Abstract
Information on the turnover and lifespan of murine γ/δ cells was obtained by administering the DNA precursor, bromodeoxyuridine (BrdU), in the drinking water and staining lymphoid cells for BrdU incorporation. For TCR-γ/δ (Vγ2) transgenic mice, nearly all γ/δ thymocytes became BrdU+ within 2 d and were released rapidly into the peripheral lymphoid tissues. These recent thymic emigrants (RTEs) underwent phenotypic maturation in the periphery for several days, but most of these cells died within 4 wk. In adult thymectomized (ATx) transgenic mice, only a small proportion of γ/δ cells survived as long-lived cells; most of these cells had a slow turnover and retained a naive phenotype. As in transgenic mice, the majority of RTEs generated in normal mice (C57BL/6) appeared to have a restricted lifespan as naive cells. However, in marked contrast to TCR transgenic mice, most of the γ/δ cells surviving in ATx normal mice had a rapid turnover and displayed an activated/memory phenotype, implying a chronic response to environmental antigens. Hence, in normal mice many γ/δ RTEs did not die but switched to memory cells.
Gamma/delta T cells comprise a minor subset of the T cells present in LNs and spleen (1, 2). Although they clearly belong to the T cell lineage, γ/δ cells differ from conventional α/β T cells in many respects. In particular, γ/δ T cells recognize antigen in a manner fundamentally different from α/β T cells. Thus, most γ/δ T cells are not MHC restricted, and antigen recognition does not require the processing pathways involved in generating peptide– MHC complexes (2). Furthermore, γ/δ T cells have been shown to directly recognize nonpeptidic antigens, including phosphorylated nucleotides and prenyl pyrophosphate (3–6). Structurally, TCR-γ/δs may be more closely related to Ig molecules than to TCR-α/βs, as suggested by primary sequence analysis (7). This differential recognition of antigen implies that γ/δ T cells may have a very different role than α/β cells in immune responses. In fact, it has been suggested that γ/δ cells may serve as a component of the innate immune system, as they have more functional similarities to macrophages and NK cells than to conventional T cells (8). A role for γ/δ cells in immune defense was suggested by their in vivo expansion in response to certain bacterial, parasitic, and viral infections (9–16). In addition, antibody depletion of γ/δ cells and experiments with the TCR gene knockout mice indicated that γ/δ cells were important in the early response against intracellular bacteria (17–19). Nevertheless, the precise function of γ/δ cells remains unclear.
Understanding of the mechanistic basis of T cell function has been aided by the examination of lymphocyte lifespan. Thus, the in vivo lifespan of α/β T cells has been extensively studied and has yielded important information about the kinetics of thymocyte development, as well as the turnover of naive and memory T cells in the periphery (20–25). In contrast, no similar information is currently available regarding the lifespan of γ/δ T cells. Here, we have investigated the lifespan of γ/δ T cells in both TCR-γ/δ transgenic and normal mice by following bromodeoxyuridine (BrdU)1 incorporation in vivo. The results suggest that γ/δ T cells have a much more rapid turnover than do α/β T cells, both during development in the thymus and after export to the peripheral lymphoid tissues.
Materials and Methods
Mice.
B6, DBA/2, and β-2-microglobulin–deficient (β2m−) mice were purchased from the rodent breeding colony at The Scripps Research Institute. G8 TCR-γ/δ transgenic mice (26) were provided by Dr. S. Hedrick (UCSD, San Diego, CA) and were backcrossed to the BALB/c background. Where indicated, mice were either thymectomized or sham operated at 5 wk of age. After the operations, the mice were given antibiotics in their drinking water and left for 4 wk before being used experimentally.
BrdU Treatment.
Mice were given BrdU (Sigma Chemical Co., St. Louis, MO) in their drinking water at a concentration of 0.8 mg/ml. BrdU was dissolved in sterile water and was changed daily.
Adoptive Transfer.
Vγ2+ T cells were purified from pooled LNs of TCR-γ/δ transgenic mice by treating cell suspensions with anti–heat-stable antigen (HSA) (J11d), anti-CD8, and anti-CD4 plus complement (27). 2.5 × 106 cells were injected intravenously in PBS into B6 or β2m− recipients that had been injected intraperitoneally with anti–NK-1.1 (PK136) 24 h earlier and irradiated (1,000 cGy) 4 h earlier. Recipients were then given BrdU for 4 d.
Antibodies and Cell Staining.
Single cell suspensions were made from pooled LNs, thymus, or spleen and were surface stained using the following antibodies: Anti-Vγ2-PE (PharMingen, San Diego, CA); anti-CD4-PE (Collaborative Biomedical Products Co., New Bedford, MA), pan anti–TCR-γ/δ–PE (PharMingen); anti-CD45RB-biotin (PharMingen); anti-HSA-biotin (J11d); anti-CD62L-biotin (Mel-14); and anti-CD44-biotin (IM7.8.1). Biotinylated antibodies were detected with streptavidin-RED613 (GIBCO BRL, Gaithersburg, MD). Staining for BrdU was done as previously described (24). In brief, after surface staining, cells were fixed with 67% ethanol for 30 min at 4°C followed by 1% paraformaldehyde plus 0.01% Tween 20 for 30 min at room temperature, incubated with 50 Kunitz units of DNAse I (Sigma Chemical Co.) for 10 min at room temperature, and then stained with anti-BrdU-FITC (Becton Dickinson, Mountain View, CA). Stained cells were analyzed on a FACScan® flow cytometer (Becton Dickinson).
Results
The turnover of γ/δ cells was examined in both TCR-γ/δ transgenic and normal mice. TCR transgenic mice will be considered first.
TCR-γ/δ Transgenic Mice
The advantage of TCR-γ/δ transgenic mice is that the numbers of γ/δ cells in these mice is far higher than in normal mice. We used the G8 TCR-γ/δ transgenic line, in which the majority of T cells (50–75%) in LNs and spleen express a Vγ2-containing TCR that reacts against gene products encoded in the T22/T10 region of the MHC (2, 26, 28). The nonclassical (class Ib) MHC antigen recognized by G8 cells is expressed in H-2b but not H-2d mice. Hence, G8 γ/δ cells are generated in H-2d mice but are deleted in the thymus of H-2b mice.
Phenotype of γ/δ Cells in Sham Thymectomized versus Adult Thymectomized Mice.
