Abstract
We report that chlamydiae, which are obligate intracellular bacterial pathogens, possess a novel antiapoptotic mechanism. Chlamydia-infected host cells are profoundly resistant to apoptosis induced by a wide spectrum of proapoptotic stimuli including the kinase inhibitor staurosporine, the DNA-damaging agent etoposide, and several immunological apoptosis-inducing molecules such as tumor necrosis factor-α, Fas antibody, and granzyme B/perforin. The antiapoptotic activity was dependent on chlamydial but not host protein synthesis. These observations suggest that chlamydia may encode factors that interrupt many different host cell apoptotic pathways. We found that activation of the downstream caspase 3 and cleavage of poly (ADP-ribose) polymerase were inhibited in chlamydia-infected cells. Mitochondrial cytochrome c release into the cytosol induced by proapoptotic factors was also prevented by chlamydial infection. These observations suggest that chlamydial proteins may interrupt diverse apoptotic pathways by blocking mitochondrial cytochrome c release, a central step proposed to convert the upstream private pathways into an effector apoptotic pathway for amplification of downstream caspases. Thus, we have identified a chlamydial antiapoptosis mechanism(s) that will help define chlamydial pathogenesis and may also provide information about the central mechanisms regulating host cell apoptosis.
Apoptosis is an active process of cellular suicide triggered by a variety of physiological and stress stimuli. Besides having an important role in normal development and tissue homeostasis, apoptosis has also been considered a primary defense against viral infection (1–3). It has also been demonstrated that apoptotic suicide by the infected cells can limit the spread of intracellular bacterial infections by provoking inflammatory responses (4) and/or by delivering the intracellular pathogens to competent professional phagocytes (5). Furthermore, induction of target cell apoptosis constitutes an essential part of antigen-specific immune effector mechanisms (6). It is therefore advantageous for intracellular pathogens to develop strategies to inhibit host cell apoptosis. In fact, many viral antiapoptotic genes have been identified (7). These include viral inhibitors for caspases such as CrmA in the cowpox virus (8) and p35 in baculovirus (9), viral Bcl-2 homologues (10–12), viral products that can modulate p53 activity (13), viral homologues of mammalian death receptors (14), and viral FLICE-inhibitory proteins (v-FLIPs) (15). Chlamydiae, which are obligate intracellular bacterial pathogens, require several days of intracellular replication and differentiation to produce sufficient infectious progeny for spread to adjacent host cells (16). Therefore, chlamydial organisms may also have evolved strategies to counteract host cell apoptosis to productively complete their obligate intracellular growth cycle.
Human chlamydial infections are recognized as the leading cause of many important sexually transmitted diseases worldwide (17), and the development of chlamydial diseases is largely due to persistent intracellular infection by the organism. Chlamydiae have a unique intracellular biphasic life cycle (16, 18). A typical chlamydial infection starts with entry of an infectious elementary body (EB) into host cells. Once internalized, an EB differentiates into a noninfectious but metabolically active reticulate body (RB), which multiplies and differentiates back to EBs. The mature EBs are then released extracellularly and spread to other potential host cells. The entire intracellular growth cycle in vitro takes ∼48–72 h and occurs within a cytoplasmic vacuole termed the chlamydial inclusion body. Since RBs are structurally fragile and not infectious, it is essential to maintain the integrity of host cells during chlamydial intracellular growth not only for supply of nutrients but also for shielding the intracellular organisms from host phagocytosis (19) and antigen-specific immune effector mechanisms (20). To achieve this, chlamydia may either sequester themselves to avoid activation of host cell apoptosis programs or actively interrupt host apoptotic pathways. It is known that infected host cells are able to respond to chlamydial attachment and intracellular growth by increasing host cell protein phosphorylation (21) and cytokine secretion (22, 23). Why, then, do the host cells often fail to activate a vital defense mechanism, apoptosis, during intracellular chlamydial infection? We proposed that intracellular chlamydial organisms are able to actively inhibit infected host cells from undergoing apoptosis. In the present study, we tested our hypothesis by evaluating whether intracellular chlamydial infection can inhibit host cell apoptosis induced by apoptotic stimuli, and explored potential mechanisms of the chlamydial antiapoptotic activity.
Apoptosis is a highly regulated cellular process that consists of diverse upstream private pathways for transducing extracellular death signals into intracellular events and a common downstream effector pathway for amplification of caspases. It has been demonstrated that different proapoptotic factors deliver death signals to host cells by different pathways (24–27) and mitochondrial cytochrome c release may be a central step connecting the diverse upstream pathways to the common effector pathway for amplification of downstream caspases (28–30). We found that chlamydia-infected host cells were profoundly resistant to apoptosis induced by both exogenous and immunological apoptosis-inducing molecules. The antiapoptotic activity was further correlated with the chlamydia-induced blockade of mitochondrial cytochrome c release and downstream caspase activation. Thus, chlamydial intracellular infection may interrupt many different upstream apoptotic pathways by blocking mitochondrial cytochrome c release.
Materials and Methods
Reagents, Cells, and Chlamydial Organisms.
Staurosporine, etoposide, penicillin G, chloramphenicol, and cycloheximide were from Sigma Chemical Co. (St. Louis, MO). Rifampin was from Calbiochem Corp. (La Jolla, CA). The following cell lines were used in this study: HeLa 229 (No. CCL-2.1; American Type Culture Collection, Rockville, MD), U937 (No. CRL-1593; American Type Culture Collection), and L929s (reference 31; provided by Dr. S. Nagata, Osaka, Japan). All cells were cultured in either RPMI (GIBCO BRL, Gaithersburg, MD) with 10% FCS (all from Intergen Company, New York) or DMEM (GIBCO BRL) with 10% FCS. The Chlamydia trachomatis serovar L2 (434/Bu), serovar C (TW3/OT), and a mouse pneumonitis agent used in this study were from Washington Research Foundation (Seattle, WA). The organisms were grown in HeLa cells and purified as described previously (32).
Chlamydia Infection.
Host cells were infected with chlamydial organisms (serovar L2 or as specified) at a multiplicity of infection (MOI) of 5 or as specified in individual experiments. For infection of adherent cells (HeLa and L929s), chlamydial organisms were allowed to attach to monolayer cells grown either on glass coverslips in 24-well plates (for microscopic observation) or in tissue culture flasks (for Western blot assay and DNA fragmentation assay) for 2 h at 37°C. The chlamydial inocula were then removed and DMEM medium with 10% FCS was added to the monolayer cultures until the infected cells were used for apoptosis induction. For infection of U937 cells, chlamydial organisms were allowed to attach to the suspension cells at 4°C for 2 h at an MOI of 20. We found that this high MOI was necessary to achieve a high infection rate since U937 cells are less susceptible to chlamydial infection (data not shown). After the chlamydial attachment, the infected cells were then grown in DMEM with 10% FCS until they were subjected to apoptosis induction.
Apoptosis Induction.
