Abstract
Monocyte chemoattractant protein 1 (MCP-1) is a CC chemokine that attracts monocytes, memory T lymphocytes, and natural killer cells. Because other chemokines have similar target cell specificities and because CCR2, a cloned MCP-1 receptor, binds other ligands, it has been uncertain whether MCP-1 plays a unique role in recruiting mononuclear cells in vivo. To address this question, we disrupted SCYA2 (the gene encoding MCP-1) and tested MCP-1–deficient mice in models of inflammation. Despite normal numbers of circulating leukocytes and resident macrophages, MCP-1−/− mice were specifically unable to recruit monocytes 72 h after intraperitoneal thioglycollate administration. Similarly, accumulation of F4/80+ monocytes in delayed-type hypersensitivity lesions was impaired, although the swelling response was normal. Development of secondary pulmonary granulomata in response to Schistosoma mansoni eggs was blunted in MCP-1−/− mice, as was expression of IL-4, IL-5, and interferon γ in splenocytes. In contrast, MCP-1−/− mice were indistinguishable from wild-type mice in their ability to clear Mycobacterium tuberculosis. Our data indicate that MCP-1 is uniquely essential for monocyte recruitment in several inflammatory models in vivo and influences expression of cytokines related to T helper responses.
Chemokines are low molecular weight secreted proteins that play a variety of roles in intercellular signaling (1). Most chemokines exert their effects on leukocytes and were first purified on the basis of their ability to attract specific leukocyte subsets in vitro. For example, monocyte chemoattractant protein 1 (MCP-1)1 was identified as a monocyte-specific chemoattractant (2–4) that was later shown to attract memory T lymphocytes and NK cells (5–7). Because of its target cell specificity, MCP-1 was postulated to play a pathogenetic role in a variety of diseases characterized by mononuclear cell infiltration, including atherosclerosis, rheumatoid arthritis, and multiple sclerosis (8–11). Support for MCP-1's importance in the physiology of inflammation comes from demonstrations in transgenic mice that it functions as a monocyte chemoattractant in vivo (12–15).
However, MCP-1's role may be neither essential nor unique because of the potential for functional redundancy. Among the known CC chemokines, MCP-1, MCP-2, MCP-3, MCP-4, MCP-5, macrophage inflammatory protein (MIP)-1α, MIP-1β, I309, and HCC-1, all have monocyte chemoattractant activity in vitro. Furthermore, monocytes express at least three cloned CC chemokine receptors, namely CCR1, CCR2, and CCR5, and even though MCP-1 binds only CCR2 with high affinity, CCR2 also binds MCP-3 and MCP-5 (16–18). With this profusion of monocyte-active chemokines and monocyte-expressed receptors, as well as ligand–receptor promiscuity in vitro, it can be legitimately asked whether a single chemokine such as MCP-1 could have an essential effect in inflammatory disease. Antibody neutralization experiments indicate that MCP-1 might play a unique role in models of granuloma formation and pulmonary inflammation (19, 20). However, these studies suffer from the usual shortcomings of antibody specificity or secondary effects of exogenously administered antibody. Therefore, to address the question of whether MCP-1 plays a unique role in inflammation in vivo, we constructed an MCP-1–deficient mouse by targeted gene disruption. Analysis of this mouse indicates that despite functional redundancy with other chemokines in vitro, MCP-1 alone is responsible for mononuclear cell infiltration in several inflammatory models in vivo.
Materials and Methods
Targeted Disruption of MCP-1.
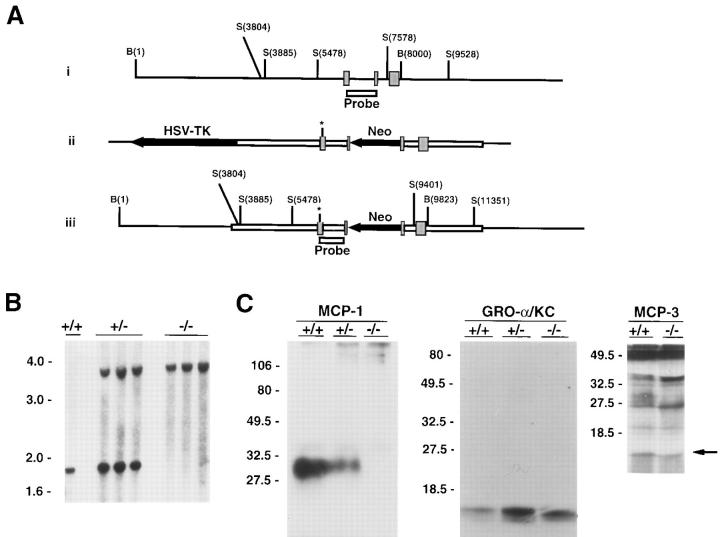
A 6600-bp genomic DNA fragment containing SCYA2 (the gene encoding MCP-1) (21, 22) was modified by introducing an XhoI linker in an HpaI site in the second exon, and the 3′ portion of the gene was cloned into the NotI–XhoI sites of pPNT (23). To modify the 5′ portion of the gene, the genomic fragment was digested with NaeI and PmlI (in the coding region of exon 1) and religated with an NheI linker to create a small deletion and an in-frame stop codon. The modified 5′ portion was then ligated into the XbaI site of pPNT to yield the targeting construct pJEKO-9, diagrammed in Fig. 1 A(ii).
