Abstract
T cell receptors on CD4+ lymphocytes recognize antigen-derived peptides presented by major histocompatibility complex (MHC) class II molecules. A very limited set of peptides among those that may potentially bind MHC class II is actually presented to T lymphocytes. We here examine the role of two receptors mediating antigen internalization by antigen presenting cells, type IIb2 and type III receptors for IgG (FcγRIIb2 and FcγRIII, respectively), in the selection of peptides for presentation to T lymphocytes. B lymphoma cells expressing recombinant FcγRIIb2 or FcγRIII were used to assess the presentation of several epitopes from two different antigens. 4 out of the 11 epitopes tested were efficiently presented after antigen internalization through FcγRIIb2 and FcγRIII. In contrast, the 7 other epitopes were efficiently presented only when antigens were internalized through FcγRIII, but not through FcγRIIb2. The capacity to present these latter epitopes was transferred to a tail-less FcγRIIb2 by addition of the FcγRIII-associated γ chain cytoplasmic tail. Mutation of a single leucine residue at position 35 of the γ chain cytoplasmic tail resulted in the selective loss of presentation of these epitopes. Therefore, the nature of the receptor that mediates internalization determines the selection of epitopes presented to T lymphocytes within single protein antigens.
Antigen receptors on CD4+ helper T lymphocytes recognize short peptides presented by class II molecules of MHC (1, 2). Antigenic peptides are generated by proteolytic degradation in the endocytic pathway, where they associate with MHC class II molecules. Only a very limited set of peptides among all the potential peptides is actually loaded onto MHC class II molecules in APCs (3). The mechanisms underlying the selection of peptides for MHC class II–restricted antigen presentation are yet unclear. However, we know that two independent complex processes of intracellular transport towards endosomes are crucial for MHC class II–restricted antigen presentation: the traffic of MHC class II molecules and the delivery of antigens (4, 5).
MHC class II intracellular transport has been analyzed in detail. Newly synthesized MHC class II molecules reach endosomes, either directly from the trans-golgi network or after a short appearance at the plasma membrane, in association to the invariant (Ii)1 chain (5). Ii is then degraded and the class II–associated Ii chain peptide (CLIP) is replaced by an antigenic peptide under the control of HLA-DM (6). It has recently become clear that an alternative, Ii chain–independent pathway for MHC class II transport to endosomes also exists. Indeed, MHC class II molecules may reach the endocytic pathway from the cell surface by endocytosis (7), due to internalization signals present in the cytosolic domain of the MHC class II β chain (8). Newly synthesized and recycling MHC class II molecules may present different peptides (9). Accordingly, we have previously shown that different antigen receptors may also selectively target antigens for presentation by either of these MHC class II presentation pathways (10).
In contrast, very little is known about the endocytic transport of antigen receptors. Physiologically, antigens are delivered to the endocytic pathway by different families of receptors, which strongly increase the efficiency of MHC class II–restricted antigen presentation (11). In B lymphocytes, surface Ig mediates both cell activation and the uptake of specific antigens (12), while the expression of a particular endocytosis-deficient receptor for the Fc portion of IgG (FcγRIIb1) prevents efficient presentation of irrelevant IgG-complexed antigens (13). Interestingly, the epitope specificity of surface Ig positively and negatively influences the presentation of various T cell epitopes (14, 15).
In monocytes and dendritic cells, receptors for IgG (FcγRs), in addition to mannose receptors, mediate antigen internalization and strongly increase the efficiency of presentation to specific T cells (16). Two different FcγRs, type IIb2 and type III (FcγRIIb2 and FcγRIII) are expressed in dendritic cells. FcγRIIb2 is a monomeric receptor that, like FcγRIIb1, mediates the inhibition of cell activation when cocrosslinked to surface Ig (13). FcγIII is an heterotrimer consisting of an α chain and a dimer of γ chains, which couple the receptor to cytoplasmic effectors of signal transduction (17).
To individually analyze the function of these two receptors, we expressed them by cDNA transfection into an FcγR-negative B cell lymphoma cell line (13, 18, 19). We have previously shown that the amino acid sequence, called immunoreceptor tyrosine kinase activation motif (ITAM), in the FcγIII-associated γ chain responsible for cell activation is also involved in receptor internalization (18, 19). In addition, mutation of either of the two tyrosine residues in the ITAM of the γ chain inhibits both cell activation and ligand internalization (19). Thus, in contrast to FcγRIIb2, which contains no ITAM, is not tyrosine phosphorylated, and does not induce cell activation, FcγRIII associates with and activates cytosolic tyrosine kinases after engagement by its ligand (18). In addition to this functional diversity of the two receptors, their expression is also selectively regulated, since TNF-α and IFN-γ increase the expression of FcγRIII and inhibit that of FcγRIIb2 in monocytes (20).
The diversity in the signals required of FcγRIIb2 and FcγRIII internalization, as well as the differential regulation of their expression in APCs, suggest that the two receptors may have different antigen-presenting functions. We here examine the ability of FcγRIIb2 and FcγRIII to induce the presentation of various T cell epitopes from two different antigens, CI λ repressor and hen egg lysozyme (HEL).
Internalization of antigen–antibody complexes through FcγRIII induced the efficient presentation of all the T cell epitopes tested, whereas FcγRIIb2 only induced the presentation of a few. Point mutation of leucine 35 to alanine (L35A) in the cytoplasmic tail of FcγRIII γ chain blocked signal transduction without affecting the internalization of immune complexes. This mutation also blocked the presentation of the epitopes that were only presented after internalization by FcγRIII. In contrast, the presentation of all the epitopes that were efficiently generated after internalization by FcγRIIb2 was not affected. Thus, the nature of the receptor that mediates antigen internalization influences the selection of the epitopes presented to T lymphocytes.
Materials and Methods
B Lymphoma Cell Lines.
The B lymphoma IIA1.6 is a type II FcγR–defective variant of A20 B lymphoma cells (21) that also lacks expression α and γ chains of type III FcγR (18). This cell line was cultured in RPMI 1640 containing 10% FCS, 10 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-mE, and 5 mM sodium pyruvate (GIBCO, Paisley, UK). FcγRII–icγ chimeras were constructed by adding the sequences encoding the cytoplasmic domain of the γ chain to cDNA encoding the extracellular and transmembrane domains of mouse FcγII (18). The cDNAs were stably expressed by transfection in the mouse B cell line IIA1.6 as previously described (18). IIAI.6 cells expressing tail minus FcγRII have been previously described (13). FcγRII–icγL35 chimeras were constructed by using PCR with the two complementary oligonucleoides GAGACATATGAGACTGCGAAGCATGAAAAACCA and TGGTTTTTCATGCTTCGCAGTCTCATATGTCTC. They introduced an alanine residue in place of the leucine at position 35 of the cytoplasmic domain of the γ chain in the FcγRII–icγ chimeras. The resulting construction was inserted in expression vectors and sequenced, and cDNA were stably expressed by transfection in the mouse B cell line IIA1.6 as previously described (18). Cell surface expression of FcγRs was measured with the rat anti–mouse FcγRII and III mAb 2.4G2 and revealed by FITC-coupled mouse anti–rat antibodies. The samples were analyzed with a FACScan® flow cytometer (Becton Dickinson, San Jose, CA).
T Cell Hybridomas.
Culturing of the T cell hybridomas and antigen presentation assays were performed in RPMI 1640 containing 10% FCS, 10 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5 × 10−5 M 2-ME. The specificity of all the CD4+ T cell hybridoma is shown in Table 1. The CI λ repressor–specific hybridomas 24.4, A128, 26.1, 9C12, and 4G2 were previously characterized (22–24). The anti-HEL T cell hybridomas B9.1 and CAB43 have been previously described (25), and Ad71, 930B2, G28C9, and 16F2QCOY (3) were obtained from Dr. E. Sercarz (University of California, Los Angeles, CA).
Table 1.
