Abstract
The brain is an immunoprivileged organ isolated from the peripheral immune system. However, it has been shown that resident cells, notably astrocytes and microglia, can express numerous innate immune molecules, providing the capacity to generate a local antipathogen system. Perforin is a cytolytic protein present in the granules of cytotoxic T lymphocytes and natural killer cells. Expression in cells other than those of the hemopoetic lineage has not been described. We report here that fetal astrocytes in culture (passages 2 to 15), astrocytoma, and adult astrocytes expressed perforin. Reverse transcriptase polymerase chain reaction followed by Southern blot was carried out using multiple specific primers and all cDNAs were cloned and sequenced. Human fetal astrocyte perforin cDNA sequence was ∼100% identical to the reported perforin cDNA cloned from T cells. Western blot analysis using monoclonal and polyclonal antiperforin peptide antibodies revealed a protein of 65 kD in both human fetal astrocyte and rat natural killer cell lysates (n = 4). Immunostaining followed by FACS® and confocal and electron microscopy analysis revealed that perforin was expressed by 40–50% of glial fibrillary acidic protein positive cells present in the fetal brain culture (n = 11). Perforin was not localized to granules in astrocytes but was present throughout the cytoplasm, probably in association with the endoplasmic reticulum. Perforin was not detected in normal adult brain tissue but was present in and around areas of inflammation (white and grey matter) in multiple sclerosis and neurodegenerative brains. Perforin-positive cells were identified as reactive astrocytes. These findings demonstrate that perforin expression is not unique to lymphoid cells and suggest that perforin produced by a subpopulation of astrocytes plays a role in inflammation in the brain.
Cytotoxic T lymphocytes (CTLs) and NK cells contain an impressive armory within their granules. Preeminent among these weapons is perforin, a granule protein which polymerizes in the membranes of target cells to form pores that contribute directly or indirectly to target cell destruction (1–3). Expression of perforin by nonlymphoid cells has not been reported. The importance of perforin in immune surveillance has been graphically demonstrated in mice in which the perforin gene has been deleted. These animals are more susceptible to viral infections and to the development of tumors (4, 5).
The brain is shielded from the immune system and contains few lymphoid cells. Therefore, defense within the brain must involve resident cells. Brain microglia subserve some of the roles of lymphoid cells and are considered to be the resident macrophage of the brain (6). However, astrocytes are by far the most abundant glial cell type in the brain and their potential role in defense has been neglected. We and others have recently shown that astrocytes express many properties usually associated with immune cells (7). For example, astrocytes can synthesize all of the components of the complement system, providing a source of complement in the brain, and express complement receptors mediating responses to complement activation products (7). In a recent survey of tissue-expressed genes by partial DNA sequencing to generate expressed sequence tags, expression of perforin mRNA was noted in the human brain (reference 8; sequence data available from EMBL/GenBank/ DDBJ under accession number AA351844). Given the paucity of lymphoid cells in the brain, we were provoked to examine whether brain cells might express perforin.
Materials and Methods
Cell Cultures and Tissues.
Primary cultures and maintenance of cell lines were carried out according to the protocol described previously (9). Human fetal brains were obtained from the Medical Research Council (MRC) Fetal Tissue Bank (Hammersmith Hospital, London, UK) and used as a source of fetal astrocytes. Cells in passages 2–15 were used in this study. Normal adult temporal lobe tissue was obtained fresh from biopsies of patients (three cases) undergoing therapeutic resection for intractible epilepsy. These were used for culture of adult glial cells as previously described (10). Glial cells from adult brain were seeded on poly l-lysine coated glass coverslip and after 3 wk in culture were used for immunocytochemistry. Two human glioma cell lines (CB193 and T98G) were also used in this study to confirm the expression of perforin by astrocyte-derived cell lines. CB193 was from Dr. B. Delpech (H. Becquerel Institute, Rouen, France) and T98G was from the American Type Culture Collection (ATCC; Rockville, MD). Human cell lines, Raji (B lymphocyte), THP1 (monocyte), and K562 (erythroleukemia) were obtained from the European Collection of Animal Cell Cultures (Salisbury, UK). Human YT (NK cell line) was obtained from Dr. G. Griffiths (University College of London, London, UK) and was cultured in the presence of recombinant interleukin 2. The rat NK cell line was available in-house and was also cultured in cytokine-supplemented medium. Brains from 1-d-old rats were used as a source of rat astrocytes. Mouse CTTL-2 cell line was obtained from Dr. J. Matthews (Department of Medicine, University of Wales College of Medicine (UWCM), Cardiff). Samples of normal human brain tissues (from three individuals with nonneurological causes of death and with postmortem delay of <12 h) were provided by the Department of Neuropathology (Dr. J.W. Neal, Neuropathology Department, UWCM). Frozen brain tissue sections from four cases of multiple sclerosis (MS;1 three semiacute plaques and one chronic plaque) were obtained from Dr. Jia Newcombe (MS Society Laboratory, London, UK). Frozen brain tissue samples from three cases of Pick's disease (PD) with Pick inclusion bodies and from three cases of Alzheimer's disease (AD) with severe β amyloid deposits were obtained from Dr. Nigel J. Cairns (MRC AD Brain Bank, Institute of Psychiatry, London, UK). Frozen brain tissue (caudate nucleus) from individuals with Huntington's disease (HD) with a high Vonsattel grade (grade 4, average of 60 CAG repeats) were diagnosed and obtained locally from Dr. J.W. Neal. All frozen brain tissues were stored at −40°C.
Antibodies and Cytokines.
The mouse monoclonal antiperforin antibody, a gift from Dr. G. Griffiths, was generated against a 16-amino acid perforin peptide encompassing residues 285–300 in human perforin, KHKMTASFHQTYRERH (14). This antibody detects human mouse, and rat perforin in the native but not the polymerized form. The polyclonal rabbit antiperforin peptide antibody was generated in-house against the COOH terminus of human perforin (LGEPPGNRSGAVW, residues 522–534). Neither of the chosen perforin peptides had any homology with the complement components C7, C8, and C9, and neither the monoclonal nor the polyclonal antibody recognized any bands in Western blots of human sera. Polyclonal rabbit anti–glial fibrillary acidic protein (GFAP) (B5) was obtained from Dr. J. Newcombe and monoclonal antibodies against brain-specific antigens (clone GA5IgG1 anti-GFAP; clone NE14IgG1; antineurofilament 200) were obtained from Sigma Chemical Co. (Poole, Dorset, UK). Mouse antigalactocerebroside (GC) was obtained from Dr. S. Piddlesden (UWCM). Mouse anti-neuron-specific enolase, (NSE; clone BBs/NC/VI-H14IgG1) was obtained from Dako Ltd. (High Wycombe, Bucks, UK). Monoclonal antimicroglia and antimacrophage (clone LN3IgG1, anti-HLA class II; clones KP1IgG1, PGM1IgG3, EBM11IgG1, anti-CD68; and clone My-4IgG2b, anti-CD14) were from Biotest (Solihull, UK), Dako Ltd., and Coulter (Luton, Beds, UK), respectively. Monoclonal anti-CD44 (clone BRIC 235IgG2b) and anti-CD59 (clone B229IgG2b) were from International Blood Group Reference Laboratory (Herts, UK). Mouse anti-CD56 (clone NCAMIgG2b) and anti-CD57 (clone HNK1IgM) were from Becton Dickinson (Oxford, UK). The hybridoma anti–human CR3 (CD11b, clone OKM1IgG2b) was purchased from the ATCC, and tissue culture supernatant was used as a source of antibody. Alkaline phosphatase– and peroxidase-conjugated goat anti–mouse and anti–rabbit immunoglobulins were from Sigma and Biorad (Hertfordshire, UK), respectively, FITC- and rhodamine-conjugated goat anti–rabbit immunoglobulins were from Harlan SeraLab (Crawley Down, Sussex, UK) and Jackson (Stratech Sci. Ltd., Luton, Beds, UK), respectively. FITC-conjugated donkey anti–mouse immunoglobulins were from Jackson and used with the rhodamine-conjugated goat anti– rabbit immunoglobulins in all double immunofluorescence experiments. RPE-conjugated goat anti–mouse immunoglobulin was from Dako and was used for FACS® analysis with the FITC-conjugated goat anti–rabbit immunoglobulins. Recombinant cytokines (IFN-γ, IL-1–β, and TNF-α) were the gift of Hoffman la Roche (Nutley, NJ) and were used at 200 IU/ml for 24 h to stimulate fetal brain astrocyte cultures. Recombinant IL-2 was provided by Dr. J. Matthews (Medicine Department, UWCM).