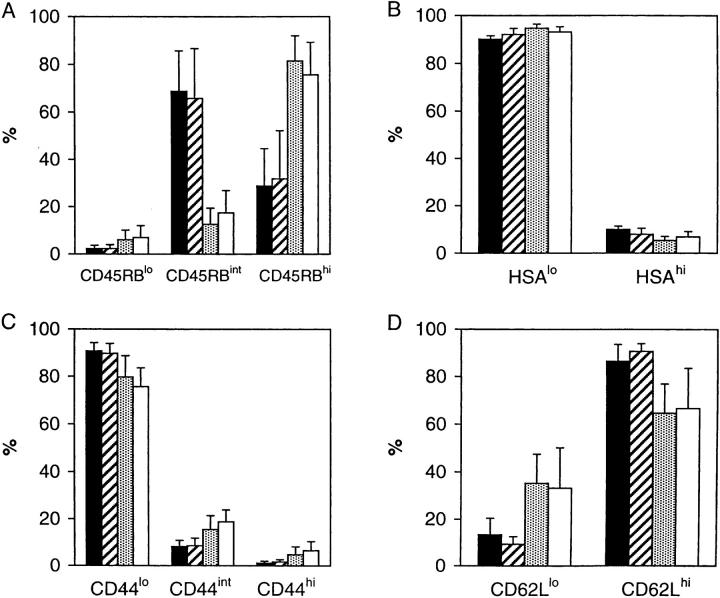
To distinguish between mature extrathymic γ/δ cells and recent thymic emigrants (RTEs), we compared adult thymectomized (ATx) versus sham thymectomized (STx) H-2d G8 mice. In terms of surface markers, the vast majority of γ/δ (Vγ2) cells in spleen and LNs of both STx and ATx mice expressed the typical HSAlo CD44lo CD62Lhi phenotype characteristic of naive T cells (Fig. 1). Cells with a memory phenotype, i.e., CD62Llo cells, were rare in STx mice (10–15%) but were more prominent in ATx mice (30–35%); however, for other markers memory-phenotype CD44hi cells and CD45RBlo cells were inconspicuous (<10%), even in ATx mice. Thus, the memory γ/δ cells in ATx mice did not express the “complete” activated/memory phenotype (CD62Llo CD44hi CD45RBlo) typical of α/β T cells (29). An unexpected difference between STx and ATx G8 mice was that CD45RBint γ/δ cells were uncommon in ATx mice (10– 15%) but a dominant population in STx mice (65–70%). Another striking finding was that total numbers of γ/δ cells in spleen and LNs were four- to fivefold lower in ATx mice than in STx mice (Fig. 2 A). These two findings suggested that the bulk of γ/δ cells in STx mice represented short-lived RTEs expressing a CD45RBint phenotype. To assess this possibility, we compared the turnover of γ/δ cells in STx versus ATx G8 mice.
Figure 1.
Phenotype of Vγ2+ T cells in STx and ATx G8 TCR-γ/δ transgenic mice. Data show proportion of Vγ2+ cells expressing different levels of (A) CD45RB, (B) HSA, (C) CD44, and (D) CD62L in pooled LNs (▪) or spleen ( ) of STx mice, or pooled LN (
) or spleen (□) of ATx mice. Data represent mean values ± SD for three (STx) or seven (ATx) mice that had been given BrdU for 4 d.
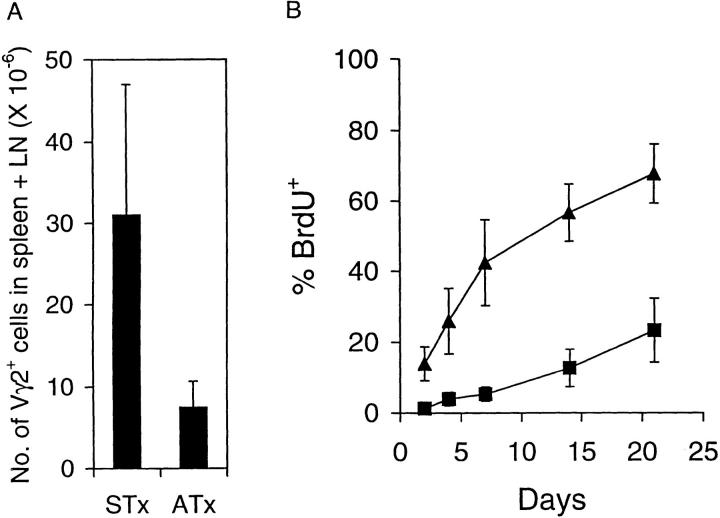
Figure 2.
Turnover of T cells in TCR-γ/δ transgenic mice. (A) Total number of Vγ2+ T cells in spleen plus pooled LNs of STx versus ATx G8 TCR-γ/δ transgenic mice. Mean cell numbers (± SD) were calculated based on total cell yields and percentage of Vγ2+ cells in 10 STx and 12 ATx mice. (B) BrdU labeling of total Vγ2+ T cells in pooled LNs in STx (▴) and ATx (▪) G8 TCR-γ/δ transgenic mice. Mice were given BrdU in their drinking water, and Vγ2+ T cells in pooled LNs were analyzed at various times. Data represent mean values ± SD for two to seven mice for each time point.
Turnover of γ/δ Cells.
T cell turnover was examined by placing mice on BrdU water for various periods and then staining lymphoid cells for surface markers versus BrdU incorporation. When total Vγ2+ cells were examined, BrdU labeling of cells in LNs (Fig. 2 B) and spleen (data not shown) occurred quite slowly in ATx mice and reached only 30% of cells by 21 d. In STx mice, by contrast, labeling was rapid; thus, 45% of cells were labeled by day 7 and 70% by day 21.
Examining the surface markers on the BrdU-labeled cells revealed distinct differences between STx and ATx mice (Fig. 3). In ATx mice, the minor subsets of CD44hi, CD45RBint, CD45RBlo, and HSAhi cells had a rapid turnover. By contrast, the major subsets of naive CD44lo, CD45RBhi, and HSAlo cells had a very slow turnover; for CD62L expression, turnover was slow for both CD62Lhi and CD62Llo cells. However, all subsets in STx mice had a more rapid turnover than in ATx mice.
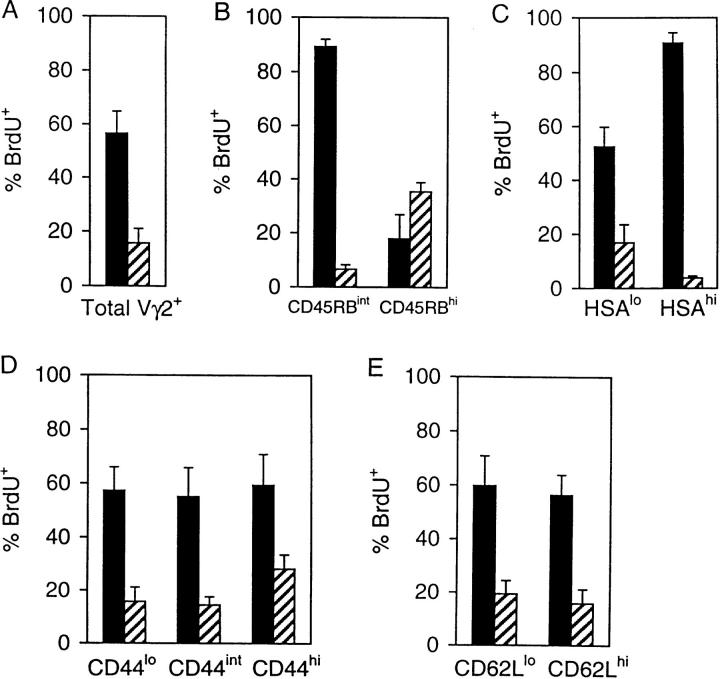
Figure 3.
