Abstract
T cells with T cell receptor (TCR) transgenes that recognized CD1 on syngeneic B cells stimulated B cells to secrete immunoglobulins in vitro. The CD4+, CD8+, or CD4−CD8− T cells from the spleen of the TCR transgenic BALB/c donors induced lupus with anti–double stranded DNA antibodies, proteinuria, and immune complex glomerulonephritis in irradiated BALB/c nude mice reconstituted with nude bone marrow. Injection of purified CD4−CD8− T cells from the marrow of transgenic donors prevented the induction of lupus by the transgenic T cells. Transgenic T cells that induced lupus secreted large amounts of interferon (IFN)-γ and little interleukin (IL)-4, and those that prevented lupus secreted large amounts of IL-4 and little IFN-γ or IL-10.
Murine lupus is an autoimmune disease with a variety of antiprotein and nonprotein autoantibodies that cause injury to several organ systems including the kidney (1–3). Cationic anti–double-stranded (ds)1 DNA antibodies are pathogenic and contribute to immune complex glomerulonephritis (4, 5). T cells play an important role in augmenting the secretion of anti–ds DNA antibodies in lupus (6, 7). It is not clear how conventional T cells that recognize peptides associated with class I and II MHC molecules provide help for B cells that secrete antibodies to nonprotein antigens. Hypothesized mechanisms of T cell help include T cell recognition of DNA-associated protein antigens, such as histones (8, 9), and recognition of peptide fragments of anti-DNA antibodies (10, 11).
Since some subsets of T cells (i.e., NK1.1+ T cells) have been reported to recognize the nonpolymorphic, class I MHC-like molecule CD1 (12, 13), and other T cells can recognize sugar and/or lipid antigens in the context of CD1 (14, 15), these anti-CD1 T cells may provide an alternative mechanism of activation and help for the secretion of antibodies to nonprotein antigens. In the current study, transgenic CD4+ and CD8+ cells that recognize CD1 on syngeneic B cells and activate them to secrete immunoglobulins were tested for their capacity to induce lupus in irradiated syngeneic (BALB/c) nude hosts. These T cells were obtained from the spleen of a line of transgenic BALB/c mice that expressed the TCR-α and -β chain genes from an anti-CD1 BALB/c T cell clone (16). The transgenic CD4+ and CD8+ T cells induced lupus in the irradiated hosts, and the majority developed severe immune complex glomerulonephritis and anti–ds DNA antibodies. On the other hand, CD4−CD8− T cells from the bone marrow (BM) of transgenic mice expressing the same TCR-α and -β chain genes prevented lupus when coinjected with inducing T cells. The latter T cells secreted large amounts of IFN-γ and little IL-4, whereas the preventive T cells secreted large amounts of IL-4 and little IFN-γ.
Materials and Methods
Transgenic and Nontransgenic Mice.
Nontransgenic BALB/c and BALB/c nu/nu mice were obtained from the breeding facility of the Department of Laboratory Animal Medicine at the Stanford University School of Medicine (Stanford, CA). Male mice, 2–3 mo old, were used in the studies. Development of the single-positive (SP; predominantly CD4+ and CD8+ T cells) and double-negative (DN; predominantly CD4−CD8− T cells) lines of TCR-α and -β chain gene transgenic mice were described in detail previously (16). Transgenic mice used in the present study were backcrossed to BALB/c mice for at least seven generations. The male transgenic mice, 2–3 mo old, were used as cell donors in the current study.
Cells and Cell Lines.
The cloned CD4−CD8−α/β T cell line, TLI-2.C4, and the B cell lymphoma (BCL)1, tumor B cell line, of BALB/c origin have been described in detail previously (17, 18). A BALB/c B cell line (A20) transfected with cDNA encoding CD1 and the nontransfected control cells were obtained from M. Kronenberg (La Jolla Institute for Allergy and Immunology, La Jolla, CA; reference 19). Spleen and BM cells were harvested as described previously (16). In some experiments, 4 × 106 BALB/c spleen cells were activated in vitro with LPS (Boivan type; Difco, Detroit, MI) at 20 μg/ml in 2 ml complete medium (see below) for 48 h, and washed before use in proliferation assays.
Monoclonal Antibodies, Immunofluorescent Staining, and Sorting.
Spleen and BM cells were stained with saturation concentrations of PE-conjugated anti-CD4 (GK1.5) and/or anti-CD8 (anti–Lyt 2) monoclonal antibodies obtained from CALTAG, Labs. (Burlingame, CA). Cells were counterstained with FITC-conjugated anti–TCR-α/β (H57-597) or anti-Vβ9 (MR10-2) monoclonal antibodies obtained from PharMingen (San Diego, CA). APC-anti-B220 (RA3-6B2) antibodies were obtained from Dr. L.A. Herzenberg (Stanford University, Stanford, CA). The staining procedures, including the use of background controls and two-color flow cytometric analysis and sorting of CD4+, CD8+, or CD4−CD8−α/β+ T cells have been described before (16, 20). In brief, combined CD4+ and CD8+ T cells (>98% purity) were obtained from the spleen of nontransgenic or SP transgenic mice by sorting cells staining positively with an FITC-conjugated monoclonal anti-Thy1.2 (53-2.1) antibody obtained from CALTAG, Labs. Sorting was performed using a FACStar® (Becton Dickinson, Mountain View, CA). CD4−CD8− T cells (>95% purity) were obtained from the spleen or BM of DN transgenic mice by sorting CD4−CD8−Thy1.2+ cells. CD4+ or CD8+ T cells (>98% purity) were obtained from SP transgenic spleen cells by sorting Thy-1.2+CD8− or Thy1.2+CD4− cells. Stained cells were analyzed using a FACScan®. Purified anti-CD1 monoclonal antibodies were obtained from PharMingen (1B1; rat IgG2b) or were made by isolating the IgM fraction of anti-CD1 hybridoma supernatants (3C11) obtained from Dr. C. Terhorst (21) using an E-Z Sep bioreactor IgM size exclusion separator kit (Pharmacia, Uppsala, Sweden) followed by separation on Biogel A–5m beads (BioRad, Hercules, CA). Staining for CD1 on cell lines was performed in two stages. In the first, cells were incubated with rat anti-CD1 monoclonal antibodies (3C11), and in the second, cells were incubated with FITC-conjugated monoclonal anti–rat IgM antibodies obtained from PharMingen. Staining for CD1 on spleen cells used biotinylated anti-CD1 antibodies and PE-streptavidin for counterstaining. Purified rat IgM myeloma protein (IR202) was obtained from Zymed (South San Francisco, CA), and purified rat IgG2b antibody controls were obtained from PharMingen.
Measurement of IgM and IgG Subclasses.
Measurements of IgM, IgG1, IgG2a, IgG2b, IgG3, and total IgG were performed using an ELISA assay with goat anti–mouse IgM plus IgG (H+L chain) antibodies (Southern Biotechnology Associates, Birmingham, AL) to capture mouse IgM and IgG, and alkaline phosphatase– labeled goat antibodies specific for Ig classes and subclasses (Southern Biotechnology Associates) for detection as described elsewhere (22).
In Vitro Proliferative Responses.
The T cell clone, TLI-2.C4, or transgenic mouse spleen cells were plated at 104 cells/well or 105 cells/well, respectively, in 96-well flat-bottomed plastic dishes. Stimulator cells including the CD1-transfected A20 and nontransfected A20 cells, BCL1 tumor cells, and LPS-activated BALB/c spleen cells were irradiated in vitro (with 4,500, 4,500, and 3,000 cGy, respectively) immediately before plating at 5 × 105 cells/well together with the cloned or transgenic T cells. Cells were cultured in RPMI-1640 medium with 10% fetal bovine serum (Hyclone, Logan, UT), 2 mM glutamine, 100 mg/ml penicillin and streptomycin, 25 mM Hepes and 10−5 M 2-mercaptoethanol (complete medium) for 48 h at 37°C in 5% CO2. [3H]thymidine (1 μCi/well) was added, and cells were harvested 24 h later. [3H]thymidine (New England Nuclear, Boston, MA) incorporation was measured in a liquid scintillation counter. In some experiments, monoclonal rat anti-CD1 monoclonal antibodies (3C11) or control rat IgM myeloma protein were added to the tissue culture media at time 0. All assays were performed in triplicate wells with responder or stimulator cells alone or together.