Figure 1.
(A i) Wild-type SCYA2 locus. The three exons encoding MCP-1 are shown as hatched boxes; the positions of BamHI and SstI restriction endonuclease sites are indicated in relation to the 5′ BamHI site; the NaeI–HpaI fragment used as a probe in Southern blotting is indicated as the open box. (ii) Targeting construct indicating the transcriptional orientation of the PGK-neo cassette inserted in the second exon of MCP-1. Asterisk denotes the site of an in-frame stop codon engineered in the first exon (see Materials and Methods). (iii) Disrupted allele. (B) Southern blot analysis of wild-type and MCP-1–deficient mice. DNA was extracted from tails of wild-type mice (+/+), and mice heterozygous (+/−) or homozygous (−/−) for the disrupted MCP-1 allele. DNA was digested using SstI and analyzed by Southern blotting using the probe indicated in A. (C) Chemokine expression in wild-type and MCP-1–deficient mice. Peritoneal macrophages from wild-type mice (+/+), and mice heterozygous (+/−) or homozygous (−/−) for the disrupted MCP-1 allele were treated with LPS and radiolabeled using [35S]methionine as described in Materials and Methods. Conditioned medium was analyzed by immune precipitation using anti–MCP-1 antiserum and the precipitates were analyzed by SDS-PAGE (left gel). The supernatants from the anti–MCP-1 precipitation were then subjected to immune precipitation using anti– murine GRO-α/KC, and these precipitates were analyzed by SDS-PAGE (middle gel). In a separate experiment, conditioned medium was analyzed by immune precipitation using anti–murine MCP-3 antiserum (right gel). Arrow indicates position of MCP-3. A small amount of cross-reactivity with MCP-1 can be discerned at ∼30 kD in supernatants from wild-type macrophages.
pJEKO-9 was introduced into J1 embryonal stem cells (24) by electroporation and cells were selected in G418 and gancyclovir as previously described (25). 2 out of 60 clones showed evidence for homologous recombination, and were injected into blastocysts using standard techniques. After reimplantation in foster mothers, only one clone resulted in chimeric mice that transmitted the disrupted allele to offspring. Mice were genotyped by Southern blot analysis of DNA extracts from tail snips using standard techniques (26). In all experiments, control wild-type mice were the same strain as MCP-1–deficient mice, namely (129Sv/J × C57Bl/6)F1, raised in identical specific pathogen-free conditions.
Immune Precipitation.
To activate peritoneal macrophages, mice were administered 1.5 ml of 4% thioglycollate broth intraperitoneally. After 72 h, cells were harvested by peritoneal lavage with 5 ml cold HBSS with 10 U/ml heparin, and plated in RPMI 1640 medium supplemented with 10% bovine calf serum. After 2 h, nonadherent cells were removed by washing and adherent cells were stimulated with 10 μg/ml LPS (Sigma Chemical Co., St. Louis, MO) for 4 h. Cells were radiolabeled with 2 mCi/ml [35S]methionine (NEN-DuPont, Boston, MA) in the presence of 10 μg/ml LPS for an additional 4 h. Medium was collected, diluted with an equal volume of RIPA buffer, and precleared with normal rabbit serum and protein A–Sepharose beads (Bio-Rad Laboratories, Hercules, CA). Immune precipitations were then performed sequentially with anti–murine MCP-1 (21) and anti-KC (27). Anti-MCP-3 (28) (a gift from R. Bravo, Bristol-Myers Squibb, Princeton, NJ) was used in a separate experiment. Similar results were obtained without thioglycollate treatment, although the levels of chemokine synthesis were much lower in wild-type and MCP-1−/− mice, suggesting that thioglycollate priming is necessary for a full response to LPS in resident peritoneal macrophages.
Thioglycollate Challenge.
8–9-wk-old male mice were administered 1 ml of 4% thioglycollate broth intraperitoneally, and 72 h later were killed by CO2 asphyxiation. Cells were recovered by peritoneal lavage and counted using a hemocytometer. Cells were applied to microscope slides using a cytospin centrifuge, stained with Diff-Quik (Baxter Healthcare Corp., McGaw Park, IL), and differential counts were obtained by morphological analysis.
Contact Hypersensitivity.
8–12-wk-old wild-type and MCP-1−/− mice were sensitized by placing 0.1 ml of 0.5% 2,4-dinitro-1-fluorobenzene (DNFB) (in 4:1 acetone/olive oil) on the shaved skin of one flank. 6 d later, naive and sensitized mice were challenged by placing 0.02 ml of 0.2% DNFB on the dorsal surface of one ear. 24 h later, thickness of treated and untreated ears was determined by an investigator blinded to treatment group using a Peacock dial gauge. Some ears were fixed in formalin, embedded in paraffin, and stained with F4/80 (Serotec, Oxford, UK) or rat IgG. 200 nucleated cells were counted in random fields of dermis and F4/80+ cells were scored by an investigator blinded to treatment group.