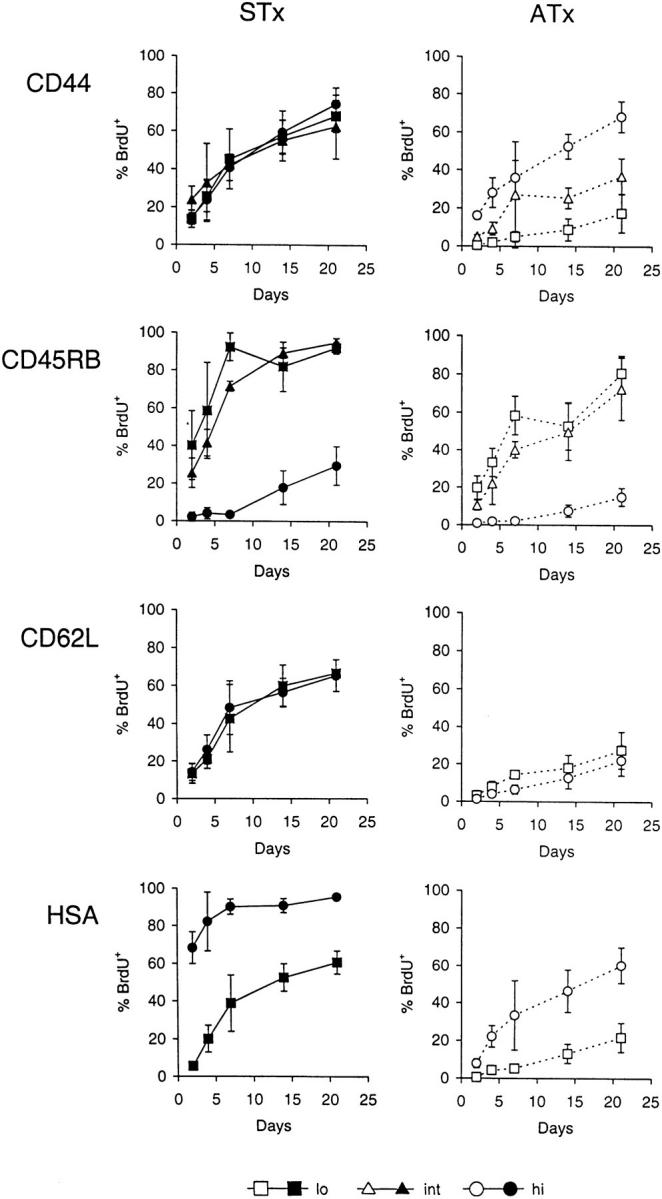
Kinetics of BrdU labeling of Vγ2+ cells in STx versus ATx G8 TCR-γ/δ transgenic mice. Mice were given BrdU continuously in their drinking water and pooled LN cells were analyzed at the indicated time points. Data show BrdU labeling of subsets of Vγ2+ cells expressing low (lo), intermediate (int), or high (hi) levels of (from top to bottom) CD44, CD45RB, CD62L or HSA in STx (left, closed symbols) or ATx (right, open symbols) TCR-γ/δ transgenic mice. Data represent mean values ± SD of two to seven mice per point.
There were two striking findings in STx mice. First, BrdU labeling of HSAhi cells was very high (70%) as early as day 2; however, labeling of HSAlo cells was very low on day 2 but reached 40% by day 7. The simplest explanation for this finding is that the RTEs released from the thymus in STx mice were initially HSAhi but then switched very rapidly to HSAlo cells. Second, BrdU labeling of the major population of CD45RBint cells in STx mice was significantly slower than for HSAhi cells but reached high levels by day 7 (70%); labeling of CD45RBhi cells, by contrast, was almost undetectable on day 7 but then rose to 30% by day 21.
These data on STx mice suggest that upon exit from the thymus RTEs were initially CD45RBint and then gradually switched to CD45RBhi cells over a period of several days. However, this transition occurred much more slowly than did the switch of HSAhi cells to HSAlo cells, which would explain why STx mice contained far more CD45RBint cells than HSAhi cells (Fig. 1). It should be noted that BrdU labeling of CD45RBhi cells was appreciably slower in ATx mice (10% at day 21) than in STx mice (30% at day 21). This finding suggests that BrdU labeling of CD45RBhi cells in STx mice was largely a reflection of the labeling of precursor cells (CD45RBint cells) within the thymus rather than postthymic division in response to environmental antigens.
Phenotype of RTEs.
Since BrdU labeling of naive phenotype cells on day 2 was conspicuous in STx mice but almost undetectable in ATx mice, it follows that the labeled cells found at day 2 in STx mice represented a relatively pure population of RTEs. As predicted from the above data, these 2 d–BrdU–labeled cells were nearly all CD45RBint rather than CD45RBhi, and most of the cells were HSAhi rather than HSAlo (Fig. 4). Typical of the naive status of RTEs, the cells were also CD44lo and CD62Lhi.
Figure 4.
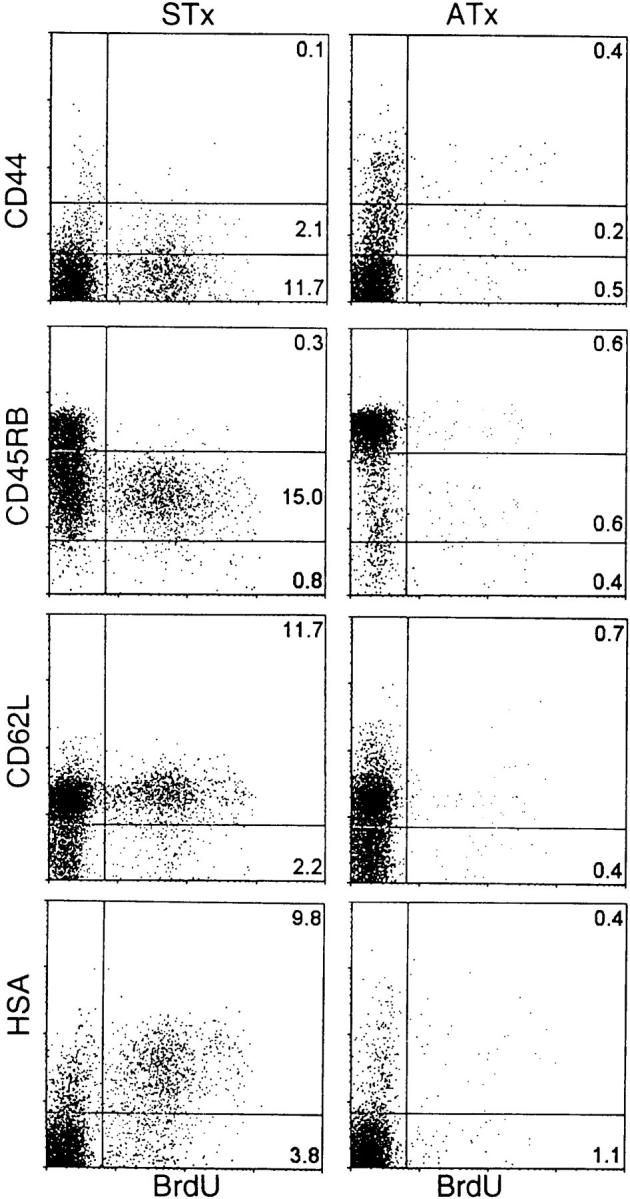
Phenotype of γ/δ recent thymic emigrants. STx (left) or ATx (right) TCR-γ/δ transgenic mice were given BrdU for 2 d. Dot plots show representative staining of Vγ2+ pooled LN cells.
