD4+ T cells. Flow cytometric analysis demonstrated that CD4+ intraepithelial T cells were increased, but T cell receptor γ/δ T cells and CD8α/α cells were not increased in the area of chronic inflammation. Increased IL-7 receptor expression in mucosal lymphocytes was demonstrated in the transgenic mice. These findings suggest that chronic inflammation in the colonic mucosa may be mediated by dysregulation of colonic epithelial cell–derived IL-7, and this murine model of chronic colitis may contribute to the understanding of the pathogenesis of human inflammatory bowel disease.
Interleukin 7 (IL-7) is a stromal cell–derived pleiotropic cytokine with cell growth–promoting activity of lymphoid precursors. IL-7 was originally described as a growth factor for precursor B cells (1–3). Subsequent in vitro studies have demonstrated that IL-7 is also a potent costimulus for immature and mature cells of the T cell lineage (4–8). Recently IL-7 messenger RNA (mRNA)1 was shown to be expressed in bone marrow stromal cells, thymus stromal cells, spleen, liver, kidney, and skin (9, 10). However, a potential role of IL-7 in peripheral lymphoid tissues remains unclear.
We have demonstrated the IL-7 mRNA expression and IL-7 protein production in human colonic epithelial cells (11). Immunohistochemical and in situ hybridization analysis have demonstrated that epithelial goblet cells are the major source of IL-7 production in the colonic mucosa. Interactions between mucosal lymphocytes and intestinal epithelial cells are thought to be crucial for maintaining mucosal immunity. Intestinal mucosal lymphocytes, including both intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs), may serve a critical role in the mucosal immune system by providing immune surveillance of epithelial cells (12–14). However, little is known about the precise mechanisms by which functional differentiation and proliferation of these cells occurs in the intestinal mucosa. We have shown that IL-7 receptor is expressed in the mucosal lymphocytes and intestinal epithelial cells, and intestinal epithelial cell–derived IL-7 may serve as a potent regulatory factor for the proliferation of these cells (11, 15). The importance of IL-7 as a mediator of local inflammatory responses remains unclear. To clarify the mechanism by which locally produced IL-7 may affect mucosal lymphocyte fraction and the role of IL-7 in colonic inflammation, we investigated transgenic mice carrying murine IL-7 cDNA and SRα promoter (16). Here we report that IL-7 transgenic mice develop chronic colitis, in concert with the expression of SRα/IL-7 transgene in the colonic mucosa. These findings favor the idea that chronic inflammation in the colonic mucosa is mediated by a colonic epithelial cell– derived IL-7.
Materials and Methods
IL-7 Transgenic Mice.
Generation of IL-7 transgenic mice has been previously described (16). PCR-amplified murine IL-7 cDNA was ligated with SRα promoter (17) and designated as SRα/IL-7. 21 founders of SRα/IL-7 transgenic mice were established by microinjection method into fertilized eggs of C57 BL6/J mice, and copy number of the transgene varied from 1 to 30. All mice were bred and housed under clean conditions with monitoring every month to ensure that they were free of specific pathogens. Transgenic lines were maintained by crossing transgenic mice with C57BL/6J mice and screening transgene-positive siblings by Southern blot analysis of their tail DNA. We used a founder mouse number 34 and its offspring, with copy number of transgenes <10. The line 34 mice developed dermatitis at earlier periods, as previously described (16).
Histological and Immunohistological Analysis.
The en block– fixed gastrointestinal tract was dissected into stomach, jejunum and ileum (small intestine), colon, and rectum. Tissue specimens were fixed in 10% formalin and embedded in paraffin. Sections were stained with hematoxylin-eosin. For immunohistochemistry, sections were fixed with 2% periodate lysine paraformaldehyde and frozen in OCT compound. Staining of sections was performed by the avidin–biotin complex method. 6 μm sections were incubated with the primary antibodies. These included anti-CD4 (L3T4), anti-CD8α (Ly-2), anti–TCR α/β (H57-597), and anti–δ chain of TCR-γ/δ (GL3) (PharMingen, San Diego, CA). Anti–murine IL-7 IgG mAb (Genzyme Corp., Cambridge, MA), anti–murine IL-7 receptor antibody (provided by S. Nishikawa, Kyoto University, Kyoto, Japan), or isotype-matched control antibody were also used. Biotinylated F(ab)2 fragments of immunoaffinity-purified antibodies were used as bridging reagents. Biotinylated antibodies were detected by immersing the sections for 40 min in a solution of streptoavidin–enzyme conjugates (Vectastain ABC kit; Vector Labs., Inc., Burlingame, CA). The localization of antigens was visualized by the incubation with diaminobenzidine solution and counterstained with methylgreen.
PCR and Southern Blotting for Murine IL-7 and IL-7 Receptor mRNA.
Cytoplasmic RNA was prepared from the indicated tissues using the RNAzol (Biotecx Lab, Houston, TX). First-strand cDNA was synthesized from 2 μg of total RNA with 1 μg oligo (dT) primer and 400 U/μl MMLV reverse transcriptase (Perkin Elmer Cetus, Norwalk, CT) by using SuperScript Preamplification System (GIBCO BRL, Gaithersburg, MD) in 20 μl of the reaction mixture. The sequences for PCR primer to detect SRα/ IL-7 transgene were: 5′ primer: 5′-CTC TAG AAC TAG TGG ATC CC-3′; 3′ primer: 5′-AGC TTC CTT TGT ATC ATC AC-3′; amplified fragment: 278 bp. Other PCR primers for murine IL-7, IL-7 receptor, and glyceraldehyde 3-phosphate dehydrogenase (G3PDH; as a house keeping gene) gene-specific amplification of cDNA in the analysis of mRNA expression by reverse transcription PCR (RT-PCR) were purchased from Clonotech (Palo Alto, CA). The sequences for IL-7 PCR primer were: 5′ primer: 5′-GCC TGT CAC ATC ATC TGA GTG CC-3′; 3′ primer: 5′-CAG GAG GCA TCC AGG AAC TTC TG-3′; amplified fragment: 496 bp. Primers for IL-7 receptor were: 5′ primer: 5′-CCC CAT AAC GAT TAC TTC AAA GGC TTC TGG-3′; 3′ primer: 5′-AGA GTT TGG CAG CAA GTC TTG ATA CAC AGG-3′; amplified fragment: 600 bp. The sequences for murine IFN-γ PCR primers were: 5′ primer: 5′ TGC ATC TTG GCT TTG CAG CTC TTC CTC ATG GC-3′; 3′ primer: 5′-TGC ACC TGT GGG TTG TTG ACC TCA AAC TTG GC-3′; amplified fragment: 365 bp. Primers for IL-4 were: 5′ primer: 5′-CCA GCT AGT TGT CAT CCT GCT CTT CTT TCT CTC G-3′; 3′ primer: 5′-CAG TGA TGT GGA CTT GGA CTG ATC TTC ATG GTG C-3′; amplified fragment: 357 bp. Primers for G3PDH were: 5′ primer: 5′-TGA AGG TCG GTG TGA ACG GAT TTG GC-3′; 3′primer: 5′-CAT GTA GGC CAT GAG GTC CAC CAC-3′; amplified fragment: 983 bp (mapping amplimers; Clonotech), used as controls. For PCR, 5 μl of cDNA was amplified in the presence of 0.5 μM of each of the 5′ and 3′ primers, and 0.5 U of Taq DNA polymerase (Ampli Tag; Perkin Elmer Cetus). PCR was performed in a DNA thermal cycler for 30 cycles (94°C for 45 s, 60°C for 45 s, and 72°C for 90 s) followed by a 7-min extension at 72°C. 9 μl of the PCR products was subjected to electrophoresis on 1.6% agarose gels and stained with 0.5 μg/ml ethidium bromide. A 100-bp DNA ladder (GIBCO BRL) was used as a marker. The specificity of the PCR products were validated by restriction enzyme digestion and Southern blot hybridization. The amplified IL-7 PCR product (496 bp) was digested by restriction enzyme EcoRI (10 U/μl; GIBCO BRL) into fragments. The predicted size of two fragments is 263 and 233 bp. For Southern blot, PCR products on the agarose gels were blotted onto nylon membrane (Biodyne; Pal BioSupport Corp., Glen Cove, NY), and hybridized with IL-7 gene-specific cDNA oligonucleotide probe (Clonotech) labeled by digoxigenin-dUTP using the DIG oligonucleotide 3′-end labeling kit (Boehringer Mannheim, Indianapolis, IN). The sequence for murine IL-7 probe was: 5′-TGT GAT ACT GTT AGT AAG TGG ACA TTG AAT-3′ (870–841). Hybridizations were done at 42°C for 18 h in a solution containing 50% formamide, 5× SCC, 0.02% SDS, 0.1% N-lauroyl sarcosine, and heat-denatured DIG-11-dUTP probe at 10 ng/ml. Blots were washed at a final stringency of 2× SSC at 68°C and processed for detection of digoxigenin-labeled oligonucleotide probes by enzyme-linked immunoassay (antidigoxigenin antibody linked to alkaline phosphatase) using the DIG nucleic acid detection kit (Boehringer Mannheim), according to the manufacturer's instructions. Subsequently, the membrane was equilibrated and then placed in a dark box containing luminogen PPD. The membrane was incubated for 2 h at 37°C and exposed for 15 min at room temperature. Negative controls (no DNAs) were incubated in each experiment and samples were run concurrently with control samples.