Presentation of Different T Cell Epitopes after Antigen Internalization through FcγRIIb2 and FcγRIII
| T cell hybridoma | Specificity | FcγRIIb2§ | FcγRIII§ | L35A§ | ||||
|---|---|---|---|---|---|---|---|---|
| 24.4 | CI/IAd 12-26 | +≳ | + | + | ||||
| 26.1 | CI/IEd 12-26 | −‡ | + | − | ||||
| A128 | CI/IAd 46-64 | + | + | + | ||||
| 4G.2 | CI/IAd 80-102 | − | + | − | ||||
| 9C12.7 | CI/IAd 12-26 | + | + | ND | ||||
| B9.1 | HEL/IEd 108-116 | − | + | − | ||||
| CAB43 | HEL/IAd 11-25 | − | + | − | ||||
| Ad71 | HEL/IAd 71-85 | − | + | ND | ||||
| 930B2 | HEL/IAd 11-25 | + | + | ND | ||||
| G28C9 | HEL/IEd 106-116 | − | + | ND | ||||
| 16F2QCOY | HEL/IEd 2-16 | − | + | ND |
+ stands for an increase (of at least 30-fold) in the efficiency of antigen presentation of immune complexes as compared to free antigen.
− denotes a similar efficiency of antigen presentation of free antigen and immune complexes.
The data reported in this table summarize the results of five independent experiments for the C1-specific hybridomas and three experiments for the HEL-specific hybridomas.
Assays for Antigen Presentation.
Antigen presentation was assessed by culturing transfected IIA1.6 cells together with specific T cell hybridomas for 18–20 h in the presence of various concentrations of λ repressor or HEL, complexed or not with two mAbs, 22D and 51F, that recognize distinct epitopes on the λ repressor (13), or F10.6.14 and F9.13.7, which recognize distinct epitopes on HEL (26). The complexes were preformed by incubating different concentrations of purified λ repressor or HEL (from 30,000 ng to 0.5 ng) with 15 μg/ml of each of two different mAbs (51F and 22D or F10.6.14 and F9.13.7) at 37°C for 15 min. The release of IL-2 by the T-cell hybridoma was determined using a CTL.L2 proliferation assay (13). Each point represents the average of duplicate samples, which varied by <5%.
Kinetics analysis was performed as previously described (10). In brief, a dose of antigen (3 μg/ml) was used for which presentation was strictly dependent on immune complex formation with the mAbs. Immune complexes were preformed at 37°C by mixing the purified λ repressor with 51F and 22D mAbs, to make a 10× mix in the culture medium. 30 μl of preformed immune complexes were added to 270 μl of APCs adjusted at 2 × 106 cells/ml and incubated at 37°C for different times. The cells were then washed twice with PBS, fixed with 0.05% glutaraldehyde for 20 min on ice, and washed again twice. Fixed cells, in duplicate samples of 100 μl, were added to 50 μl of T cell hybridomas and adjusted to 2 × 106 cells/ml. After 24 h, IL-2 production was tested as above. When indicated, APCs were preincubated for 3 h at 37°C with 10 μg/ml cycloheximide diluted from a stock solution at 10 mg/ml in water. In this case, cycloheximide was also present during the incubation times with preformed immune complexes before fixation with glutaraldehyde.
Ovalbumin immune complexes were made by mixing ovalbumin (15 μg/ml; Sigma Chemical Co., St. Louis, MO) and the IgG fraction of a rabbit antiovalbumin antiserum (50 μg/ml; Sigma Chemical Co.). The binding of these immune complexes to FcRs was controlled by immunofluorescence and FACScan® analysis. For costimulation experiments, APCs were incubated with or without ovalbumin immune complexes for 18 h and then fixed as described above. Fixed cells, in duplicate samples of 100 μl, were added to 50 μl of T cell hybridomas 24.4 and 26.1 (adjusted to 2 × 106 cells/ml) and various concentrations of CI λ repressor 12– 26 peptides. In another set of experiments, APCs were incubated either with λ repressor (30 μg/ml) and preformed ovalbumin immune complexes to stimulate FcR, or with preformed λ repressor immune complexes (as described above) and F(ab′)2 fragments of specific goat anti–mouse IgG2a antibodies (15 μg/ml; Southern Biotechnology Associated, Birmingham, AL), which do not cross-react with IgG1 anti-λ repressor mAbs 51F and 22D, and which specifically stimulate endogenous membrane IgG2a on IIA1.6 cells. After 18 h, the cells were fixed and incubated with CI λ repressor–specific T cell hybridomas 24.4 and 26.1. T cell stimulation was assayed using a CTL.L2 proliferation assay.
Assays for Cell Activation.
Cell activation through Fc receptors or endogenous membrane immunoglobulins was determined as previously described (19). In brief, triplicates of each B lymphoma cell (105 cells/well in 100 μl) were stimulated either through Fc receptors using preformed ovalbumin immune complexes, made as described above, or through endogenous membrane immunoglobulins using F(ab′)2 fragments of the IgG fraction of a rabbit anti–mouse IgG antisera (15 μg/ml). After 18 h, the supernatants were harvested and their content in I1-2 was measured using a CTL.L2 proliferation assay.
Immune Complex Internalization.
The internalization of immune complexes was assayed as previously described (19). In brief, the cells were washed once in internalization buffer (RPMI, 5% FCS, 10 mM glutamine, 5 mM sodium pyruvate, 50 mM 2-ME, and 20 mM Hepes, pH 7.4) and incubated with horseradish peroxidase (HRP) anti-HRP immune complexes for 2 h at 0°C (107 cells/ml). Immune complexes were prepared as a 10× solution in internalization buffer (HRP 50 μg/ml and a polyclonal rabbit anti-HRP antibody at 400 μg/ml) for 30 min at 37°C. After fixation of HRP immune complexes, the cells were washed three times in internalization buffer and incubated at 37°C for various times (2 × 106 cells/ml). Internalization was stopped by adding cold internalization buffer and the cells were washed once in PBS. Duplicates of each time point were either left in PBS at 4°C to measure cell surface HRP-ICs or incubated in Triton X-100 (0.1%) for 5′ at room temperature to measure the total amount of HRP-ICs. The HRP was revealed by adding substrate buffer (0.5 mg/ml OPD [Sigma Chemical Co.] and 0.12% H2O2 in 0.05 M phospho-citrate buffer, pH 5.0) at 4°C. The reaction was stopped with 6 N HCl and the change in color was determined spectrophotometrically at 492 nm.
Results
Selective Presentation of a T Cell Epitope after Antigen Internalization by FcγRIII.
We have shown previously that internalization of antigen–IgG complexes by FcγRIIb2 and III in IIA1.6 cells (an FcγR-negative B lymphoma cell line derived from A20 cells) induces presentation of the dominant epitope of the CI λ repressor (12–26 peptide on IAd) at antigen concentrations 102–104-fold lower than fluid phase uptake (13, 19). This increase in the efficiency of antigen presentation is, at least in part, due to increased antigen uptake by the APCs since endocytosis-incompetent receptors for IgG (13, 19) were deficient for both internalization of immune complexes and efficient presentation at low antigen concentrations. Although these results underline the importance of internalization in antigen presentation, they do not address the possibility that postinternalization intracellular targeting of antigen–receptor complexes is also important for the generation of certain epitopes. If this was the case, we would then expect that internalization through particular antigen receptors results in the presentation of different epitopes to specific T lymphocytes.
To test this possibility, we assessed the ability of cells expressing either FcγRIIb2 or FcγRIII (cells coexpressing the α and γ chains, which we have previously shown to present the 12–26/IAd epitope at very low antigen-IgG concentrations) to present another epitope, defined by the same 12–26 in association with I-Ed. This epitope is not presented after fluid phase antigen uptake, or in vivo after immunization with intact antigen (it is therefore a cryptic epitope), but it elicited an immune response when the mice were injected directly with the peptide (22).
