Abstract
The herpes simplex virus (HSV) infected cell protein (ICP)47 blocks CD8+ T cell recognition of infected cells by inhibiting the transporter associated with antigen presentation (TAP). In vivo, HSV-1 replicates in two distinct tissues: in epithelial mucosa or epidermis, where the virus enters sensory neurons; and in the peripheral and central nervous system, where acute and subsequently latent infections occur. Here, we show that an HSV-1 ICP47− mutant is less neurovirulent than wild-type HSV-1 in mice, but replicates normally in epithelial tissues. The reduced neurovirulence of the ICP47− mutant was due to a protective CD8+ T cell response. When compared with wild-type virus, the ICP47− mutant expressed reduced neurovirulence in immunologically normal mice, and T cell–deficient nude mice after reconstitution with CD8+ T cells. However, the ICP47− mutant exhibited normal neurovirulence in mice that were acutely depleted of CD8+ T cells, and in nude mice that were not reconstituted, or were reconstituted with CD4+ T cells. In contrast, CD8+ T cell depletion did not increase the neurovirulence of an unrelated, attenuated HSV-1 glycoprotein (g)E− mutant. ICP47 is the first viral protein shown to influence neurovirulence by inhibiting CD8+ T cell protection.
Herpesviruses are particularly adept at avoiding detection by CD8+ T lymphocytes. They often express several proteins that can independently block the MHC class I presentation pathway by which antigenic peptides are presented to CD8+ T cells. This immune evasion might be particularly important to a family of viruses that persists or establishes latent infections, often for the lifetime of an infected individual. During reactivation from the latent state, herpesviruses encounter robust, fully primed immune systems. Thus, immune evasion is probably important to reduce the effects of antiviral immunity until progeny can be produced. Herpes simplex virus (HSV)1 infected cell protein (ICP)47 blocks the transporter associated with antigen presentation (TAP), so that antigenic peptides cannot be transported into the endoplasmic reticulum, the site of assembly of MHC class I molecules (1–5). Another human herpesvirus, human cytomegalovirus, encodes at least five polypeptides that each independently inhibit the MHC class I presentation pathway (6–11). To explain this apparent redundancy, it has been suggested that these viral gene products can act in distinct tissue types to provide resistance to CD8+ T cells (5, 8). However, this has not been tested and in no case has an in vivo effect of one of these herpesvirus proteins been established.
We used a murine ocular model of HSV infection to test the effects of ICP47 on viral pathogenesis. This model has been used extensively to study HSV corneal infection, a disease that is still a leading cause of blindness. HSV-1 infection of the cornea leads to virus replication and transmission to several tissues, accompanied by leukocytic infiltration in the following sequence: (a) lesions develop in the epithelial layer of the cornea after 2 d, accompanied by predominantly PMN infiltration. These lesions heal within 2–3 d in both immunologically normal and T cell–deficient mice (12), suggesting that T cells are not important for limiting this primary disease. (b) During infection of the corneal epithelium, sensory neurons that have cell bodies in the trigeminal ganglia become infected. In ganglia, HSV-1 replication can be detected in sensory neurons, satellite cells, and Schwann cells from 2–8 d after infection, but then latency is established in neurons. However, with some strains of HSV-1, latency is incomplete, and the virus can spread from ganglia to the central nervous system and cause encephalitis. CD8+ T cells play an important role in controlling the duration of HSV-1 replication in sensory ganglia and preventing transmission to the central nervous system (13). (c) About 7 d after infection, mice develop lesions in the skin surrounding the eyelids. These lesions are significantly exacerbated in T cell–deficient mice (14), and it appears that CD4+ and CD8+, α/β TCR+ T cells (15, 16), and γ/δ TCR+ T cells (14) are important for protection from HSV-1 skin lesions. Approximately 10–14 d after infection, inflammation develops in the stromal layer of the cornea, and is regulated by CD4+ T lymphocytes (17, 18) through the elaboration of the cytokines IFN-γ and IL-2 (19–22). Therefore, HSV disease in this animal model is controlled by different T cell subsets in different tissues, but in the nervous system CD8+ T cells are particularly important.
A major consideration in using the mouse model to study the effect of ICP47 on HSV pathogenesis is the previous observations that ICP47 inhibits the murine TAP relatively poorly (3, 4, 23). Inhibition of TAP activity in mouse fibroblasts required ICP47 concentrations 50–100-fold higher than those required to inhibit TAP in human fibroblasts. These studies might militate against the use of a mouse model to study the ICP47 effect on virus virulence. However, our initial studies suggested that an ICP47− HSV mutant was less virulent in the mouse nervous system. This apparent incongruity may relate to the prominent protective role of HSV-reactive CD8+ T cells in the peripheral nervous system, and the low levels of MHC class I expression in the nervous system. Neurons are deficient in antigen presentation, expressing little or no MHC class I on their surface (24–26). However, low levels of MHC class I proteins are expressed in satellite cells and Schwann cells, which also become infected in sensory ganglia, and class I may be upregulated by HSV infection of these accessory cells (25). Therefore, it was reasonable to postulate that ICP47 might block recognition of HSV-infected host cells by CD8+ T cells in the nervous system, in a manner that cannot be discerned in assays involving cultured mouse fibroblasts. Indeed, our current findings support this hypothesis by demonstrating that an HSV-1 ICP47 deletion mutant exhibits dramatically reduced neurovirulence in mice with functional CD8+ T cells, but normal neurovirulence in CD8+ T cell–deficient mice.