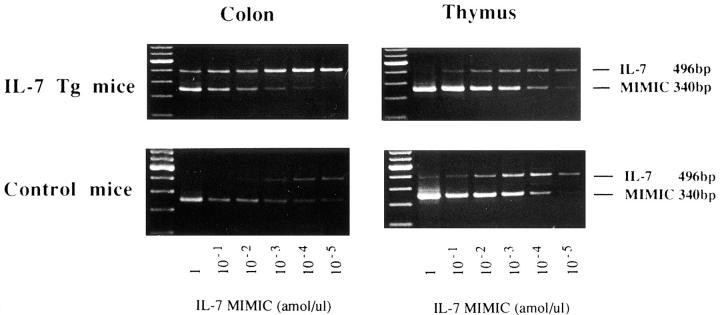
Quantitative PCR for Murine IL-7 mRNA Using MIMIC cDNA Construct.
Quantitative analysis was conducted by competitive PCR by using stepwise dilutions of competitor primer pairs as a template (PCR MIMICS; Clonotech). Two rounds of PCR amplification were performed. In the first PCR reaction, two composite primers were used. The first PCR primers were: 5′ primer: 5′-GCC TGT CAC ATC ATC TGA GTG CCC GCA AGT GAA ATC TCC TCC G-3′; 3′ primer: 5′-CAG GAG GCA TCC AGG AAC TTC TGG GGA CAA GAT ACT CAT CTG C-3′. Each composite primer has the target gene primer sequence attached to a short, 20-nucleotide stretch of sequence designed to hybridize to opposite strands of a heterologous DNA fragment. The desired primer sequences were thus incorporated during the PCR amplification. A dilution of the first PCR reaction was then amplified again using only the gene-specific primers. The size of the MIMIC was 340 bps. The yield of PCR MIMIC was then calculated and diluted to 100 attomole/μl. cDNA derived from 2 μg of total RNA was then amplified in the presence of 10-fold serial dilutions of the IL-7 MIMIC. The amount of change in IL-7 mRNA could be estimated by visually noting how much more of the MIMIC must be added to achieve an equimolar amount of product.
Preparation of Mucosal Lymphocytes and Intestinal Epithelial Cells from the Intestinal Mucosa.
We used a modification of previously established method for the isolation of mouse colonic IELs and epithelial cells (18). Intestine free of the lumen content was turned inside-out with the aid of polyethylene tubing. The inverted intestine was fastened to the tubing with a string and then cut into three to four segments. Up to 10 segments of intestines were transferred to a plastic box containing 250 ml of RPMI 1640 medium (2% FCS, 25 mM Hepes, 100 U/ml of penicillin, 100 mg/ml of streptomycin), and the box was shaken at 37°C for 45 min (150 rpm). Cell suspensions were collected in 250-ml tubes and further processed for the purification of IELs according to standard technique. In brief, the cell suspension was first passed through a glass wool column to deplete cell debris and sticky cells, and then subjected to Percoll discontinuous gradient centrifugation. After centrifugation, cells at the top of the 30% percoll solution were found to be enriched with colonic epithelial cells devoid of CD3+ cells. More than 90% of IELs were recovered at the interface of 44 and 70% percoll solutions. This new method yields IELs with minimum contamination by lymphocytes from Peyer's patches or lamina propria, and villus structure (lamina propria) without epithelium remained intact after each isolation. For isolation of LPLs from colon, mesenteric tissues and Peyer's patches were removed from intestines that were then cut open longitudinally, washed with PBS, and cut into smaller pieces. The dissected mucosa was incubated with Ca2+ Mg2+-free Hank's BSS containing 1 mM dithiothreitol (Sigma Chemical Co., St. Louis, MO) to remove mucus. The mucosa was then incubated four times in medium containing 0.75 mM EDTA (Sigma Chemical Co.). The supernatants from these incubations containing IEL population and LPLs were collected and incubated in medium with 0.02% collagenase (Worthington Biomedical Co., Freehold, NJ) and 0.01% DNase (Worthington Biomedical Co.). The fraction was pelleted twice through a 40% isotonic Percoll solution and the cells were centrifuged over Ficoll-Hypaque density gradient. The purity of resulting IELs and LPLs was analyzed by flow cytometry. Cell preparations were adequately pure, since α/β T cells made up 82–96% of the CD3+ cells in LPLs. Enriched CD4+ T cell populations were obtained by negative selection using mouse CD4+ T cell isolation columns (Isocell; Pierce Chemical Co., Rockford, IL).
Estimation of IL-7 Protein Production in Culture Supernatants of Colonic Epithelial Cells and Infiltrating Mucosal Lymphocytes.
IL-7 protein production by epithelial cells and infiltrating mucosal lymphocytes expressing IL-7 mRNA was measured by the proliferation assay of an IL-7–dependent cell line DW34 (17). Freshly isolated colonic epithelial cells, IELs and LPLs (5 × 106) were cultured in RPMI 1640 medium supplemented with fetal calf serum for 48 h. DW34 (104) cells were incubated with culture supernatants of those cells and cultured for 48 h. The cells were pulsed with 18.5 kBq of [3H]thymidine and harvested for radioactivity. The culture supernatants of colonic epithelial cells, IELs, and LPLs were treated with 10 μg/ml of mouse anti–human/mouse IL-7 mAb (Genzyme Corp.) or isotype-matched mouse Ig for 1 h before the application to DW34 cells, and concentrated using Centricon. Serial dilution (0–100 pg/ml) of rIL-7 (Genzyme Corp.) was used to calibrate IL-7 concentration.
Estimation of Cytokine Production in Culture Supernatants of Infiltrating Mucosal Lymphocytes.
Estimation of cytokine production in culture supernatants of infiltrating mucosal lymphocytes was assessed as previously described (19). To measure cytokine production, 24-well plates were coated with 10 μg/ml murine anti-CD3 antibody (clone 145-2C11; PharMingen) overnight at 4°C. Lamina propria CD4+ T cells (105) were then cultured in 1 ml of complete medium in precoated or uncoated wells, and 1 μg/ml soluble anti-CD28 antibody (clone 37.51; PharMingen) was added to the anti-CD3 antibody-coated wells. Culture supernatants were removed after 48 h and assayed for cytokine concentration. Cytokine concentrations were determined by specific ELISA kit for mouse IFN-γ, IL-2, and IL-4 (Endogen, Inc., Cambridge, MA). Optical densities were measured on an Intermet ELISA reader at a wavelength of 490 nm.