The cell lines expressing either FcγRIIb2 or FcγRIII were incubated with increasing concentrations of CI λ repressor, free or complexed to two different anti-λ repressor mAbs, as previously described (13). Complexing antigen to IgG allowed receptor-mediated antigen uptake through FcγRs. T cell hybridomas specific for either IAd- or the IEd-restricted epitopes were also included in the cultures. Antigen presentation was assessed by measuring IL-2 secretion by the T cell hybridomas.
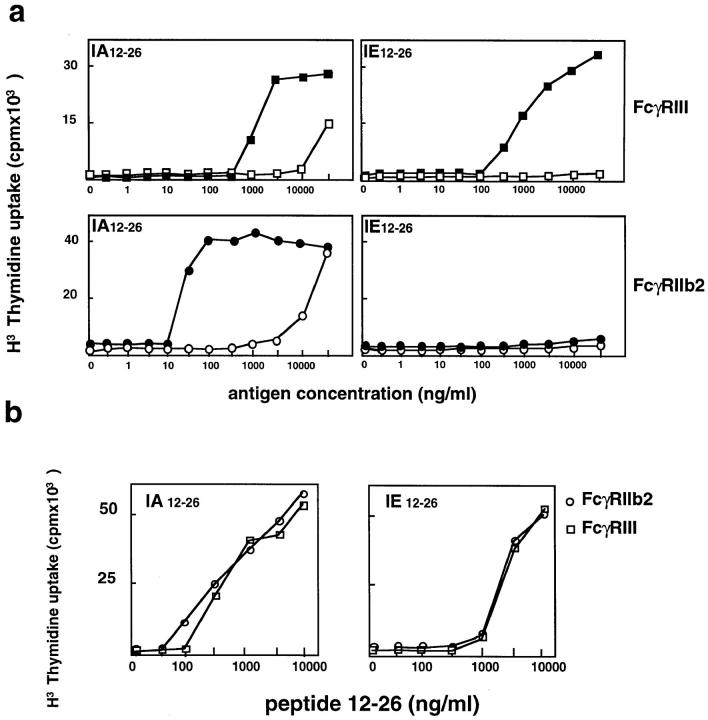
As shown in Fig. 1 A, the IEd restricted epitope was not presented after fluid phase uptake (right), whereas the IAdrestricted epitope was presented at high antigen concentrations (left). Confirming our previous results, FcγRIIb2 increased the efficiency of presentation of the IAd restricted epitope by 3–4 logs when the C1 λ repressor was complexed to IgGs (T cell activation was still observed at 30 ng/ml). Similarly, FcγRIII-mediated internalization induced presentation of this epitope at concentrations 30–100-fold lower than fluid phase uptake. Therefore, both type II and III IgG receptors increased the efficiency of presentation of this dominant IAd-restricted epitope.
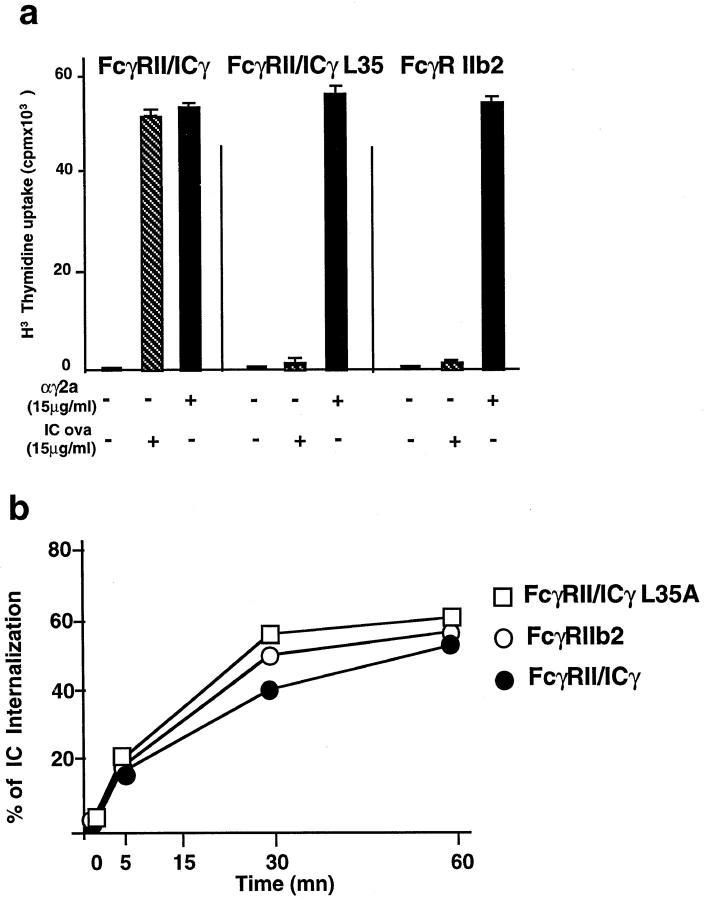
Figure 1.
Selective presentation of a cryptic epitope after antigen–IgG complexes internalization through FcγRIII. IIA1.6 cells expressing either FcγRIIb2 or FcγRIII α and γ chains were cultured in the presence of increasing concentrations of CI λ repressor (fluid phase uptake, open symbols) or CI λ repressor–IgG complexes (FcR-mediated endocytosis, filled symbols). T cell hybridomas specific for a dominant epitope (IA/12–26) or a cryptic epitope (IE/12–26) from the CI λ repressor were also included in the cultures. The secretion of IL-2 by the T cell hybrids was measured after an overnight incubation. (a) Internalization through both FcRs resulted in the efficient presentation of the dominant IAd-restricted epitope, whereas only FcγRIII-expressing cells presented the IEd-restricted epitope. (b) Both transfected cell lines presented the 12–26 peptide on IAd or IEd with identical efficiencies. The data presented are representative of results obtained in three independent experiments.
However, FcγRIII was less efficient than FcγRIIb2 (Fig. 1 A, compare lower and upper left). This difference was due to the different levels of expression of the two receptors, since FcγRII and FcγRIII were expressed at 1.6 × 105 molecules/cell and 2.8 × 104 molecules/cell, respectively (reference 19 and data not shown). When cells expressing 2–3 × 104 FcγIIb2 per cell were used, the efficiency of presentation was similar to that obtained with FcγRIII expressing cells (data not shown).
In contrast to this IAd-restricted epitope, the IEd epitope was not presented after FcγRIIb2-mediated internalization of immune complexes (Fig. 1 A, lower right). In contrast, efficient presentation of 12–26 on IEd was observed with FcγRIII-expressing cells after internalization of immune complexes (upper right). This selectivity of presentation of the IEd-restricted epitope was not due to a difference between the FcγRIIb2 and FcγRIII cell lines in the levels of surface expression of either MHC class II or other costimulatory molecules, because the 12–26 peptide was presented with similar efficiencies to the two T cell hybridomas by the two transfected cell lines (Fig. 1 B). Since engagement of FcγIII by immune complexes may, under certain circumstances (see below), induce IL-2 secretion by A20 and IIA1.6 cells (18, 27), we verified that the CI λ repressor immune complexes did not induce any IL-2 secretion in the absence of T cell hybridomas (data not shown).
Therefore, only FcγRIII-mediated internalization induced presentation of the 12–26 peptide on IEd. FcγRIIb2, which induced very efficient presentation of the same peptide on IAd, was totally inefficient for the presentation of the IEd-restricted epitope. Therefore, receptor-mediated antigen internalization is not sufficient for presentation of all T cell epitopes; the nature of the receptor mediating antigen uptake influences the selection of epitopes presented to T lymphocytes.
Presentation of the IEd-restricted Epitope Requires the FcγRIII-associated γ Chain.