Host cells with or without chlamydial infection in 24-well plates or in tissue culture flasks were treated with various apoptotic stimuli for various times in DMEM with 5% FCS as follows: HeLa cell samples were stimulated for 4 h with 1 μM staurosporine, 5 h with 300 μM etoposide, or 2 μg/ml granzyme B plus 0.12 μg/ml perforin purified as described previously (33). L929s cells were stimulated with mouse recombinant TNF-α (PharMingen, San Diego, CA) at 40 ng/ml for 8 h and U937 cells were treated with either 40 ng/ml of human recombinant TNF-α (PharMingen) for 4 h or 250 ng/ml of mouse IgM anti–human Fas antibody CH11 (Immunotech, Westbrook, ME) for 8 h in the presence of 2 μg/ml of cycloheximide. The control cells were treated with cycloheximide alone.
Apoptosis Assay.
At the end of the apoptosis induction, both the stimulated and control cells were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature followed by permeabilization with 0.5% saponin in PBS for 30 min at room temperature. The cells were then stained with 10 μM of Hoechst 33258 (Sigma Chemical Co.) for 30 min at room temperature. After washing three times with PBS, the stained cells were mounted onto slides using FluorSave Reagent (Calbiochem, La Jolla, CA) for evaluating apoptosis with a fluorescence microscope as described previously (33). Cells from five random fields for each sample were counted using a ×40 objective lens. The percent of apoptotic cells was calculated as number of apoptotic cells/number of total cells counted.
Detection of Chlamydial Antigen, Cytochrome c, and Terminal Transferase–Mediated dUTP nick-end labeling.
The various cell samples grown on glass coverslips in 24-well plates were subjected to fixation and permeabilization as described above except that cell samples for cytochrome c staining were permeabilized with 0.25% saponin. One set of coverslips was stained with the DNA dye Hoechst only as described above. A second set of coverslips was subjected to double staining to verify the presence of chromatin condensation and chlamydial antigens. The cell samples were reacted with a mixture of terminal transferase (TdT) and biotin-dUTP as described by the manufacturer (Boehringer Mannheim Canada, Laval, Canada). The biotin was visualized by reacting with avidin conjugated with FITC (Sigma Chemical Co.). The same samples were costained with a rabbit antiserum against chlamydial organisms (34) and probed with a goat anti–rabbit IgG conjugated with Cy3 ( Jackson ImmunoResearch Laboratories, West Grove, PA). A third set of coverslips was stained for both chlamydial antigens and host cytochrome c. A mouse mAb 6H2.B4 (IgG1; PharMingen) specifically recognizing native cytochrome c was reacted with the cell samples and the antibody reaction was visualized with a goat anti–mouse IgG conjugated with Cy3 ( Jackson ImmunoResearch Laboratories). The chlamydial antigens were stained with a rabbit antiserum followed by a goat anti–rabbit IgG conjugated with FITC (Sigma Chemical Co.). A fourth set of samples was doubly stained with both the Hoechst dye and the anti–cytochrome c antibody as described above. All samples with Hoechst dye staining were viewed with a ×40 and photographed with a ×100 objective lens using fluorescence microscopy as described below. The cell samples doubly stained with dUTP plus antichlamydia antiserum or with anti–cytochrome c antibody and antichlamydia antiserum were analyzed by confocal microscopy as described below.
Fluorescence and Confocal Microscopy.
Images from Hoechst stained samples were acquired under a ×100 objective lens using a Zeiss Axiophot microscope equipped with a cooled CCD camera CH250/a (Photometrics Inc., Woburn, MA) with a KAF-1400-50 sensor chip (1,317 × 1,035 pixels) driven by IPLabs Spectrum H-SU2 (version 3.0; Signal Analytics Corp., Vienna, VA) and Multiprobe 1.1 E (Signal Analytics Corp.) software on a Power Macintosh 8100. The images were then copied into Adobe photoshop files for printing. For confocal analysis, images from the doubly stained samples were acquired using a confocal fluorescence microscope equipped with an argon laser and dual detectors (Molecular Dynamics, Sunnyvale, CA.). The images were acquired with Image Space software using a ×60 objective lens and then transferred into Adobe Photoshop for printing.
DNA Fragmentation (DNA Ladder) Assay.
HeLa cells with or without infection and apoptosis induction were harvested by trypsinization. After washing with PBS, 5 × 106 cells from each sample were lysed in a lysis buffer containing 5 mM EDTA, 5 mM Tris, pH 8.0, and 0.5% Triton. The postnuclear supernatant was extracted with phenol and chloroform before precipitation with ethanol. The DNA solution was incubated at 37°C for 30 min in the presence of RNase and electrophoresed in a 3% agarose gel. Each lane was loaded with DNA sample from 1.5 × 106 cells. The gel was then stained with ethidium bromide.
Western Blot Analysis.
For Western blot analysis of caspase 3 and poly (ADP-ribose) polymerase (PARP), cells with or without chlamydial infection or apoptosis induction were lysed with a RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 1 μg/ml Aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin A, and 1 mM sodium orthovanadate) for 30 min on ice. After centrifugation at 13,000 g at 4°C for 15 min, the postnuclear supernatants were loaded on a 15% polyacrylamide gel for caspase 3 and a 10% gel for PARP detection. Each lane was loaded with the lysate from 3 × 105 cells. After electrophoresis, the gels were blotted onto nitrocellulose membrane (BioRad Lab., Hercules, CA), which was then probed with either a mouse mAb to caspase 3 (clone No. 19; Transduction Laboratories, Lexington, KY) or a mouse mAb to PARP (clone C2-10; PharMingen). Primary antibody binding was detected with a goat anti–mouse IgG-conjugated with horseradish peroxidase ( Jackson ImmunoResearch Laboratories) and visualized by enhanced chemiluminescence (ECL) as described in the manufacturer's instructions (Amersham Canada, Oakville, Canada).
For analysis of cytochrome c, a procedure described elsewhere was followed (29). Cell samples were collected by centrifugation at 600 g for 10 min at 4°C in a Beckman benchtop centrifuge. The cell pellets were washed once with ice-cold PBS and resuspended with five volumes of buffer A (20 mM Hepes-KOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM dithiothreitol, and 0.1 mM phenylmethylsulfoyl fluoride) containing 250 mM sucrose on ice for 15 min. The cells were homogenized with 15 to 20 strokes of a number 22 kontes douncer with the B pestle (Kontes Glass Company, Vineland, NJ), and the homogenates were centrifuged twice at 750 g for 10 min at 4°C. The supernatants were centrifuged at 10,000 g for 15 min at 4°C, and the resulting pellets containing mitochondria (designated as mitochondrial fraction) were resuspended in buffer A containing 250 mM sucrose and frozen in multiple aliquots at −70°C. The supernatants were further cleared of mitochondrial contamination by centrifugation at 100,000 g for 1 h at 4°C in an airfuge (Beckman, Fullerton, CA) and the resulting supernatants (designated as cytosolic fraction) were also frozen in aliqouts at −70°C. 25 μg of protein from the cytosolic fraction and 20 μg from the mitochondrial fraction for each sample were loaded in each lane of a 15% polyacrylamide gel. Cytochrome c was analyzed using the Western blot assay as described above except that a mouse mAb 7H8.2C12 (IgG2b; PharMingen) specifically recognizing the denatured form of cytochrome c was used to stain cytochrome c.