Flow Cytometry.
mAbs used included anti-CD3, anti-CD4, anti-CD8α, anti-CD8β (Ly-3.2), anti–TCR-α/β, and anti–δ chain of TCR-γ/δ (PharMingen). Flow cytometric two-color analysis carried out as described using the FACScan® (Becton Dickinson, Mountain View, CA). The data were presented as percentages of positive cells normalized to the number of total T (CD3+) cells and relative mean fluorescence. Background fluorescence was assessed by staining with control irrelevant isotype-identical mAbs.
Results
Development of Acute Colitis in the IL-7 Transgenic Mice.
IL-7 transgenic mice developed acute colitis with infiltrating neutrophils and lymphocytes at 1–3 wk of age (Fig. 1, A and B). At 1–3 wk of age when mice developed acute colitis, the SRα/IL-7 transgene and IL-7 mRNA expression in the colonic mucosa was revealed in the IL-7 transgenic mice (Fig. 1 C). IL-7 protein was also significantly expressed in the inflamed colonic mucosa of IL-7 transgenic mice (Fig. 1 D). Infiltrating T cells in the acute colitis lesion were CD4+ (Fig. 1 E) and T cell receptor γ/δ T cells in the IL-7 transgenic mice (Fig. 1 F).
Figure 1.
Transgenic mice carrying murine IL-7 cDNA by the SRα promoter developed acute colitis with infiltrating neutrophils and lymphocytes at 1–3 wk of age (A and B). At that time, the SRα/IL-7 transgene and IL-7 mRNA expression in the colonic mucosa was revealed (C). IL-7 protein was also significantly expressed in the inflamed colonic mucosa of IL-7 transgenic mice (D). Infiltrating T cells in the acute colitis lesion were mainly CD4+ T cells (E) and T cell receptor γ/δ T cells (F).
Development of Chronic Colitis in the IL-7 Transgenic Mice.
In the IL-7 transgenic founder number 34 and their progenies, diarrhea, weight loss, rectal prolapse, and remittent intestinal bleeding was observed frequently at 6–10 wk of age (Fig. 2). The fact that plural and independent SRα/ IL-7 transgenic lines developed rectal prolapse indicates that this phenomenon was caused by the transgene itself and not by the positional effect of the transgene insertion on chromosome. Wide time lags of 4–12 wk of age were observed for the onset of colitis in the mice. The final cumulative frequencies of colitis in the line 34 mice were ∼60% at 16 wk of age. Although several other lines of mice have developed rectal prolapse, the line 34 mice developed it at earlier periods and at high frequency. Therefore, the line 34 mice were used in the subsequent study.
Figure 2.
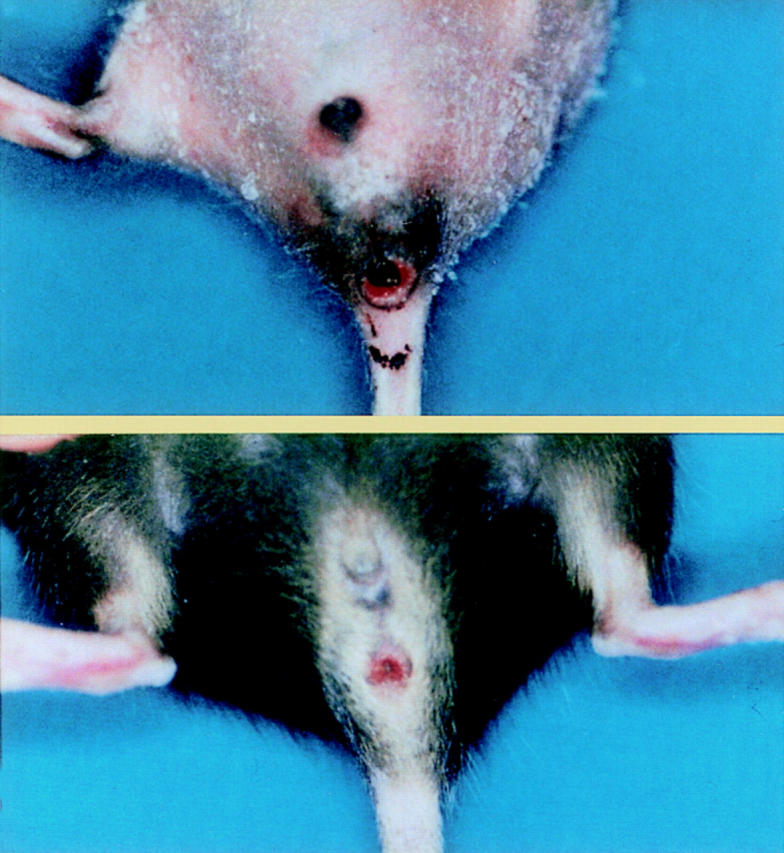
A line 34 transgenic mice carrying murine IL-7 cDNA by the SRα promoter developed rectal prolapse at 6 wk (A) and 10 wk (B). The IL-7 transgenic line 34 mice frequently showed rectal prolapse and remittent intestinal bleeding at 6–10 wk of age, suggesting the development of chronic colitis.
At 4–12 wk of age, histopathological examination of the colonic tissues revealed the development of chronic colitis in the IL-7 transgenic mice. Erosions and neutrophil infiltration were observed in the anal ring, but no ulceration was demonstrated (Fig. 3 A). The inflammatory cell infiltration and goblet cell depletion was most prominent in the rectum (Fig. 3 B), but observed throughout entire colon (Fig. 3, C and D). Crypt abscess (Fig. 3 E), paneth cell metaplasia (Fig. 3 F), and infiltration of eosinophils were observed in the lesions (Fig. 3 G). These features resembled the histopathological characteristics of ulcerative colitis in humans (20, 21). In contrast, control wild-type mice did not develop chronic intestinal inflammation at all.
Figure 3.

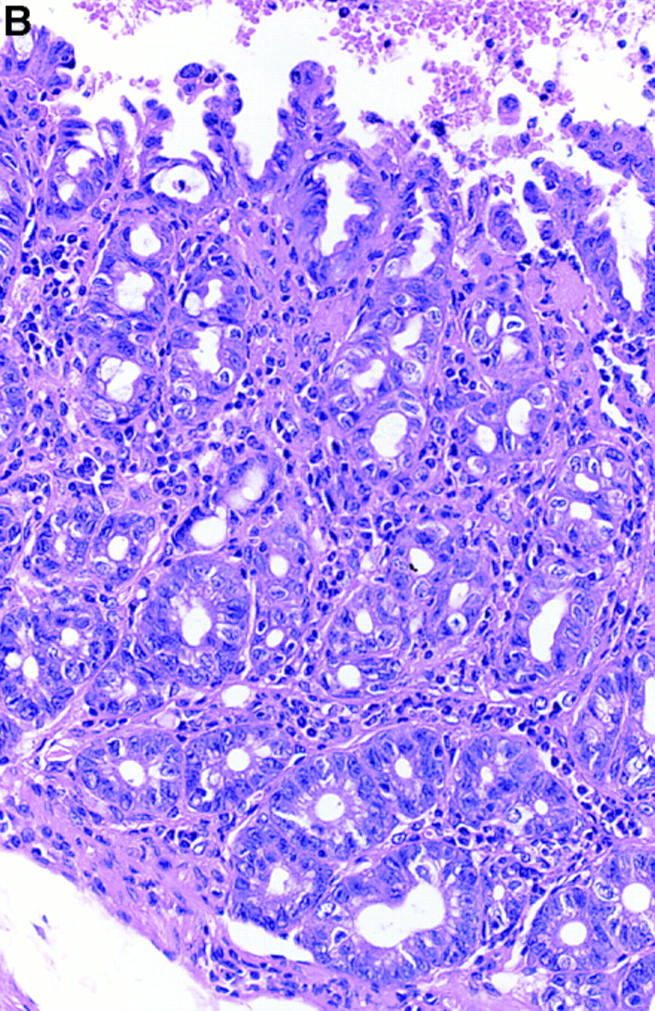


Histopathological examination (hematoxylin-eosin staining) of the colonic tissues revealed the development of chronic colitis in the IL-7 transgenic mice. Erosions and neutrophil infiltration were observed in the anal ring (original magnification: 100, A). The inflammatory cell infiltration and goblet cell depletion was most prominent in the rectum (original magnification: 200, B), but observed diffusely in the entire colon (original magnification: 100, C; original magnification: 200, D). Crypt abscess (original magnification: 200, E), paneth cell metaplasia (original magnification: 200, F), and infiltration of eosinophils (original magnification: 200, G) was observed in the lesions. These mimicked some histopathological characteristics of ulcerative colitis in humans.