Fate of RTEs.
The data on the kinetics of BrdU labeling of CD45RBint and CD45RBhi cells in STx mice (Fig. 3) suggested that division of γ/δ RTEs in the extrathymic environment was minimal. However, whether RTEs differentiated into long-lived cells or died rapidly was unclear. To examine this question, STx mice were given BrdU continuously for 14 d and then transferred to normal drinking water for a further 28 d (Fig. 5). This pulse-chase approach showed that most RTEs had only a brief lifespan. Thus, after the BrdU pulse, the percentage of total BrdU+ cells declined by ∼70% during the 28-d chase period (Fig. 5 A). Since total numbers of Vγ2 cells were largely unchanged during this period, the disappearance of BrdU+ cells could not be attributed to dilution of label.
Figure 5.
Pulse-chase BrdU labeling of Vγ2+ T cells in STx G8 TCR-γ/δ transgenic mice. Mice were given BrdU for 14 d (▪), or given BrdU for 14 d followed by normal drinking water for 28 d ( ). The percentage of BrdU+ cells in pooled LNs is shown for (A) total Vγ2+ T cells; (B) Vγ2+ T cells expressing intermediate or high levels of CD45RB; (C) Vγ2+ T cells expressing low or high levels of HSA; (D) Vγ2+ T cells expressing low, intermediate, or high levels of CD44; and (E) Vγ2+ T cells expressing low or high levels of CD62L. Data represent mean values ± SD for 7 (14 d BrdU) or 2 (14 d BrdU plus 28 d normal water) mice.
With regard to surface markers, the disappearance of ∼70% of BrdU+ γ/δ cells during the 28-d chase period applied equally to HSAlo, CD44lo, int, and hi, and CD62Llo and hi cells (Fig. 5, B–E, compare with Fig. 3). However, the loss of BrdU+ cells was much greater for the CD45RBint and HSAhi subsets (90–95%), consistent with rapid differentiation of these cells into CD45RBhi and HSAlo cells, respectively. For CD45RBint cells, it is notable that the disappearance of these cells was paralleled by a twofold increase in the proportion of BrdU+ CD45RBhi cells (Fig. 5 B), thus providing direct support for the view that CD45RBhi cells arose from CD45RBint RTE precursors. Despite this finding, the data as a whole suggest that 70% of RTEs died within 1 mo of export.
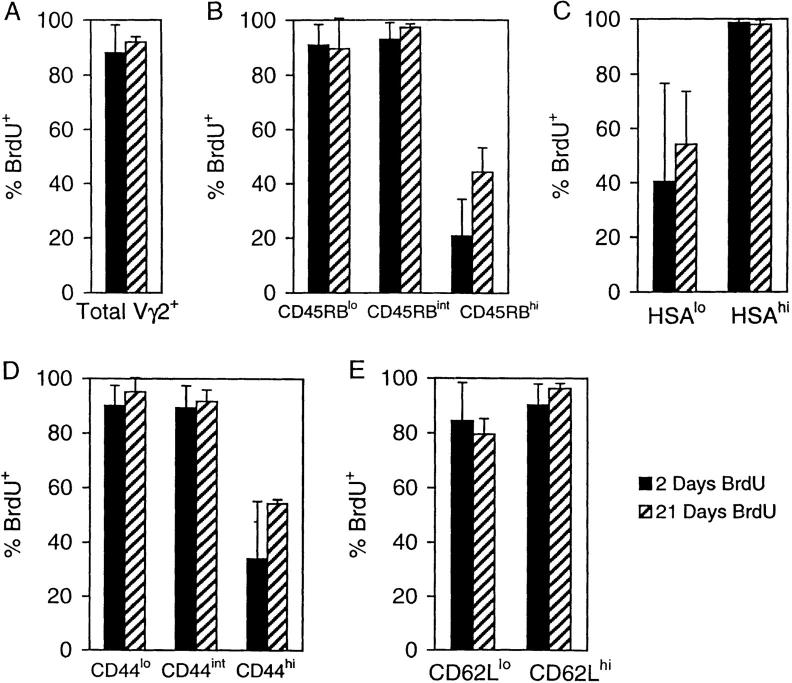
γ/δ Thymocyte Kinetics.
The observation that substantial numbers of BrdU-labeled cells appeared in the periphery of STx (but not ATx) mice within 2 d implied that the transit time of γ/δ cells through the thymus was very rapid. To examine this question directly, STx TCR-γ/δ transgenic mice were placed on BrdU and Vγ2+ cells in the thymus analyzed (Fig. 6). By day 2, 90% of total Vγ2+ cells were BrdU+ (Fig. 6 A). This rapid labeling also applied to cells having the phenotype of RTEs, i.e., to CD45RBint, HSAhi, CD44lo, and CD62Lhi cells (Fig. 6, B–E). These data indicated that Vγ2+ thymocytes had a very rapid turnover and appeared to exit the thymus very soon after division. Interestingly, continuous administration of BrdU for up to 21 d failed to label 5–10% of total Vγ2+ cells in the thymus (Fig. 6 A). These cells were predominantly CD45RBhi, HSAlo, CD44hi, and CD62Llo (Fig. 6, B–E). Two explanations could account for the presence of these cells. First, a fraction of the γ/δ cells generated in the thymus was unable to emigrate to the periphery and remained in situ for an indefinite period of time. Second, some mature γ/δ cells were able to recirculate from the periphery back to the thymus. Among α/β T cells, reentry into the thymus is restricted to activated cells (30). Whether the same restriction applies to γ/δ cells has not been studied, although it is of interest that the long-lived thymic γ/δ cells in the above experiment were CD44hi CD62Llo, a phenotype associated with activation/memory amongst α/β T cells. Therefore, it was important to examine the phenotypic changes that occurred after activation of mature γ/δ cells.
Figure 6.
Turnover of Vγ2+ thymocytes in G8 TCR-γ/δ transgenic mice. STx TCR-γ/δ transgenic mice were given BrdU for 2 (▪) or 21 ( ) d and Vγ2+ thymocytes were analyzed. (A) Total Vγ2+ thymocytes. (B) Vγ2+ CD45RB thymocyte subsets. (C) Vγ2+ HSA thymocyte subsets. (D) Vγ2+ CD44 thymocyte subsets. (E) Vγ2+ CD62L thymocyte subsets. Data represent mean values ± SD for two to three mice per point.
Activation of γ/δ T Cells.
