Abstract
Haemophilus influenzae undergoes phase variation in expression of the phosphorylcholine (ChoP) epitope, a structure present on several invasive pathogens residing in the human respiratory tract. In this study, structural analysis comparing organisms with and without this epitope confirmed that variants differ in the presence of ChoP on the cell surface–exposed outer core of the lipopolysaccharide. During nasopharyngeal carriage in infant rats, there was a gradual selection for H. influenzae variants that express ChoP. In addition, genotypic analysis of the molecular switch that controls phase variation predicted that the ChoP+ phenotype was predominant in H. influenzae in human respiratory tract secretions. However, ChoP+ variants of nontypable H. influenzae were more sensitive to the bactericidal activity of human serum unrelated to the presence of naturally acquired antibody to ChoP. Serum bactericidal activity required the binding of C-reactive protein (CRP) with subsequent activation of complement through the classical pathway. Results of this study suggested that the ability of H. influenzae to vary expression of this unusual bacterial structure may correlate with its ability both to persist on the mucosal surface (ChoP+ phenotype) and to cause invasive infection by evading innate immunity mediated by CRP (ChoP− phenotype).
Choline, a major constituent of eukaryotic membrane lipids, was previously thought to be an unusual structural feature of prokaryotes. Choline, in the form of choline phosphate or phosphorylcholine (ChoP),1 is found on the teichoic acid of Streptococcus pneumoniae and has recently been identified as a unique feature of LPS of Haemophilus influenzae (1–3). An mAb, TEPC-15, that specifically recognizes the ChoP structure has been used to show that the ChoP epitope is also expressed on pili of pathogenic Neisseriae and a protein of unknown function in Pseudomonas aeruginosa (reference 4, and Weiser, J.N., J. Goldberg, N. Pan, L. Wilson, and M. Virji, manuscript submitted for publication).
In the case of H. influenzae, choline is acquired from the environment and linked as ChoP to a hexose on the outer core region of the LPS (1, 3, 5). Since the LPS of H. influenzae lacks the multiple O-linked saccharide units characteristic of the enterobacteriaceae, ChoP is located on the cell surface. There is both inter- and intrastrain variation in structure of the LPS as a result of differences in the composition and linkage of saccharides in the outer core (6–8). An additional source of heterogeneity of the LPS is phase variation in the decoration of the LPS with the ChoP epitope. The expression of the ChoP epitope on the H. influenzae glycolipid requires the four genes of the lic1 locus (9). This locus is present in all strains in a representative survey of encapsulated and nontypable H. influenzae isolates, but is not required for normal growth in vitro (10). The first gene in lic1, licA, has homology to eukaryotic choline kinases. This gene contains an unusual feature consisting of variable numbers of tandem repeats of the sequence 5′-(CAAT)-3′ within the open reading frame (11). Variation in the number of repeat units by slipped-strand mispairing alters the alignment of initiation codons with the licA open reading frame creating a translational switch that results in spontaneous phase variation in expression of the ChoP epitope. The frequency of on-off switching in the expression of the ChoP epitope is ∼10−2–−3/generation, but varies from strain to strain depending on the length of the repetitive sequence (1).
A gene with similarity to licA has also been noted in S. pneumoniae and in various mycoplasma species, including Mycoplasma fermentans and pneumoniae (12, 13). The presence of the ChoP epitope on the cell surface of S. pneumoniae, H. influenzae, N. meningiditis, and M. pneumoniae, all major pathogens residing in the human respiratory tract, suggests that this structure may contribute to the ability of these species to occupy their niche on this mucosal surface. In S. pneumoniae, H. influenzae, N. meningitidis, N. gonorrhoeae, and P. aeruginosa there is also phase variation in the expression of the ChoP epitope (1, 14). Since these pathogens also commonly cause invasive infection, we addressed whether ChoP may contribute to the ability of organisms to reside in the human nasopharynx as well as their ability to survive in the bloodstream by evasion of humoral immunity. As a model system we have selected H. influenzae; it has already been documented that ChoP epitope expressing variants of a type b H. influenzae strain are more sensitive to the bactericidal effect of human serum than variants lacking this structure (1). It was postulated that the difference in serum sensitivity was a result of naturally acquired antibody against ChoP since the serum in this study had higher immunoglobulin G titers to LPS with the ChoP epitope compared to LPS without the ChoP epitope.
