Abstract
It has been proposed that the increase in prevalence and severity of atopic disorders inversely correlates with exposure to infectious diseases such as tuberculosis. We have investigated this issue by combining an intranasal Mycobacterium bovis–Bacillus Calmette-Guérin (BCG) infection with a murine model of allergen, (ovalbumin [OVA]) induced airway eosinophilia. BCG infection either 4 or 12 wk before allergen airway challenge resulted in a 90–95 and 60–70% reduction in eosinophilia within the lungs, respectively, compared to uninfected controls. The inhibition of airway eosinophilia correlated with a reduced level of IL-5 production by T cells from the lymph node draining the site of OVA challenge. Interestingly, BCG infection of the lung had no effect on IgG1 and IgE OVA-specific serum immunoglobulin or blood eosinophil levels. Furthermore, BCG-induced inhibition of airway eosinophilia was strongly reduced in interferon (IFN)-γ receptor–deficient mice and could be partially reversed by intranasal IL-5 application. Intranasal BCG infections could also reduce the degree of lung eosinophilia and IL-5 produced by T cells after Nippostrongylus brasiliensis infection. Taken together, our data suggest that IFN-γ produced during the T helper cell (Th)1 immune response against BCG suppresses the development of local inflammatory Th2 responses in the lung. Most importantly, this inhibition did not extend to the systemic immunoglobulin response against OVA. Our data support the view that mycobacterial infections have the potential to suppress the development of atopic disorders in humans.
Asthma is an atopic disorder characterized by activation and recruitment of eosinophils to the lung resulting in chronic swelling and inflammation of the airways. The processes leading to allergic inflammation are controlled by the Th2 lymphocytes (1–4), which secrete IL-4 and IL-5 leading to enhanced production of IgE by B cells and the generation and recruitment of eosinophils, respectively (5–9). Th2 responses predominate in individuals suffering from atopic disorders or helminthic infections (3, 6, 9).
Atopic disorders, in particular asthma, are steadily increasing, with ∼20–30% of the population in developed countries affected (10–12). The reasons for the increase are not known, although it has been noticed that the increase in atopic disorders inversely correlates with a steady decline in the extent to which the population is exposed to major human diseases such as tuberculosis, measles, whooping cough, and influenza (11, 13). One of the general features of these infectious agents is that they induce characteristic Th1 type immune responses, which lead to an immunological environment rich in IFN-γ (6, 7, 14–16). As IFN-γ is viewed as a powerful suppressive mediator of Th2 activity, the lack of frequent exposure to such infections has been speculated to increase the risk of developing atopy (6, 7, 11, 13). Supporting this hypothesis has been a recent report demonstrating an inverse relationship between atopy and infection with, or exposure to, Mycobacterium tuberculosis (13). However, these data do not confirm that mycobacterial infections reduce predisposition to atopy as genetic factors contributing to susceptibility for atopy or tuberculosis could be mutually exclusive.
In this report we address the question of whether an infection with an attenuated form of Mycobacterium bovis, Bacillus Calmette-Guérin (BCG),1 can suppress the development of type 2 immune responses in the lungs of mice. For this purpose we infected mice intranasally with BCG and investigated whether these animals could generate Th2s leading to the accumulation of eosinophils into the airways. Type 2 responses in the lung were either induced by OVA challenge (17) or by infection with the helminth Nippostrongylus brasiliensis (18). In both cases the immune response is characterized by the accumulation of eosinophils into the airways and increased IgE serum levels, and correlates with the appearance of airway hyperresponsiveness (19). Here we show that a BCG infection of the lung strongly inhibits the development of airway eosinophilia and that this effect was dependent upon IFN-γ signaling.
Materials and Methods
Mice.
C57Bl/6J mice were bred and housed at the Wellington School of Medicine Animal Facility (Wellington, New Zealand). The IFN-γR−/− and IFN-γR+/+ 129/Sv/Ev mice were a gift from M. Aguet (University of Zurich, Zurich, Switzerland; reference 20). All animals used in the experiments were between 5 and 7 wk of age. The experimental procedures were approved by the animal ethics committee and were in accordance with University of Otago (Dunedin, New Zealand) guidelines for the care of animals.
OVA-induced Airway Inflammation.
Mice were injected intraperitoneally with 2 μg OVA (Sigma Chemical Co., St. Louis, MO) in 100 μl alum adjuvant (Serva, Heidelberg, Germany) on day 0 and boosted again intraperitoneally with 2 μg OVA/alum at day 14. 14 d after the second intraperitoneal immunization, mice were anesthetized by injection of a mixture of Ketamine and Xylazine (Sigma Chemical Co.), and 100 μg OVA in a 50-μl volume of PBS was administered by intranasal inoculation. Where indicated, rIL-5 (500 Units; Genzyme Corp., Cambridge, MA) was included in the PBS containing the OVA.
Infection of Mice with BCG or N. brasiliensis.
Mice were either infected intranasally, intraperitoneally, or subcutaneously with the indicated numbers of viable CFUs of BCG (Connaught, Willowdale, Canada). Mice were anesthetized as described above, to facilitate the intranasal infection. N. brasiliensis (1,000 L3 larvae) were injected intraperitoneally to establish infection.
Detection of Different Cell Types in the Blood and Bronchoalveolar Lavage Fluid.
At the indicated time points after intranasal OVA challenge or N. brasiliensis infection, the mice were killed. Blood smears were prepared, the trachea cannulated, and bronchoalveolar lavage (BAL) performed by flushing lung and airways 3 times with 1 ml PBS. BAL cells were counted and spun onto glass slides using a cytospin (Shandon Southern Products Ltd., Asmoor, UK) together with the blood smears stained with Dif-Quik (Dade, Aguada, Puerto Rico) according to the manufacturer's instructions. Percentages of macrophages, lymphocytes, neutrophils, and eosinophils were determined microscopically using standard histological criteria.
Culture Conditions of Cells.
Single-cell suspensions from the mediastinal LNs (MLN) (2 × 106 cells/ml) of the different groups of mice were prepared and cultured in IMDM medium (Sigma Chemical Co.) supplemented with sodium bicarbonate (3.024 g/liter), 10 μg/ml streptomycin, 10 units/ml penicillin, 50 μM 2-mercaptoethanol, and 10% fetal calf serum. The cell preparations were either stimulated with 10 μg/ml purified protein derivative (PPD) from M. bovis (Commonwealth Serum Laboratories, Victoria, Australia) or in microwells coated with a monoclonal antibody to CD3ε (145-2C11, 25 μg/ml) together with 200 units/ml recombinant human IL-2 (Ciba-Geigy, Basel, CH). After 48 h, the culture supernatants were harvested and tested for the presence of cytokines by ELISA.
Proliferation Assay.
Cells were cultured in microwells in the presence of medium alone, 10 μg/ml PPD (Commonwealth Serum Laboratories) or 5 μg/ml of Con A for 40 h. Proliferative responses were determined by [3H]thymidine (0.5 μCi/well, 2 Ci/ mmol) incorporation over the last 16 h of culture.
ELISA for the Detection of Cytokines and Igs.