SRα/IL-7 Transgene mRNA Expression in the Inflamed Colonic Mucosa of IL-7 Transgenic Mice.
In the IL-7 transgenic mice, 278-bp PCR amplified products of mRNA from the SRα/IL-7 transgene were expressed in the colonic mucosa. Interestingly, the SRα/IL-7 transgene expression in the colonic mucosa was parallel with development of chronic colitis in the IL-7 transgenic mice. SRα/IL-7 transgene was expressed in the colonic mucosa of transgenic mice with chronic colitis at both 4 and 8 wk, but not expressed in the colonic mucosa of mice without colitis at 4 and 8 wk (Fig. 4 A). In the colitis lesion of transgenic mice, SRα/IL-7 transgene was expressed in the colonic epithelial cells, IELs, and LPLs (Fig. 4 B). The transgene was constitutively expressed in the thymus, spleen, kidney, brain, lung, and skin, but not in the liver and muscle as previously reported (17).
Figure 4.
The expression of SRα/IL-7 transgene in the colonic mucosa. In the IL-7 transgenic mice, 278-bp PCR amplified products of mRNA from the SRα/IL-7 transgene were expressed in the colonic mucosa. The SRα/IL-7 transgene expression in the colonic mucosa is parallel with development of colitis in the IL-7 transgenic mice. SRα/IL-7 transgene was expressed in the colonic mucosa of transgenic mice with colitis at both 4 and 8 wk, but not expressed in the colonic mucosa of mice without colitis at 4 and 8 wk (A). In the colitis lesion of transgenic mice, SRα/IL-7 transgene was expressed in the colonic epithelial cells (EC), IELs, and LPLs (B). In contrast, the transgene was constitutively expressed in the thymus, spleen, kidney, brain, lung, and skin, but not in the liver and muscle as previously reported (data not shown).
IL-7 mRNA Expression in the Colonic Mucosa of Normal Mice and IL-7 Transgenic Mice.
RT-PCR analysis demonstrated IL-7 mRNA expression in normal murine colonic tissues (Fig. 5). The specificity of amplified bands was validated by their predicted size (496 bp). To ensure the presence of the correct predicted fragments, we digested the amplified IL-7 PCR product by restriction enzyme EcoRI. As shown in Fig. 5 A, 496-bp PCR products from murine colonic mucosa was digested into two predicted fragments with 263 and 233 bp. Southern blot analysis confirmed expression of IL-7 mRNA in normal murine colonic mucosa (Fig. 5 B). IL-7 mRNA expression was detected in the colon as well as the small intestine and stomach tissues. Equivalent hybridization was observed in Southern blot analysis using cDNA prepared from five separate sets of tissue samples.
Figure 5.
The expression of IL-7 mRNA in the colonic mucosa. RT-PCR analysis demonstrated IL-7 mRNA expression in normal murine colonic tissues (A). The specificity of amplified bands was validated by their predicted size (496-bp). To ensure the correct predicted fragments are present, we digested the amplified IL-7 PCR product by restriction enzyme EcoRI. 496-bp PCR products from murine colonic mucosa were digested into two predicted fragments with 263 and 233 bp. Markers represented 100-bp DNA ladder. Southern blot analysis confirmed the expression of IL-7 mRNA in normal colonic mucosa (B). PCR products were blotted onto nylon membrane and hybridized with IL-7 gene-specific cDNA oligonucleotide probe labeled with digoxigenin. IL-7 mRNA expression was detected in the colon, and a detectable expression of IL-7 was in small intestinal tissues and stomach tissues. Markers represented the 100-bp DNA ladder. For internal standard, amplified bands for G3PDH were used as the housekeeping gene.
Overexpression of IL-7 mRNA in the Colonic Mucosal Lymphocytes but not in the Colonic Epithelial Cells in the Inflamed Colonic Mucosa of IL-7 Transgenic Mice.
To assess the change of IL-7 mRNA expression in the transgenic mice, we performed competitive PCR analysis for various tissues. As shown in Fig. 6, competitive PCR demonstrated that about a 100-fold increase in IL-7 mRNA transcripts was detected in the inflamed colonic mucosa of the IL-7 transgenic mice. In contrast, no difference of the IL-7 mRNA expression was observed in the thymus tissues between wild-type mice and IL-7 transgenic mice. We also detected increased IL-7 mRNA in the small intestine with ileitis, but the expression was quite weak compared to that in the colon with inflammation (data not shown). However, no difference of the IL-7 mRNA expression was observed in the colonic and small intestinal mucosa where no inflammation was observed. These data demonstrate that increased IL-7 mRNA expression occurs in the inflammation site of the intestinal tissues in IL-7 transgenic mice.
Figure 6.
Quantity of IL-7 mRNA expression in the colon and thymus tissues in the IL-7 transgenic mice. Competitive PCR demonstrated that 100-fold increase in IL-7 mRNA transcripts was detected in the inflamed colonic mucosa of the IL-7 transgenic mice (IL-7 Tg), as compared to those in wild-type mice (Control). On the contrary, no difference of the IL-7 mRNA expression was observed in the thymus tissues between control mice and IL-7 transgenic mice.
To assess the distribution of increased IL-7 mRNA in the inflamed colonic tissues, we examined the differential expression of IL-7 mRNA between the colonic epithelial cells and colonic mucosal lymphocytes. Colonic IELs and LPLs isolated from the inflamed colonic tissues of IL-7 transgenic mice expressed a significant amount of IL-7 mRNA (Fig. 7 A). However, IELs and LPLs from the colonic tissues of littermate mice showed no expression of IL-7 mRNA. Moreover, competitive PCR analysis demonstrated that IL-7 mRNA expression in the colonic epithelial cells was not changed in transgenic mice (Fig. 7 B). These indicated that increased IL-7 expression in the inflamed colonic mucosa of IL-7 transgenic mice was derived from overexpression of IL-7 mRNA in infiltrated mucosal lymphocytes, but not in the colonic epithelial cells. It is important to assess the correlation between the development of colitis and IL-7 mRNA expression in the colonic tissues. Increased IL-7 mRNA was observed in the colonic mucosa at 2 wk of age. However, the onset of colitis was at as early as 4 wk of age.
Figure 7.
Analysis of the differential expression of IL-7 mRNA in the colonic mucosa. PCR analysis demonstrated that colonic IELs and LPLs isolated from the inflamed colonic tissues of IL-7 transgenic mice expressed a significant amount of IL-7 mRNA (A). However, IELs and LPLs from the colonic tissues of littermate mice showed no expression of IL-7 mRNA. Interestingly, competitive PCR analysis demonstrated that IL-7 mRNA expression in the colonic epithelial cells was not changed between control mice and the transgenic mice (B).
IL-7 Protein Expression and Production in the Inflamed Colonic Mucosa of IL-7 Transgenic Mice.