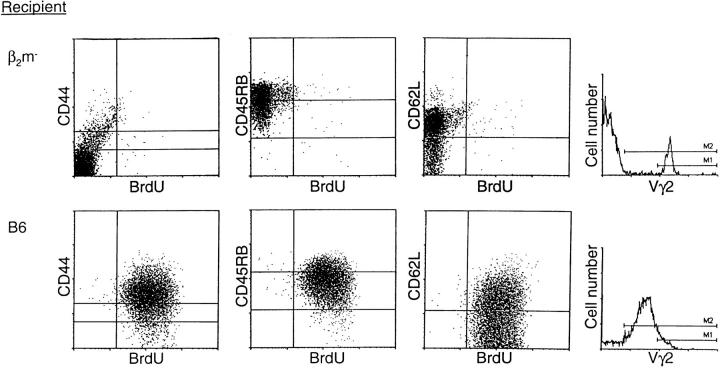
To directly determine the phenotype of activated γ/δ T cells, purified transgenic Vγ2+ LN T cells were adoptively transferred from H-2d G8 mice into heavily irradiated H-2b (B6) or β2m− mice, and the recipients were given BrdU for 4 d (Fig. 7). Since the T22/ T10 antigen is dependent upon β2m for its expression (31), one would not expect the Vγ2+ cells to be activated after transfer to the β2m− recipients. In fact, BrdU labeling of Vγ2+ cells after transfer into β2m− mice was very low. By contrast, after exposure to T22/T10 antigen for 4 d in normal B6 mice, virtually all of the injected Vγ2+ cells became BrdU+; moreover, 15-fold more Vγ2+ cells were recovered from B6 than from β2m− hosts, indicating a marked expansion of the cells to T22/T10 antigen in B6 mice. In terms of surface markers, most of the Vγ2 cells in B6 hosts displayed the typical CD44hi phenotype of activated cells. At the early time point examined, the Vγ2 cells also showed partial downregulation of CD45RB and CD62L. For obscure reasons, TCR expression on the activated Vγ2 cells in B6 hosts was appreciably lower than for the Vγ2 resting cells in β2m− hosts.
Figure 7.
Phenotype of γ/δ cells activated in vivo. Vγ2+ T cells were purified from G8 TCR-γ/δ transgenic mice and injected into either β2m− (upper) or B6 (lower) mice; recipients were treated with anti–NK-1.1 antibody and irradiated before adoptive transfer (see Materials and Methods). After injection of Vγ2+ cells, recipients were given BrdU for 4 d. Dot plots show staining of spleen cells from representative mice.
The above data indicate that exposure to specific antigen caused Vγ2 cells to divide and switch to an activated phenotype. In terms of CD44 and CD62L expression, these cells closely resembled the minor subset of activated/memory-phenotype cells found in the thymus of G8 TCR transgenic mice (see above).
γ/δ Cells in Normal Mice
The finding that the vast majority of Vγ2 cells in G8 TCR transgenic mice had a naive phenotype implied that the reactivity of this monoclonal population for typical environmental antigens was minimal. Therefore, it was considered important to examine the turnover of γ/δ cells in normal mice. Data on the phenotype and turnover of Vγ2 and total γ/δ cells in ATx and STx normal B6 mice are discussed below; the cells studied were prepared from pooled LNs and spleen. Quite similar data were seen in DBA/2 mice (data not shown).
Phenotype in STx versus ATx Mice.
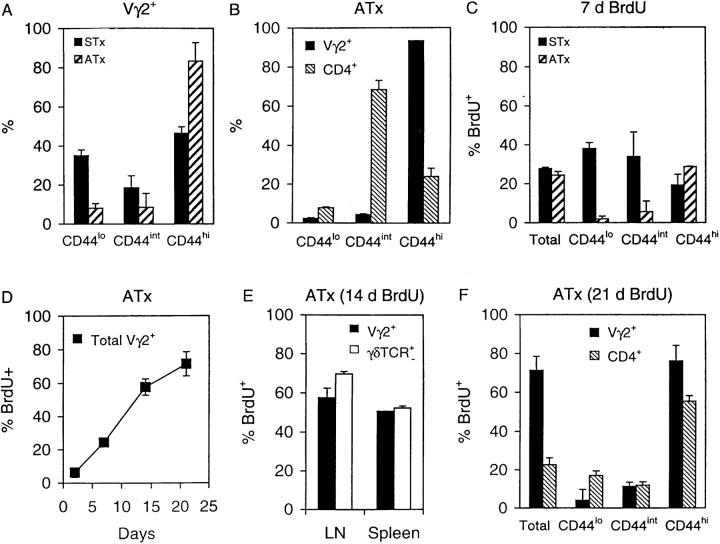
As mentioned earlier, the vast majority of Vγ2+ cells in G8 TCR transgenic mice consisted of naive phenotype cells, both in ATx and STx mice (Fig. 1). The situation in normal B6 mice was quite different (Fig. 8 A). In these mice a high proportion of Vγ2 cells in spleen and LNs displayed a CD44hi (memory) phenotype; CD44hi cells comprised ∼45% of Vγ2 cells in STx mice and 85% in ATx mice. These findings contrasted sharply with the phenotype of α/β cells (Fig. 8 B). Thus, for CD4+ cells (which consist almost entirely of α/β cells), only a small proportion of these cells (25%) were CD44hi in ATx B6 mice; the majority of CD4+ cells were CD44int, the typical phenotype of naive α/β cells.
Figure 8.
T cell turnover and phenotype in B6 mice. (A) Percentage of Vγ2+ T cells expressing low, intermediate, or high levels of CD44 in STx and ATx mice. (B) Proportion of Vγ2+ and CD4+ cells expressing low, intermediate, or high levels of CD44 in ATx mice. (C) BrdU labeling of total, CD44lo, CD44int and CD44hi Vγ2+ T cells in STx and ATx B6 mice given BrdU for 7 d. (D) Kinetics of BrdU labeling of total Vγ2+ T cells in ATx mice. (E) Comparison of BrdU labeling of Vγ2+ T cells versus total TCR-γ/δ+ T cells in ATx mice given BrdU for 14 d. (F) BrdU labeling of Vγ2+ and CD4+ T cells expressing different levels of CD44 in ATx mice given BrdU for 21 d. Data shown are for pooled LN cells and represent mean values ± SD for two to three mice per point.
Turnover in ATx Mice.
BrdU incorporation by Vγ2 cells in ATx mice was largely limited to memory phenotype CD44hi cells (Fig. 8 C). Since these cells comprised the bulk of Vγ2 cells (Fig. 8 A), the labeling of total Vγ2 cells in ATx mice was high and reached 70% by day 21 (Fig. 8 D). This high rate of labeling in ATx mice also applied to total γ/δ cells, i.e., to cells detected with a pan anti-γ/δ mAb (Fig. 8 E). For CD44 subsets, the rate of labeling of Vγ2 cells and α/β (CD4+) cells was quite similar, i.e., high for CD44hi (memory) cells and low for CD44lo/int (long-lived naive) cells (Fig. 8 F). For other markers, BrdU labeling of CD45RB subsets of Vγ2 cells in ATx B6 mice was much the same as for G8 TCR transgenic mice, i.e., high for CD45RBlo (memory) cells and low for CD45RBhi (naive) cells (data not shown). As in G8 mice, labeling of CD62Lhi and CD62Llo subsets of Vγ2 cells was quite similar (data not shown).
Turnover in STx Mice.
In marked contrast to ATx mice, the turnover of naive phenotype Vγ2 cells in STx mice was rapid. Thus, BrdU labeling of CD44lo cells in STx mice reached 40% by day 7, compared with <5% for ATx mice (Fig. 8 C). It should be emphasized that naive phenotype Vγ2 cells were a major population in STx mice. Thus, ∼50% of Vγ2 cells in STx were CD44lo/int cells, compared with only 15% in ATx mice (Fig. 8 A). The substantially higher frequency of naive phenotype Vγ2 cells in STx mice compared to ATx mice also applied to total cell numbers. Thus, total numbers of CD44lo/int Vγ2 cells in spleen plus LNs were about fourfold higher in STx than in ATx mice (data not shown). Thus, the implication is that, as in G8 TCR transgenic mice, most naive Vγ2 RTEs generated in B6 mice had a restricted lifespan.