Immunocytochemistry and Western Blotting.
The preparation of frozen tissue sections and all techniques and reagents for immunocytochemistry analysis (diaminobenzidine [DAB]/immunoperoxidase, 5-bromo-4-chloro-3indolyl phosphate [BCIP]/nitroblue tetrazolium [NBT]/immunoalkaline phosphatase, and FITC/rhodamine indirect immunofluorescence) have been previously described (9–11). Immunodetection of perforin within adherent cells and cells in suspension was carried out by FACS® and confocal and immunoelectron microscopy analysis (10, 11). The FACS® analysis protocol was adapted to detect intracellular antigen (perforin and GFAP) and included a cell permeabilization step before cell staining. Cells in suspension were fixed with 10% formaldehyde for 10 min at 4°C and after two washes in PBS cells were permeabilized with 0.05% of NP-40 in PBS with 1% BSA (NP40/PBS/BSA). After three washes with PBS, cells (105 cells/ analysis) were incubated for 1 h at 4°C with primary antibodies followed by fluorochrome-conjugated secondary antibodies, all diluted in NP40/PBS/BSA. Nonspecific binding of antibodies to cells and tissue sections was assessed in all immunocytochemical analyses using irrelevant monoclonal and polyclonal antibodies. Unstimulated fetal and adult astrocytes in culture were negative for complement factor H (FH), complement C4b binding protein (C4bp), CD11b, CD68, and C-reactive protein (CRP) when using mouse anti-FH (clone OX24IgG1, from Dr. R. Sim, MRC Immunochemistry Unit, Oxford, UK), Mouse anti-C4bp (clone H1C6IgG1, from Dr. A. Ischenko, Institute of Highly Pure Biopreparations, St. Petersburg, Russia), mouse anti-CD11b (clone OKM1), and rabbit anti-CRP (Calbiochem, Nottingham, UK), respectively. These results confirmed our previously published observations (9, 10, 12). No specific staining was obtained when brain tissue sections were incubated with mouse and rabbit anti-complement C4bp (Dr. M. Colomb, Grenoble, France), used here as irrelevant control antibodies. Fetal and adult astrocytes were GFAPhigh, CD44high, CD56high, and CD59high, and were negative for CD68, CD11b, GC, and NSE. All these cellular markers were used to identify the astrocyte cell population in vitro and in situ.
Immunocytochemical analyses were performed using either a DMLB Leica immunofluorescence/bright field microscope or a Leica TCS confocal laser scanning microscope (CLSM; Leica, Heidelberg, Germany). For CLSM, frame scanning was performed at ×400 or ×1,000 magnification and 12 optical sections were collected per field at 0.2 μm intervals from the bottom to the top of the cell. Immunogold staining analysis was performed on a Philips (Eindhoven, The Netherlands) transmission electron microscope. Preparation of cell lysates and the protocol for Western blot were previously described in detail (9).
Reverse Transcriptase PCR, Cloning, Sequencing, and Southern Blotting.
Total RNA from positive (human CTL cell line, YT) and negative (THP1, K562, Raji) control cell lines were used to test the specificity of all perforin primers by reverse transcriptase (RT) PCR. All samples were subjected to RT-PCR for β-actin and glyceraldehyde-3-phosphate dehydrogenase as positive controls and positive internal standards as previously described (9). To prevent amplification of genomic DNA, RNA samples were treated with RQ1 DNAse before the reverse transcription step (9). To further eliminate the possibility of amplification of genomic DNA, primer pairs were selected from different exons of the perforin cDNA sequence (13). Samples of RT-PCR products were separated by electrophoresis on agarose gels and blotted onto nylon membranes for Southern blotting. Two human perforin cDNA probes (15–950 bp and 15–1,610 bp) were obtained by PCR of a plasmid containing a full length human perforin cDNA insert (pHP-10, gift of Dr. J.A. Trapani, University of Melbourne, Melbourne, Australia), subcloned into the pGEM-T plasmid (Promega, Southampton, UK) and sequenced on an automated sequencer (Applied Biosystems, Inc., Warrington, UK) to confirm fidelity. Perforin cDNA probes were PCR-labelled with FITC-dUTP and used for Southern blotting according to the manufacturer's protocol (Boehringer Mannheim, Lewes, Sussex, UK). Hybridized membranes were then incubated with a rabbit anti-FITC (Dako, Bucks, UK) followed by peroxidase-conjugated anti–rabbit immunoglobulins (Biorad, Hertfordshire, UK), and were finally developed using the enhanced chemiluminescence system (Pierce and Warriner, Chester, UK).
Results
Astrocytes from Human Fetal Brain Cultures Constitutively Express Perforin mRNA.
Total RNA was isolated from six different cultures of human fetal astrocytes (passage 2–15) and subjected to RT-PCR using multiple primer sets specific for the human perforin cDNA sequence. All primer sets gave products of the predicted size from astrocyte RNA (Fig. 1 A). Perforin mRNA was also detected by RT-PCR of the two glioma cell lines CB193 and T98G (data not shown). RNA from human NK cell line (YT stimulated with IL2) gave similar results, whereas RNA from other cell lines (Raji, K562, and THP1) was consistently negative. Raji, K562, and THP1 did not express perforin mRNA even after cell stimulation with various cytokines (IFN-γ, IL1-β, and TNF-α) for a period of 24 h. Sequencing of the RT-PCR products from astrocyte RNA confirmed their identity; virtually all of the perforin coding sequence was included in these products and differed from the published sequence (13) at only one site, a C to T switch at position 490 (a silent mutation, both codons specifying histidine; Fig. 1 B). Only 1–5 × 104 cells were obtained from each adult brain culture and this low number of cells was insufficient for analysis of the expression of perforin mRNA by adult astrocytes. A compounding problem was that adult brain cultures were a mixed culture containing microglia (CD68+ cells) and fibroblasts (GFAP-negative flattened cells) and only 40–50% of cells were astrocytes. Only the immunocytochemical approach was suitable for the characterization of the expression of perforin by adult astrocytes.
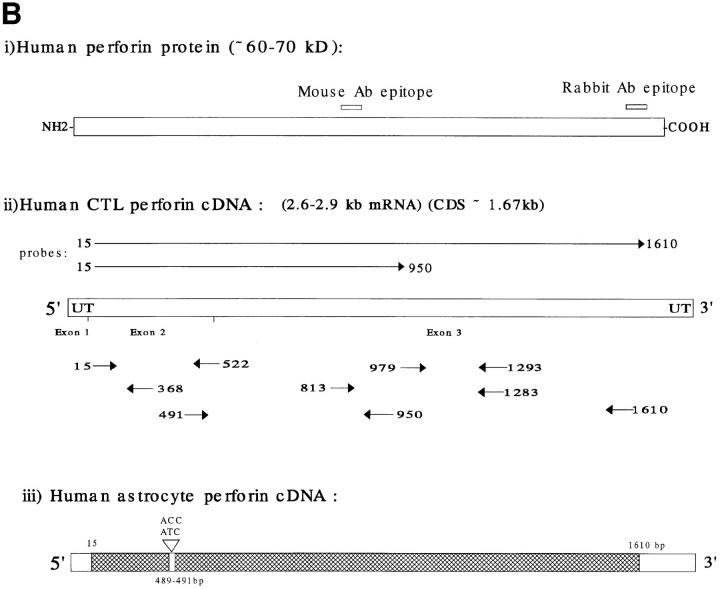
Figure 1.