For the detection of IFN-γ, IL-4, and IL-5 in the cell culture supernatants sandwich ELISAs with the following mAb recognizing two different epitopes of the respective lymphokines were used: biotinylated rat anti–mouse IFN-γ (AN-18.17.24) and unconjugated rat anti–mouse IFN-γ (R4-6A2), biotinylated rat anti–mouse IL-5 (TRFK4) and unconjugated rat anti–mouse IL-5 (TRFK5), and biotinylated rat anti–mouse IL-4 (BVD6-24G2) and unconjugated rat anti–mouse IL-4 (BVD4-1D11) (all antibodies were purchased from PharMingen, San Diego, CA). The assays were performed in polyvinyl chloride microtiter plates (Dynatech, Denkendorf, Germany) according to the instructions of the mAb manufacturer. The binding reactions were visualized with a conjugate of peroxidase-labeled streptavidin (Sigma Chemical Co.) and the substrate ABTS (Sigma Chemical Co.). Absorbance was read at 414 nm in an ELISA microplate reader (Anthos Hill, Salzburg, Austria). For quantification of the cytokines in the culture supernatants, titrations were performed with murine rIFN-γ (Sigma Chemical Co.), rIL-4, and rIL-5 (Genzyme Corp.).
OVA-specific Ig levels were determined by coating microtiter plates with either OVA (10 μg/well) or anti-IgE (1 μg/ml; the rat anti–mouse IgE mAb [3–11] was provided by C. Heusser, Novartis, Basel, Switzerland) and subsequently blocked with 10% BSA for 60 min at room temperature (RT). Twofold dilutions of serum were added and incubated for 2 h at RT. Appropriate dilutions of detecting Ab- or OVA-labeled biotin (for the detection of OVA-specific IgE) and peroxidase-labeled streptavidin (Sigma Chemical Co.) were added for 1 h at RT. Anti–mouse IgG1 was from Serotec (Oxford, UK) and anti–mouse IgG2a from PharMingen. The substrate ABTS (Sigma Chemical Co.) was used as described above.
Histological and Hematological Analysis.
Tissues from BCG-infected and OVA-immunized mice were fixed in 10% phosphate-buffered formalin for 24 h and embedded in paraffin wax. Sections (2–3 μm) were cut and stained using standard histological protocols with hematoxylin and eosin. The stained sections were visualized by light microscopy.
Enumeration of BCG in the Organs of the Infected Mice.
Organs recovered from infected mice were homogenized in sterile water containing 0.5% Tween 80 (Sigma Chemical Co.). Numbers of viable mycobacteria in the lung, liver, and spleen were determined by plating serial 10-fold dilutions of organ homogenates on Middlebrook 7H11 agar (Difco Laboratories, Detroit, MI) supplemented with 10% Middlebrook OADC enrichment (Difco Laboratories). Colonies were counted after 21 d of incubation at 37°C in 9% CO2.
Statistical Analysis.
Statistical significance was analyzed by the Student's t test.
Results
Intranasal Infection of Mice with BCG Leads to a Polarized Th1 Immune Response in the Lung.
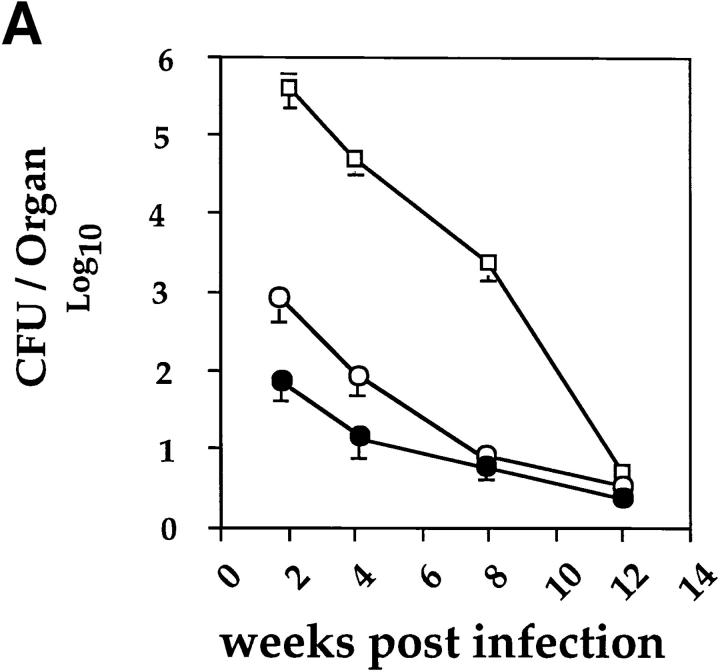
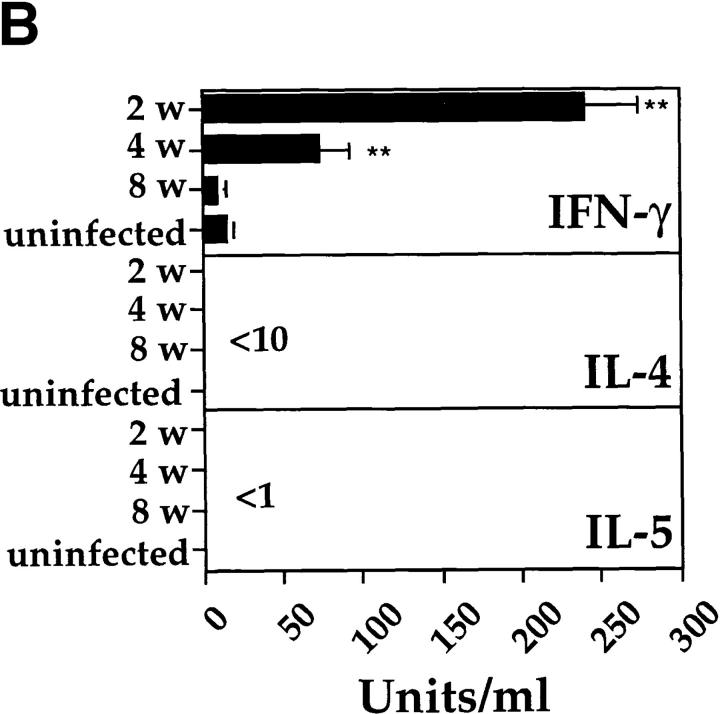
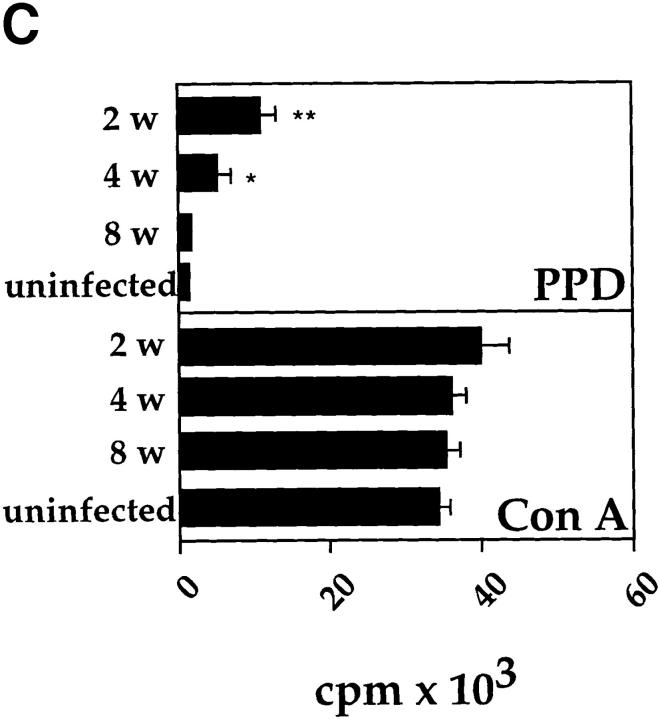
An intranasal infection model for BCG was established to investigate whether Th1 immune responses would reduce the development of Th2 responses in the lung. For this purpose, mice were anesthetized and intranasally inoculated with different infectious doses of BCG. 2–3 wk after intranasal infection with either 2 × 103, 2 × 104, or 2 × 105 CFUs of BCG, distinct granulomas could be detected within tissue sections from lungs. At doses > 5 × 105 CFUs, small granulomas were also detected in the liver and spleen (data not shown). Mice infected with BCG cleared the mycobacteria from the lung, liver, and spleen during the following weeks, with only a few persisting mycobacteria present after 12 wk of infection (Fig. 1 A). After 2, 4, or 8 wk after infection, cells isolated from the MLNs of killed mice were stimulated with PPD from M. bovis. The antigen-specific activation with PPD only resulted in the detection of IFN-γ with no IL-4 or IL-5 being detected (Fig. 1 B). No IL-4, and only very low levels of IL-5, could be detected even after in vitro polyclonal T cell stimulation with anti-CD3 and IL-2 (data not shown). The T cell response to PPD was strongest after 2 wk and had subsided by 8–12 wk of infection, as demonstrated by the kinetics of IFN-γ production by T cells and in vitro PPD-specific proliferation (Fig. 1, B and C). Taken together, the results clearly demonstrate that intranasal infection with BCG induces a Th1 immune response in the lung.