Results
Chlamydia-infected Cells Are Resistant to Apoptosis Induced by a Wide Spectrum of Stimuli.
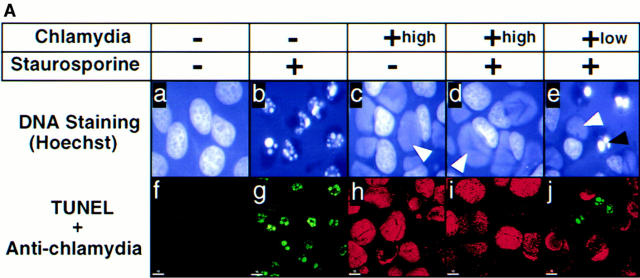
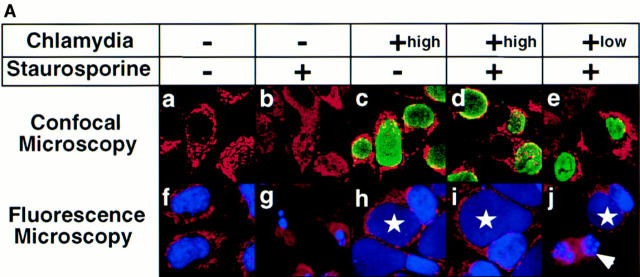
To evaluate whether intracellular chlamydial infection can inhibit host cell apoptosis, HeLa cells with or without chlamydial infection were first treated with the kinase inhibitor staurosporine, a potent apoptosis inducer (30, 35). We found that staurosporine at 1 μM efficiently induced >90% of noninfected HeLa cells to become apoptotic as revealed by both Hoechst staining of chromatin (Fig. 1 A b), the terminal transferase–mediated dUTP nick-end labeling (TUNEL) assay (Fig. 1 A g) and the appearance of a DNA ladder (see Fig. 3). However, HeLa cells infected with chlamydia were completely resistant to staurosporine by these measures (Fig. 1 A, d and i, and see Fig. 3, bottom). By evaluating samples with a low MOI for chlamydial infection (Fig. 1 A, e and j), we further observed that the antiapoptotic activity was restricted to the cells carrying the cytoplasmic chlamydial inclusion bodies. Because cells harboring the inclusion bodies were the ones that permitted chlamydial intracellular growth, this suggested that intracellular chlamydial growth was required for preventing the infected host cells from undergoing apoptosis induced by staurosporine. We also found antiapoptotic activity in cells infected with other chlamydial strains such as a C. trachomatis serovar C and a C. trachomatis mouse pneumonitis agent (data not shown), suggesting that the antiapoptotic activity is a general property of the C. trachomatis species. Since staining DNA with a Hoechst dye clearly differentiated the apoptotic from normal nuclei and also simultaneously revealed the cytoplasmic chlamydial inclusion bodies as demonstrated in Fig. 1 A c–e, we used the DNA staining assay to quantitate the apoptotic cells in the following experiments.
Figure 1.
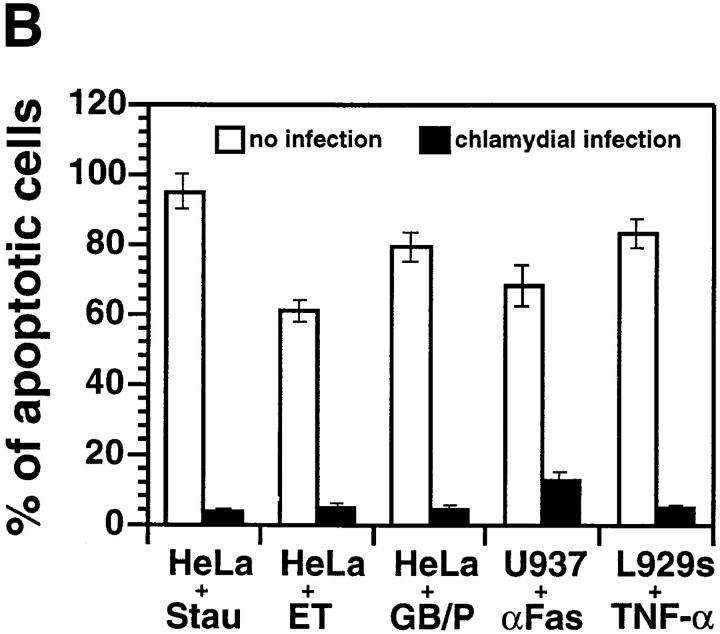
(A) Chlamydia-infected HeLa cells are resistant to apoptosis induced by staurosporine. HeLa cells with (c–e and h–j) or without (a, b, f, and g) chlamydial infection at an MOI of 5 (+high, top, c, d, h, and i) or 0.5 (+low, e and j) were treated with (b, d, e, g, i, and j) or without (a, c, f, and h) 1 μM staurosporine for 4 h. The cell samples were then either stained with Hoechst dye and viewed under a fluorescence microscope (a–e) or doubly labeled with dUTP (green) and an antichlamydial antibody (red) and viewed under a confocal microscope (f–j) as described in Materials and Methods. Hoechst dye stained both HeLa cell nuclei (black arrowhead, apoptotic nuclei) and the cytoplasmic chlamydial inclusion bodies (white arrowheads). (B) Chlamydia-infected HeLa cells are resistant to apoptosis induced by multiple apoptotic stimuli. Host cells with (solid bars) or without (open bars) chlamydial infection were stimulated with staurosporine (Stau), etoposide (ET), granzyme B/perforin (GB/P), an anti-Fas IgM antibody CH11 (αFas), or TNF-α. The cell samples were then stained with Hoechst dye. Cells from five random fields were counted under an object lens of ×40 and the percent of apoptotic cells were calculated (displayed along the y-axis). The figure shows the results from three independent experiments. The variations between the three experiments were <20% as indicated by error bars.
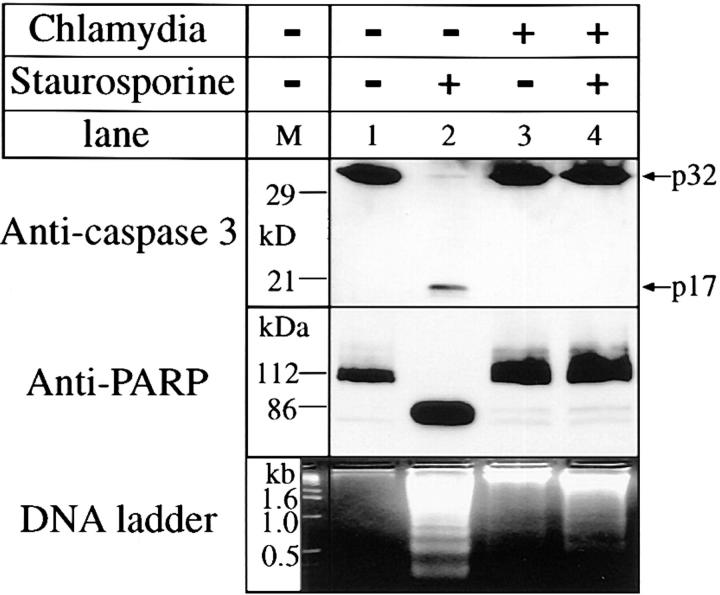
Figure 3.