Secretion of Immunoglobulin In Vitro.
Unfractionated or sorted T and/or B cells from the spleen were incubated in 96-well flat-bottomed plastic dishes in complete medium for 5 d at 37°C in 5% CO2. At the end of the culture period, supernatants were harvested and the concentrations of IgM and IgG were measured using the ELISA assay.
Induction and Monitoring of Autoimmune Disease.
2–3-mo old male BALB/c nu/nu mice were given a single dose of 800 cGy whole body irradiation from a 250 Kv x-ray source as described previously (23). BM cells with or without sorted T cells were injected intravenously within 6 h after the irradiation. Urine protein was measured on a 1 to 4+ scale using a colorimetric assay for albumin (Albustix, Miles, Inc., Elkhart, IN). Hosts were considered to have proteinuria if at least two consecutive urine samples were 2+ (100 mg/dl) or greater. Anti–ds DNA antibodies in the serum were measured using two-stage immunofluorescent staining of Crithidia luciliae organisms fixed onto glass slides (ImmunoConcepts, Sacramento, CA). Counterstaining was performed with rabbit anti–mouse IgG antibody conjugated with FITC (DAKO, San Diego, CA). A serum sample was considered positive when staining of the kinetoplasts was observed at a dilution of at least 1:40. Positive samples were confirmed by staining with biotinylated affinity-purified goat anti–mouse IgG antibodies and counterstaining with streptavidin conjugated with FITC (Vector Laboratories, Burlingame, CA). None of 12 serum samples from untreated BALB/c or BALB/c nu/nu mice were positive. Kidney tissues were evaluated by immunofluorescent staining with the rabbit anti–mouse IgG antibody conjugated with FITC using modifications of standard methods (24). Specificity for IgG deposition in glomeruli was confirmed by staining with affinity-purified goat anti–mouse IgG antibodies.
Cytokine Secretion.
Cytokine determinations were made by incubating 105 sorted T cells in 96-well round-bottomed microtiter plates in complete medium with PMA (20 ng/ml) and 1 μM ionomycin, and harvesting supernatants at 48 h. Secretion of IFN-γ, IL-10, and IL-4 was measured by enzyme-linked immunosorbent assay kits (BioSource, Camarillo, CA). IL-2 was measured using the HT-2 cell bioassay with anti–IL-4R blocking antibody (Genzyme, Cambridge, MA). Values show means of triplicate measurements of supernatants.
Results
Vβ9,Vα4.4 Transgenic T Cells That Recognize CD1 Activate B Cells to Secrete Immunoglobulins.
We established two lines of BALB/c mice that expressed the TCR-α and -β transgenes obtained from cloned CD4−CD8− T cells derived from the spleen of a BALB/c mouse (16). In one line of mice (SP) the transgenes (Vβ9-Dβ1.1-Jβ2.1 and Vα4.4-Jα24) were expressed in almost all CD4+ and CD8+ T cells, and in the other line (DN) the transgenes were expressed predominantly in CD4−CD8− T cells (16). Although the two lines of mice appeared healthy during the first year after birth, measurements of the serum immunoglobulin levels of the SP transgenic mice showed that there was an increase in the concentration of IgG as compared to nontransgenic BALB/c mice at age 3 and 6 mo of age (Table 1). However, the increase was not as great as that observed in age- and sex-matched, lupus-prone, NZB/NZW F1 mice at the two time intervals. Serum IgM levels were similar in the transgenic and nontransgenic BALB/c mice, and DN transgenic mice tested at 3 mo had IgG and IgM levels similar to that of SP transgenic mice (data not shown). Hypergammaglobulinemia was associated with an increased spontaneous secretion of IgM and IgG in vitro by the spleen cells from transgenic as compared to nontransgenic BALB/c mice (Table 1). Cells from the latter mice failed to secrete detectable levels of IgG. IgG secretion by NZB/NZW F1 mice was higher than that of the transgenic mice. Despite the increased serum IgG, none of the transgenic mice developed proteinuria or anti–ds DNA antibodies at 6 mo as did the NZB/NZW F1 mice (data not shown).
Table 1.
Concentrations of Serum IgG and Spontaneous Immunoglobulin Secretion by Spleen Cells
| Mice | Serum IgG* | Spontaneous secretion‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 mo | 6 mo | IgM | IgG | |||||
| μg/ml | ng/ml | |||||||
| BALB/c | 506 ± 184 | 1,025 ± 119 | 1,596 ± 132 | 0 ± 0 | ||||
| SPTg BALB/c | 1,684 ± 180 | 3,524 ± 182 | 3,547 ± 652 | 1,054 ± 48 | ||||
| NZB/NZW F1 | 2,792 ± 281 | 4,448 ± 1,187 | 9,394 ± 490 | 3,447 ± 1,279 | ||||
Mean ± SE of four female mice.
Female mice were 6 mo old.
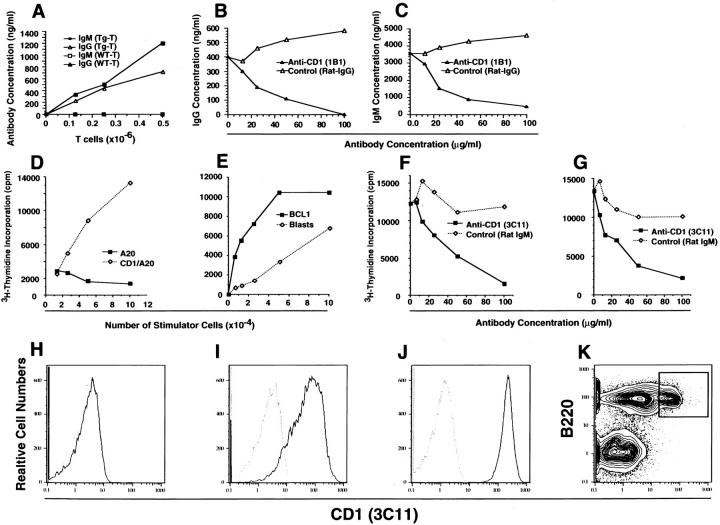
To determine the mechanism of spontaneous immunoglobulin secretion by the SP transgenic spleen cells, graded concentrations of sorted transgenic T (Thy-1+) cells were incubated with a constant number (5 × 105) of sorted nontransgenic B (B220+) cells from the spleen for five d. Fig. 1 A shows that increasing concentrations of IgM and IgG were found in the culture supernatants as the dose of transgenic T cells increased. Substitution of nontransgenic, wild-type T cells for the transgenic T cells failed to generate detectable levels of IgM and IgG. Although addition of anti–class I or II MHC antibodies failed to potently inhibit the secretion of IgM and IgG in the cultures with transgenic T cells (data not shown), the addition of an anti-CD1 monoclonal antibody (1B1; rat IgG2b) inhibited IgM and IgG secretion by >80% (Fig. 1, B and C).
Figure 1.
Mutual activation of Transgenic T cells and nontransgenic B cells. (A), 5 × 105 sorted B (B220+) cells from the nontransgenic BALB/c spleen were incubated with graded numbers of sorted T (Thy1.2+) cells from the SP transgenic (Tg) BALB/c spleen. Cells were cultured for 5 d, and concentrations of IgM and IgG in the culture supernatants were determined. In control experiments, nontransgenic, wild-type (WT), sorted T cells were substituted for transgenic T cells. (B and C) 5 × 105 sorted nontransgenic B cells and 5 × 105 sorted transgenic T cells were incubated for 5 d in the presence of anti-CD1 (1B1) antibody or isotype control (rat IgG2b), and the concentrations of IgM and IgG in culture supernatants were measured. (D) shows [3H]thymidine incorporation of 104 Vβ9, Vα4.4 cloned BALB/c T cells (parent line) after incubation with graded numbers of irradiated (4,000 cGy) CD1 transfected A20 cells or nontransfected A20 cells for 48 h. (E) [3H]thymidine incorporation of SP transgenic spleen cells (105) incubated with graded numbers of irradiated (4,000 cGy) BCL1 cells or BALB/c spleen cells activated with LPS (Blasts). (F and G), Transgenic spleen cells (105) were incubated with irradiated BCL1 cells (105) or LPS-activated spleen cells (105), respectively, in the presence of graded concentrations of anti-CD1 (3C11) antibody or isotype control. (H–J) The one-color flow cytometric analyses of nontransfected A20, CD1 transfected A20, and BCL1 cells, respectively, after staining with anti-CD1 (3Cll) antibody. Solid lines show 3Cll and dotted lines show isotype control. (K) BALB/c spleen cells after staining for B220 versus CD1 receptors. Box encloses B220+CD1hi cells.