Phenotype of RTEs.
The phenotype of RTEs in STx mice is shown in Fig. 9. When control ATx mice were placed on BrdU water for 7 d, most of the BrdU+ cells detected at days 4 and 7 were CD44hi/CD45RBlo memory cells. Labeling of these memory phenotype cells was also apparent, although to a lesser extent, in STx mice. However, like G8 mice, STx B6 mice also contained discrete populations of BrdU+ naive CD44lo and CD45RBint cells (Fig. 9). Confirming previous findings on the α/β RTEs generated in the normal thymus (24), the labeled Vγ2 RTEs found in STx B6 mice were predominantly BrdUdim rather than BrdUbright. As discussed elsewhere, the lower incorporation of BrdU by thymocytes compared to peripheral T cells presumably reflects enhanced cold target competition from DNA released by dying thymocytes (24).
Figure 9.
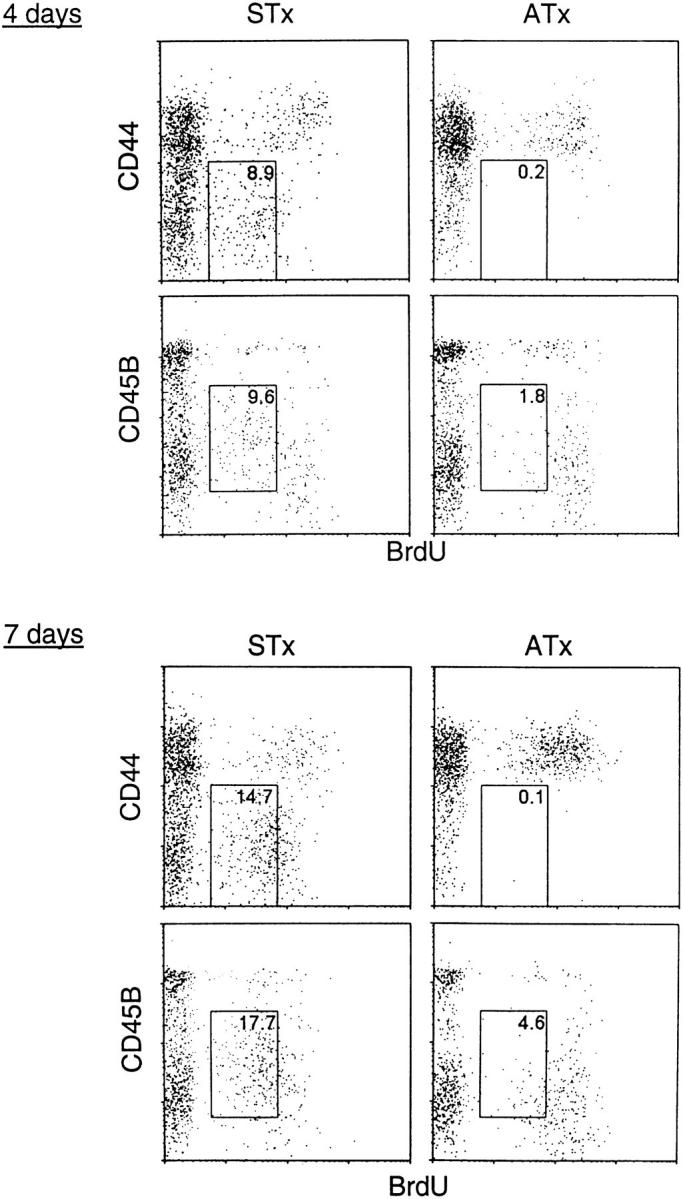
Phenotype of Vγ2+ RTEs in B6 mice. STx (left) or ATx (right) B6 mice were given BrdU for 4 d (upper) or 7 d (lower). Dot plots show representative staining of Vγ2+ pooled LN cells. The percentage of cells falling within the boxed areas is indicated.
Collectively, the above data indicate that, as in G8 TCR transgenic mice, the γ/δ cells found in the periphery of normal B6 mice comprised three broad categories of cells with different turnover rates. In STx B6 mice, ∼50% of γ/δ cells in spleen and LNs were RTEs; these cells probably incorporated BrdU exclusively in the thymus and then differentiated into typical naive resting cells in the periphery. In ATx mice, a small proportion of RTEs survived for prolonged periods as long-lived naive cells with a very slow turnover. However, in marked contrast to G8 mice, the bulk of γ/δ cells in ATx B6 mice were memory phenotype cells with a rapid turnover. This finding suggests that, after export from the thymus, most RTEs in B6 mice did not die rapidly but instead differentiated into memory cells through contact with environmental antigens. The prominent conversion of naive RTEs to memory cells in B6 mice presumably accounts for the curious observation that the turnover of total Vγ2 cells in STx and ATx B6 mice was almost identical (Fig. 8 C, left panel). Thus, numerically, the rapid turnover of memory cells in ATx mice happened to balance the rapid production of RTEs in STx mice. This was a clear contrast to G8 mice, for which the paucity of memory cells in STx mice led to much higher labeling of total Vγ2 cells in STx than in ATx mice (Fig. 2 B).
Discussion
Like α/β T cells, the γ/δ T cells found in LNs and spleen arise in the thymus and are subject to negative selection (1, 2, 8, 26, 32). Whether γ/δ cells undergo positive selection is less clear (31–37). In the case of the G8 TCR transgenic line (33) used here, and the closely-related KN6 line (34), the production of mature γ/δ cells was reported to be much lower in β2-m-negative (β2m0) mice than in β2m+ mice, implying β2m-dependent positive selection. However, another group studying G8 mice found low but significant numbers of mature γ/δ cells in β2m0 mice and concluded that the γ/δ cells generated in β2m+ H-2d mice do not undergo positive selection but instead are subject to a covert form of negative selection (35). This possibility is difficult to reconcile with the finding that the production of G8 and KN6 γ/δ cells is substantially less in β2m0 than β2m+ H-2d mice and that the residual γ/δ cells in β2m0 mice have strong reactivity for H-2b but display no detectable reactivity for H-2d even in the presence of added IL-2 (35). Moreover, the data reported here show that the RTEs in β2m+ H-2d mice have a typical naive phenotype and do not display signs of activation.
This study shows that the kinetics of thymocyte development is much more rapid for γ/δ than α/β T cells. Studies on α/β thymocytes have shown that immature CD4+8+ cells have a lifespan of ∼3.5 d, whereas the turnover of the most mature thymocyte populations is much slower; these findings apply both to normal and TCR transgenic mice (20, 21). In contrast, we show here that, at least for TCR transgenic mice, the vast majority of γ/δ thymocytes including those having the phenotype of RTEs became BrdU+ within 2 d, indicating a very rapid rate of turnover. Hence, if γ/δ cells undergo positive selection (see above), this process must occur very rapidly. On the other hand, positive selection appears to be a time-limiting step in α/β T cell development. Positive selection of α/β thymocytes occurs soon after the transition of CD4−8− cells to cortical CD4+8+ cells and induces a subset of these cells to differentiate into CD4+8− and CD4−8+ cells and migrate to the medulla (38). Although these steps in positive selection occur within several days, the subsequent export of mature α/β cells from the medulla into the extrathymic environment is slow and can take up to 1–2 wk (20). The reason for the prolonged residence of α/β thymocytes in the thymic medulla is unknown, although an obvious possibility is that additional selection steps are required before the cells are able to emigrate from the thymus. Whatever the explanation for the slow export of α/β cells, our data suggest that the release of γ/δ cells from the thymus occurs very rapidly. Thus, in G8 TCR transgenic mice, large numbers of labeled naive γ/δ cells were apparent in peripheral lymphoid tissues of STx (but not ATx) mice after only 2 d on BrdU water. Hence, in contrast to α/β cells, γ/δ cells appear to be only dependent on the thymic microenvironment for a relatively brief period during their development.