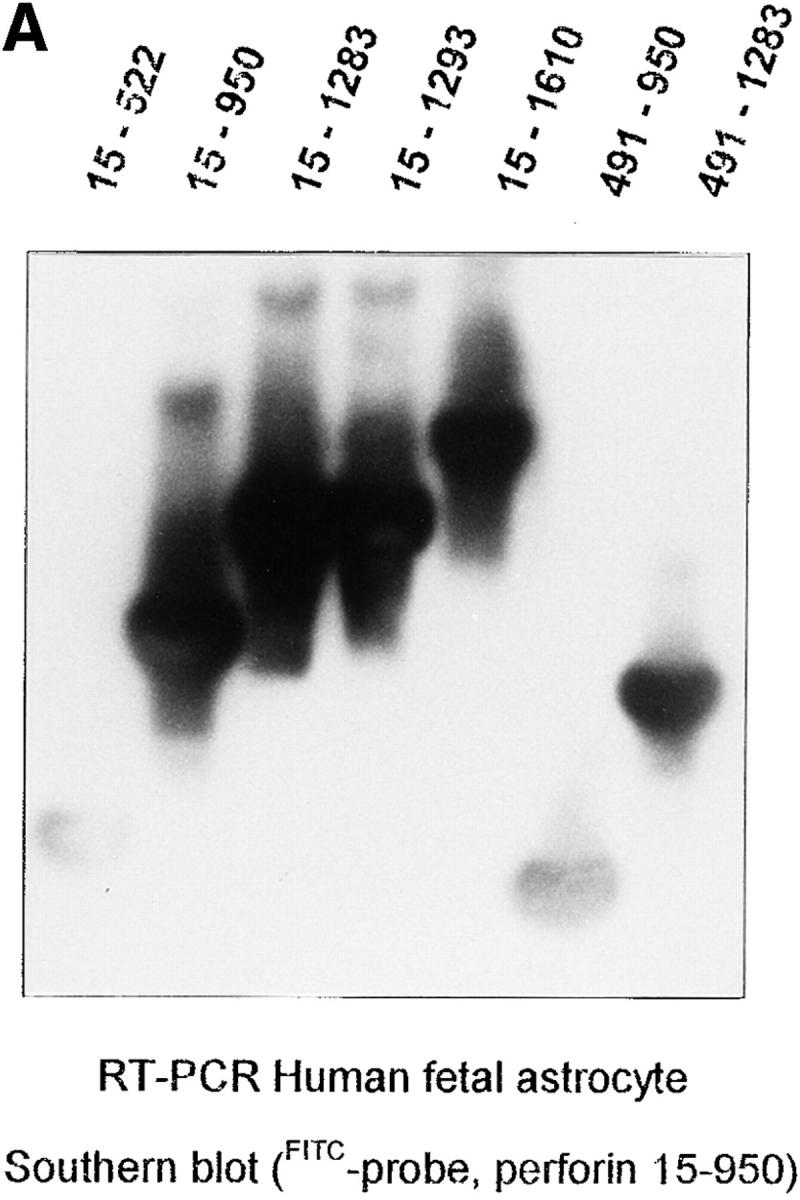
RT-PCR analysis and sequencing of astrocyte perforin cDNA. (A) Astrocyte total RNA were obtained from fetal astrocyte cultures (passage 3) and RT-PCR was carried out as previously described (9) using different primer pairs (20 bp) to amplify different regions of the perforin cDNA. The specificity of each cDNA product was assessed by Southern blotting using an FITC dUTP perforin cDNA probe (see Materials and Methods). The portion of the perforin cDNA amplified by the different primer pairs is given for each lane at the top of the figure. (B) Schematic representations of the sequence of perforin (protein and cDNA). The positions of the epitopes for the antibodies used in this study (monoclonal and polyclonal antipeptide) are indicated in i. ii shows the position of the primers and probes derived from CTL perforin cDNA (reference 13, sequence data available under EMBL/ GenBank/DDBJ under accession number M28393) and used in the analysis of astrocyte perforin cDNA. iii illustrates the astrocyte perforin cDNA sequence obtained from the cloned RT-PCR products (hatched area) on the full-length perforin cDNA. Together, these products provided >90% of the astrocyte perforin cDNA coding region. Six different batches of fetal astrocytes were used in separate experiments with identical final results. The sequence was consistent and differed from that of the published CTL perforin cDNA sequence (13) by a single residue (C490T).
Astrocytes Express Perforin Protein.
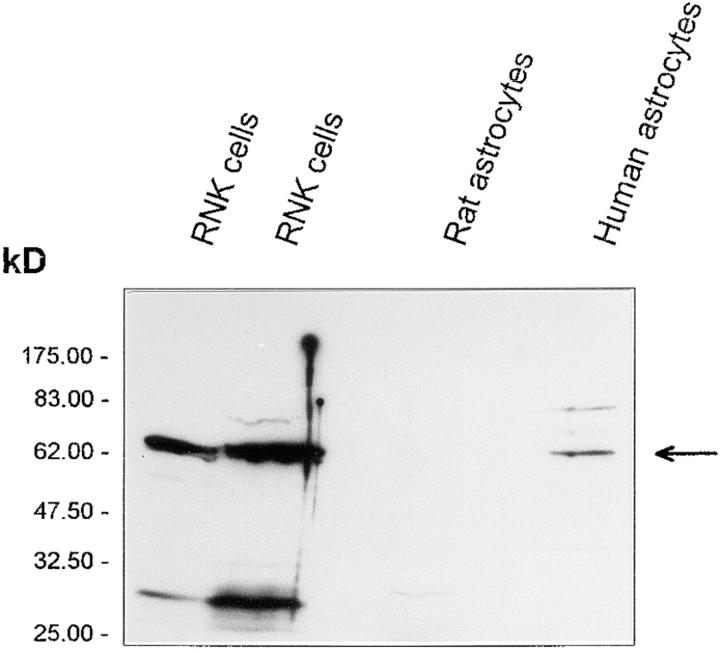
Western blotting of cell extracts of human fetal astrocytes with specific antiperforin antibodies identified a positive band with a molecular mass of ∼65 kD, identical to the major band obtained from cell extracts of rat NK cells (Fig. 2). In contrast, rat neonatal astrocytes (1–2-d-old newborn rat) did not express perforin at a level detectable by Western blotting after 15 d in culture (passage 1; Fig. 2). The same number of cells were used to prepare all cell lysates (2 × 107 cells/ml). Isolation of lytically active perforin from astrocytes was attempted to provide further proof of the identity of the molecule. Despite several attempts, no lytically active protein was obtained.
Figure 2.
Western blotting of astrocyte extracts with monoclonal antiperforin antibody. Detergent extracts of a rat NK (RNK) cell line (positive control), rat neonatal astrocytes, and human fetal astrocytes (passage 7, 2 × 107 cells/ ml) were run on SDS-PAGE (10% acrylamide gel), Western blotted, and probed with the monoclonal antiperforin antibody (0.1–0.2 μg/ml). Bound antibody was detected using goat anti–mouse immunoglobulins (1 out of 1,000) and developed using the enhanced chemiluminescence detection system. A major band at 65 kD in the rat NK cell lysates was the predicted size for perforin. A single major band of 65 kD was also detected in lysates of human fetal astrocytes (arrowed) but not in neonatal rat astrocytes (28).
Perforin Is Expressed by a Subpopulation of Astrocytes and Is Not Stored in Granules.
When mixed cultures from human fetal brain were stained with specific antiperforin antibodies, only astrocytes were stained (Fig. 3 a). The staining for perforin and GFAP was observed only when the cell membrane was permeabilized by ethanol–acetic acid treatment, suggesting that perforin was localized intracellularly. Staining was relatively homogenous throughout the cytoplasm, in clear contrast to the strong granular staining in YT NK cells, used here as a positive control (Fig. 4 d). Other cells in the culture, microglia (CD68+ cells), oligodendrocytes (GC-positive cells, representing <0.5% of the total), fibroblasts (CD68- and GFAP-negative flattened cells), and neurons (cluster of CD56high, CD44-negative cells overlying the astrocyte layer) were negative for perforin expression (data shown only for neurons, see arrow Fig. 3 a). Not all fetal astrocytes in mixed (passage 0) or pure cultures (after passage 3) were positive for perforin, as ∼20–30% of GFAP-positive cells were perforin-negative (Fig. 3, b–d). The staining of fetal astrocytes for perforin was reproducible in different cultures (n >10), the proportion of positive cells was consistent between cultures and no specific staining was obtained with irrelevant rabbit or mouse antibody (Fig. 3 e). Perforin was also expressed constitutively by two human astrocytoma cell lines, T98G and CB193 (the latter shown in Fig 4, b and c), and also by human adult astrocytes (Fig. 4, g and h) in a distribution similar to that in fetal astrocytes. The staining in human adult astrocytes was weak in comparison with that in fetal astrocytes. Staining with control irrelevant monoclonal or polyclonal antibodies was consistently negative on all cell types (Fig. 4, a, e, i, and j). When mouse and rat astrocytes (from 1–2-d-old neonates) were stained for perforin using the monoclonal antibody, a very weak staining was obtained on a small proportion of the rodent astrocytes (<20% of the mixed culture; data not shown).