Materials and Methods
Viruses and Construction of an HSV-1 ICP47-mutant.
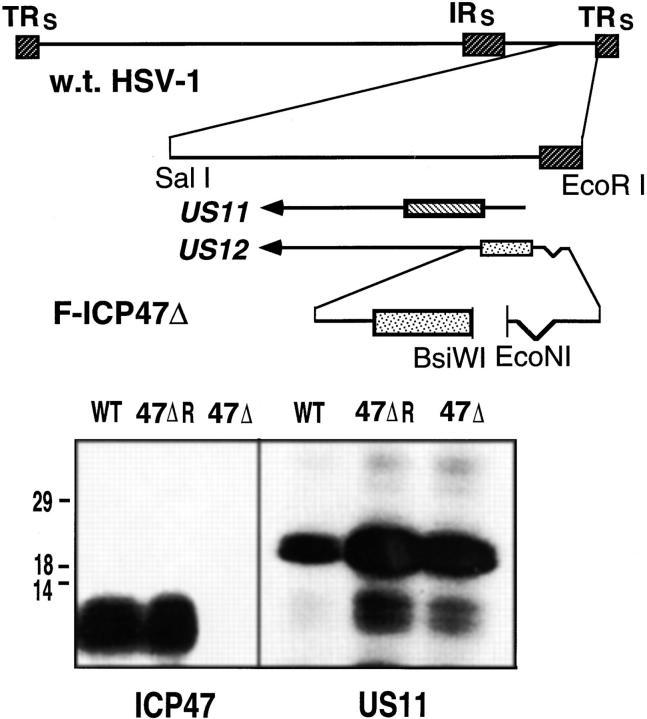
The wild-type HSV-1 strain F was obtained from Dr. Patricia Spear, Northwestern University, Chicago, IL. This virus was plaque-purified twice before construction of mutant viruses, and was propagated on Vero cell monolayers. Contruction of an HSV-1 glycoprotein E deletion mutant (F-gEβ) has been previously described (27). To contstruct an ICP47− HSV-1, a deletion of ∼10 nucleotides was introduced between an EcoNI site upstream of the ICP47 start codon and two adjacent BsiWI sites in plasmid pBR421 (28). This deletion removed the ICP47 start codon and two downstream ATG codons in the protein, and did not affect the promoter of the adjacent US11 gene. A plasmid containing this deletion was cotransfected with DNA derived from HSV-1 strain F into Vero cells as previously described (29). Using PCR to analyze viral DNA, viruses derived from the transfection were screened for the 10 nucleotide deletion. A virus, denoted F-ICP47Δ, contained the expected deletion, as confirmed by Northern blots of viral DNA. F-ICP47Δ expressed US11 protein, but did not express ICP47. F-ICP47Δ was plaque purified two additional times. To rule out the possibility of additional mutations in F-ICP47Δ, a rescued virus, able to express ICP47, was derived from F-ICP47Δ. Vero cells were cotransfected with F-ICP47Δ DNA and a plasmid, PRB421, containing wild-type ICP47 and US11 genes (28). Viruses derived from this transfection were screened by PCR and a virus, which expressed ICP47, denoted F-ICP47ΔR, and US11 was plaque purified two additional times.
Characterization of Viruses for ICP47 and US11 Expression.
To ascertain whether viruses expressed ICP47 and US11, human R970 cells were infected with wild-type HSV-1 F, F-ICP47Δ, or F-ICP47ΔR. The cells were labeled with [35S]-methionine, 3–6 h after infection for ICP47, or 7–10 h after infection for US11 expression. Cell extracts were prepared and immunoprecipitated as previously described (23). Anti-ICP47 serum was produced in rabbits injected with recombinant ICP47 from bacteria (4, 23). Anti-US11 serum has been previously described (30) and was the generous gift of Dr. Bernard Roizman, (University of Chicago, Chicago, IL).
Virus Inoculation and Evaluation of Disease.
Female A/J, BALB/c, or BALB/c athymic nude mice (Frederick Cancer Research, Frederick, MD), 6–8 wk of age, were anesthetized by intramuscular injection of 2.0 mg of ketamine hydrochloride (Vetalar; Parke-Davis, Morris Plains, NJ) and 0.04 mg of acepromazine maleate (Aveco Co., Fort Dodge, IA) in 0.1 ml of HBSS. The F strain of HSV-1, F-ICP47Δ, F-ICP47ΔR, and the gE deletion mutant F-gEβ (27) were grown in Vero cells, and intact virions were purified on Percoll (Pharmacia Biotech, Piscataway, NJ) as previously described (31). For each experiment, the virus samples were coded so that the person infecting the corneas and evaluating the corneal disease did not know which virus was used to infect the cornea. Corneas of anesthetized mice were scarified 10 times in a crisscross fashion with a sterile 30 gauge needle, and the eyes were infected topically with 3 μl of PBS containing varying amounts of HSV-1, as specified in the text. Mice were treated with 0.3% gentamicin sulfate ophthalmic solution (Genoptic; Allergen America, Puerto Rico) topically from days 4 to 11 after infection to prevent secondary bacterial infection.