Figure 1.
Intranasal infection of mice with BCG induces a Th1 response in the lung. (A) Mycobacterial clearance from mice intranasally infected with 2 × 105 CFUs of BCG organisms. The course of infection in the lung (open squares), liver (open circles), and spleen (closed circles) was followed over 12 wk after infection. Data shown are the mean bacterial counts of tissues from nine mice (three separate experiments) with standard deviations. (B) IFN-γ, IL-4, and IL-5 production by T cells from MLNs after in vitro restimulation with PPD. Single-cell suspensions (2 × 105/well) from total MLNs of control and 2-, 4-, and 8-wk postinfected mice, were stimulated in vitro for 48 h with PPD (10 μg/ml). The level of cytokines present in the supernatants was determined by ELISA. Shown are the mean values of three separate experiments with standard deviation (for each experiment lymph nodes from three mice in each group were pooled). (C) [3H]thymidine uptake by mediastinal lymphocytes of control and BCG-infected mice after stimulation with PPD or the lectin Con A. LN cells (2 × 105 cells/well) from uninfected and BCG-infected mice were incubated with medium, PPD (10 μg/ml), or Con A (5 μg/ml) for 40 h, and then pulsed with [3H]thymidine for the last 16 h of the culture period. Mean [3H]thymidine uptake of triplicates with standard deviations are shown. Uptake of [3H]thymidine was between 500 and 1,000 cpm in all cultures containing cells and medium alone. The experiments were repeated three times with similar results. *P <0.05, **P <0.0001, compared to values obtained in cultures containing cells from uninfected mice.
BCG Infection in the Lung Strongly Suppresses the Development of OVA-induced Airway Eosinophilia.
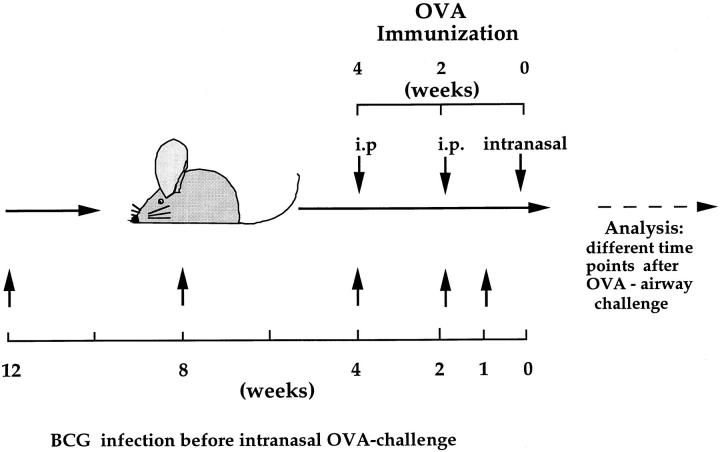
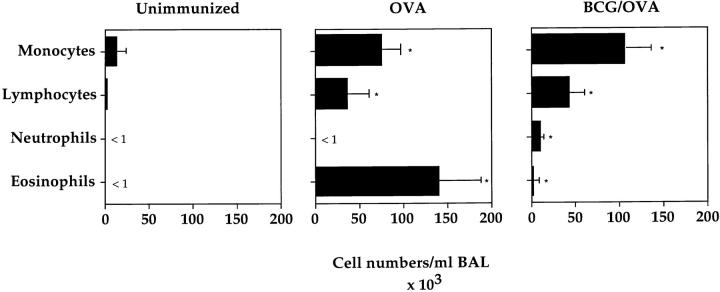
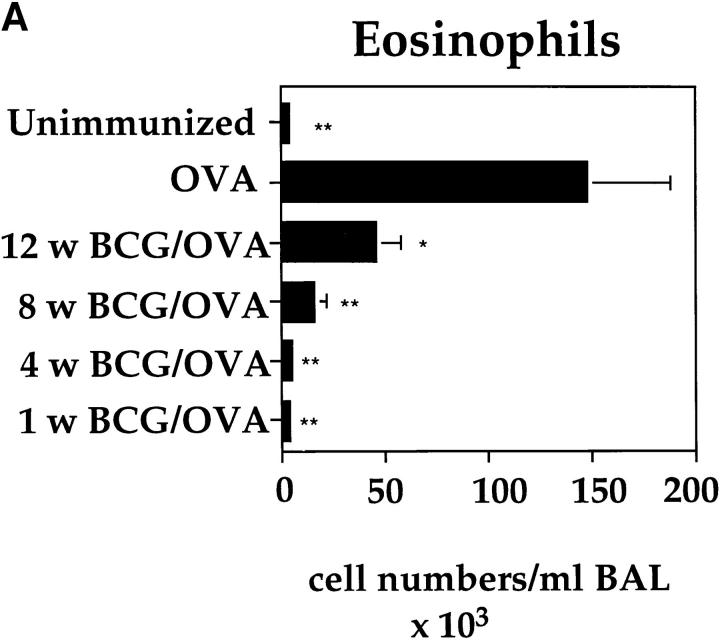
To investigate the influence of a BCG infection on the development of an OVA-specific Th2 response in the lung, an experimental protocol was developed in which BCG-infected and OVA-sensitized mice were airway challenged with OVA (Fig. 2). Intranasal infections were performed using 2 × 105 CFUs of BCG as this dose results in a strongly polarized infection in the lung, but does not cause systemic disease (see above). A BCG infection 4 wk before OVA airway challenge had a profound suppressive effect on the allergen-induced accumulation of eosinophils into the lung (Fig. 3). The decrease in eosinophils was accompanied by an increase in neutrophils, lymphocytes, and monocytes in the BAL fluid of BCG-infected animals. Histological examinations of lung tissue from mice treated with BCG and OVA or OVA alone confirmed the strong reduction in lung eosinophil numbers after BCG infection (data not shown). The closer the time of BCG infection was to the OVA airway challenge, the more pronounced the suppressive effect was on the lung eosinophilia (Fig. 4 A). Interestingly, even 12 wk past the first infection, a suppressive effect on eosinophil accumulation into the lung could be observed.