Figure 3.
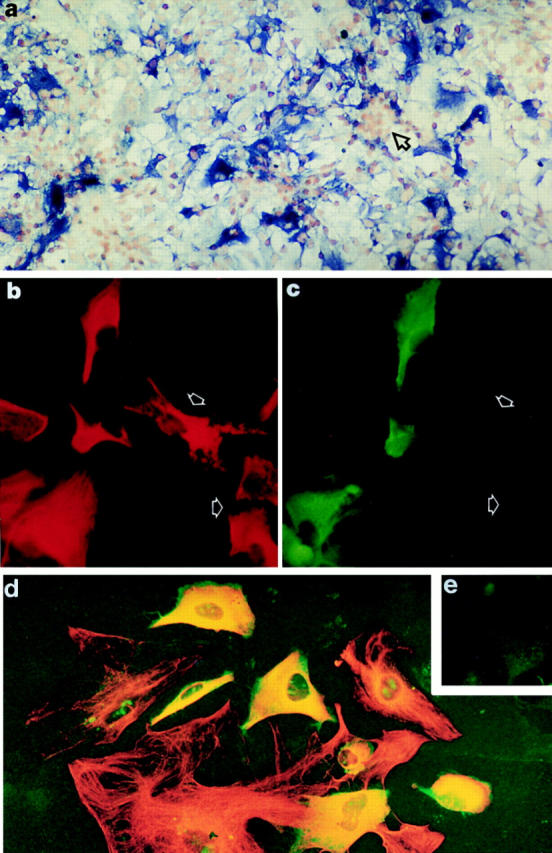
Immunodetection of perforin expressed in vitro by human fetal astrocytes. (a) In a mixed culture of cells from human fetal brain (passage 0), only astrocytes were stained with monoclonal antiperforin antibody (stained in blue, alkaline phosphatase–conjugated antibody and BCIP/NBT development), whereas fetal neurons growing in clusters (arrowed) were always negative. The staining was obtained only after permeabilization of the cell membrane (ethanol–acetic acid fixation), indicating that perforin was an intracellular protein. Original magnification: ×100. (b and c) Pure fetal astrocyte cultures (passage 3) were stained for perforin (mouse antibody, 1 μg/ml) and GFAP (rabbit antibody, 1 out of 4,000) followed by FITC-conjugated donkey anti–mouse immunoglobulins (Jackson, 1 out of 400) and rhodamine-conjugated goat anti–rabbit immunoglobulins (Jacksons, 1 out of 400). The fluorescence was photographed using filters specific for rhodamine (GFAP; red fluorescence in b) or for FITC (perforin; green fluorescence in c). The staining confirmed that GFAP-positive cells (b) were also perforin positive (c). However, it was clear that not all GFAP-positive cells expressed perforin (arrowed). Original magnification: ×500. (d) Fetal astrocyte cultures (passage 3) were stained using the same condition as described in b and c) and the fluorescence was photographed using dual exposure of both rhodamine (GFAP) and FITC (perforin). Cells positive for both fluorochromes (perforin- and GFAP-positive cells) appeared yellow (original magnification: ×500). All perforin-positive cells were GFAP-positive, but not all GFAP-positive cells were perforin-positive, indicating that only a proportion of astrocytes in culture expressed perforin at detectable levels. No perforin-positive but GFAP-negative cells were detected in fetal astrocyte cultures from passages 0 to 15. (e) No specific FITC or rhodamine staining was detected when fetal astrocyte cultures were incubated with irrelevant primary antibodies (mouse anti-FH or rabbit anti-C4bp). Original magnification: ×500.
Figure 4.
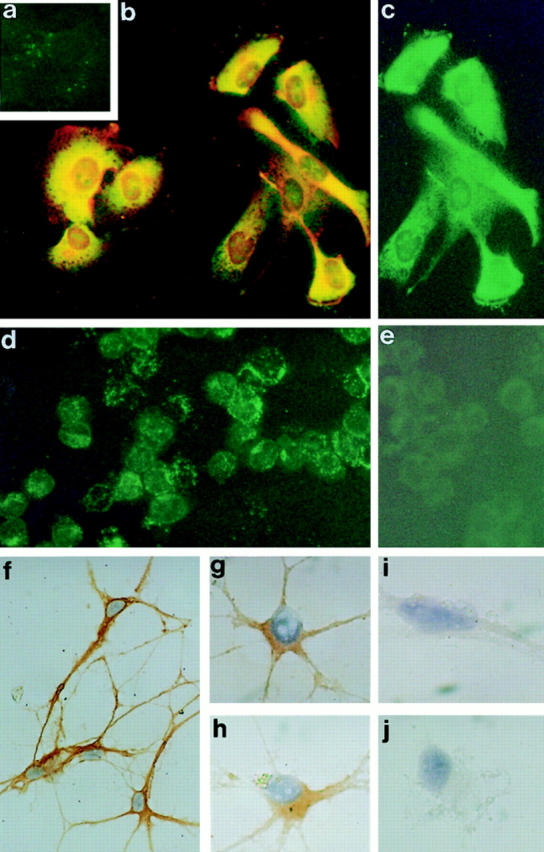
Immunostaining of human CB193 astrocytoma, YT NK cell line, and adult astrocytes for perforin. (a–c) CB193 astrocytoma was cultured on glass coverslip and stained using either irrelevant antibodies (a) or double-stained with mouse antiperforin and rabbit anti-GFAP as described in the legend of Fig. 3, b and c. The fluorescence was photographed using dual exposure (a and b). Cells positive for both fluorochromes (perforin- and GFAP-positive cells) appeared yellow. (c) Part of the same field as in b, but here the fluorescence was photographed only in the FITC channel (green fluorescence, perforin staining). All perforin-positive cells were also GFAP-positive. (d and e) YT NK cell line used here as a positive control. Cytospins were fixed in ethanol/acetic acid, and double-stained using either irrelevant antibodies (mouse and rabbit; e) or mouse antiperforin and rabbit anti-GFAP (d). The fluorescence was photographed using dual exposure. All the YT cells were perforin-positive (green fluorescence, granular staining) and GFAP-negative. No specific staining was detected using irrelevant antibodies (e). Original magnification for a–e: ×500. (f–j) Adult astrocytes cultured on glass coverslips were fixed and stained using either rabbit anti-GFAP (f ), mouse antiperforin (g and h), or irrelevant antibodies (i and j), and were counterstained with hematoxylin. Specific binding of the antibodies was detected using peroxidase-conjugated secondary antibodies (Biorad) and DAB development. Rabbit anti-GFAP gave strong cytoplasmic staining of cells with long cellular processes, confirming that these were adult astrocytes (f ). These cells were also strongly stained for CD44, CD56, and CD59 but not for CD68, GC, or NSE (data not shown). Cytoplasmic staining for perforin was weakly but reproducibly detected in the same population of cells (g and h). The staining using irrelevant antibodies was consistently negative (i and j). Original magnification for g–j: ×1,250.
Perforin expression by fetal astrocytes was confirmed by FACS® analysis of permeabilized cells using the mouse antiperforin antibody and costaining for GFAP was confirmed by using the rabbit anti-GFAP (Fig. 5 B). Between 50 and 60% of GFAP-positive cells from fetal astrocyte culture passage 3 were also stained for perforin. The human NK cell line YT and mouse CTLL-2 line were both 95–98% positive for perforin using the same antibody, and expressed on average twice the amount of perforin (as estimated by the mean of fluorescence) present in perforin-positive human fetal brain astrocytes at passage 3 (Fig. 5 A). When fetal astrocytes were stained using the rabbit anti-perforin and the mouse anti-GFAP, again 50–60% of cells were positive for both markers (data not shown). Perforin was detected by FACS® only when the cell membrane was permeabilized, confirming that perforin was not expressed on the membrane and was present only in the cytoplasm. Perforin and GFAP staining of fetal astrocytes was reproducible (n = 3). Staining for irrelevant primary antibodies, mouse anti-CD11b, and rabbit anti-CRP was always negative in FACS® analysis of fetal astrocytes (Fig. 5 B). FACS® analysis of the astrocytoma cell lines CB193 and T98G revealed that >90% of cells were perforinhigh and GFAPweak double-positive (data not shown). Insufficient cells were obtained from adult brain cultures to be analyzed by FACS® for perforin and GFAP expression.