We have shown previously that both the internalization and the increased efficiency of presentation of the IAd/12–26 dominant epitope in the CI λ repressor were dependent on the FcγRIII-associated γ chains (19). Thus, FcγRIII with a cytoplasmic domain–deleted γ chain did not internalize ligand or promote antigen presentation at low concentrations. In addition, when the cytoplasmic domain of the γ chain was fused to the extracellular and transmembrane domains of FcγRIIb2, cell activation, immune complex internalization, and efficient antigen presentation were observed (19). Therefore, we next sought to assess if the presentation of 12–26 on IEd was also dependent on the FcγRIII-associated γ chain.
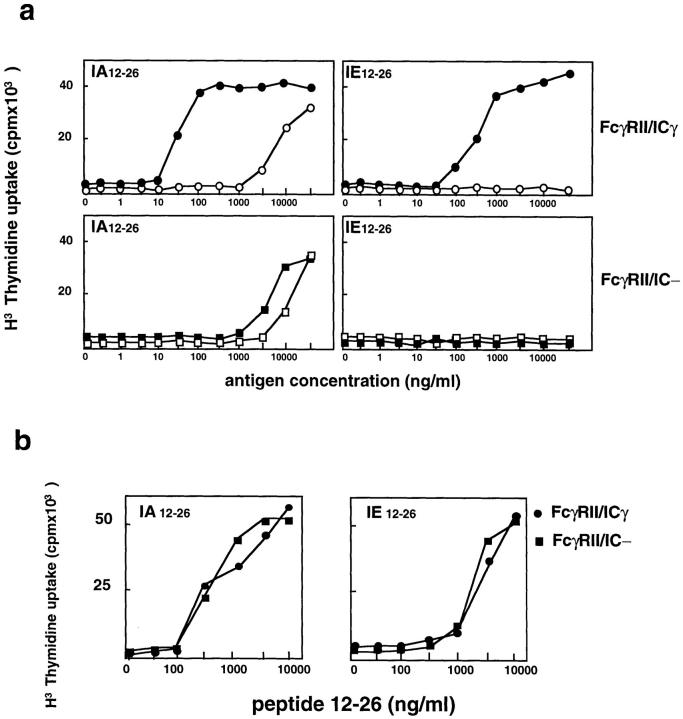
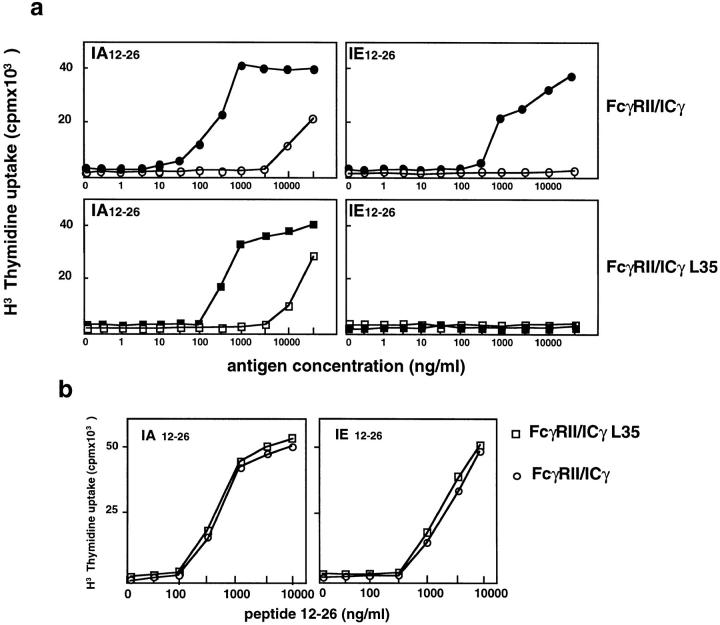
As expected, the tail-less FcγRIIb2, which was not internalized, did not allow the efficient presentation of the two epitopes (Fig. 2 A, lower panels). In contrast, when the cytoplasmic tail of the γ chain was fused to this tail-less receptor to form an FcγRII/icγ chimera, efficient presentation of both IAd/12–26 and IEd/12–26 was observed after immune complex internalization (Fig. 2 A, upper panels). The cell lines expressing the tail-less FcγRII and the FcγRII/icγ chimeras presented the 12–26 peptide with similar efficiencies (Fig. 1 B). Therefore, the cytoplasmic domain of the γ chain bears all the information required for the presentation of the IEd epitope.
Figure 2.
Presentation of the IEd-restricted cryptic epitope requires the cytoplasmic tail of the FcγRIII-associated γ chain. (a) IIA1.6 cells expressing either a tail-less FcγRII (FcγRII/ic−) or a chimera composed of the extracellular and transmembrane domains of FcγRII and the cytoplasmic tail of the FcγRIII-associated γ chain (FcγRII–icγ) were assayed for presentation of free (black symbols) and IgG-complexed (white symbols) CI λ repressor. The chimera presented both the dominant IAd-restricted epitope and the cryptic IEd-restricted epitope with high efficiency when the antigen was complexed to IgG (i.e., after receptor-mediated endocytosis). The tail-less FcγRII, in contrast, presented the dominant epitope inefficiently and was completely unable to present the cryptic epitope. (b) Both transfected cell lines presented the 12–26 peptide with similar efficiencies, indicating that the levels of IAd and IEd expression in both cell lines were the same. The data presented are representative of results obtained in five independent experiments for a and two experiments for b.
Presentation of Both IAd- and IEd-restricted Epitopes Requires Newly Synthesized MHC Class II Molecules.
Two different pathways of antigen presentation may result in the loading of antigenic peptides on MHC class II molecules: the conventional pathway requires newly synthesized and the so-called alternative pathway requires recycling MHC class II. Presentation through the conventional and alternative pathways differ by their kinetics and their sensitivity to protein synthesis inhibitors (as well as by their dependence on H2-M, the Ii chain, and the cytoplasmic tail of MHC class II molecules; references 8, 28). It has recently been shown, that these two pathways may control the presentation of different epitopes from a single antigen (9). We have also previously shown that internalization through different receptors may trigger the presentation the same epitope by either antigen presentation pathways (10). Therefore, it was possible that FcγRIIb2 and FcγRIII were also targeting antigens for presentation by different pathways and that the IEd epitope could only be generated in one of these pathways.
We tested this possibility by assessing the effect of the protein synthesis inhibitor cycloheximide on the kinetics of presentation of the IAd and IEd epitopes. FcγRIIb2- or FcγRIII-expressing cells were preincubated in cycloheximide for 3 h before addition of CI λ repressor immune complexes. After incubation of the cells at 37°C for various periods of time, the cells were fixed and T cell hybrids specific for the IAd/12–26 or IEd/12–26 epitopes were added. After an additional 18 h incubation, the amounts of IL-2 produced by the T cell hybrids were measured in the supernatants.
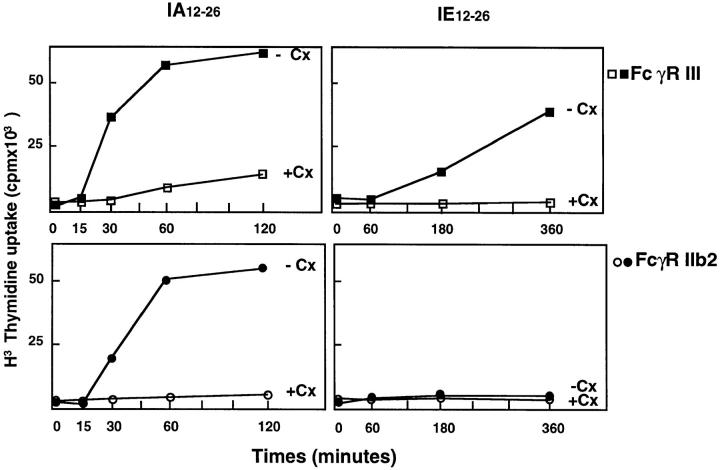
As shown in Fig. 3, presentation of the IAd epitope in FcγRII- and FcγRIII-expressing cells, as well as presentation of the IEd epitope by FcγRIII-expressing cells, all exhibited slow kinetics and were blocked by cycloheximide. These results indicate that both receptors induced antigen presentation through the conventional pathway, which requires newly synthesized MHC class II molecules. The difference in the presentation of the IEd epitope is therefore not due to the targeting of antigen to different antigen presentation pathways by the two receptors.