Effect of chlamydial infection on caspase 3 processing, PARP cleavage, and DNA fragmentation. HeLa cells with (lanes 3 and 4) or without (lanes 1 and 2) chlamydial infection and with (lanes 2 and 4) or without (lanes 1 and 3) staurosporine (1 μM) treatment were lysed for Western blot analysis using antibodies against caspase 3 (top) or PARP (middle). The caspase 3 and PARP antibody staining was developed with a secondary antibody conjugated to horseradish peroxidase followed by visualization using an ECL as described in Materials and Methods. A 3% agarose gel was used for the DNA ladder assay and ethidium bromide was used to visualize the DNA bands (bottom).
We next asked whether chlamydia-infected cells were also resistant to apoptosis induced by various immunological killing molecules, as chlamydial antiapoptotic activity may allow chlamydia to evade antigen-specific immune effector mechanisms (20). Besides analysis of apoptosis induced by the DNA-damaging agent etoposide and the kinase inhibitor staurosporine, we evaluated the effect of chlamydial infection on apoptosis initiated by three immunological killing molecules including TNF-α, anti-Fas antibody, and granzyme B/perforin. We found that chlamydial infection significantly inhibited apoptosis induced by all five stimuli, although the apoptosis induction potency of the different agents varied (Fig. 1 B). Since these reagents are known to use different “private” pathways to deliver death signals (24, 26, 27), this observation suggests that chlamydial intracellular infection may inhibit a common step that is required for apoptosis induced by all of these reagents or block multiple steps required by these proapoptotic reagents. To our knowledge, such a profound inhibition of apoptosis is unique and led us to try to elucidate the mechanism(s).
Chlamydial Antiapoptotic Activity Is Dependent on Chlamydial, but Not Host, Protein Synthesis.
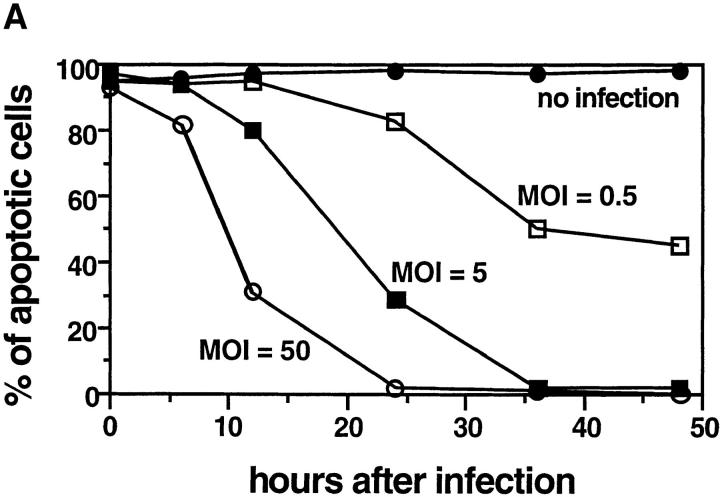
To understand the mechanisms of chlamydia-induced antiapoptotic activity, we first evaluated the kinetic relationship between the chlamydial infection dose and antiapoptotic activity. Since staurosporine is a strong apoptosis inducer and HeLa cells closely resemble the target cells of natural chlamydial infection, this system was used in the following experiments. As shown in Fig. 2 A, the time required for an infected cell to acquire resistance to induced apoptosis was dependent on the infection dose. HeLa cells infected at a higher MOI acquired antiapoptotic activity sooner than those infected at a lower MOI. For instance, at a MOI of 50, ∼70% cells were resistant to apoptosis induction 12 h after infection, whereas at a MOI of 5, ∼80% of the cells were susceptible to apoptosis induction at the same time point. Since infection at a high MOI often accelerates chlamydial intracellular growth, this observation suggests that active intracellular chlamydial growth is required for the development of antiapoptotic activity. In HeLa cultures infected with chlamydia at a MOI of 0.5, on average only one organism entered a single cell. We found that these cells became resistant after 24 h, which is the time when the chlamydial vegetative RB multiplication reaches its peak. This observation suggests that chlamydial antiapoptotic factors may be synthesized during the replication stage.
Figure 2.
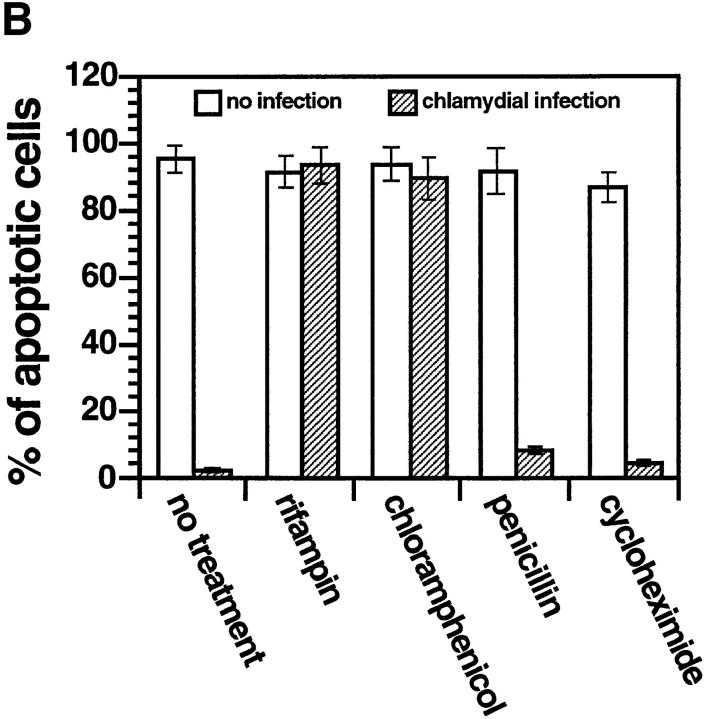
(A) Time course relationship between chlamydial infection dose and host cell apoptosis induced by staurosporine. HeLa cells were infected with chlamydial organisms with an MOI of 0 (filled circles), 0.5 (open squares), 5 (filled squares), or 50 (open circles). At various time points after infection (0, 6, 12, 24, 36, or 48 h) as indicated along the x-axis, the cells were stimulated with 1 μM staurosporine for 4 h and stained with Hoechst dye for evaluating the percentage of apoptotic cells as described in the Fig. 1 B legend. The figure shows the result from one representative experiment of three independent experiments that were performed. (B) Effect of antibiotics and cycloheximide on chlamydial antiapoptotic activity. HeLa cells infected with (hatched bars) or without (open bars) chlamydia in the presence of 1 μg/ml of rifampin, 60 μg/ml of chloramphenicol, or 100 μg/ ml penicillin G as indicated. Cycloheximide was added to the culture wells at a final concentration of 10 μg/ml 2 h before and during staurosporine stimulation. After 1 μM staurosporine stimulation for 4 h, cell samples were analyzed for percentage of apoptotic cells as described in the Fig. 1 B legend. The figure shows the result from three independent experiments. The variations between the three experiments were <20% as indicated by error bars in the figure.