Fig. 1 D shows that the BALB/c parent-cloned Vβ9, Vα4.4 T cell line proliferated in response to stimulation in vitro with irradiated CD1-transfected BALB/c B cells (A20), but not to the nontransfected A20 cells. The spleen cells from SP transgenic mice proliferated in response to irradiated LPS-activated BALB/c spleen cells and to another BALB/c B cell line, BCL1 (Fig. 1 E). These proliferative responses were potently inhibited by an anti-CD1 antibody (3C11), but not by control IgM antibody (Fig. 1, F and G). Immunofluorescent staining for CD1 receptors showed that the A20 cells did not express CD1, and the CD1-transfected A20 cells as well as the BCL1 cells expressed high levels (Fig. 1, H–J). A subset of B (B220+) cells in the nontransgenic BALB/c spleen also stained brightly for CD1 receptors, and accounted for ∼13% of nucleated cells (box, Fig. 1 K). The results suggest that the interaction between the transgenic T cells and nontransgenic B cells results in mutual activation via the CD1 receptors and the TCRs.
Injection of Transgenic T Cells into BALB/c nu/nu Mice Induces Lupus.
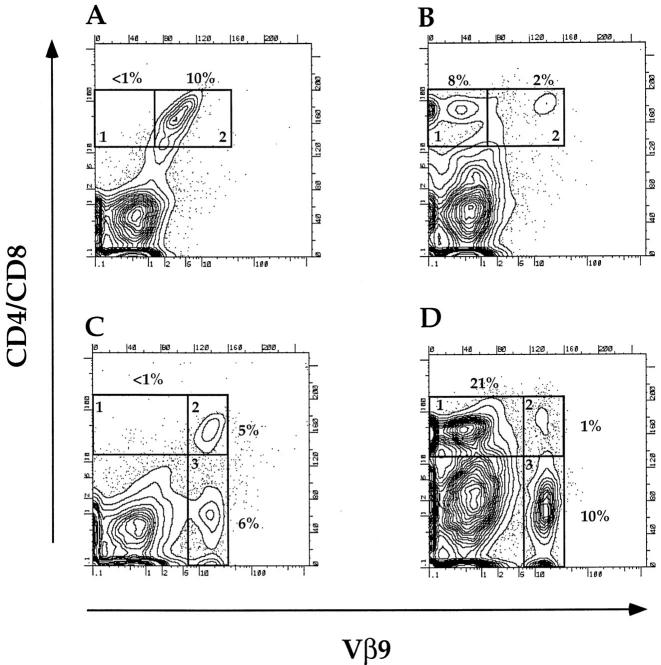
Since the transgenic T cells recognized CD1 on syngeneic B cells and were capable of stimulating IgM and IgG secretion, these T cells were tested for their capacity to induce lupus in adoptive transfer experiments. Cells from the spleen and BM of the SP transgenic mice were injected intravenously into BALB/c euthymic or nu/nu host mice after a single dose of 800 cGy whole body irradiation. As shown in Fig. 2 A, injection of 2.5 × 106 BM cells from SP transgenic mice into nu/nu hosts resulted in the appearance of CD4+ and CD8+ cells in the blood that almost exclusively expressed the Vβ9 transgene (compare boxes 1 and 2). In contrast, the large majority of CD4+ and CD8+ cells in the blood of euthymic hosts given the SP transgenic BM cells did not express the Vβ9 transgene (Fig. 2 B, boxes 1 and 2). The donor or host origin of the nontransgenic CD4+ and CD8+ cells was not determined in the current study.
Figure 2.
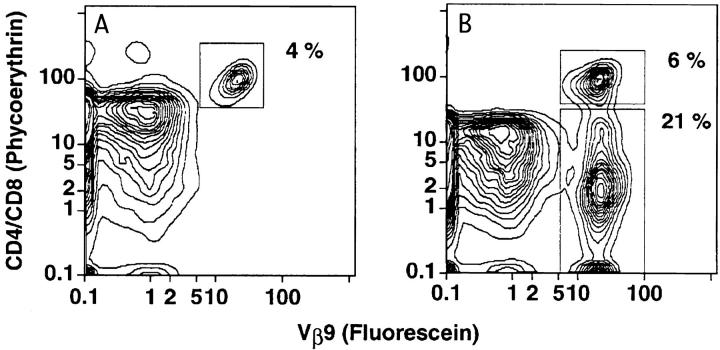
Expression of the Vβ9 transgene on T cells in the blood of irradiated BALB/c athymic or euthymic recipients given transgenic BM cells. (A and B) Two-color flow cytometric analysis of peripheral blood mononuclear cells obtained from irradiated athymic or euthymic recipients, respectively, 4 wk after the injection of 2.5 × 106 SP BM cells. Cells were stained for CD4 and CD8 receptors versus Vβ9 receptors. Boxes 1 and 2 enclose CD4+ and CD8+ cells that were Vβ9− or Vβ9+, respectively. (C and D) Two-color analysis of blood cells from athymic and euthymic recipients, respectively, 4 wk after the injection of 2.5 × 106 DN BM cells. Boxes 1, 2, and 3 enclose CD4+Vβ9− and CD8+Vβ9−, CD4+Vβ9+ and CD8+Vβ9+, and CD4−CD8−Vβ9+ cells, respectively. Panels are representative of groups of four to six recipient mice. Cells in A and B were derived from mice from two groups (athymic and euthymic) in one experiment, and cells in C and D were derived from mice from two groups in another.
Table 2 shows that the injection of 2.5 × 106 BM cells from the SP transgenic mice into 20 irradiated hosts induced ascites in 9, and proteinuria (⩾2+) and serum anti– ds DNA antibodies (titer ⩾1:40) in 15. Proteinuria did not appear until the second month after the cell injection, and all mice that developed ascites in the second or third month died by 100 d (data not shown). Autopsy of six mice with proteinuria and ascites showed that they had immune complex glomerulonephritis as judged by immunofluorescent staining of the glomeruli with anti-IgG–specific monoclonal antibodies (Fig. 3 C). Arrows show the staining of capillary loops. Fig. 3 A shows the staining pattern of the CD4+ and CD8+ Vβ9+ transgenic T cells in the BM of the SP transgenic mice, and the appearance of a recipient with ascites is shown in Fig. 3 E.
Table 2.
Fraction of BALB/c Nude Hosts with Disease Abnormalities*
| Cell source | Ascites | Proteinuria | Anti–ds DNA Antibodies | |||
|---|---|---|---|---|---|---|
| BM − SPTg | 9/20 | 15/20 | 15/20 | |||
| BM − DNTg | 0/12 | 0/12 | 0/12 | |||
| BM − BALB/c | 0/6 | 0/6 | 0/6 | |||
| BM − nude | 0/12 | 0/12 | 0/12 | |||
| BM − SPTg + 0.5 SPTg T cells | 6/6 | 6/6 | 6/6 | |||
| BM − SPTg + 0.5 DNTg T cells | 8/8 | 8/8 | 8/8 | |||
| BM − nude + 0.5 SPTg T cells | 0/8 | 8/8 | 8/8 | |||
| BM − nude + 0.5 DNTg T cells | 0/6 | 4/6 | 4/6 | |||
| BM − nude + 0.5 SPTg CD4+ T cells | 0/6 | 6/6 | 6/6 | |||
| BM − nude + 0.5 SPTg CD4+ T cells | 0/6 | 5/6 | 5/6 | |||
| BM − nude + 0.2 SPTg CD8+ T cells | 0/6 | 3/6 | 3/6 | |||
| BM − nude + 0.5 BALB/c T cells | 0/6 | 0/6 | 0/6 |
All hosts received 2.5 × 106 BM cells intravenously with or without 0.2 or 5 × 105 sorted T cells. BM-SPTg, BM cells from SP transgenic mice; BM-DNTg, BM cells from DN transgenic mice; BM–BALB/c and BM–nude, nontransgenic BM cells.