Figure 2.
Experimental design used to investigate the influence of a BCG infection on OVA-induced eosinophilia in the lung. Mice were subjected to the OVA immunization scheme (see Materials and Methods) and intranasally infected with 2 × 105 CFUs of BCG organisms 1, 4, 8, or 12 wk before intranasal OVA challenge.
Figure 3.
Intranasal infection of BCG strongly inhibits the development of airway eosinophilia. OVA-immunized mice were either intranasally infected with 2 × 105 CFUs of BCG 4 wk before OVA airway challenge or left uninfected as an OVA control (see Fig. 2). 6 d after the OVA airway challenge, a BAL was performed on both groups of mice as well as unimmunized mice. BAL cells were counted, stained with haematoxylin and eosin, and the different cell types were identified microscopically. Shown are the average numbers of cells, with standard deviation, present in the BALs of the different groups of mice (five mice per group). The experiments were repeated five times with similar results. *P <0.01, compared to values obtained in unimmunized mice.
Figure 4.
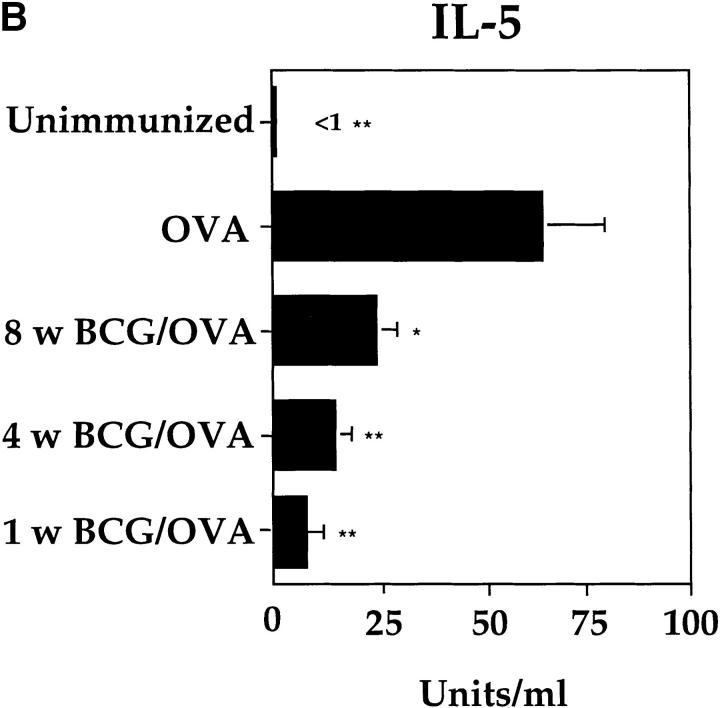
BCG-induced suppression of airway eosinophilia and reduction of IL-5 secretion by T cells is dependent upon the elapsed time from BCG infection. OVA-immunized mice were either subjected to BCG infection at 1, 4, 8, or 12 wk before OVA airway challenge or left uninfected. Age-matched mice not subjected to the OVA immunization (Unimmunized) were included as controls. 6 d after OVA airway challenge, BALs were performed on each group of mice. Shown are the average numbers of eosinophils present in the BALs of the different groups (n = 5 for each group) of mice (A). In parallel to the BALs, single-cell suspensions (2 × 105 cells/well) from MLNs of the different groups of mice were prepared and stimulated in vitro for 48 h on anti-CD3–bound plates in the presence of IL-2. Shown is the amount of IL-5 present in the MLN cultures from three individual mice per group with standard deviation (B). The experiments were repeated at least three times with similar results. *P <0.05, **P <0.0001, compared to values obtained in OVA only immunized mice.
Eosinophil recruitment and development is dependent upon the secretion of IL-5 (21, 22). Therefore, it was investigated whether the BCG induced suppression of airway eosinophilia was accompanied by reduction of Th2s producing IL-5 in the lung. We found that BCG infection 1, 4, or 8 wk before OVA challenge resulted in marked reduction in IL-5 production by restimulated T cells from the draining lymph node (Fig. 4 B). In the experiments shown in Figs. 3 and 4, responses were measured 6 d after OVA challenge, since both eosinophilia and T cell reactivity could readily be monitored at this time point. Eosinophil numbers and IL-5 production were similarly reduced when the response was analyzed 3 or 10 d after OVA challenge in mice infected with BCG 4 wk before OVA airway challenge (data not shown). These data further establish that the observed reduction of airway eosinophilia and IL-5 production by T cells was not due to a shift in the kinetics of the OVA-induced Th2 response.
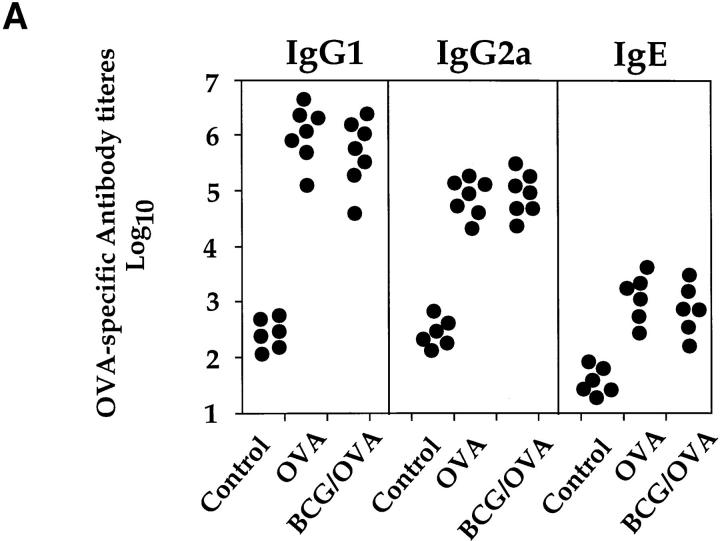
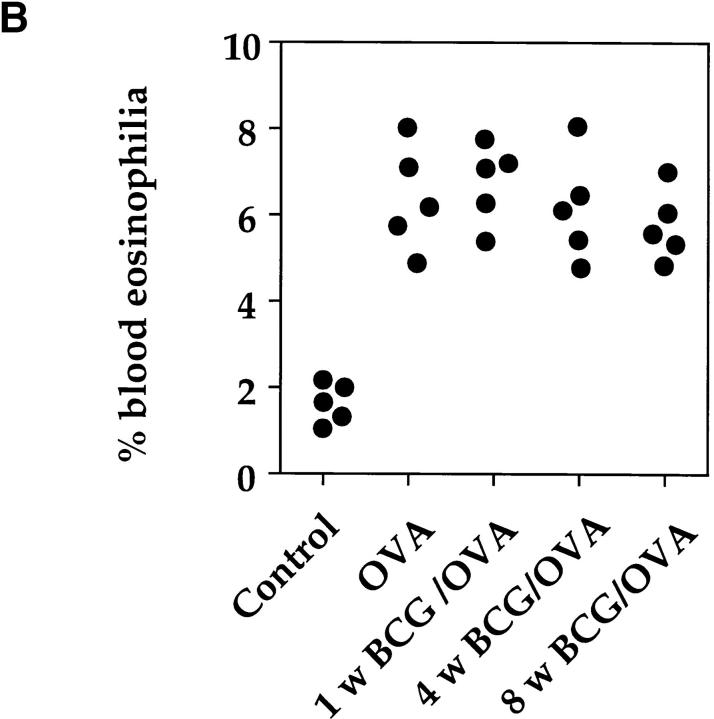
Even though BCG infection had a suppressive effect on both T cells secreting IL-5 and eosinophil accumulation into the airways, OVA-specific IgG1, IgG2a, and IgE serum levels were not reduced or altered (Fig. 5 A). Furthermore, in mice infected with BCG 1, 4, or 8 wk before OVA challenge, blood eosinophilia was not reduced compared to mice only immunized with OVA (Fig. 5 B).