It is of interest that γ/δ RTEs expressed a semimature phenotype. Thus, while RTEs resembled mature, naive γ/δ T cells in expressing low levels of CD44 and high levels of CD62L, their phenotype was immature with regard to HSA and CD45RB expression, i.e., the cells were HSAhi and CD45RBint. These findings are consistent with the report that after intrathymic injection of FITC, most of the labeled γ/δ cells released from the thymus were CD44lo HSAhi cells (39). In this study, γ/δ RTEs matured from HSAhi, CD45RBlo/int cells to HSAlo, CD45RBhi cells within 7 d after export from the thymus. It has been similarly reported that for CD4+ cells α/β RTEs are initially CD45RBint rather than CD45RBhi (24, 40). However, in contrast to γ/δ RTEs, only a small proportion of α/β RTEs express an immature HSAhi phenotype (40, 41). Therefore, a likely possibility is that those cells transiting most rapidly through the thymus, including some α/β and most γ/δ RTEs, exit as phenotypically immature cells. On this point, it is of interest that nearly all α/β RTEs in the rat emerge from the thymus as Thy-1+ CD45RC− cells and subsequently mature to a Thy-1− CD45RChi phenotype over a time span of 7 d (42). An interesting question then is whether the immature phenotype of rat α/β RTEs also reflects rapid thymocyte kinetics. No information is currently available on this point.
In addition to rapid turnover in the thymus, most naive γ/δ T cells were short-lived in LNs and spleen in both TCR-γ/δ transgenic mice and normal mice. In TCR transgenic mice, the rapid turnover occurred in the absence of antigenic stimulation and, therefore, presumably reflected the death of naive cells. Thus, a rapid rate of output of Vγ2+ cells from the thymus was balanced by a rapid loss of cells from the periphery. A high proportion of the cells appeared to die at a semimature CD45RBlo/int stage. The loss of RTEs was not an artifact of the monoclonality of the Vγ2+ population in TCR transgenic mice, since rapid turnover of these cells was also observed in normal B6 mice. Although most RTEs were short-lived, a small proportion of these cells survived to become long-lived naive cells. Thus, the majority of γ/δ cells in ATx transgenic mice, and a minority of cells in ATx B6 mice, displayed a typical naive (CD44lo HSAlo CD45RBhi) phenotype and had a very slow turnover rate. Why these particular cells were selected for survival is unclear.
In nontransgenic B6 and also DBA/2 mice, it is of interest that most peripheral γ/δ cells acquired an activated/ memory phenotype after thymectomy. Since these cells were rare in ATx TCR transgenic mice, the transition of naive to memory phenotype cells in B6 mice presumably reflected an antigen-specific response to various environmental antigens. Unlike γ/δ cells found in epithelial tissues, peripheral γ/δ T cells express diverse TCRs and, therefore, are presumed to recognize a wide array of different antigens. However, the high frequency of memory phenotype γ/δ cells in normal mice suggests that the γ/δ T cell repertoire rapidly becomes biased towards recognition of frequently encountered antigens. As a consequence, with advancing age the γ/δ T cell pool differs markedly from the α/β population in having only a very small reservoir of naive cells. In this respect, in contrast to γ/δ cells, the majority of α/β (CD4+) cells in ATx mice display a naive phenotype (24).
Although the rapid turnover of memory phenotype γ/δ cells in normal mice presumably reflected continuous or intermittent contact with antigen, some of the BrdU labeling may have represented bystander proliferation driven by cytokines, as has been observed for memory phenotype α/β cells (43). This possibility is worth considering, since IL-12 has been shown to stimulate proliferation of human γ/δ cells in vitro (44).
For α/β cells, a significant proportion of memory phenotype CD44hi cells were found to exclude BrdU for >1 mo (24). Similarly, >20% of CD44hi γ/δ cells in STx or ATx B6 mice remained BrdU− after several weeks on BrdU water (our unpublished data). For α/β cells, these BrdU− cells probably represent long-lived noncycling memory cells specific for environmental antigens. However, whether γ/δ cells carry memory is still unclear. Most functional studies pointing to a role for γ/δ cells in immune protection have focused on the primary response. Nevertheless, experiments with TCR-α/β and -γ/δ knockout mice showed that γ/δ cells do have a minor role in protection against a secondary challenge with Listeria monocytogenes (18). In addition, alloreactive γ/δ TCR transgenic mice (on a SCID background) were shown to clear antigen-expressing cells more efficiently if the mice were primed with antigen 12 d before (45). These latter studies suggest that γ/δ T cells may have some ability to mount a memory response to antigen. However, the phenotype of the cells responsible is unknown.
Acknowledgments
We thank Dr. S. Hedrick for the G8 mice.
This work was supported by grants AI-21487, AI-32068, CA-38355, and CA-25803 from the United States Public Health Service. D.F. Tough is the recipient of a Centennial Fellowship from the Medical Research Council of Canada.
Footnotes
David F. Tough's present address is The Edward Jenner Institute for Vaccine Research, Compton, Newbury, Berkshire RG20 7NN, UK.
This is publication no. 10957-IMM from the Scripps Research Institute.
Abbreviations used in this paper: β2m, β-2-microglobulin; ATx, adult thymectomized; BrdU, Bromodeoxyuridine; HSA, heat-stable antigen; RTE, recent thymic emigrants; STx, sham thymectomized.