To clarify whether increased IL-7 mRNA expression in the colonic mucosa of transgenic mice led to the local overproduction of IL-7 protein, immunohistochemical analysis using an anti–IL-7 antibody was done in the colonic mucosa. As shown in Fig. 8 A, IL-7 protein expression was demonstrated in the colonic epithelial cells and epithelial goblet cells in the colonic mucosa of normal mice. IL-7 protein expression was not changed in the colonic mucosa of IL-7 transgenic mice at 4–6 wk of age (data not shown). However, IL-7 protein expression seemed to be decreased in the goblet-depleted colonic epithelial cells in the chronic inflamed colonic mucosa at 8–10 wk of age (Fig. 8 B).
Figure 8.

Immunohistochemical analysis using rabbit anti–murine IL-7 Ab confirmed IL-7 protein expression in the human intestinal epithelial cells and epithelial goblet cells. The reactivity of anti–murine IL-7 IgG Ab but not control rabbit IgG is confined to the colonic epithelial cells and epithelial goblet cells in the normal murine intestinal mucosal tissues (A). Note that biotin-conjugated goat anti–rabbit Ab alone did not show the reactivity. IL-7 protein expression was not changed in the colonic mucosa of IL-7 transgenic mice at 4–6 wk of age (data not shown). However, IL-7 protein expression seemed to be decreased in the goblet-depleted colonic epithelial cells in the inflamed colonic mucosa of IL-7 transgenic mice at 8–10 wk of age (B).
Our previous study indicated that IL-7 protein expression by immunohistochemical technique was demonstrated only in the tissue where secreted IL-7 protein was stored and accumulated (11). Therefore, IL-7 protein production by isolated epithelial cells and infiltrating mucosal lymphocytes expressing IL-7 mRNA was assessed in the proliferation assay of an IL-7–dependent cell line DW34. Isolated epithelial cells, IELs, and LPLs from the inflamed colonic mucosa of IL-7 transgenic mice produced an increased amount of IL-7 (data not shown). These results indicated that IL-7 production was not decreased in the inflamed colonic mucosa of IL-7 transgenic mice in parallel with the increase of IL-7 mRNA, but accumulation of IL-7 protein in the colonic mucosa was decreased in the goblet cell– depleted epithelial cells.
The Infiltrating Cells in the Inflamed Colonic Mucosa Consist of CD4-positive T Cells in IL-7 Transgenic Mice.
The phenotype of the infiltrating cells was analyzed by immunohistochemistry and flow cytometry. As shown in Fig. 9, lymphoid infiltrates in the lamina propria at the colitis lesion were dominated by T cells bearing CD4. Both TCR-α/β T cells and -γ/δ T cells infiltrated in the lamina propria of colonic mucosa (data not shown). However, γ/δ intraepithelial T cells were not increased at the chronic colitic lesion (Fig. 10), though γ/δ T cells were the main components of mononuclear cells infiltrating at the dermatitis lesion of IL-7 transgenic mice as previously reported (17). Flow cytometric analysis of isolated cells from intraepithelium demonstrated that CD4+ T cells, but not CD8+ T cells were significantly increased at the colitis lesions. Infiltrating CD8+ intraepithelial T cells mainly expressed the α/β heterodimer, indicating that these cells were thymus dependent (Fig. 10).
Figure 9.

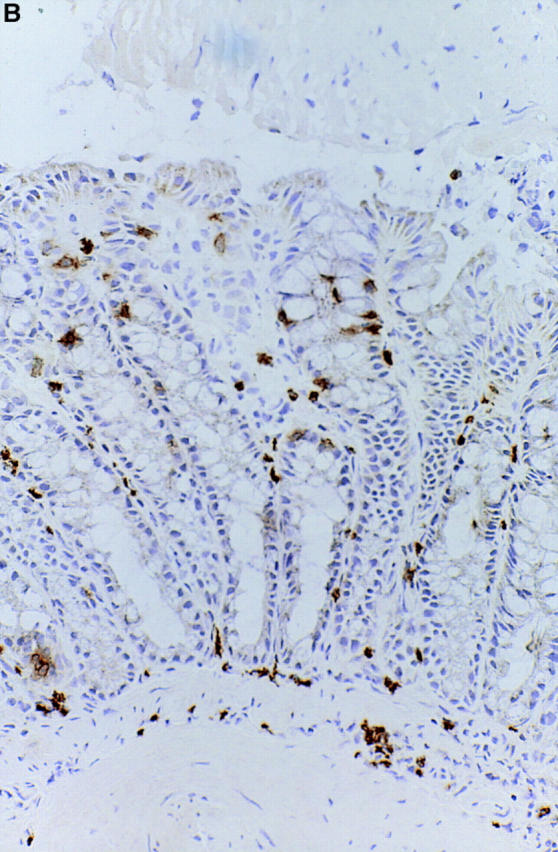
The phenotype of the infiltrating mucosal lymphocytes at the colitis lesion was analyzed by immunohistochemistry. Sections of colonic tissues from IL-7 transgenic mice were stained with anti-CD4 (A) and anti-CD8 (B) antibodies. Lymphoid infiltrates in the lamina propria at the colitis lesion were dominated by T cells bearing CD4+. Both TCR-α/β T cells and γ/δ T cells infiltrated in the lamina propria of colonic mucosa (data not shown).
Figure 10.

The phenotype of the infiltrating intraepithelial lymphocytes in the colitis lesion was analyzed by flow cytometric analysis. Flow cytometric analysis of isolated cells from IEL demonstrated that T cell receptor γ/δ IELs were not increased in the IL-7 transgenic mice (IL-7 Tg) as compared to those in control littermate mice (Control), although γ/δ T cells were the main components of mononuclear cells infiltrating in the dermatitis lesion of IL-7 transgenic mice as previously reported (17). CD4+ T cells, but not CD8+ T cells were significantly increased in the colitis lesions. Moreover, infiltrating CD8+ IELs in the IL-7 transgenic mice expressed mainly α/β heterodimers, indicating that these cells were thymus dependent. A minimum of 5,000 cells was analyzed for each histogram.
To assess the phenotype of infiltrating lamina propria CD4+ T cells in the chronic inflamed colonic mucosa of IL-7 transgenic mice, we examined CD45RB expression, cytokine mRNA expression, and protein production by these CD4+ T cells. Flow cytometric analysis of isolated cells from the intestinal mucosa demonstrated that either CD45RBhigh CD4+ or CD45RBlow CD4+ lamina propria T cells were not significantly increased in the IL-7 transgenic mice (data not shown). We also performed PCR analysis of the homogenates of the chronic inflamed colonic mucosa to assess cytokine mRNA expression, but did not find the changes in IFN-γ mRNA expression and IL-4 mRNA expression in the IL-7 transgenic mice (data not shown). We then examined cytokine protein production by isolated cells from the chronic inflamed colonic mucosa. Isolated cells were cultured and culture supernatants were analyzed for concentration of Th1 (IL-2, IFN-γ) and Th2 (IL-4) cytokines by specific ELISA. Unstimulated lamina propria CD4+ T cells from IL-7 transgenic mice and normal littermate mice produced marginal amounts of both IL-2 and IFN-γ. Interestingly, isolated lamina propria CD4+ T cells from the inflamed colonic mucosa of IL-7 transgenic mice produced significantly higher levels of both IL-2 and IFN-γ than lamina propria CD4+ T cells from littermate mice after stimulation with anti-CD3 and anti-CD28 mAbs (Fig. 11, A and B). In contrast to Th1 type cytokine production, secretion of IL-4 by unstimulated lamina propria CD4+ T cells from IL-7 transgenic mice was identical to lamina propria CD4+ T cells from littermate mice, and in anti-CD3 and anti-CD28 mAb-stimulated lamina propria CD4+ T cells from IL-7 transgenic mice, the average secretion of IL-4 was reduced compared with littermate mice (Fig. 11 C).
Figure 11.