Figure 3.
Presentation of the dominant and cryptic epitopes are blocked by cycloheximide. IIA1.6 cells expressing either FcγRII–icγ chimeras (top) or FcγRIIb2 receptors (bottom) were incubated with CI λ repressor–IgG complexes for various time periods with (+Cx) or without (−Cx) cycloheximide. The cells were then fixed with gluteraldehyde before incubation with the T cell hybridomas for 24 h. The presentation of both the dominant and the cryptic epitopes by FcγRIII, as well as presentation of the dominant epitope by FcγRIIb2, were all blocked in the presence of cycloheximide. Therefore, both the dominant and cryptic epitopes are presented by newly synthesized MHC class II molecules. (Similar results were obtained in three independent experiments).
Cell Activation Is Not Sufficient for the Presentation of the Cryptic Epitope.
Engagement of FcγRIII, but not of FcγRIIb2, induces the activation of multiple tyrosine kinases, a rise of cytosolic Ca2+ concentrations and, subsequently, the activation of the transcription of various genes (18). Therefore, the selective presentation of the cryptic epitope by cells expressing FcγRIII could be either a direct consequence of a different intracellular targeting of antigen or an indirect effect of overall cell activation. We next attempted to test the latter possibility in two different ways.
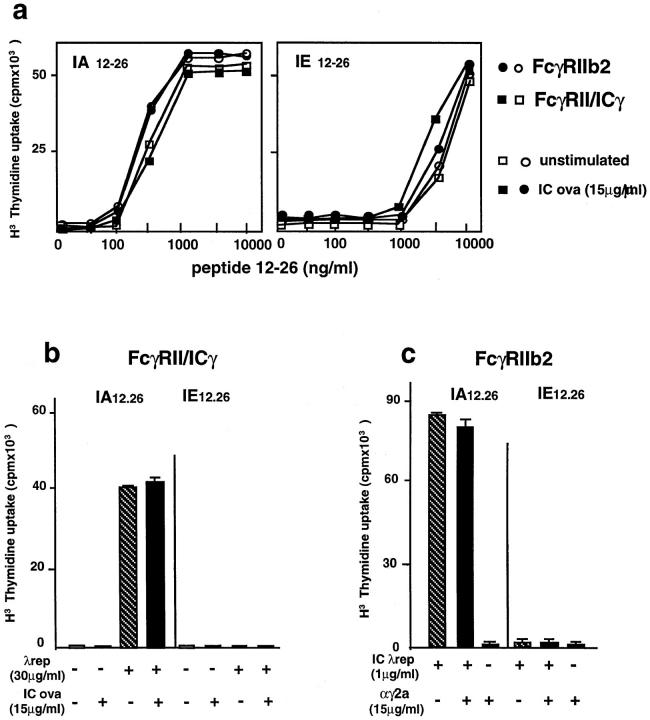
First, we tested whether cell activation by FcγRII/icγ chimera induced a change in surface expression of a putative costimulatory molecule required only for the efficient activation of some of the epitope-specific T cell hybrids. The FcγRIIb2- and FcγRII/icγ-expressing cells were incubated overnight with or without irrelevant immune complexes (ovalbumin (OVA)-anti-OVA), in order to induce cell activation (we verified that these immune complexes induced efficient cell activation using a phosphotyrosine blot assay and IL-2 secretion, data not shown). The cells were then fixed and reincubated with increasing doses of 12–26 peptide and either the IAd or the IEd 12–26 epitope-specific T cell hybrids.
As shown in Fig. 4 A, the peptide was presented to both T cell hybridomas with similar efficiencies by the unstimulated and the stimulated cells. Therefore, cell activation through the γ chain did not induce any modification in the efficiency of presentation of the 12–26 peptide to the IAd and the IEd specific T cell hybrids. The effect of antigen internalization through FcγRIII is therefore related to the intracellular processing of antigen.
Figure 4.
Cell activation per se does not induce presentation of the IEd-restricted epitope. (a) FcγRIIb2- and FcγRII–ic-γ chimera–expressing cells were incubated for 24 h with OVA–anti-OVA immune complexes. The cells were then incubated with increasing concentrations of 12–26 CI λ repressor peptide and the T cell hybridomas specific for the dominant (IAd 12–26) or cryptic (IEd 12–26) epitopes. The secretion of IL-2 in the cell culture supernatants was measured. Activation of FcγRII– icγ chimera–expressing cells with IC did not modify the efficiency of presentation of the 12–26 peptide. Similar results were obtained in three independent experiments. (b) Cell activation through FcγRII–icγ chimeras does not induce the presentation of the cryptic epitope after antigen internalization by fluid phase uptake. FcγRII–icγ–expressing cells were incubated overnight in the presence of 30 μg/ml CI λ repressor, with or without irrelevant OVA–anti-OVA immune complexes, in order to engage FcγRII–icγ chimeric receptors. The cells were then fixed and reincubated overnight with T cell hybridomas specific for the dominant or the cryptic epitopes. Engagement of FcγRII–icγ chimeric receptors did not induce the presentation of the IEd-restricted cryptic epitope. (c) Cell activation through surface IgG does not induce the presentation of the CI λ repressor cryptic epitope after immune complexes internalization through FcγRIIb2. Cells expressing FcγRIIb2 were incubated in the continuous presence of CI λ repressor immune complexes in the absence or presence of specific goat anti–mouse IgG2a F(ab)′2 fragments, in order to induce cell activation through endogenous surface IgG2a. Cell activation through sIgG2a did not induce presentation of the cryptic epitope by FcγRII2b-expressing cells. Error bars in b and c indicate the mean variation of T cell stimulation in two distinct experiments.
Second, we tested the effect of cell activation on intracellular antigen processing. FcγRII/icγ expressing cells were incubated with soluble CI λ repressor in the presence of irrelevant immune complexes (as in Fig. 4 A) in order to engage the corresponding receptors, and induce cell activation. The cells were then fixed and reincubated with either the IAd or the IEd 12–26 specific T hybridomas. As shown in Fig. 4 B, activation through FcγRII/icγ did not induce the presentation of the cryptic epitope after fluid phase antigen uptake.
However, it was still possible that both cell activation and efficient receptor-mediated antigen uptake were required together for the presentation of the IEd restricted epitope. To test this possibility, the FcγRIIb2 expressing cells were simultaneously incubated with λ repressor–containing immune complexes (to allow efficient antigen uptake) and specific anti–mouse IgG2a goat antibodies (to induce cell activation through surface immunoglobulin). The cells were then fixed and reincubated with the two T cell hybrids. As shown in Fig. 4 C, cell activation through sIgG did not induce FcγRIIb2 expressing cells to present the cryptic epitope. Therefore, overall cell activation is not sufficient to allow presentation of the IEd-restricted epitope after FcγRIIb2-mediated antigen internalization.
Mutation of Leucine 35 to Alanine in the Cytoplasmic Tail of FcγRIII-associated γ Chain Inhibits Cell Activation, but not Internalization of Immune Complexes.
We concluded from the previous series of experiments that the presentation of the IEd-restricted epitope was not an exclusive consequence of overall cell activation, but rather resulted from the selective intracellular targeting of antigen by FcγRIII. However, the intracellular traffic of membrane receptors may also be related to their signal transduction capabilities. In the case of tyrosine kinase receptors, like epidermal growth factor receptor, the kinase activity is involved in internalization (29) and endosomal trafficking (30). By analogy to PTK receptors, we reasoned that the ability of FcγRIII (or FcγRII/icγ) to activate PTKs after cross-linking (17) might influence its intracellular traffic and thereby allow the generation of the IEd-restricted epitope.