Figure 5.
BCG infection of the lung did not alter the development of OVA-specific IgG1, IgG2a, or IgE antibodies or blood eosinophilia. Mice were treated as described in the legend to Fig. 3. Serum and blood smears were prepared 10 d after OVA airway challenge and OVA-specific antibody levels were determined by ELISA (A). Values represent serum antibody titers from individual mice. Titers were determined by plotting A414 against the logarithm of the reciprocal of the serum dilution and taking the midpoint of the linear section of the sigmoid curve produced. Blood smears were stained with hematoxylin and eosin and the different cell types were identified microscopically. Shown is the percentage of eosinophils present in the blood of individual mice (B). As a control, sera and blood smears from age-matched uninfected mice were also analyzed.
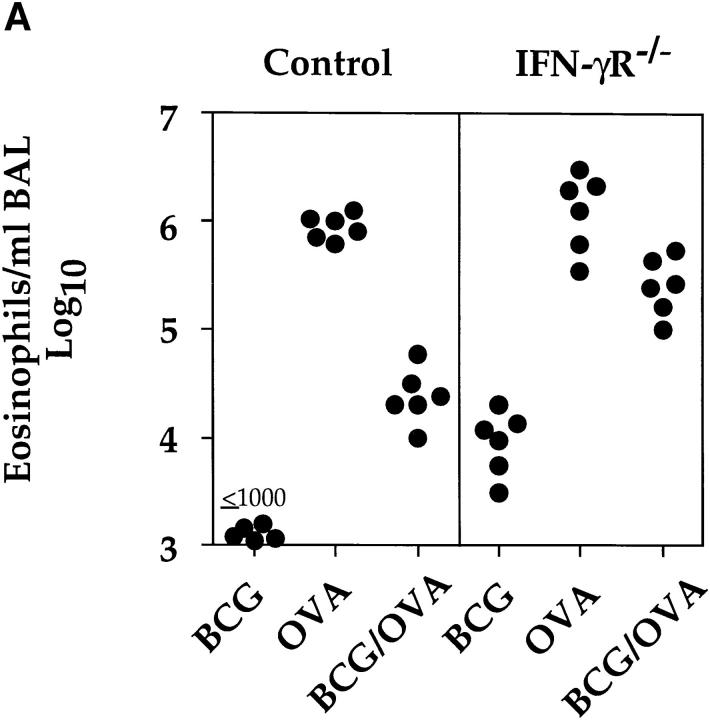
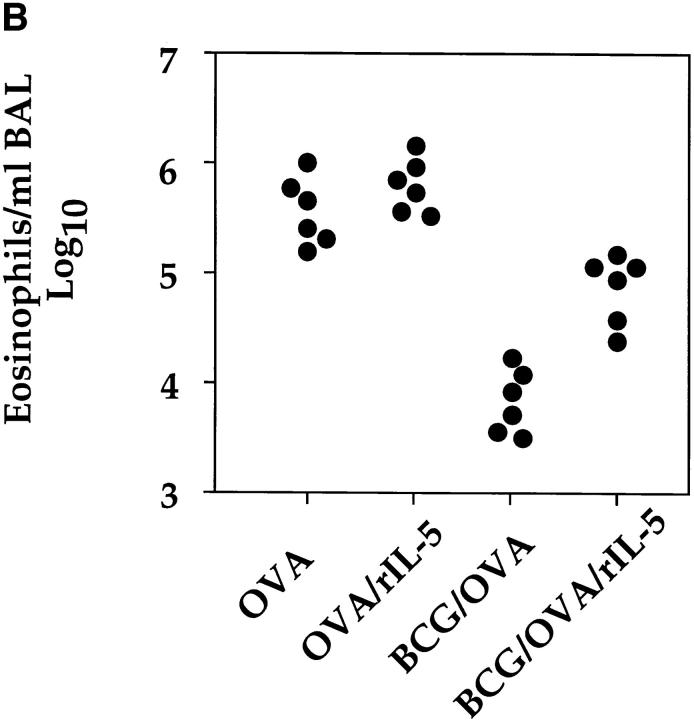
BCG-induced Suppression of Airway Eosinophilia Was Dependent upon IFN-γ Signaling and Could Be Reversed by Intranasal Administration of IL-5.
IFN-γ has been shown to inhibit the development of Th2s (23–26). To address the question of whether IFN-γ was mediating the observed inhibition of airway eosinophilia, IFN-γR−/− animals were subjected to the OVA immunization protocol and infected with BCG 2 wk before OVA challenge. The deletion of the IFN-γR strongly reduced the BCG infection-induced inhibition of airway eosinophilia (Fig. 6 A). In fact, as could be predicted, the IFN-γR−/− mice, but not controls develop a slight lung eosinophilia after BCG infection. Based on the results obtained in the IFN-γR−/− mice, the most likely explanation for our results is that IFN-γ induced by the BCG infection in the lung suppressed the development or expansion of Th2s specific for OVA and secreting IL-5. To rule out the possibility that the BCG infection directly interfered with eosinophil homing to the lung, we investigated whether the intranasal administration of IL-5 could restore accumulation of eosinophils into the airways of OVA-primed and BCG-infected mice. The inhibitory effect of a BCG infection on OVA-induced airway eosinophilia could be partially reversed by intranasal administration of IL-5 to ∼20–25% of the level observed in OVA only primed and airway-challenged mice (Fig. 6 B).
Figure 6.
BCG-induced suppression of airway eosinophilia was dependent upon IFN-γ signaling and could be reversed by the administration of IL-5. (A) 12 control (129/Sv/Ev) and 12 IFN-γR−/− mice were subjected to the OVA immunization protocol. From these mice, half were also infected with 2 × 105 CFUs of BCG organisms 2 wk before OVA airway challenge. 6 d after intranasal OVA challenge, BALs were performed and numbers of eosinophils determined as described in the legend to Fig. 3. As controls, BALs were also prepared from non–OVA-immunized wild-type 129/Sv/Ev and IFN-γR−/− mice that had been infected with 2 × 105 BCG organisms for 20 d. Shown are the numbers of eosinophils present in the BALs of six individual mice per group. (B) Four groups of mice were subjected to the OVA immunization protocol, two of which were also intranasally infected with 2 × 105 CFUs of BCG 4 wk before OVA airway challenge. OVA-primed or OVA-primed and BCG-infected mice were either intranasally challenged with OVA or with a combination of OVA and IL-5 (500 units) and the numbers of eosinophils present in the BALs were determined 6 d after airway challenge. Shown are the numbers of eosinophils present in the BALs of six individual mice per group.