Figure 3.
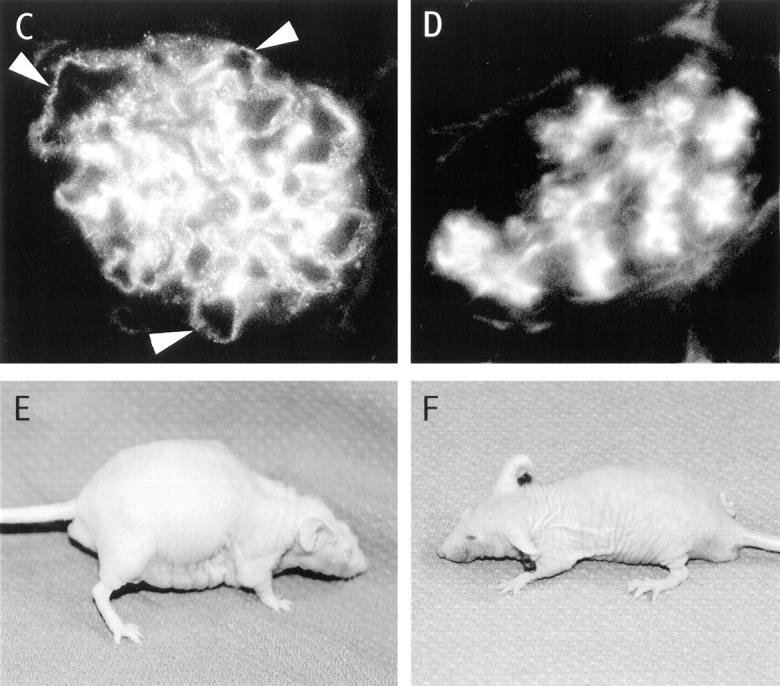
Immunohistopathology of kidneys from BALB/c nu/nu recipients given transgenic BM cells. (A and B) Two-color flow cytometric analysis of enriched T cells from the BM of SP or DN transgenic mice, respectively, stained for CD4 and CD8 receptors versus Vβ9 receptors. Upper boxes enclose CD4+ and CD8+ T cells and lower box encloses CD4−CD8− T cells. T cells were enriched by depleting B220+, Gr-1+, and Mac-1+ cells (16). (C and D) The immunofluorescent staining of histological sections of kidneys from recipients given BM cells from SP or DN transgenic mice, respectively, with FITC-conjugated anti–mouse IgG antibodies. Arrows show staining of capillary loops. E and F show the appearance of recipients given BM cells from SP or DN transgenic mice, respectively. The mouse in E has ascites and generalized edema.
The addition of 5 × 105 sorted T cells (Thy-1+) from the spleen of the SP transgenic mice to the injected BM cells induced ascites, proteinuria, and anti-ds DNA antibodies in six out of six nude hosts (Table 2), and all died by day 75 with an accelerated course of these disease abnormalities. When 2.5 × 106 BM cells from the DN transgenic line were injected into nude hosts, none developed proteinuria, anti–ds DNA antibodies, or ascites (Table 2 and Fig. 3 F). Their kidneys showed deposition of IgG in the mesangium, but not in the capillary loops (Fig. 3 D). Deposition of IgG in the mesangium was observed in control euthymic and athymic nontransgenic BALB/c mice also (data not shown). Staining of the BM cells from DN transgenic mice showed that both CD4+ and CD8+ Vβ9+ T cells as well as CD4−CD8− T cells were present (Fig. 3 B). The injection of 2.5 × 106 BM cells from euthymic or athymic nontransgenic BALB/c mice failed to induce lupus disease abnormalities (Table 2). The injection of BM cells from euthymic SP transgenic mice failed to induce anti–ds DNA antibodies, proteinuria, or ascites in eight of eight irradiated hosts that were euthymic instead of athymic (data not shown in Table 2). The failure to induce lupus was associated with the predominant appearance of nontransgenic CD4+ and CD8+ cells in the blood within 4 wk after the SP BM cell injection (Fig. 2 B). A similar predominance of non–transgenic CD4+ and CD8+ cells was observed after injection of DN transgenic BM cells into euthymic hosts (Fig. 2 D). However, when the DN BM cells were injected into athymic hosts, almost all CD4+ and CD8+ cells in the blood expressed the Vβ9 transgene as shown in Fig. 2 C. The percentages of CD4−CD8−Vβ9+ cells in the blood of both athymic and euthymic mice were similar (Fig. 2 C and D, box 3).
Injection of 5 × 105 sorted T cells from the SP transgenic spleen along with 2.5 × 106 BM cells from nontransgenic nude mice induced proteinuria and serum anti–ds DNA antibodies in eight out of eight nude hosts, but failed to induce ascites. Although BM cells from the DN transgenic mice did not induce lupus abnormalities, the addition of 5 × 105 sorted CD4−CD8− T cells from the spleen of DN transgenic mice to 2.5 × 106 BM cells from nontransgenic nude mice induced proteinuria and anti–ds DNA antibodies without ascites in four out of six nude hosts (Table 2). The sorted CD4−CD8− T cells from the DN transgenic spleen induced accelerated disease after addition to BM cells from SP transgenic mice, and eight out of eight hosts died with ascites by 75 d (Table 2).
In further experiments, sorted CD4+ or CD8+ T cells (2 or 5 × 105) from the SP transgenic spleen were tested for the ability to induce lupus abnormalities. Addition of either T cell subset to BM cells from nontransgenic nude mice induced proteinuria and anti–ds DNA antibodies in at least half of the irradiated recipients (Table 2). The CD4+ T cells appeared to be more effective on a per cell basis, since a higher proportion of hosts developed lupus abnormalities with 2 × 105 CD4+ T cells as compared to 5 × 105 CD8+ T cells. Control hosts given nontransgenic nude BM cells and sorted T cells from the spleen of nontransgenic BALB/c mice failed to show any lupus abnormalities (Table 2).
CD4−CD8− T Cells from the Transgenic Marrow Prevent Lupus.
Mixing experiments were performed to determine whether whole BM cells from DN transgenic mice affect the ability of BM cells from SP transgenic mice to induce lupus abnormalities. Hosts given a mixture of 2.5 × 106 BM cells from each source were compared to those given only SP transgenic BM cells in Table 3. Although 15 out of 20 hosts given SP transgenic BM cells alone developed proteinuria and anti–ds DNA antibodies, only two out of eight hosts given the combination of cells developed these abnormalities (P <.01; chi square test). The inhibitory activity of the DN transgenic BM cells was related to the presence of the transgenes, since substituting 2.5 × 106 BM cells from nontransgenic BALB/c mice failed to inhibit the induction of lupus abnormalities by the SP transgenic BM cells (Table 3; P >0.05).
Table 3.
Fraction of Hosts with Disease Abnormalities
| Cell source | Ascites | Pro- teinuria | Anti–ds DNA antibodies | |||
|---|---|---|---|---|---|---|
| BM − SPTg | 9/20 | 15/20 | 15/20 | |||
| BM − SPTg + BM − DNTg | 2/8 | 2/8 | 2/8 | |||
| BM − SPTg + BM − BALB/c | 3/5 | 4/5 | 4/5 | |||
| BM − SPTg + 0.25 DNTg T cells (BM) | 0/8 | 0/8 | 2/8 | |||
| BM − SPTg + 0.5 DNTg T cells (SPL) | 8/8 | 8/8 | 8/8 | |||
| BM − nude + 0.5 DNTg T cells (SPL) | 0/6 | 4/8 | 4/8 |
The inhibitory activity of the CD4−CD8− T cells in the DN transgenic marrow was tested by isolating the latter cells by flow cytometry and adding them to 2.5 × 106 unfractionated BM cells from SP transgenic mice. The CD4−CD8− T cells (2.5 × 105) were highly effective in preventing lupus abnormalities since none of the eight hosts given the combination of cells developed ascites or proteinuria and only two developed anti–ds DNA antibodies (Table 3). It is of interest that the addition of sorted CD4−CD8− T cells from the spleen (SPL) of DN transgenic mice to the BM cells from the SP transgenic mice augmented the disease abnormalities induced by the SP BM cells (eight out of eight hosts developed ascites), whereas addition of the sorted CD4−CD8− T cells from the DN marrow markedly inhibited the disease (Table 3).