Corneal epithelial disease was evaluated by instilling fluorescene drops on the eye and examining the cornea with a slit-lamp biomicroscope with a cobalt blue filter in the light path. HSV-1 replication in corneal epithelial cells results in pathognomonic dendritic-shaped lesions that stain with fluorescene. The severity of epithelial lesions was scored by a masked observer using a scale of 0 to 4+, where 1+ = punctate lesion; 2+ = dendritic lesion; 3+ = dendritic lesion with an amorphous central loss of epithelium; and 4+ = dendritic lesion with an amorphous central loss of epithelium that occupies >25% of the area of the cornea.
Corneal stromal inflammation was evaluated with a slit lamp on the basis of corneal opacity on a scale of 0 to 4+ as previously described (21). Periocular skin disease was scored on a scale of 0 to 5+, where 1+ = blepharitis (eyelid only); 2+ = <2 mm of fur loss around the eye, and no observable vesicles, 3+ = <2 mm of fur loss with vesicles, 4+ = >2 mm of fur loss around the eye, without vesicles; and 5+ = >2 mm of fur loss around the eye with vesicles. The manifestations of neurologic disease included weight loss, ruffled fur, paralysis, and death.
HSV Titration in the Infected Cornea.
Groups of 10 mice received unilateral corneal infections with either 105 or 104 PFU of wild-type HSV-1, F-ICP47Δ, or F-ICP47ΔR. 2 d later, the corneas were sterilely excised and frozen at −70°C until the time of assay. On the day of assay, the corneas were individually homogenized, submitted to three freeze–thaw cycles, and sonicated. The extracts were then centrifuged at 4,000 rpm for 30 min in a microcentrifuge, and the supernatant was assayed for infectious virus particles in a standard plaque assay on Vero cells.
In Vivo CD8+ T Lymphocyte Depletion.
Groups of A/J mice received intraperitoneal injections of 50 μg of rat mAbs specific for CD8-α (clone 2.43; American Type Culture Collection, [ATCC], Rockville, MD), or a similar dose of rat mAb to HLA-DR5 (clone SFR3-DR5; ATCC) as a control. Both mAbs were purified on a protein G–Sepharose Fastflow column (Pharmacia Biotech) and quantified with a radial immunodiffusion kit (ICN Flow Laboratories; Costa Mesa, CA). The mAb treatments were given 1 day before infection, and 2, 6, and 12 d after infection.
LD50 Assay in Athymic Nude Mice.
Groups of 6 female BALB/c athymic nude mice received corneal infections with various doses of F-ICP47Δ, F-ICP47ΔR, or the wild-type F strain of HSV-1, and survival time was recorded during a 30-d observation period.
T Cell Reconstitution of Nude Mice.
The corneas of euthymic BALB/c donor mice were infected with 105 PFU of HSV-1. 5 d later, the mice were depleted of CD4+ or CD8+ T lymphocytes by intraperitoneal injection of 250 μg of mAb to mouse CD4 (clone GK1.5; ATCC) or 50 μg of mAb to CD8-α (clone 2.43). 2 d later, draining LN cells were prepared and stimulated overnight with UV-inactivated HSV-1. The next day, the enriched CD4+ or CD8+ cells were further purified by negative selection with anti-CD4– or anti-CD8–coated magnetic beads (Dynal Inc., Lake Success, NY). This procedure routinely yielded LN cell populations in which 98–100% of the T lymphocytes were CD4+ or CD8+ as assessed by flow cytometry.
BALB/c athymic nude recipient mice were infected with F-ICP47Δ or F-ICP47ΔR HSV-1. 6 d after infection, the mice were reconstituted by a single intraperitoneal injection of 1.5 × 107 of the highly enriched CD4+ or CD8+ T lymphocytes, and the course of the disease was followed.
Results
ICP47 Does Not Affect HSV-1–induced Corneal Epithelial Disease, Stromal Inflammation, or Periocular Skin Disease.
Groups of 10 A/J mice received uniocular corneal infections with two different doses of wild-type HSV-1 strain F, F-ICP47Δ, or F-ICP47ΔR. The generation and characterization of the mutant viruses is shown diagrammatically in Fig. 1, and described in detail in Materials and Methods. 2 d after infection, all mice developed dendritic-shaped corneal epithelial lesions similar to those observed with wild-type HSV-1 (data not shown). As shown in Table 1, the severity of the corneal epithelial lesions was dose dependent, and the lesions caused by F-ICP47Δ were similar in magnitude to those caused by wild-type and F-ICP47ΔR when scored by a masked observer. Moreover, the yields of virus from corneas infected with F-ICP47Δ, F-ICP47ΔR, or wild-type HSV-1 did not differ (P = 0.79). At the higher doses of virus, corneal stromal inflammation and periocular skin disease were difficult to compare because most mice infected with F-ICP47ΔR or wild-type HSV-1 died of encephalitis. However, in mice infected with a sublethal dose (104 PFU) of wild-type F, F-ICP47Δ, or F-ICP47ΔR, there was a 50–60% incidence of stromal disease (data not shown), and the severity of this inflammation did not differ significantly (P = 0.18, Table 1). An equivalent number of mice infected with wild-type F, F-ICP47Δ, or F-ICP47ΔR developed vesicular skin lesions surrounding the eye and these lesions were of a similar magnitude (P = 0.63, Table 1). Therefore, ICP47 does not significantly impact HSV-1 replication and virulence in the eye or in the periocular skin.
Figure 1.