Cytokine protein production by isolated cells from the chronic inflamed colonic mucosa of IL-7 transgenic mice (shaded bars) and from normal mucosa of normal littermate mice (white bars). Isolated cells were cultured and culture supernatants were analyzed for concentration of Th1 (IL-2, A; IFN-γ, B) and Th2 (IL-4, C) cytokines by specific ELISA. Unstimulated lamina propria CD4+ T cells from IL-7 transgenic mice and littermate mice produced marginal amounts of both IL-2 and IFN-γ. Isolated lamina propria CD4+ T cells from the inflamed colonic mucosa of IL-7 transgenic mice produced significantly higher levels of both IL-2 and IFN-γ than lamina propria CD4+ T cells from littermate mice after stimulation with anti-CD3 and anti-CD28 mAbs. In contrast to Th1 type cytokine production, secretion of IL-4 by unstimulated lamina propria CD4+ T cells from IL-7 transgenic mice was identical to lamina propria CD4+ T cells from control littermate mice, and in anti-CD3 and anti-CD28 mAb-stimulated lamina propria CD4+ T cells from IL-7 transgenic mice, the average secretion of IL-4 was reduced compared with littermate mice. Data represent mean ± standard error from three independent experiments done in triplicate. *P <0.05, statistically significant as compared with control littermate mice by Mann-Whitney U test.
Increased IL-7 Receptor Expression in the Inflamed Colonic Mucosa of IL-7 Transgenic Mice.
To assess the effect of decreased IL-7 protein expression in the proliferation of infiltrating mucosal lymphocytes, IL-7 receptor mRNA expression was analyzed in the colonic mucosa. Southern blot analysis demonstrated that IL-7 receptor mRNA was not readily detectable in the colonic mucosa from wild-type mice. However, IL-7 receptor expression was strongly detected in the inflamed colonic mucosa of transgenic mice (Fig. 12). Receptor expression was not strong in the colonic mucosa of transgenic mice without obvious inflammation. Immunohistochemical analysis demonstrated that IL-7 receptor was mainly expressed in the colonic mucosal lymphocytes. There was no difference in the expression of IL-7 receptor in the thymus between IL-7 transgenic mice and wild-type mice (data not shown).
Figure 12.
Southern blot analysis demonstrated that IL-7 receptor (IL-7R) mRNA was not readily detectable in the colonic mucosa from normal littermate mice. However, IL-7 receptor expression was strongly detected in the inflamed colonic mucosa of transgenic mice. The specificity of the amplified bands for IL-7 receptor mRNA was validated by their predicted size (600 bp). Markers represented 100-bp DNA ladder. For internal standard, amplified bands for G3PDH were used. There was no difference in the expression of IL-7 receptor in the thymus between IL-7 transgenic mice and normal littermate mice (data not shown).
Discussion
IL-7 is a growth factor for both murine and human, T cell lineage cells. In the mouse, abundant IL-7 mRNA expression has been demonstrated in bone marrow stroma, thymus, spleen, liver, kidney, and keratinocytes (1, 9, 10). Recent studies have shown that IL-7 is expressed in human thymus, spleen, and keratinocytes (7–9), although a potential role of IL-7 in peripheral lymphoid tissues remains unclear. We have demonstrated that human intestinal epithelial cells produce IL-7 that serves as a regulatory factor for proliferation of intestinal mucosal lymphocytes expressing functional IL-7 receptor (11, 15). In this study, we demonstrated the presence of epithelial cell–derived IL-7 in the normal murine intestine.
To clarify the mechanism by which intestinal epithelial cell-derived IL-7 regulates the mucosal lymphocytes, we investigated transgenic mice carrying murine IL-7 cDNA and SRα promoter. The SRα expression system is shown to be one or two orders of magnitude more active than SV40 early promoter in a wide variety of cell types including lymphoid cells and epithelial cells, and promotes a high level of expression of various lymphokine cDNAs (17). We established transgenic mice carrying murine IL-7 cDNAs by three promoters, SRα, SV40 early, and metallothionein I (16). SRα/IL-7 transgenic mice developed severe dermatitis in 7 of 30 founder mice at 4–9 wk of age. However, SV40 early and metallothionein I/IL-7 transgenic mice never developed dermatitis in 9-mo observation periods. Interestingly, some SRα/IL-7 transgenic lines, but not SV40 early/IL-7, or metallothionein I/IL-7 transgenic mice developed anal bleeding and rectal prolapse. Therefore, we used the SRα/ IL-7 transgenic mice in this study. The fact that plural SRα/IL-7 transgenic lines developed rectal prolapse means that this phenomenon was caused by the transgene itself and not by the positional effect of the transgene insertion on chromosome.
In this study, we provide the evidence that SRα/IL-7 transgenic mice developed chronic colitis with perianal bleeding and rectal prolapse. Histological analysis showed erosions, redness, wall thickening with goblet cell-depleted epithelium, and mononuclear cell infiltration. In the gastrointestinal tract, inflammation was limited to the colon and ileum, and the most severe inflammation occurred in the rectum. The inflammatory cells then consisted of lymphocytes, plasma cells, and macrophages in the IL-7 transgenic mice. These mimicked histopathological characteristics of ulcerative colitis in humans (20, 21).
In the IL-7 transgenic mice, the SRα/IL-7 transgene was constitutively expressed in the thymus, spleen, kidney, brain, lung, and skin, but not in the liver and muscle as previously reported (16). Interestingly, development of colitis in the IL-7 transgenic mice was parallel with the SRα/IL-7 transgene expression in the colonic mucosa. Transgenic mice with SRα/IL-7 transgene expression in the colonic mucosa developed colitis, but mice without transgene in the colonic mucosa did not. This result indicated that SRα/IL-7 transgene expression in the colonic mucosa was directly related to chronic inflammation in the colon of transgenic mice. It would be interesting to clarify whether the increased expression of the transgene in the colonic mucosa induces chronic colitis or whether chronic inflammation in the colonic mucosa induces the activation of the transgene. However, conclusive evidence has not been easily obtained. The final cumulative frequencies of colitis in the line 34 IL-7 transgenic mice were not 100%, but 60%. It was quite difficult or impossible to assess which appears earlier in the same mice, the development of colitis or SRα/ IL-7 transgene mRNA expression in the colonic mucosa. However, we found that SRα/IL-7 transgene mRNA expression was not changed in the thymus, spleen, and skin without dermatitis between mice with colitis and mice without colitis (Watanabe, M., unpublished observation). Moreover, we found that 15–20% of transgenic mice with the SRα/IL-7 transgene mRNA expression in the colonic mucosa did not develop chronic colitis. The preliminary histopathological observation of colonic mucosa of these mice demonstrated that they have little infiltration of mononuclear cells in the lamina propria. On the basis of these findings, we suggest that the expression of detectable SRα/ IL-7 transgene mRNA in the colonic mucosa is a sign of developing colitis. However, we cannot deny that after initiation of colitis by the transgene expression, inflammation in the colonic mucosa also induces the activation of the transgene and eventually transgene mRNA expression is increased.
Southern blot hybridization and competitive PCR demonstrated that expression of IL-7 mRNA was increased in the inflamed colonic mucosa of the IL-7 transgenic mice. The distribution of IL-7 mRNA in the colonic tissues was quite interesting. Analysis of differential expression of IL-7 mRNA showed that colonic mucosal lymphocytes expressed a significant amount of IL-7 mRNA, but IL-7 mRNA expression in the colonic epithelial cells was unchanged in the inflamed mucosa. Therefore, increased IL-7 mRNA expression in the inflamed colonic mucosa of IL-7 transgenic mice was derived from the colonic mucosal lymphocytes. Interestingly, immunohistochemical analysis demonstrated that IL-7 protein expression in the colonic mucosa and epithelial cells seemed to be decreased at colitis lesion in the IL-7 transgenic mice. Recent studies demonstrated that intestinal epithelial cells express IL-7 receptor and IL-7 regulates the growth and function of epithelial cells by autocrine or paracrine mechanism (22, 23). Overexpression of IL-7 in mucosal lymphocytes may lead to the downregulation of IL-7 protein production in the colonic epithelial cells. However, our previous study indicated that IL-7 protein expression by immunohistochemical technique was demonstrated only in the tissue where secreted IL-7 protein was stored and accumulated (11). Therefore, we assessed IL-7 protein production by isolated cells. Isolated epithelial cells, IELs, and LPLs form the inflamed colonic mucosa of IL-7 transgenic mice produced an increased amount of IL-7. These results indicated that IL-7 production and secretion were increased in the inflamed colonic mucosa of IL-7 transgenic mice in parallel with the increase of IL-7 mRNA, but accumulation of IL-7 protein in the colonic mucosa was decreased in the colitis mucosa. We have shown that intestinal epithelial goblet cells are the major source of mucosal IL-7 in the human intestinal mucosa (11). In this study, goblet cells in the murine colon also produce IL-7. In the inflamed colonic mucosa of IL-7 transgenic mice, histopathological examination revealed the depletion of goblet cells. This result is consistent with the findings that IL-7 protein accumulation was decreased in the inflamed colonic mucosa of the transgenic mice.