To determine whether chlamydial protein synthesis is required for the antiapoptotic activity, we evaluated the effect of several antibiotics. Fig. 2 B shows that when the infected HeLa cultures were treated with either rifampin to block chlamydial transcription or chloramphenicol to block chlamydial translation, the antiapoptotic activity was completely blocked, whereas staurosporine-induced cellular apoptosis was not affected. Rifampin and chloramphenicol are known to suppress only prokaryotic and not eukaryotic protein synthesis at the concentration used in the experiment. However, treatment with penicillin G did not significantly alter the chlamydial antiapoptotic activity. Penicillin blocks the chlamydial differentiation from the noninfectious RB back to the infectious EB, but does not affect protein synthesis (36). These observations demonstrate that chlamydial protein synthesis is required for the chlamydial antiapoptotic activity.
To address the question of whether the antiapoptotic action also requires newly synthesized host proteins, we used cycloheximide, an inhibitor of host cell but not chlamydial protein synthesis. In the experiments shown in Fig. 2 B, cycloheximide treatment did not suppress apoptosis induced by staurosporine in uninfected HeLa cells and had no effect on the chlamydial antiapoptotic activity. This observation suggests that newly synthesized host proteins are not required for the chlamydial antiapoptotic activity. Chlamydia-encoded antiapoptotic factors may function by interacting with existing cellular factors. We then tried to define which step(s) of cellular apoptosis pathways is interrupted by chlamydial infection.
Activation of Caspase 3 and PARP Hydrolysis Is Inhibited by Chlamydial Intracellular Infection.
Caspase 3 is one of the key downstream members of the aspartate-specific cysteine protease family that is thought to be an essential effector of cell death (37). The targets of caspase 3 include DNA repair proteins (38), and caspase 3 is activated in the late stages of cellular apoptosis (39–41), so chlamydial antiapoptotic factors may function by inhibiting its activation. As shown in Fig. 3, p32 caspase 3 disappeared and was cleaved to a p17 in HeLa cells treated with staurosporine, confirming that staurosporine-induced apoptosis activates caspase 3 (42). However, the processing of caspase 3 induced by staurosporine in chlamydia-infected HeLa cells was completely inhibited. We evaluated the caspase enzymatic activity by measuring the cleavage of the caspase 3 substrate PARP. PARP p112 was reduced to the signature fragment p86 in cells treated with staurosporine and again this was blocked in chlamydia-infected cells. As a result of the inhibition of caspase 3 activation, nucleosomal DNA fragmentation was also suppressed in chlamydia-infected cells (Fig. 3, bottom). These observations suggest that the chlamydial antiapoptotic factors may function by preventing the activation of caspase 3. However, it is not clear whether chlamydial infection directly inhibits caspase 3 or blocks upstream steps leading to its activation.
Mitochondrial Cytochrome c Release Is Blocked by Intracellular Chlamydial Infection.
It has been recently suggested that mitochondria may play a central role in cellular apoptosis (41–45) and the release of mitochondrial cytochrome c into the cytosol may be required for activation of caspase 3 (29, 30, 41, 45, 46). We next tested whether chlamydial antiapoptotic factors can prevent cytochrome c release. Confocal microscopy was used initially to differentiate mitochondrial from cytosolic cytochrome c staining in cells treated with or without staurosporine (Fig. 4 A, a and b). We found that normal HeLa cells displayed a punctate red staining pattern, suggesting that cytochrome c in the normal HeLa cells is mainly located in mitochondria as previously suggested (29, 30, 45). However, HeLa cells stimulated with staurosporine lacked such granular staining and displayed a diffuse staining pattern, suggesting that the cytochrome c was released into the cytosol upon staurosporine stimulation, as previously demonstrated by others (29, 30). When chlamydia-infected cells were similarly stained, we found that cells bearing an intracellular chlamydial inclusion body (Fig. 4 A, c–e; inclusion bodies, green) always displayed granular staining regardless of staurosporine stimulation, suggesting that intracellular chlamydial infection can indeed block mitochondrial cytochrome c release into the cytosol. Inhibition of cytochrome c release was also found to be restricted to the cells carrying the inclusion bodies (Fig. 4 A, e and j ), which is consistent with the observation that antiapoptotic activity was restricted to the chlamydia-infected cells (Fig. 1 A, e and j). To further confirm that the cells with diffuse cytochrome c staining were apoptotic, parallel cultures were costained with both anti–cytochrome c and the DNA staining dye Hoechst and viewed under a fluorescence microscope. We found that cells with cytochrome c in mitochondria displayed normal nuclei (Fig. 4 A, f and h–j ), whereas the cells with cytosolic cytochrome c had apoptotic nuclei (g and j ). Thus, it is clear that only chlamydia-infected cells displayed normal nuclei and mitochondrial localization of cytochrome c upon staurosporine stimulation.
Figure 4.