Tuberculin-type Hypersensitivity.
8–12-wk-old wild-type and MCP-1−/− mice were sensitized by injecting 0.05 ml of a 3% solution of the O-succinimide ester of 4-hydroxy-3-nitrophenyl acetyl (NP-O-Su) in DMSO in two sites on the ventral flank, followed by 0.1 ml of borate buffered saline (pH 8.6) in the dorsal midline. 7 d later, naive and sensitized mice were challenged by injecting 0.025 ml of 3% NP-O-Su freshly diluted at 1:20 in PBS (pH 7.8) into one footpad. 24 h later, thickness of treated and untreated footpads were determined by an investigator blinded to treatment group using a Peacock dial gauge.
Schistosoma mansoni Egg–induced Pulmonary Granulomata.
Mice were sensitized by intraperitoneal injection of 3,000 viable Schistosoma mansoni eggs. 2 wk later, synchronous pulmonary granulomata were induced by intravenous injection of 3,000 viable eggs as previously described (20). At 2, 4, and 8 d after embolization, lungs were inflated with formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin, and granuloma area was measured by an investigator blinded to treatment group using a computerized morphometer (The Morphometer; Woods Hole Educational Associates, Woods Hole, MA).
Cytokine Production by Splenocytes.
Mice were sensitized by injection with Schistosome egg antigen (SEA). 2 wk later, splenocytes were isolated and cultured in RPMI 1640 with 10% fetal calf serum alone or with 10 μg/ml SEA. 24 h later medium was collected and concentrations of IL-2, IFN-γ, IL-5, and IL-10 were determined using specific ELISAs (Genzyme Corp., Boston, MA).
Infection with Mycobacterium tuberculosis.
Mice were injected intravenously with 105 CFU of the H37Rv strain of Mycobacterium tuberculosis. Four wild-type and four MCP-1−/− mice were killed at days 10, 20, 50, and 80 after infection, and homogenates of spleen, lung, and liver were plated on enriched agar (Middlebrook 7H11; Difco Laboratories, Detroit, MI). Colonies were counted after 3–4 wk of growth.
Results and Discussion
Gene Targeting and MCP-1 Expression.
Fig. 1 A describes the gene targeting strategy for MCP-1. The frequency of homologous recombination in embryonic stem (ES) cells was ∼3% and resulted in an MCP-1 allele that was disrupted by a neomycin resistance cassette in the second exon (in a transcriptional orientation opposite to that of MCP-1) and a linker with an in-frame stop codon in the first exon. Two targeted ES clones were injected into blastocysts, but only one resulted in a live birth after transfer to foster mothers. This chimera passed the disrupted allele to its progeny which were intercrossed to produce mice homozygous for the disrupted allele (Fig. 1 B) in the expected Mendelian proportion.
To test whether MCP-1 expression had been disrupted, peritoneal macrophages were isolated from wild-type mice and from mice heterozygous or homozygous for the disrupted allele. Fig. 1 C shows that LPS-stimulated macrophages from wild-type mice secreted MCP-1 protein, a 25–35 kD microheterogeneous glycoprotein (21, 29), while macrophages from homozygous mice secreted no detectable MCP-1. Macrophages from heterozygous mice secreted intermediate amounts. The absence of MCP-1 secretion by macrophages from homozygous mice was not due to absence of peritoneal macrophages since the resident macrophage population was similar in number to wild-type mice (see below). In addition, stimulated macrophages from homozygous mice secreted wild-type amounts of other chemokines such as GRO-α/KC and MCP-3 (Fig. 1 C), as well as MIP-1α (data not shown).
Phenotype of Unchallenged MCP-1–deficient Mice.
Litter size and sex distribution of MCP-1−/− mice were indistinguishable from wild-type mice. MCP-1−/− mice developed normally and had the same life span as wild-type mice. Their hematologic profiles were also similar. In addition, MCP-1–deficient mice had normal numbers of Küpffer cells and alveolar macrophages as determined by immunohistochemical staining with F4/80 (data not shown).
Monocyte Recruitment in Response to Nonspecific Stimuli.
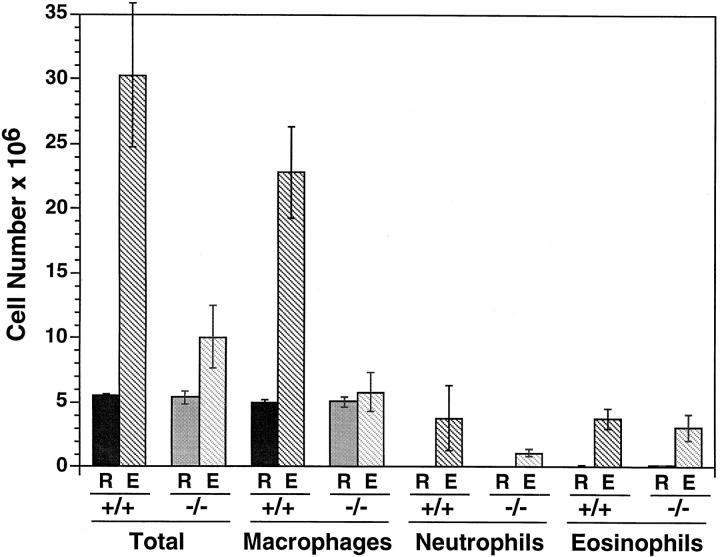
In response to intraperitoneal thioglycollate instillation, wild-type mice develop peritonitis that, after 72 h, consists primarily of monocytes and macrophages. Fig. 2 shows that before challenge, peritoneal lavage of wild-type and MCP-1– deficient mice recovered ∼5 × 106 cells/mouse, of which ∼95% were macrophages. This indicates that, similar to resident macrophage populations in liver and lung, MCP-1 is not required for the establishment of resident peritoneal cells.