How does mucosal lymphocyte–produced IL-7 in the colonic mucosa of IL-7 transgenic mice affect the proliferation of mucosal lymphocytes? We have demonstrated IL-7 mRNA expression and protein production by human colonic epithelial cell line HT29-18-N2 cells after they differentiated into the goblet cell phenotype (Watanabe, M., unpublished observation). However, these cell lines produced marginal amounts of IL-7 in the culture supernatants though these high levels of expressed IL-7 mRNA. The puzzle regarding the low IL-7 protein production is not specific to the HT29 sublines. As other investigators discussed (9), almost all of the cells and cell lines that have been found to produce IL-7 release IL-7 protein only at low levels. The case is the most extreme in the human cells, since it was difficult to detect IL-7 protein production even in the SK-HEP cell line that was used to clone the cDNA for the human IL-7 (24). These observations suggested that IL-7 protein may be rapidly degraded. Locally produced IL-7 from IELs and LPLs in the colonic mucosa may be also degraded or internalized when it does not accumulate in the colonic mucin of the goblet cells. Therefore, we suggest that accumulation of IL-7 protein in the mucus of the goblet cells in the colonic mucosa is crucial for regulating the proliferation of mucosal lymphocytes.
The importance of IL-7 as a mediator of local inflammatory responses remains unclear. However, a possible role for IL-7 in mucosal inflammation has been recently suggested by the finding of decreased IL-7 mRNA expression and IL-7 protein accumulation in the colonic epithelium in inflamed mucosa of patients with ulcerative colitis (Watanabe, M., unpublished observation). We have also demonstrated that IL-7 mRNA expression and IL-7 protein accumulation was significantly increased in the colonic mucosa of patients with acute Salmonella infectious colitis, where goblet cell depletion was not observed. In the IL-7 transgenic mice, decreased IL-7 protein accumulation in the colonic epithelial cells was demonstrated after the onset of colitis. These results favor the idea that the decrease of IL-7 protein accumulation due to the deletion of goblet cells involves the chronic inflammation of the intestinal mucosa.
Lymphoid infiltrates in the lamina propria at the colitis lesion of IL-7 transgenic mice were dominated by CD4+ T cells. Analysis of cytokine production by anti-CD3 and anti-CD28 mAb-stimulated lamina propria CD4+ T cells from chronic inflamed colonic mucosa of IL-7 transgenic mice showed elevated levels of IL-2 and IFN-γ production. In contrast, IL-4 secreted by those lamina propria T cells were decreased in IL-7 transgenic mice. These results indicate that Th1 type CD4+ T cells were dominated in the infiltrating lamina propria T cells in the chronic inflamed colonic mucosa of IL-7 transgenic mice. This Th1 pattern of cytokine production from lamina propria CD4+ T cells was also observed in the inflamed colonic mucosa of mice with the hapten reagent 2, 4, 6-trinitrobenzene sulfonic acid–induced colitis (19). Flow cytometric analysis of isolated cells from intraepithelium demonstrated that CD4+ T cells, but not CD8+ T cells were increased at the colitis lesions. Interestingly, infiltrating CD8+ T cells mainly expressed the α/β heterodimer, indicating that these cells were thymus dependent. TCR-γ/δ intraepithelial T cells were not increased, although Uehira et al. reported that transgenic mice constitutively expressing IL-7 developed severe dermatitis and double negative γ/δ T cells were the main components of the infiltrating mononuclear cells at the dermatitis lesion (16). Recent evidence of an extrathymic pool of TCR-α/β mucosal lymphocytes in the gut suggests that intestinal epithelial cells may share some differentiation-inducing capacities with thymic epithelial cells, leading to in situ TCR rearrangement of extrathymically derived T cells (25). We have recently demonstrated the presence of a novel lymphoid tissue, cryptopatches (CP), in the murine intestinal mucosa where clusters of IL-7 receptor+ c-kit+ Thy-1+ lympho-hematopoietic progenitor developed in IL-7–dependent fashion (26). These results indicated that IL-7 may mediate an extrathymic pathway of mucosal T cell proliferation. Therefore, overexpression of IL-7 protein in the colitis mucosa of acute stage and decrease of IL-7 protein accumulation in goblet cell–depleted colonic mucosa of the chronic colitis may disturb IL-7–dependent proliferation of lymphocytes and progenitors in the mucosal tissues.
We have demonstrated that mucosal T cells in the normal murine as well as human colonic mucosa constitutively express the receptor for IL-7. In the present study, IL-7 receptor mRNA expression was strongly detected in the inflamed colonic mucosa of IL-7 transgenic mice, but not readily detectable in the colonic mucosa from normal littermate mice. Receptor expression was not strong in the colonic mucosa of transgenic mice without obvious inflammation. These findings indicated that the infiltrating mucosal lymphocytes in the colonic lesion of IL-7 transgenic mice highly expressed the IL-7 receptor. Interestingly, IL-7 receptor expression was quite marked in the mucosal lymphocytes of severely inflamed colonic mucosa from patients with ulcerative colitis (Watanabe, M., unpublished observation). These results raise the possibility that decreased mucosal IL-7 protein accumulation observed in the colonic mucosa with chronic inflammation of IL-7 transgenic mice may result in the increase of infiltrating mucosal lymphocytes expressing the IL-7 receptor. Previous studies have shown that IL-7 stimulates the proliferation of human mature T cells after exogenous stimulation in short time culture, suggesting that IL-7 receptor is not expressed by resting PBLs, but expressed after activation. Therefore, our results indicate that mucosal lymphocytes are activated in the transgenic mice. These activated T cells may produce regulatory factors such as cytokines that augment the inflammation process.
It has been reported that IL-7 acts synergistically with anti-CD3 Ab to stimulate proliferation of human T cells in the thymus and peripheral blood. However, our previous studies demonstrated that IL-7 inhibited anti-CD3–induced proliferation of mucosal T cells in the normal colonic mucosa. Moreover, we showed that IL-7 alone inhibited the growth of mucosal lymphocytes isolated from the inflamed colonic mucosa (Watanabe, M., unpublished observation). These suggested that IL-7 may inhibit the proliferation of activated and IL-7 receptor–expressing mucosal T cells in the intestinal mucosa.