Generation of an HSV-1 ICP47 deletion mutant. The genome of HSV-1 is depicted in the upper panel of the figure. A 100- nucleotide deletion in the HSV US12 gene, which encodes ICP47, was created. The deletion removes the ICP47 start codon and two downstream ATG codons, but does not affect the promoter of the adjacent US11 gene. Cells were cotransfected with a plasmid containing the deletion, and viral DNA derived from HSV-1 (strain F). Virus progeny were screened for the mutation by performing PCR on samples of viral DNA. A virus, denoted F-ICP47Δ, contained the deletion in the US12 gene. A rescued derivative of F-ICP47Δ, denoted F-ICP47ΔR, was produced by cotransfecting cells with F-ICP47Δ DNA and a plasmid containing the wild-type US12 gene. In the lower panel, cells were infected with wild-type HSV-1, F-ICP47Δ, or F-ICP47ΔR, then radiolabeled with [35S]methionine. ICP47 was immunoprecipitated from cell extracts using a rabbit anti-ICP47 serum, and US11 protein was immunoprecipitated using an anti-US11 serum.
Table 1.
Effect of ICP47 on HSV-1 Corneal and Skin Disease Severity
| Infection* | Corneal Disease | Skin Disease | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Epithelial‡ | Stromal§ | Virus Yield‖ (PFU × 103) | ||||||||
| Strain | Dose (PFU) | |||||||||
| mean ± SEM | mean ± SEM | mean ± SEM | mean ± SEM | |||||||
| Wild-type | 1.0 × 105 | 3.0 ± 0 | N/A** | 5.7 ± 1.6 | N/A | |||||
| Wild-type | 1.0 × 104 | 1.2 ± 0.4 | 2.4 ± 2.1 | 4.9 ± 2.6 | 2.4 ± 1.0 | |||||
| F-ICP47Δ | 1.0 × 105 | 3.0 ± 0 | 2.3 ± 2.0 | 4.1 ± 1.3 | 2.2 ± 1.0 | |||||
| F-ICP47Δ | 1.0 × 104 | 1.1 ± 0.3 | 2.8 ± 2.9 | 3.2 ± 1.9 | 2.2 ± 1.0 | |||||
| F-ICP47ΔR | 1.0 × 105 | 3.0 ± 0 | N/A | 3.9 ± 1.9 | N/A | |||||
| F-ICP47ΔR | 1.0 × 104 | 1.2 ± 0.4 | 2.7 ± 1.7 | 3.6 ± 2.7 | 2.4 ± 1.6 | |||||
One cornea of each mouse was infected with HSV-1 F strain (wild-type), and ICP47 deletion mutant derived from F (F-ICP47Δ), or a revertant of ICP47Δ (F-ICP47ΔR).
Size of the epithelial lesion, scored on a scale of 0–4+.
Degree of corneal opacity, scored on a scale of 0–4+.
Corneas were excised 2 d after infection, and extracted virus was quantified in a standard plaque assay.
Severity of periocular skin disease scored on a scale of 0–5+.
Mice died before disease fully developed.
ICP47 Does Influence HSV-1 Neurovirulence and Lethality in the Mouse.
In contrast to the effects seen in the skin and eye, the ICP47− mutant was less able to cause neurologic symptoms and death. In 3 experiments, each involving 10 mice, 80% of the mice infected with wild-type HSV-1 experienced hind limb paralysis and loss of motor coordination along with ruffled fur and obvious weight loss by 10 d, and 76% succumbed to the infection by day 12 (Fig. 2). Similarly, 68% of the mice infected with F-ICP47ΔR showed neurologic symptoms and 61% died by day 13 (Fig. 2). By contrast, only 24% of the mice infected with F-ICP47Δ displayed neurologic symptoms and died of the infection (Fig. 2).
Figure 2.
ICP47 enhances neurovirulence in mice. In 3 experiments, groups of 10 female A/J mice were depleted of CD8+ T cells by injection of anti-CD8 mAb (solid bars), or were mock depleted by injection of control mAb (gray bars). The mice received uniocular corneal infections with 105 PFU of wild-type HSV-1 F strain, F-ICP47Δ, or F-ICP47ΔR, or F-gEβ. The survival rate was determined after a 30-d observation period, although all deaths occurred 12–14 d after infection. The *** indicates that the survival rate was significantly increased (P < 0.001) in immunologically normal mice infected with the F-ICP47Δ deletion mutant when compared with one of the following: CD8+ T cell–depleted mice infected with F-ICP47Δ; immunologically normal mice infected with wild-type F; or immunologically normal mice infected with F-ICP47ΔR.
ICP47 Augments HSV-1 Neurovirulence in the Presence, but not in the Absence, of CD8+ T Lymphocytes.
To determine if CD8+ T cells were responsible for the reduced neurovirulence of F-ICP47Δ, mice were depleted of CD8+ T cells by treatment with anti-CD8-α mAb, as described in the Materials and Methods section. This treatment protocol routinely resulted in >98% depletion of CD8+ cells in LN as assessed by flow cytometric analysis. As shown in Fig. 2, CD8+ T cell depletion dramatically increased neurological disease and mortality in mice infected with F-ICP47Δ, but had little effect on the mortality caused by wild-type HSV-1 or F-ICP47ΔR.
A gE-mutant (F-gEβ), also derived from the HSV-1 F strain, is also compromised in its ability to induce encephalitis in immunologically normal mice (27). However, CD8+ T cell depletion did not increase the neurovirulence of F-gEβ (Fig. 2). Thus, although deletion of several HSV genes can reduce neurovirulence, ICP47 is unique in that its effect on neurovirulence is only seen in the presence of functional CD8+ T lymphocytes.