We attempted to address this possibility by searching for mutations that differentially affected internalization and cell activation by the γ chain. An alanine scan of the region of the ITAM (Amigorena, S., and C. Bonnerot, unpublished results) was performed by sequentially introducing single point mutations in the cytoplasmic tail of FcγRII/icγ. The corresponding cDNAs were expressed stably in the IIA1.6 B lymphoma cells and the resulting transfectants were tested for their ability to induce cell activation and ligand internalization after receptor cross-linking by anti-FcγR mAbs (Amigorena, S., and C. Bonnerot, unpublished results). Thus, we found that mutation of leucine in position 35 to alanine (FcγRII/icγL35A) completely abolished cell activation without affecting receptor internalization.
As shown in Fig. 5 A, FcγRII–icγL35A chimera–expressing cells have lost the ability to secrete IL-2 after engagement of the receptor by immune complexes, whereas secretion of IL-2 was normal after engagement of surface IgG in the same cells. In contrast, internalization of immune complexes was not affected by the mutation (Fig. 5 B). Therefore, leucine 35 in the γ chain cytoplasmic domain is required for cell activation but dispensable for receptor internalization.
Figure 5.
FcγRII–icγL35 receptors have lost the ability to induce cell activation but are still efficiently internalized. (a) The secretion of IL-2 by the IIA1.6 cells was measured after engagement of the transfected FcγRII–icγ or FcγRII–icγL35 chimeras or FcγRIIb2 by OVA–anti-OVA immune complexes. As we have previously shown, FcγRII–icγ but not FcγRIIb2 induce the secretion of IL-2 after binding to immune complexes. The L35 mutation inhibits the induction of IL-2. (b) Internalization of the HRP–anti-HRP immune complexes was measured as described in Materials and Methods. The three transfected cell lines internalized immune complexes with similar kinetics and efficiencies. Data are representative of two independent experiments.
Internalization through FcγRIII Induces the L35-dependent Presentation of Multiple Epitopes that were Not Presented after Internalization through FcγRII.
Since FcγRII–icγL35A was incapable of stimulating IL-2 production or inducing activation of PTKs (Amigorena, S., and C. Bonnerot, unpublished results) but was normally internalized, we next tested its ability to induce the presentation of the IAd- and IEd-restricted 12–26 peptide. As shown in Fig. 6 A, FcγRII–icγL35A–expressing cells presented soluble CI λ repressor IAd 12–26 epitope with the same efficiency as FcγRIII-expressing cells. When the cells were incubated with antigen–IgG complexes, both FcγRIII–icγ– and FcγRII– icγL35A–expressing cells presented the IAd epitope with high efficiency. In contrast, only FcγRII–icγ–expressing cells presented the IEd 12–26 epitope, whereas absolutely no presentation of this epitope was observed after immune complexes internalization by FcγRII–icγL35A–expressing cells (Fig. 6 A, lower panels). The efficiencies of presentation of the 12–26 peptide to the IAd and IEd-specific T cell hybridomas were similar for the FcγRII–icγ– and the FcγRII– icγL35A–expressing cells (Fig. 6 B). The mutation of leucine 35 therefore affected the presentation of the IEd 12–26 epitope much more drastically than the presentation of the same peptide with IAd.
Figure 6.
Selective effect of mutation of leucine 35 to alanine on the presentation of different epitopes. (a) The presentation of the dominant and cryptic epitopes of CI λ repressor were measured on IIA1.6 cells expressing either FcγRII–icγ or FcγRII–icγL35 receptors as described in Fig. 1. Mutation of leucine 35 to alanine did not significantly affect the presentation of the IAd-restricted epitope. In contrast, it completely inhibits presentation of the IEd-restricted epitope. (b) The two transfected cell lines present the 12–26 peptide with the same efficiency to the T cell hybridomas specific for both epitopes. The data presented are representative of results obtained in three independent experiments.
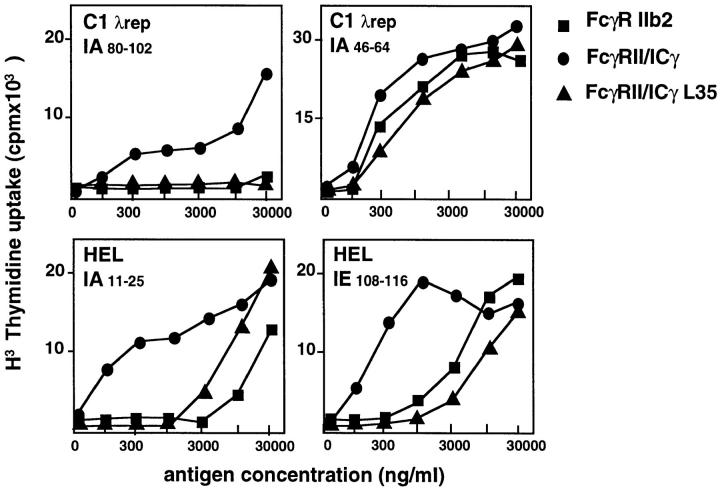
The two epitopes described this far concern the same or overlapping peptides (included in the 12–26 region) associated to either IAd or IEd. We next tested whether the same effect was found using two other IAd restricted epitopes, IAd 46–64 and IAd 80–102. As shown in Fig. 7 (upper right), the 46–64 epitope was efficiently presented after internalization by both FcγRIIb2 and FcγRII–icγ. In contrast, the 80–102 peptide was only presented after internalization by the FcγRII–icγ. As in the case of the IEd 12–26 epitope, the IAd 80–102 epitope was not at all presented after internalization by FcγRIIb2. In addition, as in the case of the IEd 12–26 and IAd 12–26, the presentation of the IAd 80– 102, but not of IAd 46–64 epitope, was blocked by the L35A mutation.
Figure 7.
Mutation of leucine 35 inhibits presentation of the FcγRIII-restrained epitopes, but does not affect presentation of the epitopes also presented after internalization by FcγRII. IIA1.6 cells expressing FcγRIIb2, FcγRII–icγ, or FcγRII–icγL35 were incubated with increasing concentrations of CI λ repressor immune complexes or HEL immune complexes. The presentation of two epitopes of the CI λ repressor and two epitopes of HEL were measured with the corresponding T cell hybridomas. For these four epitopes, FcγRII–icγL35 receptors behave like FcγRIIb2 and not like FcγRII–icγ chimeras. The same results were obtained in three independent experiments.
Is the difference in the repertoire of epitopes presented after FcγRIII or FcγRII–icγ antigen internalization limited to CI λ repressor? To answer this question, we tested a panel of six T cell hybridomas specific for various epitopes of HEL. The efficiency of presentation of IgG-complexed HEL to the different T cell hybrids was compared for the FcγRIIb2– and the FcγRII–icγ–expressing cells. As shown in Fig. 7 (lower panels), the IAd 11–25 and IEd 108–116 epitopes were efficently presented only after internalization by FcγRII–icγ–expressing cells. As in the case of the CI λ repressor IAd 12–26 and IAd 46–64 epitopes, the L35A mutation inhibited the efficient presentation of these two epitopes.
As summarized in Table 1, internalization by FcγRII–icγ chimeras allowed the efficient presentation of all epitopes tested, whereas FcγRIIb2 only presented a subset of these epitopes. For all the other epitopes tested, FcγRIIb2 either allowed no presentation or induced presentation at the same high antigen concentrations as those which also induced presentation after fluid phase uptake. As a general rule, the epitopes which were efficiently presented after internalization by either FcγRIII or FcγRIIb2 were also efficiently presented by the L35 mutant receptors. In contrast, in the case of the epitopes that were presented after FcγRIII and FcγRII–icγ, but not presented after internalization by FcγRIIb2, the L35 mutation inhibited presentation. In all cases, the L35 mutant receptors behaved like the FcγRIIb2 in terms of epitope presentation.