Materials and Methods
Bacterial Strains, Media, and Chemicals.
H. influenzae strains used for this study included H233, a nontypable clinical isolate (Strain A860516) obtained from the collection of Dr. Loek van Alphen (University of Amsterdam, The Netherlands). A kanamycin-resistant encapsulated type b strain (Eagan) and a mutant of this strain with a deletion/insertion spanning the four genes in lic1 were used in animal experiments (15, 16). Type b strain RM7004 was used for structural analysis (9). H. influenzae strains were grown in brain heart infusion broth supplemented with 1.5% fildes enrichment with or without 1% agar (Difco Laboratories, Detroit, MI). When specified, a chemically defined medium was used with laboratory strain Rd, for which this medium is suitable (17). Chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Structural Analysis of LPS.
LPS from TEPC-15–reactive and nonreactive colonies of strain RM7004 were isolated from BHI broth-grown cells by the hot phenol-water extraction procedure (8). Purified LPS were analyzed directly for sugar composition by complete acid hydrolysis and gas liquid chromatography of the derived acetylated reduced aldoses (8). O-deacylated LPS were prepared by mild hydrazine treatment for comparative analysis by electrospray ionization–mass spectrometry (ESI-MS) and 1H nuclear magnetic resonance (NMR) spectroscopy (18). ESI-MS was performed in the negative ion mode on a VG Quattro Mass Spectrometer (Micromass, Manchester, UK) by direct infusion of samples in 25% aqueous acetonitrile containing 0.5% acetic acid. 1H NMR spectra were recorded at 500 MHz on samples in D2O at 37°C on a Bruker AMX 500 spectrometer with acetone (methyl resonance: δ 2.225 ppm) as the internal chemical shift standard.
Colony Immunoblotting.
Colonies lifted onto nitrocellulose were immunoblotted to separate phase variants as previously described (1). The ChoP epitope on colonies lifted onto nitrocellulose was detected using a 1:10,000 dilution of mAbs against ChoP, TEPC-15, or HAS (Statens SerumInstitut, Copenhagen, Denmark), followed by alkaline phosphatase–conjugated anti– mouse IgA or IgM, respectively. In samples obtained from nasal washes, 20–200 colonies from each pup were immunoblotted to determine the proportion of ChoP+ and − colonies. After determining the phenotype with respect to ChoP expression, a single colony was used to inoculate 5-ml BHI broth cultures for extraction of chromosomal DNA for use as a template for the PCR by a published method (19).
Western Blot Transfer and Immunoblotting.
Electrotransfer of proteins separated on 12% SDS-PAGE onto Immobilon-P (Millipore Co., Bedford, MA) and Western blotting were carried out as previously described (1). Depletion of C-reactive protein (CRP) and/or IgG from the pooled normal human serum (NHS) was confirmed by Western blot analysis using an mAb to human CRP and polyclonal serum against human IgG, respectively.
Serum Bactericidal Assays.
Complement-mediated serum bactericidal activity was determined in NHS pooled from six random adult donors and stored at −80°C. Assays were performed with 20 μl of a suspension of midlog phase organisms (OD620 0.3–0.4) diluted to 105 CFU/ml with HBSS (GIBCO BRL, Gaithersburg, MD), 60 μl HBSS, 100 μl PBS, and 20 μl pooled NHS. After incubation for 60 min at 37°C with rotation, the assay was stopped by cooling to 4°C and dilutions were made for quantitative culture. To calculate the percentage of survival, colony counts were compared to controls in which complement activity had been eliminated by prior heating of the NHS to 56°C for 30 min. IgG was removed from NHS using a protein G column according to the manufacturer's instructions (Pharmacia Biotech AB, Uppsala, Sweden). CRP was removed from the NHS by incubation at 4°C for 30 min with an equal volume of ChoP-coupled agarose beads (Pierce Chemical Co., Rockford, IL) that had been washed in 0.02 M Tris (pH 7.2), 0.15 M NaCl, and 10 mM CaCl2. Purified human CRP was dialyzed in PBS to remove sodium azide and its concentration was determined using the Micro BCA protein assay (Pierce Chemical Co.). The purity of the human CRP was confirmed by SDS-PAGE. The requirement for Ca2+ in CRP-mediated serum killing was tested by preincubation of cells in CRP (5 μg/ml) for 15 min at 37°C in either the buffer described above or the same buffer in which 2 mM EDTA was substituted for the 10 mM CaCl2. After washing the cells in HBSS to remove unbound CRP, the cells were used in a bactericidal assay using CRP-depleted NHS as a source of complement activity.