Figure 2.
Thioglycollate elicitation in wild-type and MCP-1–deficient mice. Total and differential leukocyte counts were obtained on the resident peritoneal cells of six wild-type and six MCP-1−/− mice. An additional six wild-type and six MCP-1−/− mice were challenged with intraperitoneal thioglycollate. After 72 h, elicited cells were collected by lavage and analyzed for total cell and differential leukocyte count. Essentially no lymphocytes or mast cells were seen in either genotype. R, resident cells; E, elicited cells. Error bars indicate SEM. Differences in numbers of elicited neutrophils and eosinophils between wild-type and MCP-1−/− mice were not statistically significant by Student's t test.
72 h after thioglycollate administration, wild-type mice were observed to have a sixfold increase in the number of cells in their peritoneal cavities (Fig. 2). These cells consisted of a small number of neutrophils and eosinophils elicited over days 1–3, but most of the increase was due to monocytes and macrophages. In contrast, MCP-1−/− mice experienced only a doubling of total intraperitoneal cell number due to an increase in neutrophils and eosinophils that was statistically similar to the increase seen in wild-type mice. However, the MCP-1−/− mice showed essentially no recruitment of monocytes or macrophages to their peritonea.
In this model of peritonitis, both neutrophil and monocyte accumulation depend on the expression of selectins (30, 31), β2 integrins (32), LFA-1 (33, 34), intracellular adhesion molecule–1 (ICAM-1) (35), and platelet-endothelial cell adhesion molecule–1 (PECAM-1) (36). Although the absence of elicited monocytes in MCP-1−/− mice could be due to deficient adhesion molecule expression, elicitation of neutrophils and eosinophils in numbers identical to those in wild-type mice suggest that adhesion molecule expression was intact. We did not test for monocyte accumulation after 72 h, and it is possible that other chemokines might compensate for MCP-1 at later time points. However, at 72 h, when maximal monocyte recruitment normally occurs, monocyte accumulation clearly depends on MCP-1.
Delayed-type Hypersensitivity Responses.
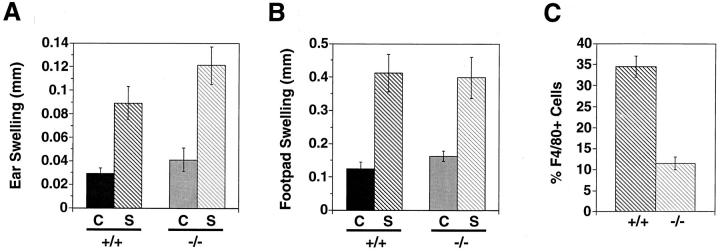
To test the role of MCP-1 in a contact hypersensitivity response, mice were sensitized with DNFB, then challenged by application of the hapten on the skin of the ear. Fig. 3 A shows that sensitized wild-type and MCP-1−/− mice experienced the same increase in ear swelling compared to nonsensitized mice of matched genotype. Similarly, in a tuberculin-type delayed-type hypersensitivity (DTH) model, sensitized mice were challenged by footpad injection of NP-O-Su. Again, as shown in Fig. 3 B, sensitized wild-type and MCP-1−/− mice had the same amount of footpad swelling compared to nonsensitized mice. These results indicate that MCP-1 is not required for the component of the DTH response that produces edema.
Figure 3.
DTH responses in wild-type and MCP-1–deficient mice. (A) Contact hypersensitivity. Six wild-type and six MCP-1−/− mice were sensitized with DNFB as described in Materials and Methods. 6 d later, sensitized mice as well as six naive wild-type and six naive MCP-1−/− mice were challenged by application of DNFB to one ear. 24 h later, the difference in thickness between treated and untreated ears was determined. C, control naive mice; S, sensitized mice; +/+, wild-type; −/−, MCP-1−/−. Error bars indicate SEM. The difference in ear swelling between sensitized wild-type and sensitized MCP-1−/− mice was not statistically significant. Similar results were observed in males and females. (B) Tuberculin-type hypersensitivity. 10 wild-type and 11 MCP-1−/− mice were sensitized with NP-O-Su as described in Materials and Methods. 7 d later, sensitized mice as well as nine naive wild-type and nine naive MCP-1−/− mice were challenged by injecting NP-O-Su in one footpad. 24 h later, the difference in thickness between injected and noninjected footpads was determined. Annotations are the same as in A. Similar results were observed in males and females. (C) F4/80+ cells in contact hypersensitivity lesions. Contact hypersensitivity challenges were performed as described in A, and ears from sensitized mice were harvested 24 h after challenge. Sections were stained and the proportion of F4/80+ cells from two animals in each group were determined. Error bars indicate SEM. These results are typical of two independent experiments.