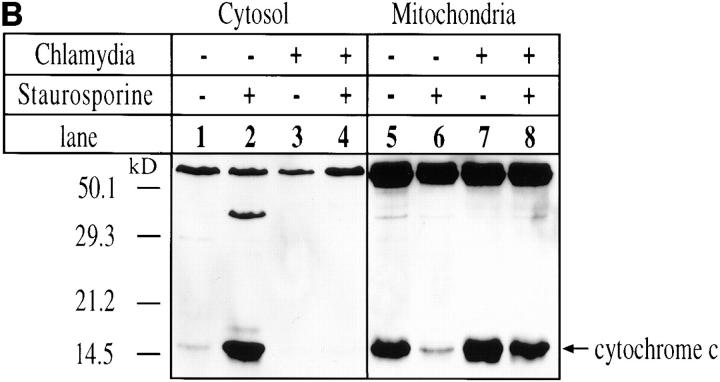
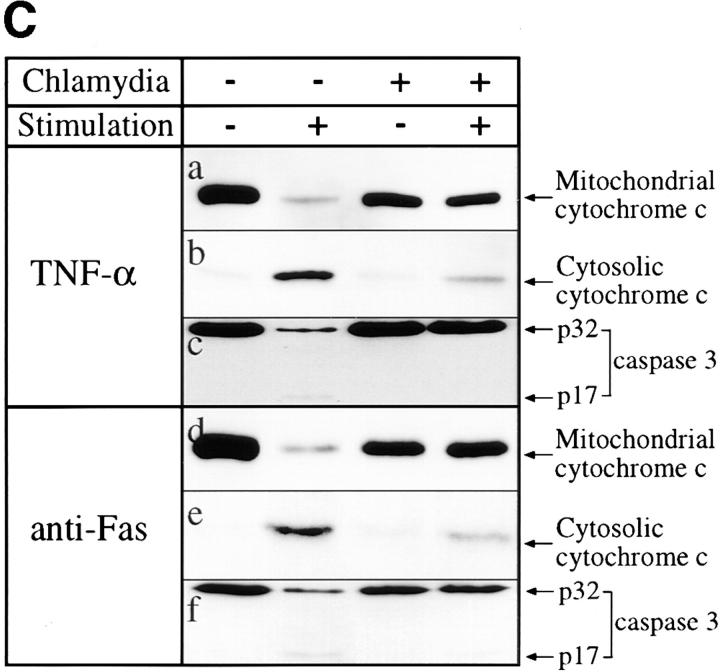
(A) Immunofluorescence analysis of the effect of chlamydial infection on cytochrome c distribution. HeLa cells were infected with chlamydial organisms and treated with staurosporine (1 μM) as described in the Fig. 1 A legend. The cells were then either stained for cytochrome c (red) and chlamydial antigens (green) and viewed under a confocal microscope (a–e) or stained for cytochrome c (red) and DNA (blue; apoptotic nuclei, white arrowheads; chlamydial inclusion bodies, white stars) with Hoechst dye and viewed under a fluorescence microscope (f–j). Punctate red staining, mitochondrial localization; diffuse red staining, cytosolic localization. (B) Western blot analysis of chlamydial inhibition of mitochondrial cytochrome c release induced by staurosporine. HeLa cells were infected with chlamydial organisms and treated with staurosporine (1 μM) as described in the Fig. 3 legend. The cell samples were then fractionated into cytosol (lanes 1–4) and mitochondrial (5–8) fractions, and analyzed by Western blot analysis. A mouse mAb that specifically recognizes denatured cytochrome c was used to stain the blot and a secondary antibody conjugated with horseradish peroxidase was used to detect the first antibody binding. The antibody reaction was visualized with ECL as described in Materials and Methods. The arrow denotes the position of cytochrome c and the high molecular bands may represent proteins that cross-reacted with the anti–cytochrome c antibody. (C) Chlamydial infection suppresses mitochondrial cytochrome c release induced by TNF-α and anti-Fas antibody cross-linking. U937 cells were infected with chlamydial organism at an MOI of 20. 30 h after infection, the cells were treated with either 40 ng/ml of human TNF-α for 4 h (a–c) or a mouse IgM antibody (CH11) against human Fas at 250 ng/ml for 8 h (d–f ). Both treatments were carried out in the presence of 2 μg/ml of cycloheximide. The control cell samples were treated with cycloheximide alone. The treated cell samples were then fractionated into cytosolic (b and e) and mitochondrial fractions (a and d ) for Western blot analysis as described in the Fig. 4 B legend. c was from the b blot; after stripping, the b blot was restained with an anti–caspase 3 antibody. f was similarly restained after stripping e.
The blockade in mitochondrial cytochrome c release by intracellular chlamydial infection was further confirmed biochemically by Western blot (Fig. 4 B). In this experiment, cell samples were separated into cytosolic and mitochondrial fractions as described in Materials and Methods. The fractionated samples were then subjected to Western blot analysis. By comparing the cytochrome c signal from mitochondrial fractions with that from cytosolic fractions, we found that most of the cytochrome c was detected in the mitochondrial fraction from normal HeLa cells, whereas in staurosporine-treated cells the majority of the cytochrome c was detected in the cytosolic fraction. This confirms the finding using fluorescence staining of cytochrome c (Fig. 4 A) and is consistent with previous reports of cytochrome c release during apoptosis (29, 30). In chlamydia-infected cells, most of the cytochrome c was detected in the mitochondrial fractions even after staurosporine stimulation, which suggests that mitochondrial cytochrome c release is blocked by chlamydial infection.
We next tested whether the inhibition of immunological apoptosis-inducing factors by chlamydia also prevents mitochondrial cytochrome c release. As shown in Fig. 4 C, we found that both TNF-α and anti-Fas antibody treatment in the presence of cycloheximide-induced mitochondrial cytochrome c release (Fig. 4 C, a, b, d, and e) and chlamydial infection significantly blocked the release. The chlamydial blockade of mitochondrial cytochrome c release was correlated with chlamydial inhibition of caspase 3 activation (Fig. 4 C, c and f ).
Discussion
We have demonstrated that chlamydia-infected host cells are profoundly resistant to apoptosis induced by all apoptotic stimuli tested. Although many viral antiapoptotic factors have been described (7, 47), there has been no previous reports of bacterial antiapoptotic activity, although it is known that host cells are able to respond to intracellular bacterial invasion with apoptosis (48, 49). These include organisms such as Shigella flexneri (50), Listeria monocytogenes (51), Mycobacterium tuberculosis (52), and Salmonella typhimurium (53). Although the mechanisms of apoptosis induced by bacterial intracellular invasion have not yet been elucidated, in some cases the host apoptotic response can facilitate the killing of intracellular bacteria localized in cytoplasmic vacuoles by either provoking an inflammatory response (4, 54) or by delivering the intracellular pathogens to competent professional phagocytes (5). Therefore, chlamydial antiapoptotic activity may have evolved for suppressing the host apoptotic response to the initial intracellular invasion. As a result, the host cells can continuously supply nutrients for chlamydial growth and the infected cells may shield the intracellular organisms from host phagocytosis (19). Since chlamydial infection also confers the infected cells with a profound resistance to various apoptosis-inducing molecules of immunological origin, the infection may protect the intracellular pathogens from attack by host antigen-specific immune responses (20, 55). We have recently found that cells infected with chlamydia are resistant to lysis by a cytotoxic T lymphocyte hybridoma cell line (Hu, H., H. Lu, and G. Zhong, manuscript in preparation). Thus suppression of host cell primary defense (apoptotic responses) and evasion of host antigen-specific immune attack as a result of the potent antiapoptotic may set the conditions for persistent chlamydial infection.
The mechanism(s) by which chlamydial infection inhibits host cell apoptosis was further studied. Antiapoptotic activity correlated well with chlamydia-induced blockade of mitochondrial cytochrome c release and downstream caspase activation. This suggests that chlamydial intracellular infection interrupts many different upstream apoptotic pathways by blocking mitochondrial cytochrome c release. A central role of mitochondria in converting various private apoptotic pathways to a common effector pathway of final amplification of caspase activation (43, 56) has recently been proposed based on the following evidence. (a) Induction of mitochondrial permeability transition (PT) can cause nuclear apoptosis, whereas prevention of PT can impede apoptosis in both a cell-free system and intact cells (57). (b) Many proapoptotic factors including Fas are able to induce mitochondrial PT and apoptosis-inducing factor release (28, 58). (c) Mitochondrial cytochrome c release is necessary for the cytosolic activation of caspase 3 and further nuclear apoptosis (30, 41, 46). (d ) Many proapoptotic factors including staurosporine (29), etoposide (45), actinomycin D (45), cisplatinum (59) and Fas (60) stimulate mitochondrial cytochrome c release. (e) Overexpression of mitochondrial Bcl-2 or Bcl-xL blocks the mitochondrial PT change (58), cytochrome c release (29, 45, 61, 62), and apoptosis (44, 58, 62). The present study also provides additional support for the model that suggests an essential role of mitochondria in regulating apoptosis. We have demonstrated that a wide spectrum of apoptotic reagents can induce both mitochondrial cytochrome c release and apoptosis. Blocking of mitochondrial cytochrome c release by chlamydial intracellular infection can prevent downstream caspase activation and nuclear apoptosis. Therefore it is clear that understanding the mechanisms of chlamydial antiapoptotic activity will not only help define the basis of chlamydial pathogenesis, but also provide new information on the central mechanisms regulating host apoptosis.