Figure 5.
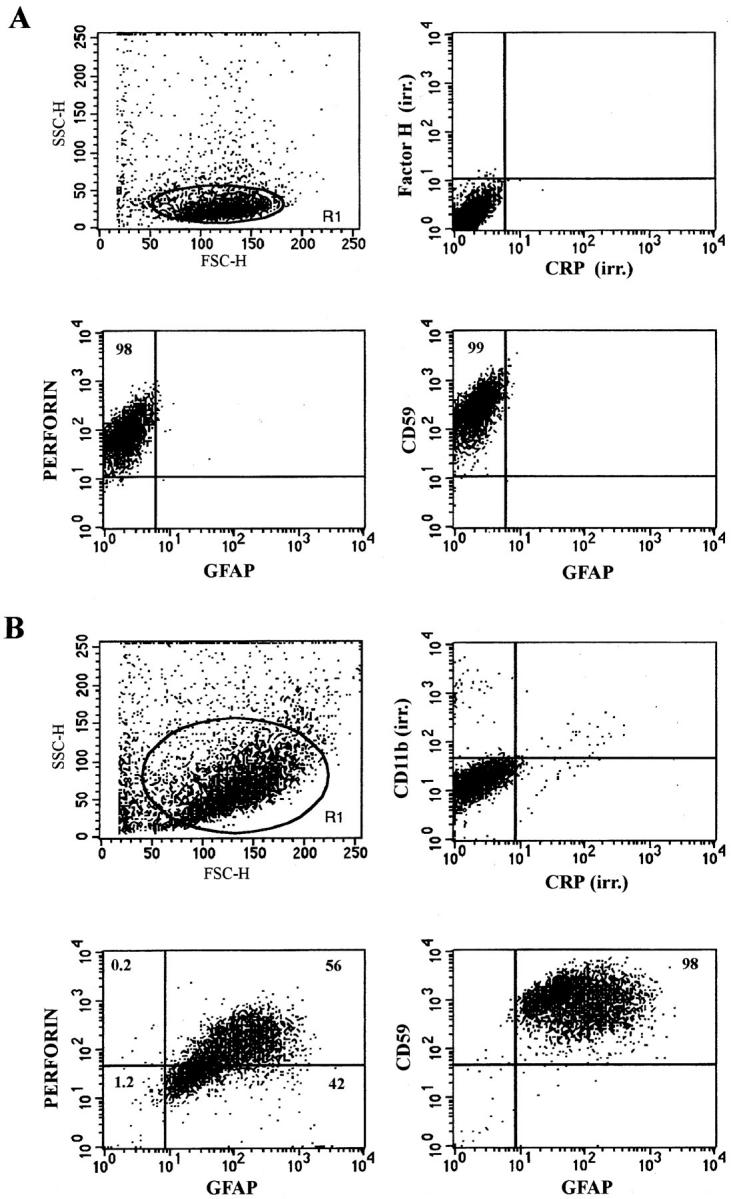
FACS® analysis of YT NK cells and fetal astrocytes double stained for perforin and GFAP. Cells in suspension were fixed and permeabilized and then simultaneously stained for perforin and GFAP using mouse antiperforin (0.2 μg/100 μl/105 cells) and rabbit anti-GFAP (1/ 200) followed by recombinant PE–conjugated goat anti-mouse immunoglobulins (Dako, 1/100) and FITC-conjugated goat anti–rabbit immunoglobulins (SeraLab, 1/100). FITC fluorescence and RPE fluorescence were measured on a Becton Dickinson Facscan. Data are represented as dot plots of a gated population R1 for each cell type (A) YT cells were 98% perforin positive but GFAP negative. Irrelevant (irr.) monoclonal (Factor H) or polyclonal (CRP) antibodies gave no staining. (B) Fetal astrocytes were 98% positive for GFAP and 56% of the GFAP-positive cells were also positive for perforin. Irrelevant antibodies were again negative. Staining for CD59 was used here as a positive control.
Indirect immunofluorescence staining and confocal microscopy analysis confirmed that perforin staining in fetal astrocytes was not associated with the cell membrane but was diffusely distributed throughout the cytoplasm (Fig. 6, b and c), in clear contrast with the granular cytoplasmic staining of human YT and mouse CTL cells (Fig. 6, e and f ). At the electron microscopic level, perforin staining in fetal astrocytes was cytoplasmic (Fig. 7, e and f ), again contrasting with the distribution in mouse CTL, where perforin was restricted to dense cytoplasmic granules (Fig. 7 b) as previously reported (14, 15). The distribution pattern for astrocyte perforin in electron micrographs was suggestive of an association with intracellular membrane networks.
Figure 6.
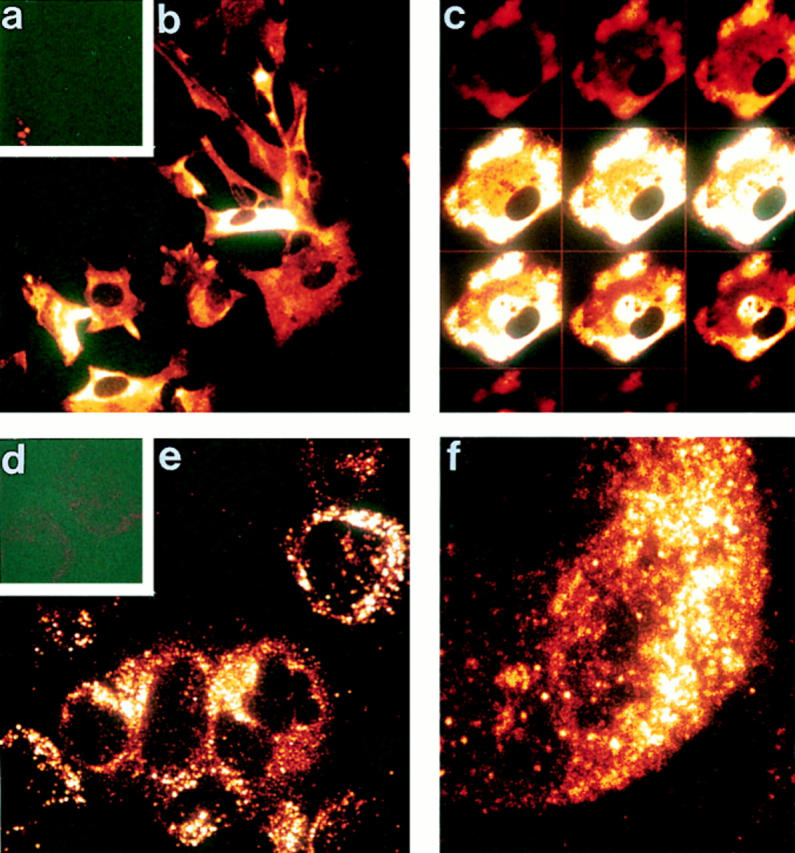
Confocal microscopy confirms cytoplasmic localization of perforin in fetal astrocytes. Fetal astrocytes and YT NK cells were permeabilized/fixed on glass coverslips and stained using either an irrelevant antibody (mouse anti-FH; a and d) or mouse anti-perforin (1 μg/ml) followed by FITC-conjugated donkey anti–mouse immunoglobulins (Jackson, 1/400). Fluorescence was analyzed on Leica TCS confocal microscope. (a–c) Fetal astrocytes: a, negative control; b, extended focus image of a field of fetal astrocytes stained for perforin; c, gallery of individual section scans through a single fetal astrocyte stained for perforin. The lack of membrane staining and cytoplasmic location of label is clear in the gallery. (d–f ) YT cells: d, negative control; e, extended focus image of a field of YT cells stained for perforin (note the granular staining pattern); f, high power view from e. Original magnification: ×500 for a, b, d, and e and ×1,250 for c and f.
Figure 7.