However, examination of infiltrates in contact hypersensitivity responses showed that the proportion of F4/80+ cells in the lesions of MCP-1−/− mice was decreased threefold compared to wild-type mice (Fig. 3 C). Furthermore, while the total number of elicited cells per unit area appeared to be lower in MCP-1−/− mice, the number of neutrophils appeared to be the same (data not shown). Thus MCP-1 is required for eliciting a full complement of mononuclear cells in a DTH response but not for generating edema, suggesting that other cell types, such as neutrophils, may be responsible for this manifestation of DTH (37). This may be particularly relevant in the mouse where neutrophils comprise a much larger proportion of the infiltrate compared to humans. A similar dissociation between cell recruitment and vascular leak has been observed in P-selectin–deficient mice (38).
The presence of DTH-associated swelling in MCP-1−/− mice indicates that sensitization occurred without MCP-1. This may mean that MCP-1 is not necessary for activation of dendritic cells or for their migration into draining lymph nodes, which is a notable finding because of the evidence from some laboratories that MCP-1 can attract dendritic cells (15, 39). Our data suggest that this property of MCP-1 may not be relevant in the two models of DTH used in this study.
Response to Schistosoma mansoni Eggs.
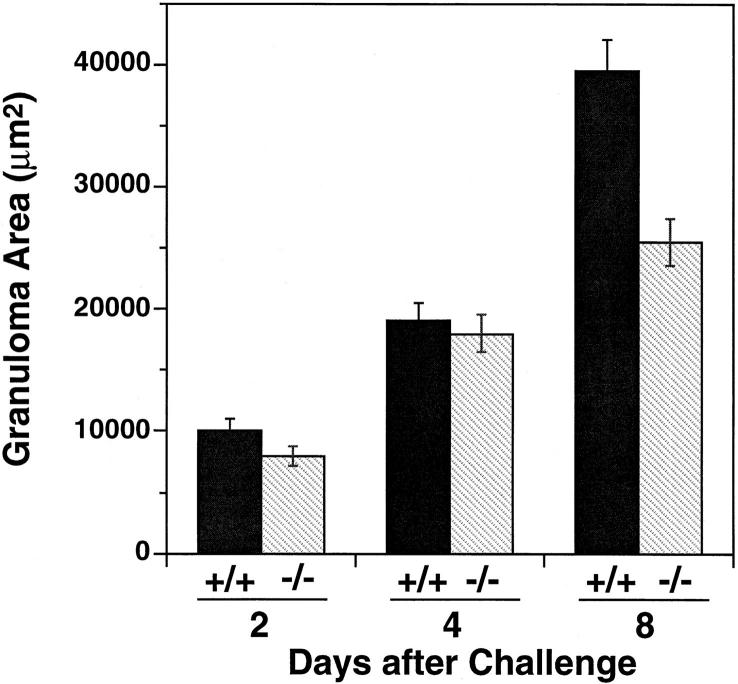
To examine MCP-1's role in a different hypersensitivity system, we challenged sensitized mice by intravenous administration of Schistosoma mansoni eggs to elicit the synchronous formation of secondary granulomata around eggs embolized to the pulmonary vasculature. It had been previously demonstrated that administration of anti–MCP-1 antibodies reduced the size of secondary pulmonary granulomata in this model by ∼40% (20). Consistent with that finding, Fig. 4 shows that the size of secondary granulomata 8 d after challenge in MCP-1−/− mice was also ∼40% smaller than granulomata in wild-type mice, thereby genetically confirming the importance of MCP-1 in this process.
Figure 4.
Secondary granuloma formation in response to Schistosoma mansoni eggs in wild-type and MCP-1−/− mice. Synchronous pulmonary granulomata were induced in chronically infected wild-type (+/+) or MCP-1−/− (−/−) mice as described in Materials and Methods. At the indicated time after egg injection, lungs from five to six mice were collected and the granuloma area was determined. Differences between wild-type and MCP-1−/− mice were significant at day 2 (P <0.01) and day 8 (P <0.001) by Student's t test. Error bars indicate SEM. At least 20 granulomata were measured per mouse.
Secondary granulomatous responses have been suggested to be predominantly controlled by Th2 cells (40–42). To examine the effect of MCP-1 on the development of T helper cells in this model, splenocytes from sensitized mice were tested for cytokine secretion in response to SEA in vitro. As shown in Table 1, IL-4 and IL-5 production were significantly reduced in MCP-1−/− splenocytes compared to wild-type splenocytes. However, IFN-γ secretion was also 59% lower in MCP-1−/− mice (but with a P value of only 0.08), whereas IL-2 and IL-10 expression were unchanged (as were the proliferative responses of splenocytes to SEA [data not shown]). Thus the absence of MCP-1 altered patterns of cytokine expression in sensitized mice, although the defect was not restricted to Th2 cytokines.
Table 1.