Cytokine Profile of Transgenic T Cells That Induce or Prevent Lupus.
Since some subsets of transgenic T cells induced and some prevented lupus in the adoptive hosts, the cytokine profiles of the purified T cell subsets from the spleen and BM were studied to determine whether different patterns were associated with the different functions. Sorted CD4+ and/or CD8+ T cells from the spleen of the SP transgenic mice as well as sorted CD4−CD8− T cells from the spleen and BM of the DN transgenic mice were incubated in vitro for 48 h with ionomycin and PMA added to the tissue culture medium. The supernatants were harvested thereafter, and assayed for the concentration of IL-2, IL-4, IL-10, and IFN-γ. The T cell subsets that induced lupus (CD4+, CD8+, CD4+ and CD8+, CD4−CD8− T cells from the spleen) showed a Th1-like cytokine secretion pattern with high levels of IFN-γ and IL-2 and relatively low levels of IL-4 (Table 4). The concentration of IFN-γ in the supernatants from these inducing T cell subsets varied from 5–64-fold greater than that of IL-4. On the other hand, the sorted CD4−CD8− T cells from the BM showed a Th2-like cytokine secretion pattern with high levels of IL-4 and relatively low levels of IFN-γ and IL-2. The secretion of IL-4 was about sixfold higher than that of IFN-γ. The level of secretion of IL-10 was not associated with the capacity of the T cell subsets to induce or prevent disease. It is of interest that CD4−CD8− BM T cells that prevented disease did not secrete IL-10. In addition, the secretion of IL-10 did not follow a classical Th1 or Th2 pattern (25, 26). Some inducing T cell subsets that secreted large amounts of IFN-γ also secreted large amounts of IL-10 (CD4+ T cells), and other T cell subsets that secreted predominantly IL-4 (CD4− CD8− marrow T cells) secreted no IL-10.
Table 4.
Cytokine Secretion by Subsets of Transgenic T Cells
| Transgenic T cell source | IL-10* | IL-2 | IL-4 | IFN-γ | IFN-γ/IL-4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pg/ml | IU/ml | pg/ml | pg/ml | ratio | ||||||
| SP spleen − CD4+ and CD8+ T cells | 49 ± 6 | 285 ± 17 | 24 ± 1 | 439 ± 11 | 18 | |||||
| SP spleen − CD4+ T cells | 560 ± 5 | 537 ± 15 | 126 ± 6 | 614 ± 27 | 5 | |||||
| SP spleen − CD8+ T cells | 117 ± 38 | 339 ± 4 | 9 ± 1 | 547 ± 61 | 64 | |||||
| DN spleen − CD4−CD8− T cells | 0 ± 0 | 755 ± 25 | 9 ± 9 | 254 ± 2 | 30 | |||||
| DN BM − CD4−CD8− T cells | 0 ± 0 | 2 ± 1 | 506 ± 43 | 80 ± 4 | 0.16 |
Mean and standard errors of four determinations.
Serum Levels of Immunoglobulins in BALB/c nu/nu Recipients.
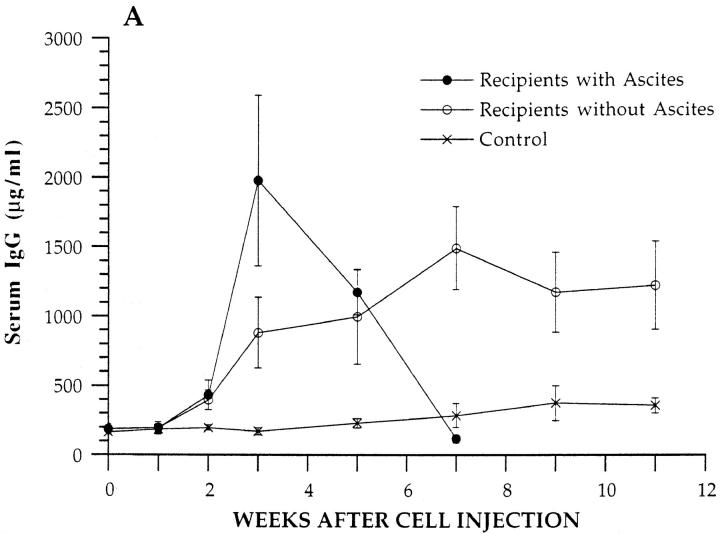
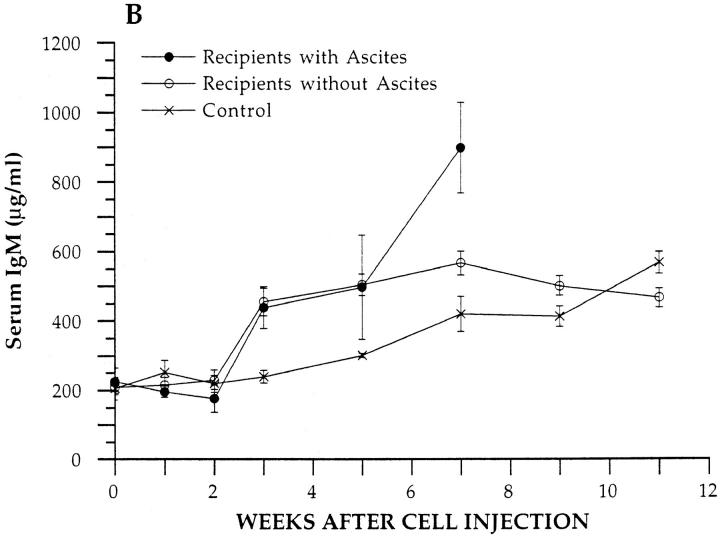
Since levels of serum IgM and IgG are elevated in mice with hereditary lupus (1–3) and in the transgenic donor mice, the kinetics of changes in serum IgM and IgG concentrations were determined in BALB/c nu/nu recipients given unfractionated BM from SP transgenic mice or from control nontransgenic BALB/c nu/nu mice. Fig. 4 A shows the mean levels of serum IgG at different time points in the transgenic cell recipients that developed ascites and in those that did not. Levels in ascitic recipients are shown for only 7 wk due to deaths thereafter. Recipients that developed ascites showed a rapid rise of ∼10-fold in serum IgG as compared to baseline during the first 3 wk after cell injection, and the peak level was ∼2,000 μg/ml. The peak level was statistically significantly different from baseline (P <0.001), as judged by the comparison of independent means using the two-tailed Students t test. Thereafter, the levels fell rapidly and returned to baseline or below at 7 wk. Recipients that did not develop ascites showed a progressive slower rise in serum IgG, and the plateau level of ∼1,500 μg/ml observed at 7 wk was significantly increased as compared to baseline (P <0.001). Control recipients showed a slight rise in serum IgG that was not statistically significant (P >0.05) at the peak. At 3 wk, IgG levels in both groups of experimental recipients were significantly increased as compared to control levels (P <0.001), and the experimental group with ascites was significantly increased as compared to the experimental group without ascites (P <0.01). Levels of serum IgM were statistically significantly increased (P <0.01) at weeks three, five, and seven in transgenic T cell recipients as compared to controls, but experimental and control levels converged by 9 wk (Fig. 4 B).
Figure 4.
Serum IgM and IgG concentrations in BALB/c nu/ nu recipients. Concentrations of serum IgM and IgG were measured at serial time points in experimental recipients given an injection of 2.5 × 106 BM cells from SP transgenic mice or in control recipients given 2.5 × 106 BM cells from nontransgenic BALB/c nu/nu mice. Each point represents the mean of four experimental mice that developed ascites, six experimental mice that did not develop ascites, or four control mice. Bars show standard errors of the means. A and B show IgG and IgM levels, respectively.