We are now doing the precise time course studies concerning development of colitis, IL-7 protein expression in the colonic mucosa, and phenotype of infiltration lymphocytes in the IL-7 transgenic mice. IL-7 transgenic mice developed acute colitis with infiltrating neutrophils and lymphocytes at 1–3 wk of age. At that time, IL-7 protein was significantly expressed in the inflamed colonic mucosa of IL-7 transgenic mice, but then decreased with depletion of goblet cells. Accumulation of IL-7 protein in the colonic mucosa was significantly decreased at 8–12 wk of age. We also demonstrated that at 1–3 wk of age, infiltrating T cells in the acute colitis lesion were γ/δ T cells. This result is consistent with the findings that γ/δ T cells were accumulated at the acute dermatitis lesion of IL-7 transgenic mice (16), and is contrasted with the our findings that α/β T cells were infiltrated in the chronic colitis lesion of the IL-7 transgenic mice. Therefore, we supposed that at 1–3 wk, IL-7 transgenic mice developed γ/δ T cell–mediated acute colitis due to the overexpression of IL-7 protein, but with the inflammation IL-7 protein–producing goblet cells decreased and accumulation of IL-7 protein in the colonic mucosa decreased in the goblet cell–depleted epithelial cells. Since we demonstrated that mucosal IL-7 regulates the proliferation of mucosal lymphocytes by apoptotic mechanism (Watanabe, M., unpublished observation), decreased accumulation of IL-7 protein may lead to the chronic inflammation of colon in the IL-7 transgenic mice. These results are consistent with our recent findings that IL-7 protein expression was increased in the inflamed colonic mucosa of acute Salmonella enterocolitis and initial attack of ulcerative colitis, but significantly decreased at the chronic colitis lesion in the relapsing-remission type and chronic continuous type of ulcerative colitis (Watanabe, M., unpublished observation).
In this paper, we demonstrate that the dysregulation of mucosal IL-7–mediated immune regulation results in the acute and chronic colitis in IL-7 transgenic mice. This colitis mimicked several histopathological characteristics of ulcerative colitis in humans. A major limitation in understanding the pathogenesis responsible for the mucosal injury observed in human inflammatory bowel disease has been due to the lack of animal models that possess the histopathological features of human disease. A series of valuable new models that spontaneously arise in gene knockout mice gives a major impact in our understanding of the mechanisms that lead to chronic intestinal inflammation (27–29). Although the mechanism by which these mice developed chronic colitis is unclear, series of the study using these mice and our IL-7 transgenic mice may lead to the understanding of the pathogenesis of human inflammatory bowel disease.
Acknowledgments
This study was supported in part by Grants-in-Aid from the Japanese Ministry of Education, Culture and Science, the Japanese Ministry of Health and Welfare, and Keio University Medical Science Fund.
Footnotes
The authors would like to express special thanks to Professors Daniel Podolsky, Lloyd Mayer, and Sadakazu Aiso for critical comments; Drs. Noriaki Watanabe, Yasuo Hosoda, Nagamu Inoue, and Hiromasa Takaishi for technical assistance; and Miss Reiko Fujisaki for manuscript preparation.
Abbreviations used in this paper: G3PDH, glyceraldehyde 3-phosphate dehydrogenase; IEL, intraepithelial lymphocyte; LPL, lamina propria lymphocyte; mRNA, messenger RNA; RT-PCR, reverse transcriptase PCR.
References
- 1.Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer AE, Mosley B, March CJ, Urdal DL, Gillis S, et al. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin RG, Lupton S, Schmierer A, Hjerrild KJ, Jerzy R, Clevenger W, Gillis S, Cosman D, Namen AE. Human interleukin 7: molecular cloning and growth factor activity on human and murine B-lineage cells. Proc Natl Acad Sci USA. 1989;86:302–306. doi: 10.1073/pnas.86.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrissey PJ, Goodwin RG, Nordan RP, Anderson D, Grabstein KH, Cosman D, Sims J, Lupton S, Acres B, Reed SG. Recombinant interleukin 7, pre–B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989;169:707–716. doi: 10.1084/jem.169.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chazen GD, Pereira GMB, LeGros G, Gillis S, Shevach EM. Interleukin 7 is a T-cell growth factor. Proc Natl Acad Sci USA. 1989;86:5923–5927. doi: 10.1073/pnas.86.15.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch PA, Namen AE, Goodwin RG, Armitage R, Cooper MD. Human IL-7: a novel T cell growth factor. J Immunol. 1989;143:3562–3567. [PubMed] [Google Scholar]
- 6.Armitage RJ, Namen AE, Sassenfeld HM, Grabstein KH. Regulation of human T cell proliferation by IL-7. J Immunol. 1990;144:938–941. [PubMed] [Google Scholar]
- 7.Goodwin RG, Friend D, Ziegler SF, Jerzy R, Falk BA, Gimpel S, Cosman D, Dower SK, March CJ, Namen AE, Park LS. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990;60:941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- 8.Appasamy PM. Interleukin-7: biology and potential clinical applications. Cancer Invest. 1993;11:487–499. doi: 10.3109/07357909309018880. [DOI] [PubMed] [Google Scholar]
- 9.Heufler C, Topar G, Grasseger A, Stanzl U, Koch F, Romani N, Namen AE, Schuler G. Interleukin 7 is produced by murine and human keratinocytes. J Exp Med. 1993;178:1109–1114. doi: 10.1084/jem.178.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiles MV, Ruiz P, Imhof BA. Interleukin-7 expression during mouse thymus development. Eur J Immunol. 1992;22:1037–1042. doi: 10.1002/eji.1830220424. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M, Ueno Y, Yajima T, Iwao Y, Tsuchiya M, Ishikawa H, Aiso S, Hibi T, Ishii H. Interleukin-7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Invest. 1995;95:2945–2953. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balk SP, Ebert EC, Blumenthal RL, McDermott FV, Wucherpfenning KW, Landau SB, Blumberg RS. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991;253:1411–1415. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- 13.Panja A, Blumberg RS, Balk SP, Mayer L. CD1d is involved in T cell–intestinal epithelial cell interactions. J Exp Med. 1993;178:1115–1119. doi: 10.1084/jem.178.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janeway CA, Jr, Jones B, Hayday A. Specificity and function of T cells bearing γ receptors. Immunol Today. 1988;9:73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe, M., Y. Ueno, and T. Hibi. 1996. Intestinal epithelial cell–derived interleukin 7 as a regulatory factor for the intestinal mucosal lymphocytes. In Essentials of Mucosal Immunology. M. Kagnoff and H. Kiyono, editors. Academic Press, Inc., San Diego. 279–290.
- 16.Uehira M, Matsuda H, Hikita I, Sakata T, Fujiwara H, Nishimoto H. The development of dermatitis infiltrated by γ/δ T cells in IL-7 transgenic mice. Int Immunol. 1993;5:1619–1627. doi: 10.1093/intimm/5.12.1619. [DOI] [PubMed] [Google Scholar]
- 17.Takabe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SRα promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa H, Li Y, Abeliovich A, Yamamoto S, Kaufmann SH, Tonegawa S. Cytotoxic and interferon γ-producing activities of γ/δ T cells in the mouse intestinal epithelium are strain dependent. Proc Natl Acad Sci USA. 1993;90:8204–8208. doi: 10.1073/pnas.90.17.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neurath MF, Brian IF, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podolsky DK. Inflammatory bowel disease. Part I. N Engl J Med. 1991;325:928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 21.Podolsky DK. Inflammatory bowel disease. Part II. N Engl J Med. 1991;325:1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- 22.Kiyono, H., K. Fujihashi, M. Yamamoto, T. Hiroi, M. Coste, S. Kawabata, P. Boyaka, and J. R. McGhee. 1996. Intraepithelial γ/δ T cells and epithelial cell interactions in the mucosal immune system. In Essentials of Mucosal Immunology. M. Kagnoff and H. Kiyono, editors. Academic Press, Inc., San Diego. 194–205.
- 23.Reinecker HK, Podolsky DK. Human intestinal epithelial cells express functional cytokine receptors sharing the common γc chain of the interleukin 2 receptor. Proc Natl Acad Sci USA. 1995;92:8353–8357. doi: 10.1073/pnas.92.18.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodwin, R.G., and A.E. Namen. 1991. Interleukin-7. In The Cytokine Handbook. A. Thomson, editor. Academic Press, Inc., San Diego. 191–200.
- 25.Rocha B, Vassali P, Guy-Grand D. The Vβ repertoire of mouse gut homodimeric a CD8+ intraepithelial T cell receptor α/β+ lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991;173:483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+IL-7R+Thy1+lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn R, Loher J, Rennick D, Rajewsky K, Muller W. Interleukin-10–deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 29.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:275–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]