We do not yet understand how chlamydial intracellular infection blocks mitochondrial cytochrome c release. Chlamydiae may preserve mitochondrial function and prevent mitochondrial cytochrome c release by three potential mechanisms. (a) Chlamydial infection may block the upstream steps that can perturb mitochondrial function. It has been suggested that different proapoptotic and/or stress factors may use different mechanisms to deliver signals to mitochondria (24, 27, 43). To achieve a wide spectrum of antiapoptosis activity, chlamydiae would have to produce multiple antiapoptotic factors to deal with these many individual mechanisms. This would be very inefficient, although possible. (b) Chlamydial infection may upregulate expression of mitochondrial Bcl-2 or Bcl-2–like molecules, as these mitochondrial membrane proteins are able to prevent cytochrome c release and caspase 3 activation. However, overexpression of Bcl-2 does not always protect from apoptosis induced by Fas cross-linking (63–65). Furthermore, overexpression of Bcl-XL failed to block apoptosis in U937 cells induced by TNF-α (26). We have examined Bcl-2 protein levels and they were not elevated in chlamydia-infected cells (data not shown). It is therefore not likely that the chlamydial antiapoptotic activity is solely mediated by host Bcl-2 or Bcl-2–like molecules. However, we can not completely rule out the possibility that Bcl-2 or other molecules of this family participate in chlamydial antiapoptotic activity. (c) Chlamydia may produce novel antiapoptotic factors. The chlamydial antiapoptotic activity requires chlamydial growth and is dependent on chlamydial, but not host protein synthesis. Among the many viral antiapoptotic factors so far identified (7, 47), the ones with the broadest effects are the viral homologues of mammalian Bcl-2 such as the BHRF1 protein from Epstein-Barr virus. BHRF1 can inhibit apoptosis induced by both exogenous stimuli, including DNA-damaging agents (66), the calcium ionophore ionomycin (10), and immunological apoptosis-inducing molecules such as TNF-α and Fas (67). These observations suggest that a microbial homologue of apoptosis suppressors such as Bcl-2 may be more potent than endogenous mammalian Bcl-2 in regulating apoptosis. We therefore hypothesize that chlamydial organisms produce potent antiapoptotic factors. Efforts to identify the potential chlamydial antiapoptotic factor(s) are under way.
Footnotes
We thank Drs. Ronald N. Germain and Robert C. Brunham for helpful discussions and reading the manuscript.
This work was supported by the Medical Research Council (MRC) of Canada. G. Zhong is the recipient of an MRC scholarship.
Address correspondence to Guangming Zhong, Department of Medical Microbiology, University of Manitoba, 508-730 William Ave., Winnipeg, Manitoba, Canada R3E OW3. Phone: 204-789-3835; Fax: 204-789-3926; E-mail: gmzhong@cc.umanitoba.ca
Abbreviations used in this paper: EB, chlamydial infectious elementary body; ECL, enhanced chemiluminescence; MOI, multiplicity of infection; PARP, poly(ADP-ribose) polymerase; PT, permeability transition; RB, chlamydial noninfectious reticulate body.
References
- 1.Shen Y, Shenk TE. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 2.Antoni BA, Sabbatini P, Rabson AB, White E. Inhibition of apoptosis in human immunodeficiency virus–infected cells enhances virus production and facilitates persistent infection. J Virol. 1995;69:2384–2392. doi: 10.1128/jvi.69.4.2384-2392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JC, Chen HH, Wei HL, Chao YC. Superinfection-induced apoptosis and its correlation with the reduction of viral progeny in cells persistently infected with Hz-1 baculovirus. J Virol. 1993;67:6989–6994. doi: 10.1128/jvi.67.12.6989-6994.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fratazzi C, Arbeit RD, Carini C, Remold HG. Programmed cell death of Mycobacterium avium serovar4–infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997;158:4320–4327. [PubMed] [Google Scholar]
- 6.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 7.Teodoro JG, Branton PE. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 9.Bertin J, Mendrysa SM, LaCount DJ, Gaur S, Krebs JF, Armstrong RC, Tomaselli KJ, Friesen PD. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J Virol. 1996;70:6251–6259. doi: 10.1128/jvi.70.9.6251-6259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus–coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revilla Y, Cebrian A, Baixeras E, Martinez C, Vinuela E, Salas ML. Inhibition of apoptosis by the African swine fever virus Bcl-2 homologue: role of the BH1 domain. Virology. 1997;228:400–404. doi: 10.1006/viro.1996.8395. [DOI] [PubMed] [Google Scholar]
- 12.Nava VE, Cheng EH, Veliuona M, Zou S, Clem RJ, Mayer ML, Hardwick JM. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XW, Gibson MK, Vermeulen W, Yeh H, Forrester K, Sturzbecher HW, Hoeijmakers JH, Harris CC. Abrogation of p53-induced apoptosis by the hepatitis B virus X gene. Cancer Res. 1995;55:6012–6016. [PubMed] [Google Scholar]
- 14.Schreiber M, Sedger L, McFadden G. Distinct domains of M-T2, the myxoma virus tumor necrosis factor (TNF) receptor homolog, mediate extracellular TNF binding and intracellular apoptosis inhibition. J Virol. 1997;71:2171–2181. doi: 10.1128/jvi.71.3.2171-2181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 16.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grayston JT, Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 18.Hackstadt T, Fischer ER, Scidmore MA, Rockey DD, Heinzen RA. Origins and functions of the chlamydial inclusion. Trends Microbiol. 1997;5:288–293. doi: 10.1016/S0966-842X(97)01061-5. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan D, Krahling S, Williamson P, Schlegel RA. Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol Biol Cell. 1997;8:767–778. doi: 10.1091/mbc.8.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moretta A. Molecular mechanisms in cell-mediated cytotoxicity. Cell. 1997;90:13–18. doi: 10.1016/s0092-8674(00)80309-8. [DOI] [PubMed] [Google Scholar]
- 21.Birkelund S, Johnsen H, Christiansen G. Chlamydia trachomatis serovar L2 induces protein tyrosine phosphorylation during uptake by HeLa cells. Infect Immun. 1994;62:4900–4908. doi: 10.1128/iai.62.11.4900-4908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magee DM, Smith JG, Bleicker CA, Carter CJ, Bonewald LF, Schachter J, Williams DM. Chlamydia trachomatis pneumonia induces in vivo production of interleukin-1 and -6. Infect Immun. 1992;60:1217–1220. doi: 10.1128/iai.60.3.1217-1220.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, Fierer J, Stephens RS, Kagnoff MF. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydiainfection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enari M, Hug H, Hayakawa M, Ito F, Nishimura Y, Nagata S. Different apoptotic pathways mediated by Fas and the tumor-necrosis-factor receptor. Cytosolic phospholipase A2 is not involved in Fas-mediated apoptosis. Eur J Biochem. 1996;236:533–538. doi: 10.1111/j.1432-1033.1996.t01-1-00533.x. [DOI] [PubMed] [Google Scholar]