The levels of serum IgG subclasses were measured at the peak IgG time points in the experimental and control recipients. Table 5 shows that experimental recipients with ascites had about a 5-fold increase in IgG1 and a 13-fold increase in IgG2a, as compared to control recipients (P <0.001). Levels of IgG2b and IgG3 were not significantly different (P >0.05). Experimental recipients without ascites had about a 4-fold increase in IgG1, and a 5-fold increase in IgG2a, as compared to controls (P <0.001). IgG2b levels were significantly increased also in these recipients (P <0.05), but IgG3 levels were not (P >0.05). Peak total IgG levels (measured independently with an anti-IgG–specific antibody, that was not specific for subclasses) were not statistically significantly different between the experimental groups with and without ascites (P >0.05), but the IgG2a level in mice with ascites was significantly increased (P <0.05) as compared to experimental mice without ascites. Recipients given a combination of BM cells from SP transgenic mice and DN T cells from the BM of DN transgenic mice showed surprisingly high levels of serum IgG1 (mean: 3,016 μg/ml), which were about 4-fold increased as compared to recipients with ascites given only BM cells from SP transgenic donors (P <0.05; Table 5). Recipients given the combination of cells had significant elevations of IgG2a as compared to controls (P <0.05), but IgG2a levels were significantly reduced as compared to recipients given only SP BM cells (P <0.05). All recipients given only SP BM cells shown in Table 5 had elevated levels (⩾1:40) of anti– ds DNA antibodies and proteinuria, but none of the recipients in Table 5 given the combination of cells had elevated anti–ds DNA antibodies or proteinuria. The serum levels of IgG subclasses in unirradiated BALB/c nu/nu mice are shown for comparison.
Table 5.
Peak Serum IgG Subclass Concentrations in BALB/c nu/nu Recipients
| Donor cells* | IgG1‡ | IgG2a | IgG2b | IgG3 | Togal IgG | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| μg/ml | μg/ml | μg/ml | μg/ml | μg/ml | ||||||
| BM − SPTg (ascites) (n = 4) | 747 ± 90 | 671 ± 131 | 84 ± 15 | 165 ± 20 | 1,920 ± 635 | |||||
| BM − SPTg (no ascites) (n = 6) | 570 ± 176 | 272 ± 25 | 216 ± 66 | 133 ± 12 | 1,525 ± 201 | |||||
| BM − SPTg + DN Tg T cells (BM) (n = 4) | 3,016 ± 1,028 | 185 ± 43 | 141 ± 60 | 128 ± 30 | 3,420 ± 619 | |||||
| BM − nude (n = 4) | 157 ± 28 | 51 ± 20 | 123 ± 9 | 125 ± 14 | 357 ± 54 | |||||
| Control nude§ (n = 6) | 99 ± 21 | 16 ± 2 | 79 ± 10 | 171 ± 16 | 231 ± 31 |
Recipients were given 2.5 × 106 BM cells with or without 2.5 × 105 T cells.
Mean ± SE.
Unirradiated BALB/c nu/nu mice.
Discussion
The genes that encode the polymorphic MHC antigens have been shown to play an important role in hereditary lupus of NZB/NZW mice, since the H-2z haplotype of the NZW parent line contributes to disease susceptibility (27). The mechanism of this contribution is presumably via T cell recognition of pathogenic self-peptides presented by the polymorphic MHC molecules. However, pathogenic autoantibodies such as cationic anti–ds DNA IgG antibodies are directed to nonprotein antigens. T cells that recognize peptides derived from nucleosomes or from anti-DNA antibodies have been postulated to augment the secretion of these anti-DNA antibodies (9, 10). An alternative pathway of T cell–induced polyclonal activation of B cells and/or help for the secretion of autoantibodies to nonprotein antigens (i.e., nucleotides, phospholipids, and phosphodiesters) in lupus is via T cell recognition of the nonpolymorphic MHC class I–like, CD1 molecule (12–15). Some T cells (NK1.1+ T cells) in mice have been reported to recognize CD1 itself, and T cell clones have been reported to recognize glycolipid antigens in association with CD1 (12–15).
In this study, T cells with Vα4.4 and Vβ9 TCR-α and -β transgenes stimulated nontransgenic B cells to secrete IgM and IgG in vitro via engagement of the CD1 receptors on the B cells. The TCR transgenic mice developed hypergammaglobulinemia, and their spleen cells spontaneously secreted IgM and IgG in vitro. Nevertheless, the transgenic mice did not develop overt lupus, and had no detectable anti–ds DNA antibodies or proteinuria. The anti-CD1 transgenic T cells differed from most NK1.1+ T cells that recognize CD1, since the latter express an invariant Vα14-Jα281 gene that is associated with Vβ2, Vβ7, or Vβ8 (13, 28). Transgenic mice that express the invariant Vα14 gene have an increased percentage of NK1.1+ T cells in the lymphoid tissues, and the transgenic T cells secrete high levels of IL-4, as do NK1.1+ T cells (29). The SP and DN lines of transgenic mice that express the anti-CD1 Vα4.4 and Vβ9 combination contained a heterogeneity of transgenic T cell subsets including CD4+, CD8+, and CD4−CD8− phenotypes (16). Stimulation of the sorted subsets in vitro with PMA and ionomycin showed a heterogeneity of cytokine secretion patterns, none of which were classical Th1 or Th2 patterns. Sorted CD4+, CD8+, and CD4−CD8− transgenic T cells from the spleen secreted large amounts of IFN-γ and IL-2, and small amounts of IL-4. In that respect, transgenic cells were similar to Th1 T cells. However, unlike Th1 cells, the transgenic CD4+ T cells secreted large amounts of IL-10.
Although the splenic CD4−CD8− transgenic T cells secreted large amounts of IFN-γ and little IL-4, the CD4− CD8− transgenic T cells in the bone marrow showed the opposite pattern with little IFN-γ and large amounts of IL-4. The marrow T cells were not typical of Th2s, since they secreted no IL-10. The CD4−CD8− T cells in the spleen and marrow shared the same DN phenotype and the same TCR transgenes, but differed in their cytokine secretion pattern. Previous studies suggested that the splenic DN T cells were derived from a thymus-derived maturation pathway, whereas those in the marrow were derived from an extrathymic maturation pathway (16). This may account for the differences in their cytokine secretion pattern.
The injection of SP BM cells containing CD4+ and CD8+ transgenic T cells into the irradiated BALB/c nude hosts induced overt lupus with anti–ds DNA antibodies, increased serum levels of IgG, immune complex glomerulonephritis, proteinuria, and ascites. Injection of BM cells from nontransgenic athymic or euthymic BALB/c mice failed to induce any lupus abnormalities. Addition of sorted CD4+, CD8+, or CD4−CD8− transgenic T cells from the spleen to nontransgenic nude BM cells induced anti–ds DNA antibodies and proteinuria in most recipients without ascites. The most severely affected hosts were those given a combination of SP transgenic BM cells containing transgenic CD4+ and CD8+ T cells, and sorted CD4+ and CD8+ T cells from the SP transgenic spleen or sorted CD4−CD8− T cells from the DN transgenic spleen. All of these hosts developed ascites, and died by 75 d. Both CD4+ and CD4−CD8− T cells from the spleen of mice with hereditary lupus have been reported to augment the secretion of anti–ds DNA antibodies in vitro (6).
Although the transgenic T cells induced overt lupus in adoptive nude hosts, the transgenic donor mice did not develop lupus nor did adoptive euthymic hosts. In the case of the transgenic donors, thymus-dependent regulatory cells expressing endogenous TCR genes may inhibit the development of lupus. In the case of euthymic hosts, nontransgenic T cells of either host or donor origin were predominant. In contrast, almost all T cells in athymic hosts expressed the Vβ9 transgene and developed lupus. Previous studies have shown that irradiated thymectomized hosts given a combination of nontransgenic BM cells and TCR transgenic T cells show a marked expansion of the transgenic T cells when the transgenic TCR ligand (antigen) is coinjected (30). However, the antigen-induced expansion of the transgenic T cells is markedly inhibited by the presence of the thymus in the adoptive hosts, and the outgrowth of thymus-dependent nontransgenic T cells competes effectively with the transgenic T cells. Thus, the presence of the thymus in the current model may inhibit the expansion of T cells expressing transgenic TCR-α or -β chain genes that recognize CD1, and favor those that express endogenous α or β chain genes.