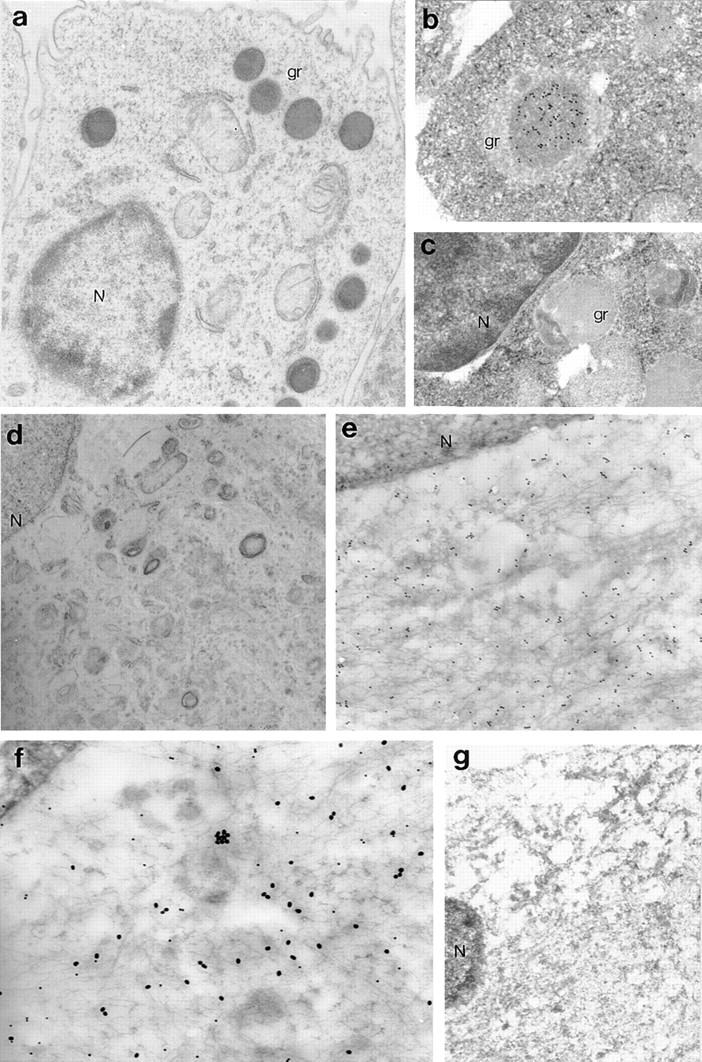
Electron microscopic analysis of perforin distribution in human fetal astrocytes and mouse CTL. Cells were fixed, immunogold stained, and processed for electron microscopy as previously described (12). The mouse cytotoxic T cell line (CTLL2) stained strongly for perforin in a granular cytoplasmic distribution (a, original magnification: ×10,000). At higher power (b, original magnification: ×22,000) the granules were more clearly defined and it was apparent that there was little or no staining outside the granules. No specific staining of mouse CTLL2 was obtained using irrelevant mouse anti-FH (c, original magnification: ×20,000). Astrocytes did not contain detectable cytoplasmic granules (d, original magnification: ×10,000) and immunogold staining demonstrated that perforin was localized to the cytoplasm, perhaps in association with intracellular membranous structures (e, original magnification: ×22,000). The nucleus (N) was not stained above background. Double-immunostaining of cells for perforin (small gold particles) and GFAP (large gold particles) confirmed that all perforin-positive cells were also GFAP-positive (f, original magnification: ×30,000). No specific staining was obtained when fetal astrocytes were stained with irrelevant mouse and rabbit primary antibodies (anti-FH and anti-CRP, respectively; g, original magnification: ×13,000). gr, granules.
Perforin Is Expressed by Reactive Astrocytes in Inflamed Human Central Nervous System Tissues.
The finding that perforin is expressed by astrocytes and astrocyte cell lines in vitro provoked us to examine whether perforin was also expressed by these cells in vivo. Three samples of normal adult brain (temporal brain) and two of fetal brain were analyzed in this study. There was no evidence of brain inflammation in these samples as indicated by the absence of astrogliosis (GFAP and CD44 staining) or microgliosis (LN3 and CD14 staining). None of the normal human adult (Fig. 8 b) or fetal brain frozen tissue sections (data not shown) stained with antiperforin antibody, indicating that this molecule was not expressed in normal brain and confirming a previous report using a different monoclonal antiperforin antibody (16). However, brain sections from several unrelated pathologies, all presenting evidence of ongoing inflammation with marked gliosis, including MS (Fig. 9, c, e, and f ), AD (Fig. 9, h and i), Huntington's disease (HD, Fig. 9 k), and PD (Fig. 9 m), were positive for perforin, and the staining was associated with large, GFAP-positive cells with a morphological appearance typical of reactive fibrillary astrocytes (Fig. 9, a, g, j, and l). The perforin-positive cells were confirmed as astrocytes by double-staining of brain sections with rabbit anti-GFAP in MS brain (Fig. 9, p–r; Fig. 10, a–d), AD (Fig. 10, e and f ), PD, and HD (data not shown). Reactive astrocytes in chronic and semiacute MS were strongly stained for perforin and no specific staining was detected when irrelevant antibodies were applied on the tissue sections (mouse anti-FH and rabbit anti-C4bp, Fig. 9, b, d, and o). In comparison, perforin staining on astrocytes was weaker in the three different neurodegenerative disorders examined. In MS, the majority but not all of the GFAP-positive reactive astrocytes expressed perforin in their cytoplasm; interestingly, another population of small, rounded cells were also found to be strongly perforin positive but GFAP-negative (Fig. 9, c, e, and p, indicated by the arrow). These small cells were present only in MS (plaque and perivascular localization) but not in neurodegenerative disorders (in temporal lobe and caudate nucleus). We suspect that these cells represent perforin-positive NK and/or CTL that are infiltrating the brain tissue in MS (for review see reference 17).
Figure 8.
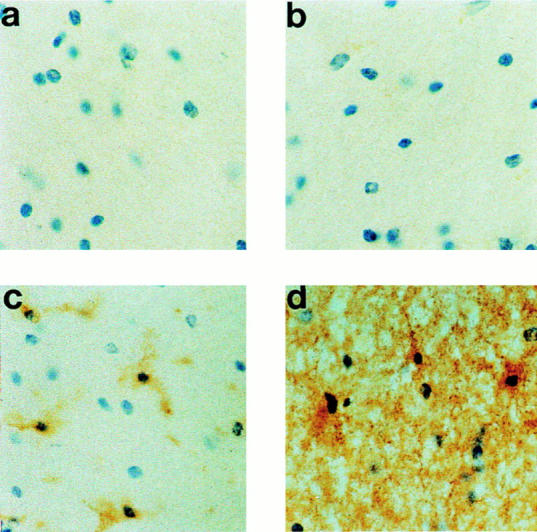
Immunodetection of perforin in normal human brain tissue. Frozen tissue sections were stained (a) using an irrelevant antibody (OX23, mouse antifactor H) or with mouse antibody against (b) perforin, (c) HLA class II (clone LN3), or (d) CD44 (clone BRIC222) DAB/immunoperoxidase protocol and were counterstained using hematoxylin. No specific staining for perforin was obtained in normal surgical brain, whereas quiescent microglia and astrocytes were identified respectively by the HLA class II and CD44 stainings (original magnification: ×500).
Figure 9.