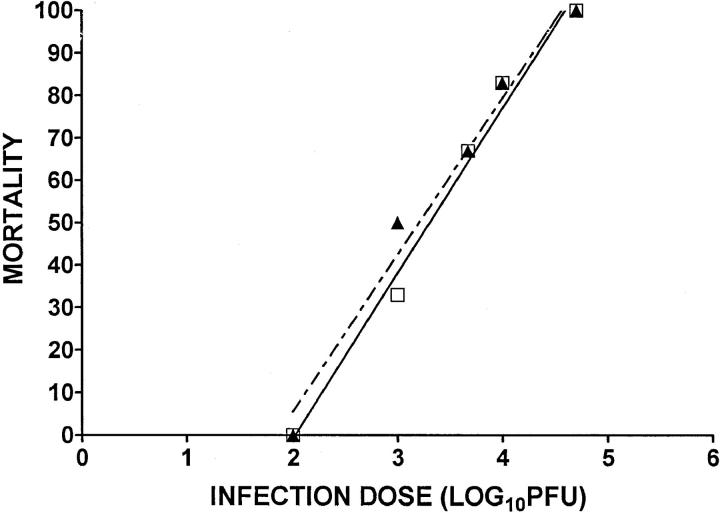
To determine whether CD4+ T cells contribute to the reduced neurovirulence, we initially attempted similar in vivo depletion experiments involving an anti-CD4 mAb, but these studies were inconclusive because depletion of CD4+ T cells caused significant reductions in CD8+ T cell infiltration into ganglia (data not shown). We chose to circumvent this problem by performing adoptive transfers into athymic nude mice. The LD50 of F-ICP47Δ (2,014 PFU) was not significantly different from the LD50 of F-ICP47ΔR (1,596 PFU) in nude mice (Fig. 3). Thus, ICP47 does not alter virus replication or neurovirulence in the absence of T lymphocytes.
Figure 3.
ICP47 does not enhance neurovirulence in T cell– deficient mice. Groups of six BALB/c athymic nude mice received corneal infections with various doses of F-ICP47Δ (▴) or F-ICP47ΔR (□). The mortality rate was observed over a 30-d observation period. The symbols represent the percentage of mortality at each infectious dose. The linear regression lines are shown for each data set. There is no significant difference in the slopes (P = 0.6604) or the elevations and intercepts (P = 0.3738) of the two lines.
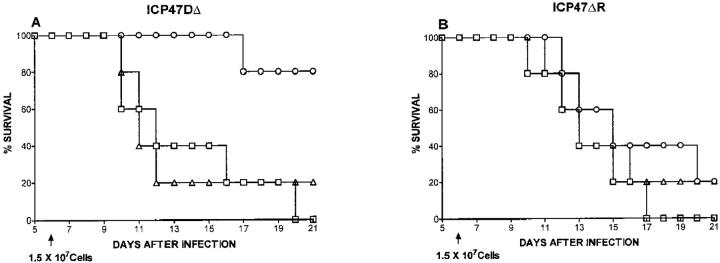
To determine whether CD4+ and CD8+ T cells influenced neurovirulence, highly enriched populations of CD4+ or CD8+ T cells, obtained from the LNs of HSV-infected mice, were transferred into nude mice. Adoptive transfer of CD8+ T cells significantly enhanced the survival of F-ICP47Δ–infected mice, but did not significantly alter survival of F-ICP47ΔR–infected mice (Fig. 4). Adoptive transfer of CD4+ T cells did not significantly influence the survival of mice that were infected with either virus. These data clearly establish that ICP47 augments HSV neurovirulence in mice by specifically inhibiting a protective CD8+ T cell response. With ICP47 in place, the F strain of HSV-1 is relatively resistant to the effects of CD8+ cells in the nervous system.
Figure 4.
Adoptive transfer of CD8+, but not CD4+, T cells inhibits neurovirulence of the ICP47− mutant in nude mice. Groups of five BALB/c athymic nude mice received corneal infections with 105 PFU of F-ICP47Δ HSV-1 (A), or F-ICP47ΔR HSV-1 (B). 6 d later, the control group (□) received no cells, or the mice received 1.5 × 107 lymph node cells from HSV-1– infected euthymic BALB/c mice that were highly enriched for CD4+ (▵) CD8+ (○) T lymphocytes. Survival of the recipient mice was followed through a 30-d observation period. Adoptive transfer of CD8+ cells conferred a significant survival advantage for mice infected with the F-ICP47Δ strain (P = 0.0186), but not for mice infected with the F-ICP47ΔR strain (P = 0.3962). Adoptive transfer of CD4+ cells did not significantly alter (P >0.05) survival of mice infected with either virus.
Discussion
CD8+ T cells can potentially recognize the broad array of HSV-1 polypeptides that are produced in virus-infected cells. However, there is substantial evidence that HSV avoids detection by CD8+ T cells, and the anti-HSV CD8+ T cell response is often weak in humans (for review see reference 5). ICP47 is an HSV immediate early protein, expressed very early after infection, that can inhibit CD8+ T cell recognition of infected cells (23). Recent studies have established clearly that ICP47 can bind with high affinity to human TAP and inhibit the transport of peptides into the endoplasmic reticulum (3, 4). The implication of this finding is that ICP47 can inhibit the expression of HSV antigens in the context of MHC class I on the surface of infected cells. This is a potentially important adaptation of the virus to the immune defenses of the host. Unfortunately, the contribution of ICP47 to viral virulence cannot be readily determined in humans. Such studies require an animal model in which both the HSV-1 disease pattern and the protective effect of T lymphocytes is characterized.