Binding of CRP to H. influenzae Variants.
ChoP+ and − variants were grown to OD620 = 0.4, washed in PBS, and resuspended in 0.02 M Tris (pH 7.2), 0.15 M NaCl, and either 10 mM CaCl2 or 2 mM EDTA. Purified human CRP (1.0 μg/108 cells) was allowed to bind to the cells in the presence of 2.5% CRP-depleted NHS for additional buffering and to block nonspecific binding. After 15 min at 37°C with slow rotation, the cells were washed in PBS and an aliquot was removed and treated at 100°C in gel loading buffer for separation in 12% SDS-PAGE and Western blot analysis. CRP was detected using an mAb to human CRP followed by an alkaline phosphatase–conjugated anti–mouse immunoglobulin.
Choline Incorporation.
H. influenzae was radiolabeled by adding [3H]choline (New England Nuclear Co., Boston, MA) to the chemically defined medium (final concentration: 0.25 μCi/ml). H. influenzae was grown to an OD620 of 0.3 and washed three times in an equal volume of PBS. Aliquots were removed for colony immunoblotting and for determination of total cellular protein. The remainder of the sample was used to determine the incorporation of the label in whole cells.
Infant Rat Model of Nasopharyngeal Colonization.
Synchronized pregnant Sprague-Dawley rats were purchased from Taconic Farms (Germantown, N.Y.). 5-d-old infant rats were randomized among litters. For intranasal inoculations, 10 μl of PBS-washed midlog phase organisms adjusted to a density of 108 CFU/ml were inoculated into the left anterior naris. Animals receiving different phenotypic variants were housed in separate cages. Colony counts were performed to ensure the inocula were of the desired density. The nasopharynx was cultured by the slow instillation of 20–40 μl of sterile PBS into the left naris and withdrawal of the initial 10 μl discharge from the right naris. This ensured that the fluid had passed through the nasopharynx. To assess the quantity of organisms in nasal washes, serial dilutions in PBS were plated on supplement brain heart infusion agar containing kanamycin (20 μg/ml) to diminish the growth of non-Haemophilus contaminants.
Genotypic analysis of H. influenzae in human respiratory tract secretions.
Human respiratory tract secretions obtained from expectorated sputum or bronchoscopy were stored at −80°C and were included only if shown subsequently to have a predominant growth of H. influenzae on culture. Specimens containing purulent sputum were from nonbacteremic adult patients hospitalized with pneumonia. The diagnosis of H. influenzae pneumonia was based on microscopic examination of gram-stained sputum that revealed >20:1 ratio of inflammatory to epithelial cells and profuse gram-negative coccobacilli with only rare or no other organisms (20). DNA was extracted from 50 μl of homogenized specimen by the addition of 1 μl of 1 M Tris-HCl (pH 7.6) and 1 μl of proteinase K (5 μg/ml) followed by incubation at 35°C for 60 min and then for 20 min at 100°C. This DNA was used as a template to amplify the 5′ region of lic1 using Taq polymerase and primers 5′-TCGAATCCGCAAAAGTCACCATTTATTGTGAAG-3′ (forward) and 5′-TGGAATTCGTCCGCCTAATATGCCAGATAAC-3′ (reverse) by PCR. Conditions for PCR included thirty cycles in 1–2 mM Mg2+, denaturing at 94°C for 40 s, annealing at 50°C for 40 s and extension at 60°C for 90 s. The number of CAAT repeats was determined on the gel-extracted PCR product by sequencing across this region using primer 5′-TATTACATAATCTTTCAGCT-3′.