Cytokine Secretion by Splenocytes Challenged with SEA (pg/ml)
| IL-4 | IL-5 | IL-10 | IL-2 | IFN-γ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MCP-1+/+ (4) | 58 ± 12 | 2100 ± 540 | 276 ± 88 | 492 ± 96 | 1953 ± 544 | |||||
| MCP-1−/− (4) | 11 ± 1.6 | 1000 ± 190 | 288 ± 43 | 510 ± 63 | 820 ± 176 | |||||
| P value | 0.028 | 0.048 | NS | NS | 0.08 |
Mice were sensitized with SEA and spleens were harvested 2 wk later. Splenocytes were challenged in vitro with SEA and the concentration of indicated cytokine in conditioned medium was determined 24 h later by ELISA. Numbers in parentheses indicate the number of mice tested. P values were determined by Student's t test.
It remains to be determined whether this phenotype reflects a direct influence of MCP-1 on Th development (43) or an indirect influence of other abnormalities that may be present in MCP-1−/− mice. For example, NK cells are a likely source of IFN-γ in splenocytes of sensitized mice (44), and there may be fewer NK cells in MCP-1−/− spleens. It is not inconceivable that MCP-1 has important influences on the basal population of lymphoid tissues.
Response to Mycobacterium tuberculosis.
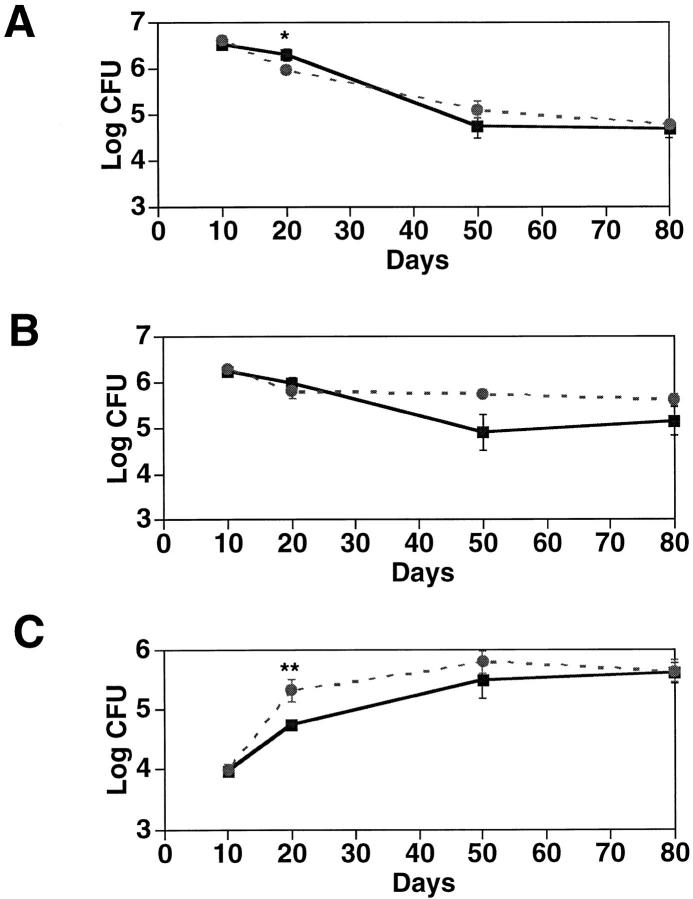
The preceding experiments demonstrated that lack of MCP-1 causes deficiencies in cellular recruitment to DTH and granulomatous lesions. This raised the question of whether MCP-1 also plays a role in systemic inflammatory challenges. To test this idea, we intravenously inoculated mice with 105 CFU of the virulent Mycobacterium tuberculosis strain H37Rv. At various times after challenge, CFU in lung, liver, and spleen were counted. Fig. 5 shows that MCP-1−/− mice cleared organisms from spleen and lung slightly less efficiently than wild-type mice at early time points, but by 80 days after inoculation the two genotypes were indistinguishable. Thus macrophages in these organs are capable of suppressing infection by this systemically administered pathogen even in the absence of MCP-1.
Figure 5.
CFU in organs of wild-type and MCP-1–deficient mice inoculated with Mycobacterium tuberculosis. 16 wild-type and 16 MCP-1−/− mice were injected intravenously with 105 CFU of Mycobacterium tuberculosis. At the indicated days after infection, 4 mice from each group were sacrificed and homogenates of liver (A), spleen (B), and lung (C) were plated. CFU are indicated from wild type (black square) and MCP-1−/− (gray circle) mice. Error bars indicate SEM. *P ⩽0.05, **P ⩽0.025 by Student's t test.
It is possible that this resistance to mycobacteria reflects intact Th1-like responses in MCP-1−/− mice. This interpretation would be consistent with results from an experimental model in which intravenously injected SEA-coupled beads, but not purified protein derivative (PPD)–coupled beads, elicited MCP-1 expression in pulmonary granulomata, and anti–MCP-1 treatment decreased the size of the SEA-induced, but not the PPD-induced, granulomata (45). These results differ from responses in transgenic mice expressing MCP-1 under the control of the mouse mammary tumor virus LTR (46). Those mice had high serum levels of MCP-1 and were deficient in clearing intravenously administered mycobacteria. Thus, persistent ambient MCP-1 may cause defects in macrophage function that are distinct from those caused by absence of MCP-1. For example, high serum levels of MCP-1 may downregulate another receptor on monocytes in addition to CCR2.