BM cells containing SP transgenic CD4+ and CD8+ T cells induced lupus in most nude recipients, but BM cells from DN transgenic mice containing transgenic CD4+, CD8+, and CD4−CD8− T cells failed to induce lupus in any recipients. Mixing experiments showed that the latter BM cells ameliorated lupus disease abnormalities induced by SP transgenic BM cells. Sorted transgenic CD4−CD8− T cells derived from the DN BM were very effective in preventing disease induced by SP BM cells, and none of the hosts given a combination of these cells developed proteinuria during the 100-d observation period.
BALB/c nude hosts given BM from SP transgenic donors had levels of serum IgG that were ∼10-fold higher than those of hosts given BM from nontransgenic BALB/c nude donors. Experimental recipients had significant increases in both IgG1 and IgG2a subclasses, and those with ascites had the highest levels of IgG2a. The latter mice showed a rapid decline in serum IgG levels after a transient peak, just as mice with hereditary lupus show a decline after the development of ascites (3). This may be due to loss of IgG in the urine and intestines, and to the effects of systemic illness on IgG synthesis. Although BM from DN transgenic mice failed to induce lupus and sorted CD4− CD8− T cells from the DN BM cells protected in mixing experiments, the combination of SP BM cells and protective CD4−CD8− T cells induced significant elevations in serum IgG1 and significant reductions of serum IgG2a as compared to recipients given SP BM alone. Recipients given the combination of cells had little anti–ds DNA antibodies or proteinuria. Thus, severity of disease is associated with the development of anti–ds DNA antibodies and with elevated serum IgG2a as has been observed in hereditary lupus (5, 31).
The ability of the different transgenic T cell subsets to induce or prevent lupus was correlated with their cytokine secretion pattern, and as in other autoimmune diseases mediated by T cells, the ratio of secretion of IFN-γ to IL-4 was important (32–35). In particular, T cells that induced disease had a high ratio of IFN-γ to IL-4 secretion, and those that prevented disease had a high ratio of IL-4 to IFN-γ secretion (33, 35). It is likely that the latter ratio contributed to the very high IgG1 levels and reduced IgG2a levels in recipients given suppressive cells, and may have contributed to the inhibition of anti–ds DNA antibody secretion. In experimental allergic encephalomyelitis, IL-4 has been shown to play a direct role in ameliorating the disease (36) and, in NOD mice, introduction of an IL-4 transgene regulated by the insulin promoter renders the animals resistant to the development of disease (37). In addition, introduction of an IL-4 transgene into the (NZW × C57BL/6. Yaa)F1 mice prevents the development of lupus in this strain (38). It is not clear how IL-4 plays a role in ameliorating an antibody-mediated disease such as lupus. One possibility is that IL-4 regulates isotype switching so that pathogenic IgG2a anti–ds DNA antibodies like those found in hereditary lupus (22, 38) are reduced.
In the case of hereditary murine lupus, administration of IL-10 worsens the disease and administration of anti–IL-10 antibodies ameliorates the disease (39). The effects of anti– IL-10 antibodies may be related to the regulation of TNF-α secretion since endogenous secretion of TNF-α is increased in lupus after injection of the antibodies (39). Amelioration of disease by anti–IL-10 antibodies can be blocked by injection of anti–TNF-α antibodies (39). Administration of IFN-γ worsens lupus, and injection of anti–IFN-γ antibodies ameliorates the disease (40, 41). Thus, IFN-γ and IL-10 on one hand, and TNF-α on the other, play opposing roles in regulating the disease. It is not surprising that T cells that secrete high levels of IFN-γ and IL-10 and low levels of IL-4 such as the transgenic anti-CD1 CD4+ T cells may induce or worsen lupus after activation of B cells via their CD1 receptors. On the other hand, the transgenic BM CD4−CD8− T cells that secrete high levels of IL-4 and low levels of IFN-γ and no IL-10 would have been predicted to ameliorate disease based on their cytokine secretion pattern. CD4−CD8− T cells from the BM of nontransgenic mice, as well as cloned “natural suppressor” CD4−CD8− T cells (including those that express the transgenic Vβ9, Vα4.4 receptors) have been reported to ameliorate acute lethal graft versus host disease (17, 42, 43).
One hypothesis that explains the current experimental results is that the transgenic TCRs engage/cross-link CD1 itself or CD1 associated with an endogenous lipid or nucleotide fragments on the surface of B cells, and activate the latter cells to secrete IgG antibodies including pathogenic anti–ds DNA antibodies that cause glomerulonephritis. The engagement of the TCRs activates the transgenic T cells to proliferate and secrete cytokines using previously described pathways involving CD40–CD40 ligand, and B7-1/B7-2-CD28 interactions (44, 45). The cytokine secretion pattern of the T cells plays a critical role in regulating the B cell activation even when the TCR of the T cell subsets and the CD4 and CD8 receptor expression are identical. The target B cells that express high levels of CD1 appear to be a distinct subset in the spleen as judged by their immunofluorescent staining pattern. The characteristics of this subset, and the role of this B cell subset and anti-CD1 T cells in hereditary or spontaneous lupus remain to be elucidated. It is of interest that MRL/lpr mice lacking CD1 receptors due to a disruption of the gene encoding β2 microglobulin do not develop hereditary lupus and that lupus induced by the injection of anti–ds DNA idiotype protein is prevented by disruption of the β2 microglobulin gene (46, 47). In addition, MRL/gld/gld, and NZB/NZW F1 mice lose a subset of T cells (NK1.1+Vα14) that recognizes CD1 and secretes high levels of IL-4 just before lupus develops (48). Anti-Vα14 monoclonal antibodies injected into MRL/lpr mice exacerbates the development of lupus, and depletes this T cell subset (48). The latter subset shows two characteristics (recognition of CD1 and high level secretion of IL-4) with the CD4−CD8− T cell subset in the marrow that prevented lupus in this study.
Footnotes
This work was supported by a grant from The American Lupus Society, a research grant from the Arthritis Foundation, and a National Institutes of Health grant AI-40093. D. Zeng was supported in part by a fellowship from Activated Cell Therapy, Inc. We thank Drs. G. Rolink and B. Kotzin for advice concerning measurement of serum immunoglobulins, A. Mukhopadhyay for technical assistance, and V. Cleaver for preparation of the manuscript.
Address correspondence to Samuel Strober, Division of Immunology and Rheumatology, Rm. S105B, Stanford University School of Medicine, 300 Pasteur Dr., Stanford, CA 94305-5111. Phone: 650-723-6500; Fax: 650-725-6104; E-mail: sstrober@leland.stanford.edu
Abbreviations used in this paper: BCL, B cell lymphoma; BM, bone marrow; cGy, centiGray; DN, double-negative; ds, double-stranded; SP, single-positive.
References
- 1.Theofilopoulous AN, Kofler R, Singer PA, Dixon FJ. Molecular genetics of murine lupus models. Adv Immunol. 1989;46:61–109. doi: 10.1016/s0065-2776(08)60651-3. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida S, Castles JJ, Gershwin ME. The pathogenesis of autoimmunity in New Zealand mice. Semin Arthritis Rheum. 1990;19:224–242. doi: 10.1016/0049-0172(90)90002-w. [DOI] [PubMed] [Google Scholar]
- 3.Hahn, B.H. 1993. Animal models of systemic lupus erythematosus. In Dubois Lupus Erythematosus. 4th ed. D.J. Wallace and B.H. Hahn, editors. Philadelphia, PA: Lea and Febiger, Philadelphia. 157–177.