Discussion
The mechanisms of selection of T cell epitopes within complex protein antigens are poorly understood. Yet this choice is critical, on one the hand for shaping the T cell repertoire during thymic positive and negative selection, and, on the other hand, for the development of efficient immune responses. Our results show that the T cell epitopes that we have analyzed may be classified into two discrete categories. The first category consists of the epitopes that were efficiently presented after internalization by either FcγRII or FcγRIII (which in most cases were also presented, although less efficiently after fluide phase uptake), and the second, of the epitopes that were only presented after internalization by FcγRIII, or the FcγRII– icγ chimeras (at least two of these epitopes were cryptic, i.e., not presented after immunization of mice with intact antigen).
Why, then, did FcγRIII and FcγRIIb2/γ-chain chimeras induce the presentation of epitopes that were not presented after internalization via FcγRIIb2 or fluid phase uptake? The selectivity in the presentation of different epitopes between receptors is certainly not due to quantitative differences in the uptake of antigen by the presenting cells, because (a) both FcγRIII and the FcγRII–chain chimeras were expressed at lower levels than FcγRIIb2; (b) all receptors mediated the internalization of immune complexes with similar kinetics and efficiencies (Fig. 5 B); and (c) FcγRIIb2 increased the efficiency of presentation of several epitopes to the same extent or even to greater extents than FcγRIII and the FcγRII–icγ chimera (Figs. 1 and 2). Therefore, the selectivity in epitope presentation must reside in a qualitative difference between the two receptors.
One of the qualitative differences between type II and III FcγRs is in the induction of cell activation. The γ chains have been shown to mediate activation through FcγRIII, due to a conserved ITAM, present in its cytoplasmic tail. In contrast, FcγRIIb2 bears no ITAM and does not induce cell activation. Upon activation through FcγRIII, IIA1.6 cells produced a cytokine that supports the growth of the CTLL.2 IL-2–dependent cell line (18). We checked very carefully that the IL2 production measured by the CTLL.2 cells in our antigen presentation experiments was due to the T cell hybrids and not to the presenting cells. If the presenting cells were incubated with CI λ repressor or HEL immune complexes in the absence of T lymphocytes, background proliferation of the CTLL.2 cells was observed (data not shown). In addition, all the experiments were repeated with paraformaldehyde-fixed APCs, a treatment that completely prevents cytokine secretion (Fig. 3 and data not shown). Under these conditions, only the T cell hybrids can produce IL-2.
The coactivation experiments (Fig. 4) show that presentation of the FcγRIII-restricted epitopes was not a consequence of overall cell activation. The most convincing result from this point of view may be the one shown in Fig. 4 C. When immune complexes were internalized by FcγRIIb2-expressing cells in the continuous presence of F(ab)′2 fragments of anti-IgG antibodies, which efficiently induced cell activation through mIgG (18), no presentation of the FcγRIII-restricted epitopes was observed. It could be argued here that cell activation through sIgG might be qualitatively different from cell activation through FcγRIII. Although we can not completely exclude this possibility, we found that direct antigen internalization though sIg (using A20 B cells expressing anti-DNP sIg and DNP-derivatized CI λ repressor) allowed presentation of the same epitopes as FcγRIII (data not shown). This shows that although presentation of FcγRIII-restricted epitopes also occurred after antigen internalization by sIg, overall cell activation through sIg did not induce the presentation of those epitopes when the antigen was internalized through FcγRIIb2. Therefore, antigen must be physically associated to the receptor for the efficient presentation of the FcγRIII-restricted epitopes.
The simplest and most direct explanation for these results is that FcγRIII targeted antigens to a particular intracellular compartment that FcγRIIb2 can not access. Several of our experiments support this possibility and, taken together, shed light on various aspects of this putative transport path. First, the differential generation of T cell epitopes after internalization by FcγRIIb2 and III was not due to selective antigen targeting to the conventional and recycling presentation pathways, since presentation of both dominant and cryptic epitopes had slow kinetics and were blocked by cycloheximide. This was an important possibility to test because it has recently been shown that the epitopes presented by both pathways may be different (9).
Second, a single point mutation (of leucine 35 to alanine) blocked the presentation of FcγRIII-restricted epitopes, without affecting the presentation of the epitopes efficiently presented after FcγRIIb2 internalization. In other words, the chimeric FcγRII–icγL35A behaved exactly like FcγRIIb2 in terms of epitope presentation. Interestingly, L35A mutation was first identified as blocking cell activation, but not receptor internalization. Therefore, like FcγRIIb2, L35A chimeric receptors are not capable of inducing activation of cytosolic PTKs. These results suggest that association to tyrosine kinases or tyrosine phosphorylation of the receptor itself is required for the efficient generation of the FcγRIII-restricted epitopes.
In the case of PTK receptors, like the epidermal growth factor receptor, the kinase activity has been shown to directly affect intracellular transport, since in addition to internalization (29) transport from endosomes to lysosomes requires the kinase activity (30). Although the FcγRIII-associated γ chain contains no intrinsic PTK activity, it has been shown to associate to Syk after phosphorylation of its ITAM by PTK of the src family (17). In addition, for ITAM-containing receptors like FcγRIII, association to Syk is required for transport from endosomes to lysosomes (Amigorena, S., and C. Bonnerot, unpublished results). However, transport to lysosomes is unlikely to be determinant for presentation of the FcγRIII-restricted epitopes, since FcγRIIb2 efficiently mediated transport of immune complexes to Percoll heavy, degradative compartments (31).
Several recent studies have analyzed the intracellular locations where peptide loading occurs and showed that multiple endocytic compartments may be involved (32–34). However, little is known about the actual influence of the intracellular compartments where peptide loading occurs on the generation of particular T cell epitopes (34). Indeed, besides the affinity of peptides for MHC class II molecules, other factors are critical for the formation of T cell epitopes. The proteolytic generation of peptides, or their degradation depends on specific proteolytic enzymes. The loading of different peptides is differentially affected by HLA-DM (35). Therefore, the endosomal environment most likely determines the nature of the peptides loaded onto MHC class II molecules (34).
We have previously shown that a particular population of vesicles in the cells used here, class II vesicles or CIIV, are an important site of peptide loading (36, 37). Therefore, it is possible that FcγRIIb2 and FcγRIII (or FcγRII– icγ) have differential accessibilities to CIIV, thus accounting for the generation of different peptides after internalization by these two receptors. Directly testing this possibility will require an extensive analysis of FcγRIIb2 and FcγRIII intracellular transport, which will next be undertaken.
Physiologically, FcγRII and FcγRIII are expressed on professional APCs such as macrophages and dendritic cells (20, 38). Furthermore, the relative rates of expression of these two receptors is regulated by cytokines such as IFN-γ and TNF-α (20). Our results therefore suggest that the selection of peptides presented to T cells depends on the antigen receptors expressed by the APCs which are themselves dependent on their microenvironment.
The consequences of antigen receptor expression on the presentation of different epitopes may also be relevant to autoimmunity. Indeed, it has recently been proposed that only dominant epitopes from autoantigens participate in thymic selection, since cryptic epitopes would not be presented under normal conditions (3, 39). T cells specific for cryptic epitopes from autoantigens therefore are not tolerized and may become pathogenic if cryptic epitopes are presented in the periphery for any particular reason. In the recent past, several groups have shown various mechanisms for unveiling cryptic epitopes in vitro (for review see reference 39). Interestingly, after downregulation of a membrane receptor antigen by its ligand, cryptic epitopes may be revealed (40). Changes in the hierarchy of epitope presentation may also occur in different APCs or when antigen is complexed to other proteins (such as antibodies) (15). Our results indicate a novel mechanism of cryptic epitope unveiling, i.e., receptor-mediated antigen uptake. The participation of FcγRIII in revealing cryptic epitopes in vivo, as well as the possible involvement of FcRs in epitope spreading and in autoimmunity, have now to be evaluated.