The role of T lymphocytes in controlling the multiple disease manifestations that result from HSV-1 corneal infection are well characterized. CD8+ T cells do not play an essential role in controlling HSV disease in the cornea or skin. Corneal epithelial disease is resolved with normal kinetics in the absence of CD4+ and CD8+ T cells (12). CD4+ T cells regulate corneal stromal inflammation (18, 32). Disease in the skin surrounding the eye, which begins ∼7 d after the primary infection in the cornea, is controlled by CD4+ or CD8+ α/β+ TCR T cells (15, 33), and by γ/ δ+ TCR T cells (14). However, in the sensory ganglia CD8+ T cells apparently play a dominant role in controlling HSV-1 replication and in preventing its transmission to the central nervous system and subsequent establishment of lethal encephalitis (13). Thus, it was reasonable to assume that a viral protein that specifically inhibits CD8+ T cell function might not dramatically affect HSV-1 disease in the cornea or skin of the mouse, but might nonetheless influence viral neurovirulence and lethality.
The disease pattern seen in immunologically normal mice after corneal infection with F-ICP47Δ HSV-1 was consistent with the hypothesis that ICP47 confers resistance to CD8+ T cell responses in the nervous system, but is less effective in the cornea and skin. ICP47 deletion did not influence the capacity of the virus to replicate in and destroy corneal epithelial cells. Thus, when compared with the parental F strain or the F-ICP47ΔR revertant, the F-ICP47Δ mutant produced corneal epithelial lesions of comparable severity, and similar virus titers were detected in the infected tissue. Moreover, the F-ICP47Δ mutant, a rescued version of the mutant, and wild-type HSV-1 all produced mild skin disease in immunologically normal mice, and severe skin disease in T cell–deficient mice. In contrast to what was seen at the periphery, the ICP47− HSV caused little or no neurologic disease and encephalitis, while the wild-type HSV-1 and the rescued virus killed most animals after causing encephalitis.
Since CD8+ T cells help control virus infection in the nervous system, one could argue that any HSV mutant that was “crippled” because it replicated less efficiently, either at the periphery or in the nervous system, might replicate better in the absence of CD8+ T cells. Although this is possible, we consider it unlikely for several reasons. First, HSV-1 thymidine kinase and γ34.5 mutants do not cause neurologic disease or encephalitis in either normal or immunologically compromised mice (34, 35). There are defects in replication of these viruses in cells that constitute the nervous system, and the inability in these viruses to replicate is not overcome by removing T cells. Second, we showed here that a gE− HSV-1 is unable to cause neurologic disease whether or not mice are depleted of CD8+ T cells. This mutant can replicate normally in neurons and other cells, but can not spread efficiently from cell to cell, and spreads poorly from the eye to the brain (27, 36). Therefore, it is not surprising that CD8+ T cell depletion cannot rescue this defect. Third, reconstitution with CD4+ T cells did not alter the course of disease caused by ICP47− HSV-1 in nude mice. Protection from lethal HSV-1 infections in mice can be controlled by both CD4+ and CD8+ T cells, and in one study a dominant role for CD4+ cells was described (16). Therefore, the ICP47− HSV is not just a “weak” virus with defects in replication that can be overcome by inhibiting host immunity. Instead, it appears that ICP47 augments neurovirulence by specifically subverting a protective CD8+ T cell response.
The capacity of HSV ICP47 to block CD8+ T cell recognition of infected target cells varies in different species and in different types of cells from the same species. Thus, HSV-infected human B cells and HSV-infected mouse fibroblasts are lysed by HSV-specific CTLs, whereas HSV-infected human fibroblasts are not lysed by the same CTLs (23). Moreover, the inhibitory effect of ICP47 on CTL recognition of human fibroblasts could be overcome by exposure of these cells to IFN-γ, presumably through its capacity to augment the basal level of antigen processing and presentation of these cells (23). These findings suggest that the capacity of ICP47 to block CD8+ T cell protection of various HSV-infected tissues is determined by a variety of factors, including: (a) how efficiently ICP47 inhibits TAP function in that species; (b) the basal level of antigen processing and presentation in cells of the infected tissue; and (c) the cytokine milieu within the infected tissue. HSV-1 ICP47 is a relatively poor inhibitor of murine TAP function (3, 4). However, in nervous tissue with impaired capacity to process and present antigens in the context of MHC class I, even a modest inhibition of TAP function might have a profound effect on a protective CD8+ T cell response. Assuming a comparable basal level of antigen processing and presentation in the mouse and human nervous system, one would predict a more profound effect of ICP47 on HSV-1 neurovirulence in humans due to its increased binding affinity for human TAP.