- 4.Tsao BP, Ebling FM, Roman C, Panosian-Sahadian N, Calame K, Hahn BH. Structural characteristics of the variable regions of immunoglobulin genes encoding a pathogenic autoantibody in murine lupus. J Clin Invest. 1990;85:530–540. doi: 10.1172/JCI114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Keefe TL, Datta SK, Imanishi-Kari T. Cationic residues in pathogenic anti-DNA autoantibodies arise by mutations of a germline gene that belongs to a large VH gene subfamily. Eur J Immunol. 1992;22:619–624. doi: 10.1002/eji.1830220302. [DOI] [PubMed] [Google Scholar]
- 6.Datta SK, Patel H, Berry D. Induction of a cationic shift in IgG anti-DNA autoantibodies. Role of T helper cells with classical and novel phenotypes in three murine models of lupus nephritis. J Exp Med. 1987;165:1252–1268. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ando DG, Sercarz EE, Hahn BH. Mechanisms of T and B cell collaboration to the in vitro production of anti-DNA antibodies in the NZB/NZW F1murine SLE model. J Immunol. 1987;138:3185–3190. [PubMed] [Google Scholar]
- 8.Hardin JA, Thomas JO. Antibodies to histones in systemic lupus erythematosus. Localization of prominent autoantigens on histone H1 and H2B. Proc Natl Acad Sci USA. 1983;80:7410–7414. doi: 10.1073/pnas.80.24.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebling FM, Tsao BP, Singh RR, Sercarz EE, Hahn BH. A peptide derived from an autoantibody can stimulate T cells in the (NZB/NZW) F1 mouse model of systemic lupus erythematosus. Arthritis Rheum. 1993;36:355–364. doi: 10.1002/art.1780360311. [DOI] [PubMed] [Google Scholar]
- 11.Singh RR, Kumar V, Ebling FM, Southwood S, Sette A, Sercarz EE, Hahn BH. T cell determinants from autoantibodies to DNA can upregulate autoimmunity in murine systemic lupus erythematosus. J Exp Med. 1995;181:2017–2027. doi: 10.1084/jem.181.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkeiwicz RR. CD1 recognition by mouse NK1+T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald HR. NK1.1+ T cell receptor-αβ+cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckman EM, Procelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted αβ+T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 15.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 16.Cheng L, Dejbakhsh-Jones S, Liblau R, Zeng D, Strober S. Different patterns of TCR transgene expression in single-positive and double-negative T cells. J Immunol. 1996;156:3591–3601. [PubMed] [Google Scholar]
- 17.Strober S, Dejbakhsh-Jones S, Van Vlasselaer P, Duwe G, Salimi S, Allison J P. Cloned natural suppressor cell lines express the CD3+CD4−CD8−surface phenotype and the α, β heterodimer of the T cell antigen receptor. J Immunol. 1989;143:1118–1122. [PubMed] [Google Scholar]
- 18.Gronowicz ES, Doss CA, Howard FD, Morrison DS, Strober S. An in vitro line of the B cell tumor BCL1can be activated by LPS to secrete IgM. J Immunol. 1980;125:976–980. [PubMed] [Google Scholar]
- 19.Kim KJ, Nero GB, Laskov R, Merwin RM, Logan WJ, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 20.Dejbakhsh-Jones S, Okazaki H, Strober S. Similar rates of production of T and B lymphocytes in the bone marrow. J Exp Med. 1995;181:2201–2211. doi: 10.1084/jem.181.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990;250:679–682. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- 22.Reininger L, Radaszkiewicz T, Kosco M, Melchers F, Rolink AG. Development of autoimmune disease in SCID mice populated with long-term in vitro proliferating (NZB × NZW) F1pre-B mice. J Exp Med. 1992;176:1343–1353. doi: 10.1084/jem.176.5.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palathumpat V, Holm B, Dejbakhsh-Jones S, Strober S. Treatment of BCL1leukemia by transplantation of low density fractions of allogeneic bone marrow and spleen cells. J Immunol. 1992;148:3319–3326. [PubMed] [Google Scholar]
- 24.Striker, L.J., J.L. Olson, and Gary E. Striker. 1990. Handling and preparation of specimens. In The Renal Biopsy. J.L. Bennington, consulting editor. W.B. Saunders Company, Philadelphia. 39–49.
- 25.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 26.Powrie F, Coffman RL. Cytokine regulation of T cell function: potential for therapeutic intervention. Immunol Today. 1993;14:270–274. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- 27.Drake CG, Rozzo SJ, Vyse TJ, Palmer E, Kotzin BL. Genetic contributions to lupus-like disease in (NZB × NZW) F1 mice. Immunol Rev. 1995;144:51–74. doi: 10.1111/j.1600-065x.1995.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 28.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of MHC class I–specific CD4+ and CD4−CD8−T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bendelac A, Hunziker RD, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med. 1996;184:1285–1293. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 31.Slack JH, Hang L, Barkley J, Fulton RJ, D'Hoostelaere L, Robinson A, Dixon FJ. Isotypes of spontaneous and mitogen-induced autoantibodies in SLE-prone mice. J Immunol. 1984;132:1271–1275. [PubMed] [Google Scholar]
- 32.Racke MK, Dhib-Jalbut S, Cannella B, Albert PS, Raine CS, McFarlin DE. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transferring growth factor beta–1. J Immunol. 1991;146:3012–3017. [PubMed] [Google Scholar]
- 33.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 34.Inobe JI, Chen Y, Weiner HL. In vivo administration of IL-4 induces TGF-beta–producing cells and protects animals from experimental autoimmune encephalamyelitis. Ann NY Acad Sci. 1996;778:390–392. doi: 10.1111/j.1749-6632.1996.tb21153.x. [DOI] [PubMed] [Google Scholar]
- 35.Steinman L. A few autoreactive cells in an autoimmune infiltrate control a vast population of nonspecific cells: a tale of smart bombs and infantry. Proc Natl Acad Sci USA. 1996;93:2253–2256. doi: 10.1073/pnas.93.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw MK, Lorens JB, Dhawan A, Dalcanto R, Tse HY, Tran AB, Bonpane C, Eswaran SL, Brocke S, Sarvetnick N. Local delivery of interleukin-4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:1711–1714. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in non-obese (NOD) mice. J Exp Med. 1996;184:1093–1099. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santiago ML, Fossati L, Jacquet C, Muller W, Izui S, Reininger L. Interleukin-4 protects against a genetically linked lupus-like autoimmune syndrome. J Exp Med. 1997;185:65–70. doi: 10.1084/jem.185.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1mice. J Exp Med. 1994;179:305–310. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engleman EG, Sonnenfeld G, Dauphinee M, Greenspan JS, Talal N, McDevitt HO, Merigan TC. Treatment of NZB/NZW F1 hybrid mice with Mycobacterium bovisstrain BCG or type II interferon preparations accelerates autoimmune disease. Arthritis Rheum. 1981;24:1396–1402. doi: 10.1002/art.1780241110. [DOI] [PubMed] [Google Scholar]
- 41.Jacob CH, van der Meide PH, McDevitt HO. In vivo treatment of (NZB × NZW) F1lupus-like nephritis with monoclonal antibody to γ interferon. J Exp Med. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strober S, Palathumpat V, Schwadron R, Hertel-Wulff B. Cloned natural suppressor cells prevent lethal graft versus host disease. J Immunol. 1987;138:699–703. [PubMed] [Google Scholar]
- 43.Palathumpat V S, Dejbakhsh-Jones, Holm B, Strober S. Different subsets of T cells in the adult mouse bone marrow and spleen induce or suppress acute graft versus host disease. J Immunol. 1992;149:808–817. [PubMed] [Google Scholar]
- 44.Grewal IS, Flavell RA. The role of CD40 ligand in co-stimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Gool SW, Vandenberghe P, de Boer M, Ceuppens JL. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev. 1996;153:47–83. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 46.Christianson GJ, Blankenburg RL, Duffy TM, Panka D, Roths JB, Marshak-Rothstein A, Roopenian DC. β2-microglobulin-dependence of the lupus-like autoimmune syndrome of MLR-lprmice. J Immunol. 1996;156:4932–4939. [PubMed] [Google Scholar]
- 47.Mozes E, Kohn LD, Hakim F, Singer DS. Resistance of MHC class I–deficient mice to experimental systemic lupus erythematosus. Science. 1993;261:91–93. doi: 10.1126/science.8316860. [DOI] [PubMed] [Google Scholar]
- 48.Mieza MA, Itoh T, Cui JQ, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, et al. Selective reduction of Vα14+NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]