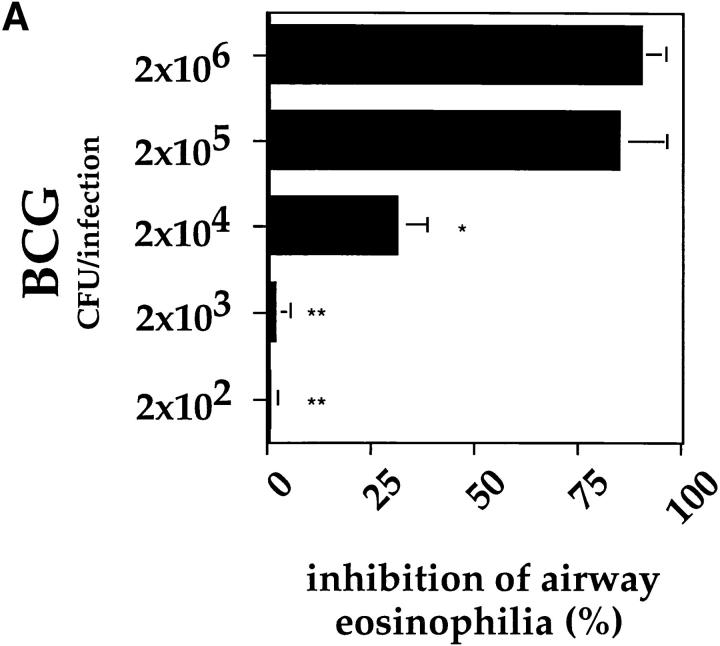
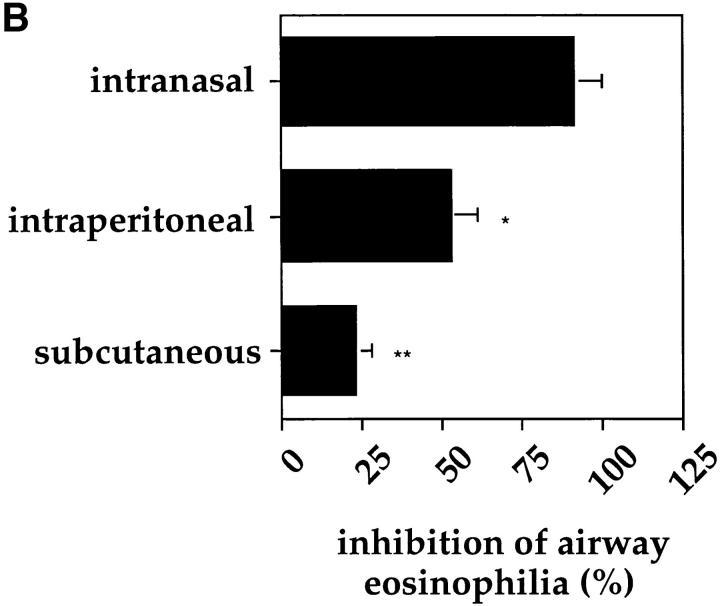
Reduction of Airway Eosinophilia Was Dependent upon the Dose of Mycobacteria Used and the Route of Infection.
In all experiments shown so far, a standard dose of 2 × 105 BCG organisms was used for the intranasal infections. To assess the number of BCG organisms necessary to induce the suppressive effect on the development of airway eosinophilia, mice were infected with different doses of BCG and subjected to the OVA immunization protocol. The use of 2 × 106 CFUs of BCG for infection 4 wk before OVA challenge did not result in a higher degree of suppression than did a dose of 2 × 105 organisms. However, the suppressive effect was already markedly reduced using 2 × 104 and no longer detectable using 2 × 103 organisms (Fig. 7 A). Moreover, the inhibitory effect of BCG infection on the accumulation of eosinophils into the airways was dependent upon the route of infection. Intranasal infection was superior to intraperitoneal or subcutaneous infection in its ability to reduce airway eosinophilia (Fig. 7 B). Taken together, these experiments clearly demonstrate that the reduction in airway eosinophilia was both dependent upon the amount of BCG organisms used and the route of infection.
Figure 7.
Inhibition of airway eosinophilia was both dependent upon the dose and route of BCG infection. Mice were subjected to the OVA immunization protocol and infected with different doses of BCG (A) or with 2 × 105 CFUs of BCG organisms either intranasally, intraperitoneally, or subcutaneously (B). All infections were performed 4 wk before OVA airway challenge. 6 d after OVA airway challenge BALs were prepared and treated as described in the legend to Fig. 3. Shown is the mean inhibition with standard deviation of the response from five age-matched mice. The experiments were repeated three times with similar results. *P <0.01, **P <0.0001, compared to percentage of inhibition using 2 × 106 BCGs (A) or intranasal infection (B).
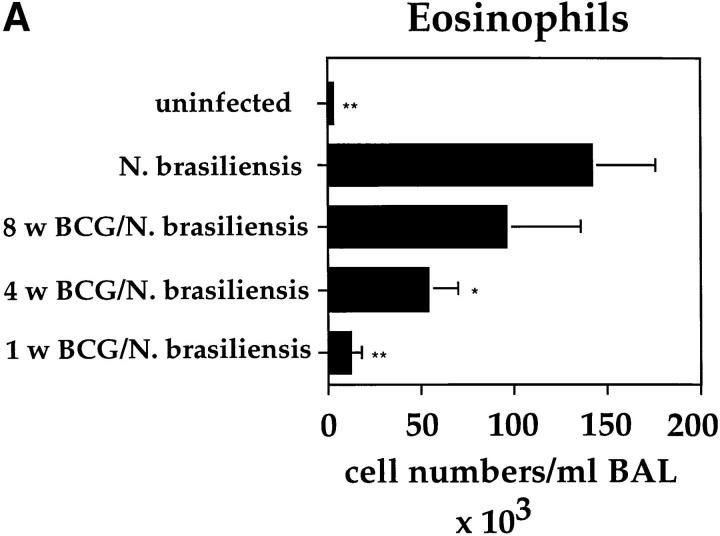
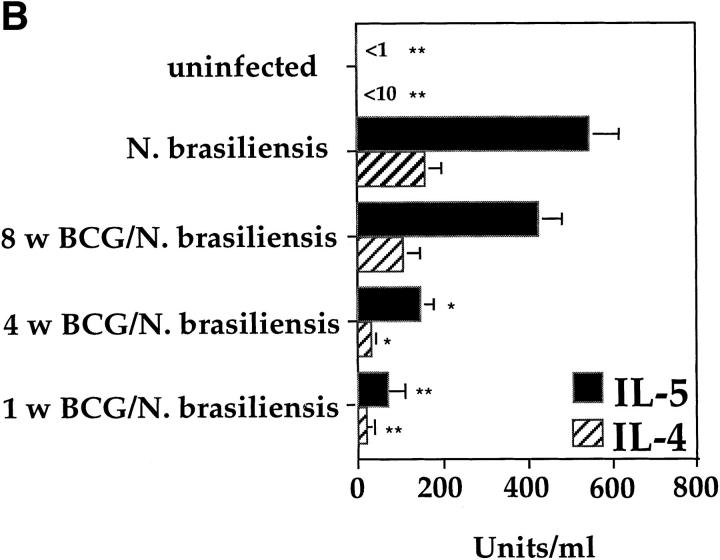
BCG Infection in the Lung Partially Suppressed the Development of Airway Eosinophilia Induced by N. brasiliensis Infection.