The mechanism by which CD8+ T cells protect HSV-infected sensory ganglia is not clear. A previous study showed increased production of MHC class I transcripts in satellite cells, Schwann cells, and neurons of spinal ganglia after HSV-1 infection of the skin (24). MHC class I protein was subsequently expressed on the satellite and Schwann cells, but not on the neurons. CD8+ T cells infiltrate the trigeminal ganglia of mice ∼7 d after corneal infection with wild-type HSV-1, and some are seen in direct apposition to satellite cells but not neurons in areas of viral antigen expression (37). We propose that direct interaction with CD8+ T cells might result in destruction of the infected satellite cells, but with simultaneous release by the CD8+ cells of antiviral cytokines that control HSV-1 replication in nearby neurons. This theory is consistent with the observations that, (a) the cytokines IFN-γ and TNF-α can inhibit HSV replication (38, 39), and (b) these cytokines are produced in the trigeminal ganglion during acute and latent infection (37, 40–42). Thus, HSV-specific CD8+ T cells could control virus by cytotoxic mechanisms acting on replaceable satellite cells, and by noncytotoxic mechanisms in neurons that cannot be regenerated.
The effect of ICP47 on the pattern of MHC class I expression in HSV-1–infected trigeminal ganglia is currently unknown. By inhibiting the upregulation of MHC class I expression on satellite cells, ICP47 could reduce the effectiveness of the CD8+ T cell response. Alternatively, ICP47 might inhibit the direct interaction of CD8+ cells with infected neurons by contributing to the failure of infected neurons to express MHC class I protein, despite production of MHC class I heavy and light chain RNA. Either of these mechanisms could inhibit CD8+ T cell control of HSV-1 replication in sensory neurons, favoring viral transmission to the brain and induction of lethal encephalitis. Although the mechanism remains unclear, our data clearly establish that HSV ICP47 can inhibit a CD8+ T cell response that prevents lethal encephalitis following from corneal infection.
Wild-type strains of HSV vary markedly in their capacity to induce neurologic disease in mice. Observations in both mice (13, 14, 43, 44) and humans (45) have also established that susceptibility to HSV encephalitis is augmented when the host immune system is compromised. Thus, resistance to encephalitis probably results from a balance between the neurovirulence properties of the infecting virus and the strength of the host immune response. In general, this balance is maintained, as encephalitis is rare in humans. Our studies establish for the first time that HSV can influence this balance by producing a protein, ICP47, that inhibits an important host defense mechanism within the nervous system. Our results show clearly that a viral immune evasion strategy can act in a restricted tissue-specific manner and yet dramatically alter the outcome of disease. In addition, our observations underscore the difficulties associated with extrapolating from in vitro biochemical analyses to in vivo effects on viral disease. Based on studies in mouse fibroblasts, one might have predicted that ICP47 would not influence HSV disease in mice.
Acknowledgments
This work was supported by National Institutes of Health grants EY-05945 (to R.L. Hendricks), and EY-11245 (to D.C. Johnson), Core grant EY-01792 (to R.L. Hendricks); an unrestricted research grant from Research to Prevent Blindness, Inc., New York; and by the Lions of Illinois Foundation, Maywood, IL. R.L. Hendricks is a Research to Prevent Blindness Senior Scientific Investigator.
Footnotes
Abbreviations used in this paper: gE, glycoprotein E; HSV, herpes simplex virus; ICP, infected cell protein; TAP, the transporter associated with antigen presentation.
K. Goldsmith and Wei Chen contributed equally to this paper.
References
- 1.Früh K, Ahn K, Djaballah H, Sempe P, van Endert PM, Tampe R, Peterson PA, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 2.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 3.Ahn K, Meyer TH, Uebel S, Sempé P, Djaballah H, Yang Y, Peterson PA, Früh K, Tampé R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus protein ICP47. EMBO (Euro Mol Biol Organ) J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 4.Tomazin R, Hill AB, Jugovic P, York I, van Endert P, Ploegh HL, Andrews DW, Johnson DC. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO (Eur Mol Biol Organ) J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson, D.C., and A.B. Hill. 1998. Herpesviruses and immune evasion. Curr. Top. Microbiol. Immunol. In press. [DOI] [PubMed]
- 6.Hengel H, Koopmann JO, Flohr T, Muranyi W, Goulmy E, Hammerling GJ, Koszinowski UH, Momburg F. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity. 1997;6:623–632. doi: 10.1016/s1074-7613(00)80350-7. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler H, Thale R, Lucin P, Muranyi W, Flohr T, Hengel H, Farrell H, Rawlinson W, Koszinowski UH. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-golgi compartments. Immunity. 1997;6:57–66. doi: 10.1016/s1074-7613(00)80242-3. [DOI] [PubMed] [Google Scholar]
- 8.Ahn K, Gruhler A, Galocha B, Jones TR, Wiertz EJ, Ploegh HL, Peterson PA, Yang Y, Früh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 9.Ahn K, Angulo A, Ghazal P, Peterson PA, Yang Y, Fruh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci USA. 1996;93:10990–10995. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert MJ, Riddel SR, Plachter B, Greenberg PD. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature. 1996;388:720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- 11.Jones TR, Hanson LK, Sun L, Slater JS, Stenberg RM, Campbell AE. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J Virol. 1995;69:4830–4841. doi: 10.1128/jvi.69.8.4830-4841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Tang Q, Hendricks RL. Ex vivo model of leukocyte migration into herpes simplex virus– infected mouse corneas. J Leukocyte Biol. 1996;60:167–173. doi: 10.1002/jlb.60.2.167. [DOI] [PubMed] [Google Scholar]