- 25.Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 26.Erhardt P, Cooper GM. Activation of the CPP32 apoptotic protease by distinct signaling pathways with differential sensitivity to Bcl-xL. J Biol Chem. 1996;271:17601–17604. doi: 10.1074/jbc.271.30.17601. [DOI] [PubMed] [Google Scholar]
- 27.Shi L, Chen G, MacDonald G, Bergeron L, Li H, Miura M, Rotello RJ, Miller DK, Li P, Seshadri T, et al. Activation of an interleukin 1 converting enzyme–dependent apoptosis pathway by granzyme B. Proc Natl Acad Sci USA. 1996;93:11002–11007. doi: 10.1073/pnas.93.20.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F, Reed JC, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95– and ceramide–induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 31.Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 32.Zhong GM, Reid RE, Brunham RC. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatiswith synthetic peptides. Infect Immun. 1990;58:1450–1455. doi: 10.1128/iai.58.5.1450-1455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L, Mai S, Israels S, Browne K, Trapani JA, Greenberg AH. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J Exp Med. 1997;185:855–866. doi: 10.1084/jem.185.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong GM, Brunham RC. Antigenic determinants of the chlamydial major outer membrane protein resolved at a single amino acid level. Infect Immun. 1991;59:1141–1147. doi: 10.1128/iai.59.3.1141-1147.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weil M, Jacobson MD, Coles HS, Davies TJ, Gardner RL, Raff KD, Raff MC. Constitutive expression of the machinery for programmed cell death. J Cell Biol. 1996;133:1053–1059. doi: 10.1083/jcb.133.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbour AG, Amano K, Hackstadt T, Perry L, Caldwell HD. Chlamydia trachomatishas penicillin-binding proteins but not detectable muramic acid. J Bacteriol. 1982;151:420–428. doi: 10.1128/jb.151.1.420-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henkart PA. ICE family proteases: mediators of all apoptotic cell death? . Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 38.Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 40.Vaux DL. CED-4–the third horseman of apoptosis. Cell. 1997;90:389–390. doi: 10.1016/s0092-8674(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 41.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegansCED-4, participates in cytochrome c–dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsen MD, Weil M, Raff MC. Role of Ced-3/ICE–family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 44.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis (published erratum 8:934) Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 45.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 46.Kluck RM, Martin SJ, Hoffman BM, Zhou JS, Green DR, Newmeyer DD. Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO (Eur Mol Biol Organ) J. 1997;16:4639–4649. doi: 10.1093/emboj/16.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillet G, Brun G. Viral inhibition of apoptosis. Trends Microbiol. 1996;4:312–317. doi: 10.1016/0966-842x(96)10047-0. [DOI] [PubMed] [Google Scholar]
- 48.Finlay BB, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 49.Zychlinsky A, Sansonetti PJ. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? . Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 50.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneriinduces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 51.Rogers HW, Callery MP, Deck B, Unanue ER. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 52.Rojas M, Barrera LF, Puzo G, Garcia LF. Differential induction of apoptosis by virulent Mycobacterium tuberculosisin resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J Immunol. 1997;159:1352–1361. [PubMed] [Google Scholar]
- 53.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimuriuminvasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laochumroonvorapong P, Paul S, Elkon KB, Kaplan G. H2O2 induces monocyte apoptosis and reduces viability of Mycobacterium avium–M. intracellularewithin cultured human monocytes. Infect Immun. 1996;64:452–459. doi: 10.1128/iai.64.2.452-459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henkart PA, Williams MS, Zacharchuk CM, Sarin A. Do CTL kill target cells by inducing apoptosis? . Semin Immunol. 1997;9:135–144. doi: 10.1006/smim.1997.0063. [DOI] [PubMed] [Google Scholar]
- 56.Petit PX, Zamzami N, Vayssiere JL, Mignotte B, Kroemer G, Castedo M. Implication of mitochondria in apoptosis. Mol Cell Biochem. 1997;174:185–188. [PubMed] [Google Scholar]
- 57.Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boise LH, Thompson CB. Bcl-x(L) can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Proc Natl Acad Sci USA. 1997;94:3759–3764. doi: 10.1073/pnas.94.8.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S, Weichselbaum R, et al. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage–induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krippner A, Matsuno-Yagi A, Gottlieb RA, Babior BM. Loss of function of cytochrome c in Jurkat cells undergoing fas-mediated apoptosis. J Biol Chem. 1996;271:21629–21636. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]
- 61.Adachi S, Cross AR, Babior BM, Gottlieb RA. Bcl-2 and the outer mitochondrial membrane in the inactivation of cytochrome c during Fas-mediated apoptosis. J Biol Chem. 1997;272:21878–21882. doi: 10.1074/jbc.272.35.21878. [DOI] [PubMed] [Google Scholar]
- 62.Kim CN, Wang X, Huang Y, Ibrado AM, Liu L, Fang G, Bhalla K. Overexpression of Bcl-X(L) inhibits Ara-C–induced mitochondrial loss of cytochrome c and other perturbations that activate the molecular cascade of apoptosis. Cancer Res. 1997;57:3115–3120. [PubMed] [Google Scholar]
- 63.Chiu VK, Walsh CM, Liu CC, Reed JC, Clark WR. Bcl-2 blocks degranulation but not fas-based cell-mediated cytotoxicity. J Immunol. 1995;154:2023–2032. [PubMed] [Google Scholar]
- 64.Memon SA, Moreno MB, Petrak D, Zacharchuk CM. Bcl-2 blocks glucocorticoid- but not Fas- or activation-induced apoptosis in a T cell hybridoma. J Immunol. 1995;155:4644–4652. [PubMed] [Google Scholar]
- 65.Strasser A, Harris AW, Huang DC, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO (Eur Mol Biol Organ) J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarodi B, Subramanian T, Chinnadurai G. Epstein-Barr virus BHRF1 protein protects against cell death induced by DNA-damaging agents and heterologous viral infection. Virology. 1994;201:404–407. doi: 10.1006/viro.1994.1309. [DOI] [PubMed] [Google Scholar]
- 67.Kawanishi M. Epstein-Barr virus BHRF1 protein protects intestine 407 epithelial cells from apoptosis induced by tumor necrosis factor alpha and anti-Fas antibody. J Virol. 1997;71:3319–3322. doi: 10.1128/jvi.71.4.3319-3322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]