However, in vitro ligand binding experiments suggest that MCP-1's sole cloned receptor is CCR2 (an early assignment of MCP-1 to CCR4 [47] has not been reliably reproduced [48]). Recently, two models of CCR2-deficient mice constructed by gene targeting have been described, and both share several features with the MCP-1– deficient model reported here, including a selective defect in macrophage elicitation in response to intraperitoneal thioglycollate (49, 50). This suggests that CCR2 is probably MCP-1's sole receptor in vivo as well. Interestingly, one of these models (49) demonstrated a relatively selective defect in Th1 responses, which differs from suggestions using antibody neutralization or TCR transgenic T cells in vitro that MCP-1 influences Th2 responses (43, 45). However, those CCR2-deficient mice also showed defects in IL-4 and IL-5 secretion as well as IFN-γ secretion, similar to our mice. This indicates, again, that MCP-1's role as an immunoregulatory molecule is complex and not restricted to type 1 or type 2 helper T cells. When examined in vivo, MCP-1's influence on acquired immunity may be due to its attraction of specific leukocyte subsets to secondary lymphatic organs rather than a direct effect on T lymphocyte differentiation.
In summary, the major finding from our work concerns chemokine redundancy. In spite of the existence of many CC chemokines that attract monocytes in vitro, our data show that loss of MCP-1 alone is sufficient to impair monocyte trafficking in several different models at the times we examined. In addition, absence of MCP-1 results in profound alterations in cytokine secretion by splenocytes sensitized to SEA. This suggests that chemokine redundancy as defined by receptor binding in vitro may not be relevant in vivo, where specificity is achieved by timing and levels of expression. For example, while many CC chemokines can attract monocytes, only MCP-1 appears to be expressed at high levels in the peritoneum in response to thioglycollate. Thus, strategies designed to disrupt a single chemokine– receptor pair may be beneficial. This will depend on demonstrating an essential role for a specific chemokine in disease models, an approach that is now feasible using MCP-1−/− mice.
Footnotes
The authors thank Dr. Arlene Sharpe for blastocyst injections; Dr. Abul Abbas for helpful comments and encouragement; Drs. Rolf Freter and Charles Stiles for the 5′ genomic MCP-1 fragment; Dr. Maja Sirotkovic for technical help; and the staff of the Animal Resource Division at Dana-Farber Cancer Institute for its superb care and handling of the mice used in this study. We also appreciate the administrative support of Laurie Geronimo.
This work was funded by National Institutes of Health grants CA-53091, HL-35276, and HL-51366. B.J. Rollins is a Scholar of the Leukemia Society of America.
Address correspondence to Barrett Rollins, Dana-Farber Cancer Institute, 44 Binney St., Boston, MA 02115. Phone: 617-632-3896; Fax: 617-632-5417; E-mail: barrett_rollins@dfci.harvard.edu
Abbreviations used in this paper: DNFB, 2,4-dinitro-1-fluorobenzene; DTH, delayed-type hypersensitivity; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; SEA, Schistosome egg antigen.
References
- 1.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 2.Valente AJ, Graves DT, Vialle-Valentin CE, Delgado R, Schwartz CJ. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988;27:4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu JI, Leonard EJ. Purification and amino acid analysis of two human glioma–derived monocyte chemoattractants. J Exp Med. 1989;169:1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allavena P, Bianchi G, Zhou D, van Damme J, Jilek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein–1, –2, and –3. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 6.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+mobilization, and enzyme release. J Immunol. 1996;156:322–327. [PubMed] [Google Scholar]
- 7.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yla-Herttuala S, Lipton BA, Rosenfeld ME, Sarkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein–1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, Burdick MD, Pope RM, Strieter RM. Enhanced production of monocyte chemoattractant protein–1 in rheumatoid arthritis. J Clin Invest. 1992;90:772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB (Fed Am Soc Exp Biol) J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 12.Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA. Controlled recruitment of monocytes/macrophages to specific organs through transgenic expression of MCP-1. J Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- 13.Grewal IS, Rutledge BJ, Fiorillo JA, Gu L, Gladue RP, Flavell RA, Rollins BJ. Transgenic monocyte chemoattractant protein–1 (MCP-1) in pancreatic islets produces monocyte-rich insulitis without diabetes: abrogation by a second transgene expressing systemic MCP-1. J Immunol. 1997;159:401–408. [PubMed] [Google Scholar]
- 14.Gunn MD, Nelken NA, Liao X, Williams LT. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol. 1997;158:376–383. [PubMed] [Google Scholar]
- 15.Nakamura K, Williams IR, Kupper TS. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J Invest Dermatol. 1995;105:635–643. doi: 10.1111/1523-1747.ep12324061. [DOI] [PubMed] [Google Scholar]
- 16.Combadière C, Ahuja SK, Van Damme J, Tiffany HL, Gao JL, Murphy PM. Monocyte chemoattractant protein-3 is a functional ligand for CC chemokine receptors 1 and 2B. J Biol Chem. 1995;270:29671–29675. doi: 10.1074/jbc.270.50.29671. [DOI] [PubMed] [Google Scholar]