- 13.Simmons A, Tscharke DC. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor–γ/δ cells protect mice from herpes simplex virus type 1–induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith PM, Wolcott RM, Chervenak R, Jennings SR. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-γ (IFN-γ) Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 16.Manickan E, Rouse BT. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell–deficient mouse models. J Virol. 1996;69:8178–8179. doi: 10.1128/jvi.69.12.8178-8179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newell CK, Martin S, Sendele D, Mercadal CM, Rouse BT. Herpes simplex virus–induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989;63:769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendricks RL, Tumpey TM. Contribution of virus and immune factors to herpes simplex virus type 1 induced corneal pathology. Investig Ophthalmol Vis Sci. 1990;31:1929–1939. [PubMed] [Google Scholar]
- 19.Hendricks RL, Tumpey TM, Finnegan A. IFN-γ and IL-2 are protective in the skin but pathologic in the corneas of HSV-1–infected mice. J Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 20.Niemialtowski M, Rouse B. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J Immunol. 1992;148:1864–1870. [PubMed] [Google Scholar]
- 21.Tang Q, Hendricks RL. IFN-γ regulates PECAM-1 expression and neutrophil infiltration into herpes simplex virus–infected mouse corneas. J Exp Med. 1996;184:1435–1447. doi: 10.1084/jem.184.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Q, Chen W, Hendricks RL. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J Immunol. 1997;158:1275–1283. [PubMed] [Google Scholar]
- 23.York IA, Roop C, Andrews DW, Riddell SR, Graham FL, Johnson DC. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 24.Pereira RA, Tscharke DC, Simmons A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells, and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. J Exp Med. 1994;180:841–850. doi: 10.1084/jem.180.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann H, Cavalie A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 26.Rall GF, Mucke L, Oldstone MBA. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I–expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavromara-Nazos P, Silver S, Hubenthal-Voss J, McKnight JL, Roizman B. Regulation of herpes simplex virus 1 genes: α gene sequence requirements for transient induction of indicator genes regulated by β or late (γ 2) promoters. Virology. 1986;149:152–164. doi: 10.1016/0042-6822(86)90117-0. [DOI] [PubMed] [Google Scholar]
- 28.Ligas MW, Johnson DC. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roller RJ, Roizman B. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J Virol. 1992;66:3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendricks RL, Sugar J. Lysis of herpes simplex virus–infected targets. II. Nature of the effector cells. Cell Immunol. 1984;83:262–270. doi: 10.1016/0008-8749(84)90305-8. [DOI] [PubMed] [Google Scholar]
- 31.Newell CK, Sendele D, Rouse BT. Effects of CD4+ and CD8+T-lymphocyte depletion on the induction and expression of herpes simplex stromal keratitis. Reg Immunol. 1989;2:366–369. [PubMed] [Google Scholar]
- 32.Nash AA, Jayasuriyz A, Phelan J, Cobbold SP, Waldmann H. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system . J Gen Virol. 1987;68:825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34A.Valyi-Nagy T, Fareed MU, O'Keefe JS, Gesser RM, MacLean AR, Brown SM, Spivack JG, Fraser NW. The herpes simplex virkus type 1 strain 17+ gamma 34.5 deletion mutant 1716 is avirulent in SCID mice . J Gen Virol. 1994;75:2059–2063. doi: 10.1099/0022-1317-75-8-2059. [DOI] [PubMed] [Google Scholar]
- 34.Valyi-Nagy T, Gesser RM, Raengsakulrach B, Deshmane S, Randazzo BP, Dillner AJ, Fraser NW. A thymidine kinase-negative HSV-1 strain establishes a persistent infection in SCID mice that features unconrolled peripheral replication but only marginal nervous system involvement. Virology. 1994;199:484–490. doi: 10.1006/viro.1994.1150. [DOI] [PubMed] [Google Scholar]
- 35.Dingwell KS, Doering LC, Johnson DC. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type I corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feduchi E, Alonso MA, Carrasco L. Human γ interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989;63:1354–1359. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobsen H, Mestan J, Mittnacht S, Dieffenbach CW. β interferon subtype 1 induction by tumor necrosis factor. Mol Cell Biol. 1989;9:3037–3042. doi: 10.1128/mcb.9.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantin EM, Hinton DR, Chen J, Openshaw H. γ interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halford WP, Gebhardt BM, Carr DJJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 41.Shimeld C, Whiteland JL, Williams NA, Easty D, Hill TJ. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and immune cell infiltration. J Gen Virol. 1996;77:2583–2590. doi: 10.1099/0022-1317-77-10-2583. [DOI] [PubMed] [Google Scholar]
- 42.Ikemoto K, Pollard RB, Fukumoto T, Morimatsu M, Suzuki F. Small amounts of exogenous IL-4 increase the severity of encephalitis induced in mice by the intranasal infection of herpes simplex virus type 1. J Immunol. 1995;155:1326–1333. [PubMed] [Google Scholar]
- 43.Mitchell BM, Stevens JG. Neuroinvasive properties of herpes simplex virus type 1 glycoprotein variants are controlled by the immune response. J Immunol. 1996;156:246–255. [PubMed] [Google Scholar]
- 44.Nahmias, A.J., and R.M. Coleman. 1984. The significance of herpes simplex virus infections in humans. In Immunobiology of Herpes Simplex Virus Infection. B.T. Rouse and C. Lopez, editors. CRC Press, Inc., Boca Raton, FL. 1–8.