References
- 1.Raulet DH. The structure, function and molecular genetics of the γ/δ T cell receptor. Annu Rev Immunol. 1989;7:175–207. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- 2.Schild H, Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, Bluestone JA, Matis L, Draper RK, Chien YH. The nature of major histocompatibility complex recognition by γδ T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 3.Constant P, Davodeau F, Peyrat M-A, Poquet Y, Puzo G, Bonneville M, Fournie J-J. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic nonpeptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 5.Burk MR, Mori L, De Libero G. Human Vγ9-Vδ2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol. 1995;25:2052–2058. doi: 10.1002/eji.1830250737. [DOI] [PubMed] [Google Scholar]
- 6.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 7.Rock EP, Sibbald PR, Davis MM, Chien Y-H. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boismenu R, Havran WL. An innate view of γδ T cells. Curr Opin Immunol. 1997;9:57–63. doi: 10.1016/s0952-7915(97)80159-8. [DOI] [PubMed] [Google Scholar]
- 9.Carding SR, Allan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by γ/δ+T cells. J Exp Med. 1990;172:1225–1231. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Paoli P, Gennari D, Martelli P, Cavarzerani V, Comoretto R, Santini G. γδ T cell receptor–bearing lymphocytes during Epstein-Barr virus infection. J Infect Dis. 1990;161:1013–1016. doi: 10.1093/infdis/161.5.1013. [DOI] [PubMed] [Google Scholar]
- 11.Ho M, Webster HK, Tongtawe P, Pattanapanyasat K, Weidanz WP. Increased γδ T cells in acute P. falciparummalaria. Immunol Lett. 1990;25:139–141. doi: 10.1016/0165-2478(90)90105-y. [DOI] [PubMed] [Google Scholar]
- 12.Modlin TL, Pirmez C, Hofman FM, Torigian V, Uyemura K, Rea TH, Bloom BR, Brenner MB. Lymphocytes bearing antigen-specific γδ T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 13.Ohga S, Yoshikai Y, Takeda Y. Sequential appearance of γδ T- and αβ-bearing T cells in the peritoneal cavity during an i.p. infection with Listeria monocytogenes. . Eur J Immunol. 1990;20:533–538. doi: 10.1002/eji.1830200311. [DOI] [PubMed] [Google Scholar]
- 14.Rosat J-P, McDonald HR, Louis JA. A role for γδ T cells during experimental infection of mice with Leishmania major. . J Immunol. 1993;150:550–555. [PubMed] [Google Scholar]
- 15.Roussilhon C, Agrapart M, Ballet J-J, Bensussan A. T lymphocytes bearing the γδ T cell receptor in patients with acute P. falciparummalaria. J Infect Dis. 1990;162:283–285. doi: 10.1093/infdis/162.1.283-a. [DOI] [PubMed] [Google Scholar]
- 16.Takada H, Hiromatsu K, Matsuzaki G, Muramori K, Nomoto K. Peritoneal γδ T cells induced by Escherichia coliinfection in mice. Correlation between Thy-1 phenotype and host minor lymphocyte-stimulating phenotype. J Immunol. 1993;151:2062–2069. [PubMed] [Google Scholar]
- 17.Skeen MJ, Ziegler HK. Induction of murine peritoneal γ/δ T cells and their role in resistance to bacterial infection. J Exp Med. 1993;178:971–984. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SHE. Different roles of α/β and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 19.Ladel CH, Hess J, Daugelat S, Mombaerts P, Tonegawa S, Kaufmann SHE. Contribution of α/β and γ/δ T lymphocytes to immunity against Mycobacterium bovisBacillus Calmette Guerin: studies with T cell receptor–deficient mutant mice. Eur J Immunol. 1993;25:838–846. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- 20.Egerton M, Scollay R, Shortman K. Kinetics of mature T-cell development in the thymus. Proc Natl Acad Sci USA. 1990;87:2579–2582. doi: 10.1073/pnas.87.7.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66:533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 22.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michie CA, McLean A, Alcock A, Beverley PCL. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 24.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Boehmer H, Hafen K. The life span of naive α/β T cells in secondary lymphoid organs. J Exp Med. 1993;177:891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dent AL, Matis LA, Hooshmand F, Widacki SM, Bluestone JA, Hedrick SM. Self-reactive γδ T cells are eliminated in the thymus. Nature. 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 27.Sprent J, Schaefer M. Properties of purified T cell subsets. I. In vitro responses to class I vs. class II H-2 alloantigens. J Exp Med. 1985;162:2068–2088. doi: 10.1084/jem.162.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weintraub BC, Jackson MR, Hedrick SM. Gamma delta T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. J Immunol. 1994;153:3051–3058. [PubMed] [Google Scholar]
- 29.Sprent J. Immunological memory. Curr Opin Immunol. 1997;9:371–379. doi: 10.1016/s0952-7915(97)80084-2. [DOI] [PubMed] [Google Scholar]
- 30.Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Kaer L, Wu M, Ichikawa Y, Ito K, Bonneville M, Ostrand-Rosenberg S, Murphy DB, Tonegawa S. Recognition of MHC TL gene products by γδ T cells. Immunol Rev. 1991;120:89–115. doi: 10.1111/j.1600-065x.1991.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 32.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 33.Wells FB, Gahm SJ, Hedrick SM, Bluestone JA, Dent A, Matis LA. Requirement for positive selection of γδ receptor–bearing T cells. Science. 1991;253:903–905. doi: 10.1126/science.1831565. [DOI] [PubMed] [Google Scholar]
- 34.Pereira P, Zijlstra M, McMaster J, Loring JM, Jaenisch R, Tonegawa S. Blockade of transgenic γδ T cell development in β2-microglobulin deficient mice. EMBO (Eur Mol Biol Organ) J. 1992;11:25–31. doi: 10.1002/j.1460-2075.1992.tb05023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweighoffer E, Fowlkes BJ. Positive selection is not required for thymic maturation of transgenic γδ T cells. J Exp Med. 1996;183:2033–2041. doi: 10.1084/jem.183.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correa I, Bix M, Liao NS, Zijlstra M, Jaenisch R, Raulet D. Most γδ T cells develop normally in β2-microglobulin-deficient mice. Proc Natl Acad Sci USA. 1992;89:653–657. doi: 10.1073/pnas.89.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bigby M, Markovitz JS, Bleicher PA, Grusby MJ, Simha S, Seibrecht M, Wagner M, Nagler-Anderson C, Glimcher LH. Most γδ T cells develop normally in the absence of MHC class II molecules. J Immunol. 1993;151:4465–4475. [PubMed] [Google Scholar]
- 38.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 39.Kelly KA, Pearse M, Lefrancois L, Scollay R. Emigration of selected subsets of γδ+T cells from the adult murine thymus. Int Immunol. 1993;5:331–335. doi: 10.1093/intimm/5.4.331. [DOI] [PubMed] [Google Scholar]
- 40.Gabor MJ, Godfrey DI, Scollay R. Recent thymic emigrants are distinct from most medullary thymocytes. Eur J Immunol. 1997;27:2010–2015. doi: 10.1002/eji.1830270827. [DOI] [PubMed] [Google Scholar]
- 41.Kelly KA, Scollay R. Analysis of recent thymic emigrants with subset- and maturity-related markers. Int Immunol. 1990;2:419–425. doi: 10.1093/intimm/2.5.419. [DOI] [PubMed] [Google Scholar]
- 42.Hosseinzadeh H, Goldschneider I. Recent thymic emigrants in the rat express a unique antigenic phenotype and undergo post-thymic maturation in peripheral lymphoid tissues. J Immunol. 1993;150:1670–1679. [PubMed] [Google Scholar]
- 43.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 44.Ueta C, Kawasumi H, Fujiwara H, Miyagawa T, Kida H, Ohmoto Y, Tsuyuguchi I. Interleukin-12 activates human γδ T cells: synergistic effect of tumor necrosis factor–α. Eur J Immunol. 1996;26:3066–3073. doi: 10.1002/eji.1830261237. [DOI] [PubMed] [Google Scholar]
- 45.Spaner D, Migita K, Ochi A, Shannon J, Miller RG, Pereira P, Tonegawa S. γδ T cells differentiate into a functional but nonproliferative state during a normal immune response. Proc Natl Acad Sci USA. 1993;90:8415–8419. doi: 10.1073/pnas.90.18.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]