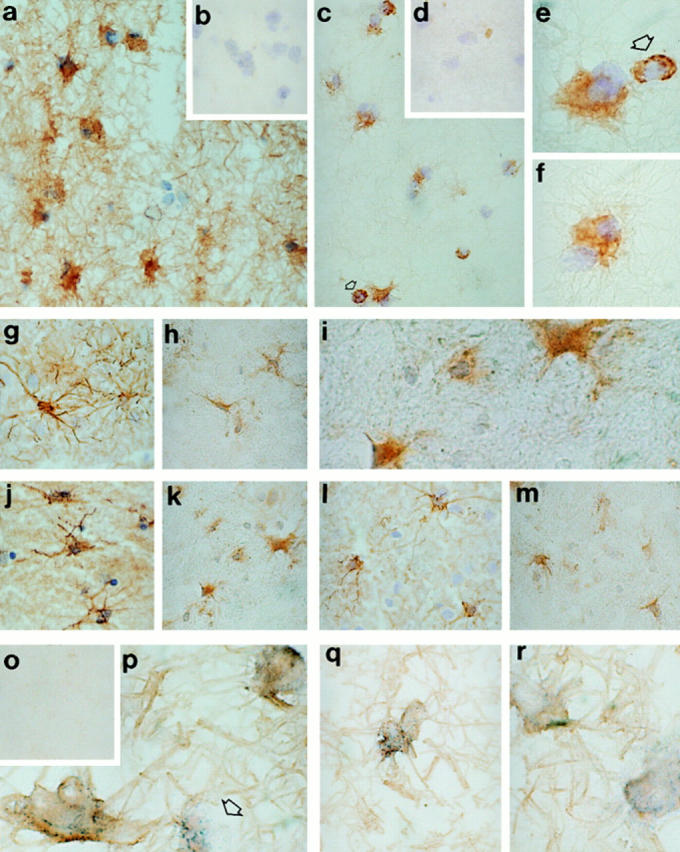
Immunodetection of perforin in inflamed human brain tissue. Frozen tissue sections were stained either for perforin (mouse antibody; c, e, f, h, i, k, and m), GFAP (rabbit antibody; a, g, j, and l) or irrelevant antibodies (b and d) using a DAB/immunoperoxidase protocol (12) and counterstained using hematoxylin. The diseases examined were MS (a–e and o–r), AD (g–i), HD (j and k), and PD (l and m). In MS brain most reactive astrocytes (GFAP-positive cells, a, original magnification ×500) were stained for perforin (c, original magnification: ×500) and (e and f, original magnification: ×1,250). No specific staining of MS tissue sections was obtained with irrelevant antibodies (rabbit anti-CRP [b] and mouse anti-FH [d], original magnification: ×500). Small perforin-positive, GFAP-negative cells were also identified in MS sections (2–3 cells/field at ×500 original magnification, panels c and e, indicated by the arrow). These cells had no astrocytic features and were found in the inflammatory infiltrate as well as in the demyelinating plaque. Perforin-positive–reactive astrocytes were also indentified in brain from AD (h and i, temporal lobe), HD (k, caudate), and PD (m, temporal lobe). MS tissue (acute plaque) was also double stained for perforin and GFAP without counterstaining (p–r). The majority of GFAP positive cells (DAB development, brown staining) were also perforin positive (BCIP/NBT development, blue precipitates). The arrow in p indicates a single perforin-positive, GFAP-negative cell. Tissue sections incubated with the appropriate control antibodies were completely negative (o). Original magnification: ×1,250 except for panel o, original magnification: ×500.
Figure 10.
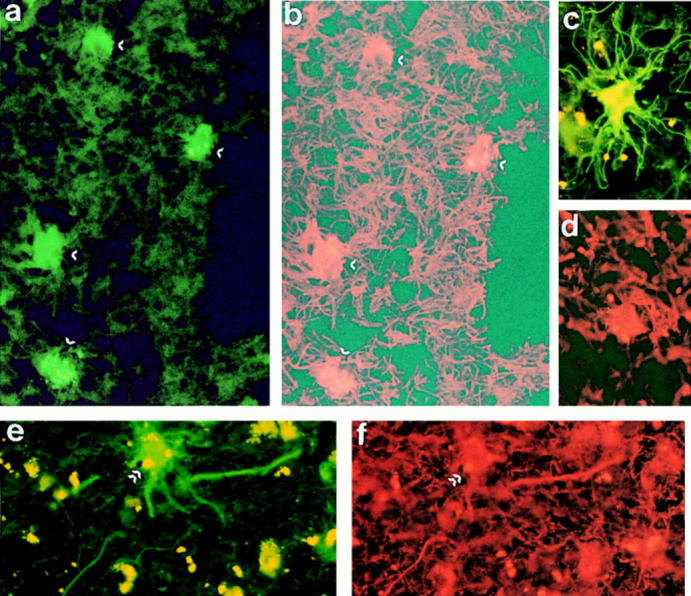
Double immunofluorescence staining of MS and AD brains for perforin and GFAP. Frozen sections of MS (a–d) and AD (e and f ) brain were stained using mouse antiperforin (FITC, green fluorescence) and rabbit anti-GFAP (rhodamine, red fluorescence). In both diseases, all perforin-positive cells (a, c, and e) were also GFAP positive (b, d, and f ) (original magnification: ×1,250). Arrows in a and b and e and f indicate the same cells in the two exposures. No staining was obtained with irrelevant antibodies (data not shown).
Discussion
In this paper, we demonstrate by diverse methods that cultured human fetal astrocytes, adult astrocytes, and astrocytoma cell lines constitutively express perforin. Astrocyte perforin was indistinguishable from that in T and NK cell lines upon Western blotting and the cDNA sequences were essentially identical. In contrast to T and NK cell lines, astrocyte perforin was not contained within cytoplasmic granules but was present throughout the cytoplasm, possibly in association with the endoplasmic reticulum. Despite numerous attempts and the relative abundance of perforin in fetal astrocytes, we were unable to isolate lytically active perforin from these cells. We believe that this failure was a consequence of the limited numbers of primary astrocytes that could be generated and the fact that human perforin has extremely low activity in comparison with rat or mouse perforin in the standard erythrocyte lysis assay system (our unpublished observations). Indeed, we have been unable to purify lytically active perforin even from human NK lines, although we have successfully purified active rat and mouse perforin.
We also show that perforin is expressed in inflamed human brain in diverse pathological states but is not expressed in normal noninflamed fetal or adult brain. This latter observation suggests that products of inflammation cause the upregulation of perforin expression in vivo and we are currently examining in vitro whether stimulation with inflammatory cytokines or other effector molecules can trigger changes in the expression of perforin or induce its release from astrocytes. Perforin expression in vivo is seen only in the presence of pathology and specifically in association with inflammation. It would be of interest to know when during the course of the pathology expression of perforin occurs. To address this issue we are currently attempting to characterize the expression of perforin in rodent brains during the induction of experimental neuropathology, including experimental demyelination, brain infection, and stroke. In these models we will be able to analyze during the time course of the disease not only the cellular source of perforin but also the site of expression in the central nervous system. These studies will help to explain the role of perforin expression by astrocytes in the development of the various pathologies.
The cytoplasmic localization of perforin in astrocytes presents a conundrum. In cytotoxic T cells, dogma states that perforin is contained within granules that are released only after binding to target cell and only into the intracellular space at the binding site. This event induces a localized permeability of the target membrane, allowing delivery of other granule constituents, which induce killing, into the target cell (1, 2). However, a second pathway of perforin release has been demonstrated; a constitutive pathway in which newly synthesized perforin is released directly from the cell without prior inclusion in granules (18). This constitutive pathway can mediate lytic killing of bystander cells, indicating that perforin secreted in this manner is functional. Perforin expressed in insect cells is also lytic despite the absence of granules in these cells (19). Therefore, it is possible that perforin is secreted constitutively by reactive astrocytes and mediates cytotoxic and cytolytic effects on surrounding cells.
The physiological role of astrocyte perforin is likely to be in immune defense and remodeling in the brain. The absence of neurological deficit in perforin knockout mice indicates that the latter activity is not essential to normal brain development (4, 5). In pathology, reactive astrocytes may be induced to synthesize and secrete perforin in an undirected, constitutive manner, placing resident brain cells at risk. Astrocytes are relatively resistant to the lytic effects of perforin (our unpublished data). However, oligodendrocytes, the myelin-producing cells in the brain, and neurons are known to be extremely susceptible to perforin damage (20, 21). Perforin generated locally by astrocytes may thus contribute to the loss of oligodendrocytes in demyelination and of neurons in neurodegeneration.
A second killing strategy used by cytotoxic T cells involves interaction of Fas ligand with Fas on the target (3). It has recently been reported that glial cells in MS brain and in brain tumors express de novo Fas ligand and it has been suggested that the interaction of glial Fas ligand with Fas on target cells (notably on autoreactive T cells) might contribute to the control of immune defense in the brain (22, 23). Although it was not clear from this report which glial cell type expressed Fas ligand, the astrocyte is a prime candidate. We are currently analyzing expression of Fas ligand by perforin-positive astrocytes in vitro and in vivo and examining the role of cytokines in the expression of these molecules. Preliminary data indicates that astrocytes may indeed express Fas ligand, further extending the similarity with cytotoxic T cells. In addition, it has been recently shown that oligodendrocytes express high level of Fas and that anti-Fas monoclonal antibody or purified Fas-ligand induced oligodendrocyte necrosis but not programmed cell death (24). It was proposed that elevated Fas expression by oligodendrocytes in MS might contribute to the loss of the myelinating cells by interaction of Fas on the target cell with Fas-ligand expressed by infiltrating CTLs and glia (24). Reactive astrocytes in MS may thus be capable of killing oligodendrocytes through secretion of perforin and/or through the Fas–Fas-ligand system. Endocytosis of oligodendrocytes by reactive astrocytes in MS brain has been described for many years (25–27). Whether this represents a mechanism for the clearance of dead oligodendrocytes or a fundamental component of the proposed killing process remains to be ascertained.