- 17.Franci C, Wong LM, Van DJ, Proost P, Charo IF. Monocyte chemoattractant protein–3, but not monocyte chemoattractant protein–2, is a functional ligand of the human monocyte chemoattractant protein–1 receptor. J Immunol. 1995;154:6511–6517. [PubMed] [Google Scholar]
- 18.Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)–5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med. 1997;185:99–109. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flory CM, Jones ML, Warren JS. Pulmonary granuloma formation in the rat is partially dependent on monocyte chemoattractant protein 1. Lab Invest. 1993;69:396–404. [PubMed] [Google Scholar]
- 20.Chensue SW, Warmington KS, Lukacs NW, Lincoln PM, Burdick MD, Strieter RM, Kunkel SL. Monocyte chemotactic protein expression during schistosome egg granuloma formation. Sequence of production, localization, contribution, and regulation. Am J Pathol. 1995;146:130–138. [PMC free article] [PubMed] [Google Scholar]
- 21.Rollins BJ, Morrison ED, Stiles CD. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci USA. 1988;85:3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freter RR, Irminger JC, Porter JA, Jones SD, Stiles CD. A novel 7-nucleotide motif located in 3′ untranslated sequences of the immediate-early gene set mediates platelet-derived growth factor induction of the JEgene. Mol Cell Biol. 1992;12:5288–5300. doi: 10.1128/mcb.12.12.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tybulewicz VLJ, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-ablproto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 24.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 25.Lu B, Gerard NP, Kolakowski LF, Jr, Bozza M, Zurakowski D, Finco O, Carroll MC, Gerard C. Neutral endopeptidase modulation of septic shock. J Exp Med. 1995;181:2271–2275. doi: 10.1084/jem.181.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 28.Heinrich JN, Ryseck RP, Macdonald-Bravo H, Bravo R. The product of a novel growth factor-activated gene, fic, is a biologically active “C-C”-type cytokine. Mol Cell Biol. 1993;13:2020–2030. doi: 10.1128/mcb.13.4.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst CA, Zhang YJ, Hancock PR, Rutledge BJ, Corless CL, Rollins BJ. Biochemical and biological characterization of murine MCP-1: identification of two functional domains. J Immunol. 1994;152:3541–3549. [PubMed] [Google Scholar]
- 30.Watson SR, Fennie C, Lasky LA. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimaera. Nature. 1991;349:164–167. doi: 10.1038/349164a0. [DOI] [PubMed] [Google Scholar]
- 31.Tedder TF, Steeber DA, Pizcueta P. L-selectin–deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O'Brien WE, Beaudet AL. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993;151:1571–1578. [PubMed] [Google Scholar]
- 33.Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1–deficient mice. J Clin Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmits R, Kundig TM, Baker DM, Shumaker G, Simard JJ, Duncan G, Wakeham A, Shahinian A, van der Heiden A, Bachmann MF, et al. LFA-1–deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sligh JE, Jr, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J Exp Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen CG, Thomsen MK, Gesser B, Thomsen PD, Deleuran BW, Nowak J, Skodt V, Thomsen HK, Deleuran M, Thestrup-Pedersen K, et al. The delayed-type hypersensitivity reaction is dependent on IL-8. Inhibition of a tuberculin skin reaction by an anti–IL-8 monoclonal antibody. J Immunol. 1995;155:2151–2157. [PubMed] [Google Scholar]
- 38.Subramaniam M, Saffaripour S, Watson SR, Mayadas TN, Hynes RO, Wagner DD. Reduced recruitment of inflammatory cells in a contact hypersensitivity response in P-selectin–deficient mice. J Exp Med. 1995;181:2277–2282. doi: 10.1084/jem.181.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukocyte Biol. 1996;60:365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- 40.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, Heiny S, Lewis F, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine Schistosomiasis mansoni. . J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 41.Wynn TA, Eltoum I, Cheever AW, Lewis FA, Gause WC, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. . J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 42.Boros DL. The role of cytokines in the formation of the schistosome egg granuloma. Immunobiology. 1994;191:441–450. doi: 10.1016/S0171-2985(11)80450-X. [DOI] [PubMed] [Google Scholar]
- 43.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 44.Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoniand exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel SL. Role of monocyte chemoattractant protein–1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol. 1996;157:4602–4608. [PubMed] [Google Scholar]
- 46.Rutledge BJ, Rayburn H, Rosenberg R, North RJ, Gladue RP, Corless CL, Rollins BJ. High level monocyte chemoattractant protein–1 expression in transgenic mice increases their susceptibility to intracellular pathogens. J Immunol. 1995;155:4838–4843. [PubMed] [Google Scholar]
- 47.Power CA, Meyer A, Nemeth K, Bacon KB, Hoogewerf AJ, Proudfoot AEI, Wells TNC. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 48.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell–directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 49.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RVJ, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]