The Th2 response in the lung induced by OVA immunization leads to the secretion of relatively low levels of IL-4 and IL-5 by T cells from the lung or MLNs after in vitro stimulation (Fig. 4 B and data not shown). We next investigated whether BCG infections could also suppress the development of a strong natural Th2 response induced through the infection with the helminth N. brasiliensis. Intranasal BCG infections were able to suppress accumulation of eosinophils into the lung after N. brasiliensis infection (Fig. 8 A). However, the accumulation of eosinophils into the lung was only strongly inhibited when the BCG infection was done 1 wk before the N. brasiliensis infection. Infection with BCG before N. brasiliensis only reduced eosinophil numbers by ∼50% and only 30% when performed 4 and 8 wk before the helminth infection, respectively. The suppression of airway eosinophil accumulation correlated strongly with a marked reduction in IL-4 and IL-5 production by T cells from the MLNs, measured after in vitro stimulation on anti-CD3–coated plates (Fig. 8 B). It clearly appears that the closer the BCG infection was to that of N. brasiliensis, the less IL-4 and IL-5 could be detected in the cultures of T cells from the MLNs.
Figure 8.
Intranasal infection with BCG reduces airway eosinophilia and the secetion of IL-4 and IL-5 by T cells after infection with the helminth N. brasiliensis. Mice were intranasally infected with 2 × 105 CFUs of BCG at the different time points indicated before intraperitoneal infection with 1,000 L3 larvae of N. brasiliensis. 10 d after N. brasiliensis infection, BAL and MLN cultures were prepared. Shown are the average numbers with standard deviations of eosinophils present in the BALs of five individual mice per group (A). MLN cells from the different groups of mice were stimulated in vitro for 48 h on anti-CD3–bound plates in the presence of IL-2. Shown is the mean amount of IL-4 and IL-5 produced by T cells in the MLN cultures from five individual mice per group with standard deviations (B). The experiments were repeated three times with similar results. *P <0.01, *p<0.001, compared to values obtained in cultures containing cells from mice only infected with N. brasiliensis.
Interestingly, the suppressive effect of a BCG infection on a developing Th2 response in the lung was more pronounced in the model of OVA-induced airway eosinophilia than after N. brasiliensis infection. This demonstrates that BCG infection can very efficiently inhibit the development of airway eosinophilia induced by allergens, but not as significantly as that induced by a helminth infection (Figs. 4 and 8). These findings suggest that the amount of IFN-γ induced by infection with BCG is sufficient to suppress the relatively weak Th2 response induced by an OVA immunization, but not the strong Th2 response induced by N. brasiliensis infection.
Discussion
The results presented in this report clearly demonstrate that BCG infection of the lung suppressed the development of OVA-induced airway eosinophilia and support the hypothesis that a reduction of infectious diseases may contribute to the increase in severity and prevalence of atopic disorders in humans. The closer in time the BCG infection was to OVA challenge, the greater the degree of suppression of eosinophil accumulation in the airways. The inhibition of eosinophil accumulation in the airways correlated with a reduction in the capacity of draining LN T cells to produce IL-5 upon in vitro restimulation. Conversely, the stronger the inhibition of airway eosinophilia, the less IL-5 could be detected. Furthermore, the inhibitory effect of BCG on airway eosinophilia was dependent upon the dose of infection, with BCG numbers ⩾2 × 105 CFUs resulting in maximal inhibition. The route of infection also influenced BCG-induced suppression of airway eosinophilia, with intranasal infection being superior to intraperitoneal or subcutaneous infection in its ability to reduce airway eosinophilia.
We used IFN-γR−/− mice to investigate whether IFN-γ was the Th1-related cytokine responsible for the observed inhibition of airway eosinophilia. IFN-γR−/− mice receiving both the OVA immunization protocol and infection with BCG developed profound airway eosinophilia indicating that the BCG-induced inhibition was mediated by IFN-γ. However, the level of eosinophilia in the IFN-γR−/− mice infected with BCG was somewhat lower than that detected in mutant mice only subjected to the OVA immunization protocol. This indicates that other factors besides IFN-γ may play a role in the observed inhibition of airway eosinophilia. Further analysis of the immune response against BCG in IFN-γR−/− mice revealed that a background lung eosinophilia also developed, which was not observed in uninfected mutant or control mice. The Th2 response was characterized by T cells secreting IL-5 after PPD-specific activation in vitro, lung and blood eosinophilia, and increased IgE serum levels (Erb, K.J., J. Kirman, B. Delahunt, and G. Le Gros, manuscript in preparation). This suggests that IFN-γ expression generally leads to the inhibition of Th2 immune responses in vivo. Our observations are supported by previous reports showing that IFN-γ suppresses the development of Th2s both in vitro and in vivo (23–26). Furthermore, IFN-γ–mediated suppression of Th2 responses in the lung has also been documented (27–29). In view of these reports, the most likely explanation for our results is that the production of IFN-γ during an active BCG infection blocks the expansion of Th2s secreting IL-5 in the lung. Alternatively, the BCG infection could directly interfere with the homing of Th2s into the lung. Impaired homing of Th2s into inflamed sites dominated by Th1 responses has recently been reported (30). Irrespective of the underlying mechanism, reduction of IL-5 production in the lung seems to be one of the major factors responsible for the observed inhibition of airway eosinophilia, since accumulation of eosinophils into the airways is highly dependent upon IL-5 (21, 22) and administration of IL-5 into the lung at least partly restored airway eosinophilia (20–25% of the levels observed in control mice). However, since IL-5 administration into the lung did not totally restore eosinophil accumulation into the airways, we cannot rule out that the BCG infection also induced the production of some unknown factor thus contributing to the observed suppression of airway eosinophilia. Furthermore, MacLean et al. recently reported that the production of the chemokine eotaxin, which leads to the accumulation of eosinophils, was dependent upon Th2s (31). The incomplete restoration of airway eosinophilia after IL-5 administration may therefore also reflect an eotaxin deficit induced by the BCG infection in the lung via the suppression of Th2 development. Interestingly, BCG infection 12 wk before OVA challenge still resulted in a strong inhibition (60–70%) of airway eosinophilia. At this time point, both the inflammatory and T cell response against BCG had already largely subsided with only a few persisting BCG organisms still detected in the lung.
One important therapeutic implication from our study is that the suppressive effect of BCG infection on the OVA-specific Th2 response was localized to the lung and did not influence blood eosinophil or OVA-specific IgG1 or IgE serum levels. This demonstrates that intranasal inoculation with BCG only suppressed the local Th2 immune response induced after OVA airway challenge and not the systemic one induced by the intraperitoneal immunization with OVA in alum adjuvant. In this context, it is important to note that OVA airway challenge is not necessary to induce the systemic Th2 response since blood eosinophilia and OVA-specific IgE are already detectable before OVA-intranasal challenge (our unpublished observation).