In summary, we report that astrocytes in vitro and in vivo in the inflamed brain express perforin, a cytolytic protein which had previously been considered to be T and NK cell–specific. We suggest that astrocytes may use CTL-like mechanisms (perforin and Fas/Fas-ligand) to destroy pathogens in the brain and that these mechanisms may also contribute to the loss of glia and neurons in diverse neuropathologies.
Footnotes
The authors thank Dr. Gillian M. Griffiths for the generous gift of the monoclonal and polyclonal antiperforin antibodies and the human NK cell line YT and Jean Hopkins for her technical help. We also thank Dr. Jim W. Neal for normal and HD brains and helpful discussions.
This work was supported by grants from the Medical Research Council (P. Gasque), the Wellcome Trust (B.P. Morgan), the Arthritis and Rheumatism Council (J. Jones), and the Welsh Scheme for the Development of Health and Social Research (S.K. Singhrao).
Address correspondence to Dr. Philippe Gasque, University of Wales College of Medicine, Department of Medical Biochemistry, Brain Inflammation and Immunity Group (BIIG), Tenovus Building, Heath Park, Cardiff, CF4 4XX, UK. Phone: 44-1-222-744236; Fax: 44-1-222-744305; E-mail: wmbpg@cardiff.ac.uk
Abbreviations used in this paper: AD, Alzheimer's disease; BCIP, 5-bromo-4-chloro-3indolyl phosphate; C4bp, C4B binding protein; CRP, C-reactive protein; DAB, diaminobenzidine; FH, complement factor H; GC, galactocerebroside; GFAP, glial fibrillary acidic protein; HD, Huntington's disease; MS, multiple sclerosis; NBT, nitroblue tetrazolium; NSE, neuron-specific enolase; PD, Pick's disease; RT, reverse transcriptase.
References
- 1.Podack ER, Hengartner H, Lichtenheld MG. A central role of perforin in cytolysis? . Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 2.Liu CC, Walsh CM, Young JDE. Perforin: structure and function. Immunol Today. 1995;16:194–201. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 3.Tschopp J, Hofman K. Cytotoxic T cells: more weapons for new targets? . Trends Microbiol. 1996;4:91–94. doi: 10.1016/0966-842X(96)81522-8. [DOI] [PubMed] [Google Scholar]
- 4.Kagi D, Lederman B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 5.VandenBroek MF, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJM, Zinkernael RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrikant P, Benveniste EN. The central nervous system as an immunocompetent organ. Role of glial cells in antigen persentation. J Immunol. 1996;157:1819–1822. [PubMed] [Google Scholar]
- 7.Moregan BP, Gasque P. Expression of complement in the brain: role in health and disease. Immunol Today. 1996;10:461–466. doi: 10.1016/0167-5699(96)20028-f. [DOI] [PubMed] [Google Scholar]
- 8.Adams, M.D., A.R. Kerlavage, R.D. Fleischmann, R.A. Fuldner, C.J. Bult, N.H. Lee, E.F. Kirkness, K.G. Weinstock, J.D. Gocayne, O. White, et al. 1995. Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence. Nature. 377 (6547 Suppl.):3–174. [PubMed]
- 9.Gasque P, Chan P, Fontaine M, Ischenko A, Lamacz M, Gotze O, Morgan BP. Identification and characterization of the complenent C5a anaphylatoxin receptor on human astrocytes. J Immunol. 1995;155:4882–4889. [PubMed] [Google Scholar]
- 10.Gasque P, Singhrao SK, Neal JW, Gotze O, Morgan BP. Expression of the receptor for complement C5a (CD88) is upregulated on reactive astrocytes, microglia, and endothelial cells in inflamed human central nervous system. Am J Pathol. 1997;150:31–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Gasque P, Ischenko A, Legoedec J, Mauger C, Schouft MT, Fontaine M. Expression of the complement classical pathway by human glioma in culture. J Biol Chem. 1993;268:25068–25074. [PubMed] [Google Scholar]
- 12.Singhrao SK, Neal JW, Gasque P, Morgan BP, Newman J. Role of complement in the aetiology of Pick's disease. J Neuropathol Exp Neurol. 1996;55:578–593. doi: 10.1097/00005072-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Shinkai Y, Yoshida MC, Maeda K, Kobata T, Maruyama K, Yodoi J, Yagita H, Okumura K. Molecular cloning and chromosomal assignment of a human perforin (PFP) gene. Immunogenetics. 1989;30:452–457. doi: 10.1007/BF02421177. [DOI] [PubMed] [Google Scholar]
- 14.Baetz K, Isaaz S, Griffiths GM. Loss of cytotoxic T lymphocyte function in Chediak-Higashi syndrome arises from a secretory defect that prevents lytic granule exocytosis. J Immunol. 1995;154:6122–6131. [PubMed] [Google Scholar]
- 15.Ojcius DM, Zheng LM, Sphicas EC, Zychlinsky A, Young JDE. Subcellular localization of perforin and serine esterase in lymphokine-activated killer cells and cytotoxic T cells by immunogold labeling. J Immunol. 1991;146:4427–4432. [PubMed] [Google Scholar]
- 16.Hameed A, Olsen KJ, Cheng L, Fox WM, III, Hruban RH, Podack ER. Immunohistochemical identification of cytotoxic lymphocyte using human perforin monoclonal antibody. Am J Pathol. 1992;140:1025–1030. [PMC free article] [PubMed] [Google Scholar]
- 17.Wucherpfennig KW, Weiner HL, Hafler DA. T-cell recognition of myelin basic protein. Immunol Today. 1991;12:277–282. doi: 10.1016/0167-5699(91)90126-E. [DOI] [PubMed] [Google Scholar]
- 18.Isaaz S, Baetz K, Olsen K, Podack E, Griffiths GM. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. 1995;25:1071–1079. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- 19.Liu CC, Persechini PM, Young JDE. Expression and characterization of functionally active recombinant perforin produced in insect cells. J Immunol. 1996;156:3292–3300. [PubMed] [Google Scholar]
- 20.Jones J, Frith S, Piddlesden S, Morgan BP, Compston DAS, Campbell AK, Hallett MB. Imaging Ca2+changes in individual oligodendrocytes attacked by T-cell perforin. Immunology. 1991;74:572–57. [PMC free article] [PubMed] [Google Scholar]
- 21.Rensing-Ehl A, Malipiero U, Irmler M, Tschopp J, Constam D, Fontana A. Neurons induced to express major histocompatibility complex class I antigen are killed via the perforin and not the Fas (Apo-1/CD95) pathway. Eur J Immunol. 1996;26:2271–2274. doi: 10.1002/eji.1830260945. [DOI] [PubMed] [Google Scholar]
- 22.Dowling, P., G. Shang, S. Raval, J. Menonna, S. Cook, and W. Husar. 1996. Involvement of the CD95 (Apo-1/Fas) receptor/ligand system in multiple sclerosis brain. J. Exp. Med. 184;1513–1518. [DOI] [PMC free article] [PubMed]
- 23.Saas P, Walker PR, Hahne M, Quiquerez AL, Schnuriger V, Perrin G, French L, VanMeir EG, DeTribolet N, Tschopp J, Dietrich PY. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? . J Clin Invest. 1997;99:1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med. 1996;184:2361–2370. doi: 10.1084/jem.184.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghatak NR. Occurrence of oligodendrocytes within astrocytes in demyelinating lesions. J Neuropathol Exp Neurol. 1992;51:40–46. doi: 10.1097/00005072-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Wu E, Brosnan CF, Raine CS. SP40,40 immunoreactivity in inflammatory CNS lesions displaying astrocyte/oligodendrocyte interactions. J Neuropathol Exp Neurol. 1993;52:129–134. doi: 10.1097/00005072-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Raine CS. The Norton lecture: a review of the oligodendrocyte in the multiple sclerosis lesion. J Neuroimmunol. 1997;77:135–152. doi: 10.1016/s0165-5728(97)00073-8. [DOI] [PubMed] [Google Scholar]
- 28.Rogers CA, Gasque P, Piddlesden SJ, Okada N, Holers VM, Morgan BP. Expression and function of membrane regulators of complement on rat astrocytes in culture. Immunology. 1996;88:153–161. doi: 10.1046/j.1365-2567.1996.d01-637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]