Footnotes
We are grateful to Drs. Ming-Zong Lai and Eli E. Sercarz for providing us with several anti-CI λ repressor and anti-HEL T cell hybridomas, to P. Benaroch and G. Langsley for critically reading the manuscript and to all members of the CJF-INSERM 95-01 for useful discussions.
This work was supported by grants from the INSERM, Institut Curie, the Association de Recherche contre le Cancer (ARC), and the Ligue Nationale Contre le Cancer. L. Gapin was supported by ARC and Pasteur-Weizman fellowships and V. Briken by an E.C. fellowship.
Address correspondence to Sebastian Amigorena, INSERM CJF 95-01, Institut Curie, Section Recherche, 12 rue Lhomond, 75005, Paris, France. Phone: 33-01-42-34-63-89; Fax: 33-01-42-34-63-82; E-mail, s.amigorena@curie.fr
Abbreviations used in this paper: Ii, invariant chain; ITAM, immunoreceptor tyrosine kinase activation motif; HEL, hen egg lysozome; HRP, horseradish, peroxidase; PTK, protein tyrosine kinase.
References
- 1.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 3.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 5.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 6.Roche, P.A. 1995. HLA-DM: an in vivo facilitator of MHC class II peptide loading. 3:259–262. [DOI] [PubMed]
- 7.Reid PA, Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990;346:655–657. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- 8.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 9.Guangming Z, Romagnoli P, Germain RN. Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J Exp Med. 1997;185:429–438. doi: 10.1084/jem.185.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnerot C, Lankar D, Hanau D, Spehner D, Davoust J, Salamero J, Fridman WH. Role of B cell receptor Ig alpha and Ig beta subunits in MHC class II–restricted antigen presentation. Immunity. 1995;3:335–347. doi: 10.1016/1074-7613(95)90118-3. [DOI] [PubMed] [Google Scholar]
- 11.Lanzavecchia A. Antigen uptake and accumulation in antigen-specific B cells. Immunol Rev. 1987;99:39–51. doi: 10.1111/j.1600-065x.1987.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 12.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 13.Amigorena S, Bonnerot C, Drake JR, Choquet D, Hunziker W, Guillet JG, Webster P, Sautes C, Mellman I, Fridman WH. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 14.Watts C, Lanzavecchia A. Suppressive effect of antibody on processing of T cell epitopes. J Exp Med. 1993;178:1459–1463. doi: 10.1084/jem.178.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–1963. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony–stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 18.Bonnerot C, Amigorena S, Choquet D, Pavlovich R, Choukroun V, Fridman WH. Role of associated gamma-chain in tyrosine kinase activation via murine Fc gamma RIII. EMBO (Eur Mol Biol Organ) J. 1992;11:2747–2757. doi: 10.1002/j.1460-2075.1992.tb05340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amigorena S, Salamero J, Davoust J, Fridman WH, Bonnerot C. Tyrosine-containing motif that transduces cell activation signals also determines internalization and antigen presentation via type III receptors for IgG. Nature. 1992;358:337–341. doi: 10.1038/358337a0. [DOI] [PubMed] [Google Scholar]
- 20.Weinshank RL, Luster AD, Ravetch JV. Function and regulation of a murine macrophage-specific IgG Fc receptor, FcγRα. J Exp Med. 1988;167:1909–1925. doi: 10.1084/jem.167.6.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones B, Tite JP, Janeway CA. Different phenotypic variants of the mouse B cell tumor A20/2J are selected by antigen- and mitogen-triggered cytotoxicity of L3T4-positive, IA-resticted T cell clones. J Immunol. 1986;136:348–356. [PubMed] [Google Scholar]
- 22.Guillet JG, Lai MZ, Briner TJ, Smith JA, Gefter ML. Interaction of peptide antigens and class II major histocompatibility complex antigens. Nature. 1986;324:260–262. doi: 10.1038/324260a0. [DOI] [PubMed] [Google Scholar]
- 23.Lai MZ, Ross DT, Guillet JG, Briner TJ, Gefter ML, Smith JA. T lymphocyte response to bacteriophage lambda repressor cI protein. Recognition of the same peptide presented by Ia molecules of different haplotypes. J Immunol. 1987;139:3973–3980. [PubMed] [Google Scholar]
- 24.Li WF, Fan MD, Pan CB, Lai MZ. T cell epitope selection: dominance may be determined by both affinity for major histocompatibility complex and stoichiometry of epitope. Eur J Immunol. 1992;22:943–949. doi: 10.1002/eji.1830220410. [DOI] [PubMed] [Google Scholar]
- 25.Cibotti R, Cabaniols JP, Pannetier C, Delarbre C, Vergnon I, Kanellopoulos JM, Kourilsky P. Public and private Vβ T cell receptor repertoires against hen egg white lysozyme (HEL) in nontransgenic versus HEL transgenic mice. J Exp Med. 1994;180:861–872. doi: 10.1084/jem.180.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper M, Lema F, Boulot G, Poljack RJ. Antigen specific and cross-reactivity of monoclonal anti-lysozyme antibodies. Mol Immunol. 1987;2:97–108. doi: 10.1016/0161-5890(87)90081-2. [DOI] [PubMed] [Google Scholar]
- 27.Justement LB, Kreiger J, Cambier JC. Production of multiple lymphokines by the A20.1 B cell lymphoma after cross-linking of membrane Ig by immobilized anti-Ig. J Immunol. 1989;143:881–889. [PubMed] [Google Scholar]
- 28.Pinet V, Malnati MS, Long EO. Two processing pathways for the MHC class II–restricted presentation of exogenous influenza virus antigen. J Immunol. 1994;152:4852–4860. [PubMed] [Google Scholar]
- 29.Lamaze C, Schmid SL. Recruitment of epidermal growth factor receptors into coated pits requires their activated tyrosine kinase. J Cell Biol. 1995;129:47–54. doi: 10.1083/jcb.129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- 31.Mellman I, Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol. 1984;98:1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellino F, Germain RN. Extensive trafficking of MHC class II–invariant chain complexes in the endocytic pathway and appearance of peptide-loaded classs II in multiple compartments. Immunity. 1995;2:73–88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 33.Brachet V, Raposo G, Amigorena S, Mellman I. Ii chain controls the transport of major histocompatibility complex class II molecules to and from lysosomes. J Cell Biol. 1997;137:51–65. doi: 10.1083/jcb.137.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin JP, Chu R, Harding CV. Early endosomes and a late endocytic compartment generate different peptide–class II MHC complexes via distinct processing mechanisms. J Immunol. 1997;158:1523–1532. [PubMed] [Google Scholar]
- 35.Kropshofer H, Vogt AB, Moldenhaur G, Hammer J, Blum JS, Hammerling GJ. Editing of HLA-DR peptide repertoire by HLA-DM. EMBO (Eur Mol Biol Organ) J. 1996;15:6144–6154. [PMC free article] [PubMed] [Google Scholar]
- 36.Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- 37.Amigorena S, Webster P, Drake J, Newcomb J, Cresswell P, Mellman I. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med. 1995;181:1729–1741. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Astier A, de la Salle H, de la Salle C, Bieber T, Esposito-Farese ME, Freund M, Cazenave JP, Fridman WH, Teillaud JL, Hanau D. Human epidermal Langerhans cells secrete a soluble receptor for IgG (Fc gamma RII/ CD32) that inhibits the binding of immune complexes to Fc gamma R+cells. J Immunol. 1994;152:201–212. [PubMed] [Google Scholar]
- 39.Lanzavecchia A. How can cryptic epitopes trigger autoimmunity? . J Exp Med. 1995;181:1945–1948. doi: 10.1084/jem.181.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salemi S, Caporossi AP, Boffa L, Longobardi MG, Barnaba V. HIVgp120 activates autoreactive CD4-specific T cell responses by unveiling of hidden CD4 peptides during processing. J Exp Med. 1995;181:2253–2257. doi: 10.1084/jem.181.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]