Szabo et al. recently published a report showing that IL-4 downmodulated the expression of the IL-12R β chain on T cells in vitro (32). The authors suggest that this downmodulation leads to the generation of Th2s, since they are no longer responsive to IL-12–mediated signaling. Importantly, the presence of IFN-γ inhibited this IL-4–induced effect, thereby leading to the development of only Th1s. The observed strong inhibition of the OVA-induced Th2 response in the lung could be due to this IFN-γ–mediated effect. However, the results of the N. brasiliensis experiments lead us to the conclusion that the suppressive effect of a BCG infection on airway eosinophilia can at least in part be overridden by the induction of a strong Th2 response. It is therefore tempting to speculate that the balance between IFN-γ and IL-4 present at the site of T cell activation determines whether Th1 and or Th2 cells are generated, with high IL-4 levels being able to overcome the inhibitory effect of IFN-γ on Th2 development in vivo. Supporting this view are results showing that infection of mice with respiratory syncytial virus, which induces the production of IFN-γ, did not inhibit eosinophilic inflammation into the airways after OVA sensitization (33). Inhibition of airway eosinophilia might therefore be limited to mycobacterial infections that induce strong and relatively long lasting IFN-γ responses in the lung (14, 15).
In conclusion, our data demonstrate that a localized BCG infection in the lungs of mice can inhibit the accumulation of eosinophils into the airways and reduce the amount of IL-5 secreted by the T cells from the MLNs, and that the inhibitory effect was at least to a great degree dependent upon IFN-γ signaling and could be partially reversed by the presence of IL-5 in the lung. Importantly, an allergic type Th2 response, induced by OVA immunization, was more efficiently suppressed than an infectious type induced by a helminth infection. Our results support the hypothesis that infectious diseases of the lung may help decrease the severity and prevalence of atopic disorders in humans. Furthermore, BCG immunization of children may potentially be helpful in reducing the risk of developing severe asthma, which is strongly associated with eosinophil-induced inflammation (3). However, the results presented in this report suggest that for BCG to have the most pronounced effect, it should be administered directly into the lung. A recent report demonstrating that subcutaneous immunization of children with BCG had no inhibitory effect on the development of asthma, suggests that this view may be true (34).
Footnotes
This work was supported by an AIDS Scholarship, Bundesministerium für Bildung und Forschung (Germany) to Klaus J. Erb, and a Wellcome Trust Research Fellowship to Graham LeGros.
The authors would like to thank Paige Lacy, Annelise Schimpl, and especially Paul Horrocks for the critical reading of this manuscript.
Address correspondence to Klaus Erb, Zentrum für Infektionsforschung, Universität Würzburg, Röntgenring 11, D-97070 Würzburg, Germany. Phone: 49-931-312474; Fax: 49-931-312578; E-mail: zinf036@rzroe.uni-wuerzburg.de
Abbreviations used in this paper: BAL, bronchoalveolar lavage; BCG, Bacillus Calmette-Guérin; MLN, mediastinal LN; PPD, purified protein derivative; RT, room temperature.
References
- 1.Carballido JM, Carballido-Perrig N, Terres G, Heusser CH, Blaser K. Bee venom phospholipase A2-specific T cell clones from human allergic and non-allergic individuals: cytokine patterns change in response to the antigen concentration. Eur J Immunol. 1992;22:1357–1363. doi: 10.1002/eji.1830220605. [DOI] [PubMed] [Google Scholar]
- 2.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 3.Del Prete G. Human Th1 and Th2 lymphocytes: their role in the pathophysiology of atopy. Allergy. 1992;47:450–455. doi: 10.1111/j.1398-9995.1992.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 4.Erb KJ, LeGros G. The role of Th2 type-CD4+ T cells and CD8+ T cells in asthma. Immunol Cell Biol. 1996;74:206–209. doi: 10.1038/icb.1996.29. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone: definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 6.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 7.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets? . Curr Opin Immunol. 1996;8:336–342. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 8.Sher A, Gazzinelli RT, Oswald IP, Clerici M, Kullberg M, Pearce EJ, Berzofsky JA, Mosmann TR, James SL, Morse HB. Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 9.Romagani S. Induction of Th1 and Th2 responses: a key role for the “natural” immune response? . Immunol Today. 1992;13:379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 10.Bräbäck L, Breborowiez A, Dreborg S, Knutsson A, Pieklik H, Björksten B. Atopic sensitization and respiratory symtoms among Polish and Swedish school children. Clin Exp Allergy. 1994;26:621–623. doi: 10.1111/j.1365-2222.1994.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 11.Cookson WOCM, Moffatt MF. Asthma: an epidemic in the absence of infection? . Science. 1997;275:41–42. doi: 10.1126/science.275.5296.41. [DOI] [PubMed] [Google Scholar]
- 12.Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? . Thorax. 1994;49:171–174. doi: 10.1136/thx.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 14.Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. . J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 16.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 17.Harris N, Peach R, Naemura J, Linsley PS, LeGros G, Ronchese F. CD80 costimulation is essential for the induction of airway eosinophilia. J Exp Med. 1997;185:177–182. doi: 10.1084/jem.185.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopf M, Le GG, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 19.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 21.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, Matthaei KI. IL-5–deficient mice have a developmental defect in CD5(+) B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 22.Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 23.Gajewski TF, Fitch FW. Anti-proliferative effect of IFN-gamma in immune regulation: IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- 24.Donckier V, Abramowicz D, Bruyns C, Florquin S, Vanderhaeghen ML, Amraoui Z, Dubois C, Vandenabeele P, Goldman M. IFN-gamma prevents Th2 cell–mediated pathology after neonatal injection of semiallogenic spleen cells in mice. J Immunol. 1994;153:2361–2368. [PubMed] [Google Scholar]
- 25.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon gamma–deficient mice infected with Leishmania major. . J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parronchi P, De Carli M, Manetti R, Simonelli C, Sampognaro S, Piccinni MP, Macchia D, Maggi E, Del PG, Romagnani S. IL-4 and IFN (alpha and gamma) exert opposite regulatory effects on the development of cytolytic potential by Th1 or Th2 human T cell clones. J Immunol. 1992;149:2977–2983. [PubMed] [Google Scholar]
- 27.Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lack G, Bradley KL, Hamelman E, Renz H, Loader J, Leung DYM, Larsen G, Gelfand EW. Nebulized IFN-gamma inhibits the development of secondary allergic responses in mice. J Immunol. 1996;157:1432–1439. [PubMed] [Google Scholar]
- 29.Li X, Chopra RK, Chou T, Schofield BH, Wills-Karp M, Huang S. Mucosal IFN-gamma gene transfer inhibits pulmonary allergic responses in mice. J Immunol. 1996;157:3216–3219. [PubMed] [Google Scholar]
- 30.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 31.MacLean JA, Ownbey R, Luster AD. T cell– dependent regulation of eotaxin in antigen-induced pulmonary eosinophilia. J Exp Med. 1996;184:1461–1469. doi: 10.1084/jem.184.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin 12 receptor beta 2 subunit expression in developing Th1 and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced sensitization to allergen. J Clin Invest. 1997;100:226–233. doi: 10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alm JS, Lilja G, Pershagen G, Scheynlus A. Early BCG vaccination and development of atopy. Lancet. 1997;350:400–403. doi: 10.1016/S0140-6736(97)02207-1. [DOI] [PubMed] [Google Scholar]