Results
Structural Analysis of Variants in Expression of the ChoP Epitope.
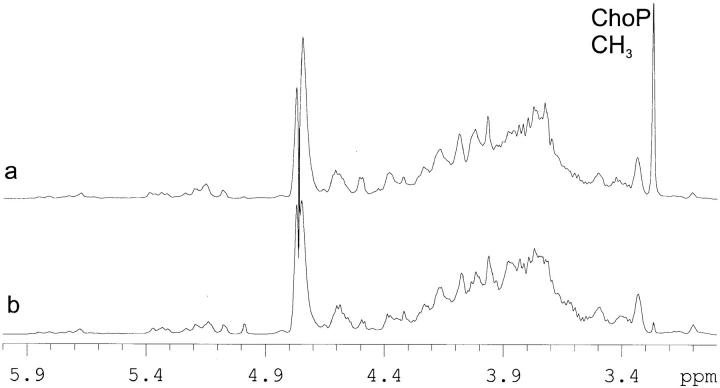
Comparative analysis of LPS from TEPC-15–reactive and –nonreactive colonies confirmed that the presence of ChoP on the outer core region of the LPS accounted for the difference. This was achieved by ESI-MS and 1H NMR analysis of samples of O-deacylated LPS from a type b strain. A notable feature of the 1H NMR that was particularly informative is the intense singlet at ca 3.25 ppm due to the ChoP methyl protons (Fig. 1); a signal of significant intensity is observed only in the O-deacylated LPS containing the ChoP epitope. It is now well established that H. influenzae express a heterogeneous mixture of LPS molecules. A structural model has been advanced, comprised of a conserved heptose-containing inner core trisaccharide moiety attached via phosphorylated 3-deoxy-d-manno-octulosonic acid to a lipid A component in which each of the heptose residues can provide a point for elongation by hexose containing oligosaccharide chains or for attachment of noncarbohydrate substituents (3, 8). ESI-MS of O-deacylated LPS from TEPC-15–nonreactive colonies revealed a series of related structures differing in the number of hexose residues (Table 1) in which populations of LPS glycoforms containing three to nine hexose residues were identified. An additional parallel series of ions corresponding to subpopulations of glycoforms containing ChoP substituents was detected only for O-deacylated LPS from TEPC-15–reactive colonies. In a lic2 mutant of this type b strain, detailed structural studies have established the molecular environment in which the ChoP structure is expressed (5).
Figure 1.
1H NMR spectra of O-deacylated LPS from TEPC-15–reactive (a) and –nonreactive (b) colonies of H. influenzae strain RM7004. The strong signal at ca 3.35 ppm in a is indicative of the presence of ChoP. This signal is present only to the extent of ca 1% in b. Spectra were recorded at 500 MHz in D2O containing 2 mM perdeutero EDTA and 10 mg/ml perdeutero SDS.
Table 1.
Negative Ion ESI-MS Data and Proposed Compositions for O-deacylated LPS from TEPC-15–nonreactive and –reactive Colonies of Type b H. influenzae (RM 7004)
| LPS glycoform | Observed [M-3H]3− ions* | Molecular mass | Proposed composition | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TEPC-15 non-reactive | TEPC-15 reactive | |||||||||
| Observed | Calculated‡ | |||||||||
| Hex3 | 812.0 | 812.1 | 2439.0 | 2439.2 | Hex3 × Hep3 × Pea1 × KDO1 × P1 × LipidA-OH | |||||
| 866.7 | 2603.1 | 2604.3 | ChoP × Hex3 × Hep3 × PEA1 × KDO1 × P1 × | |||||||
| LipidA-OH | ||||||||||
| Hex4 | 865.9 | 865.7 | 2601.1 | 2601.3 | Hex3 × Hep3 × Pea1 × KDO1 × P1 × LipidA-OH | |||||
| Hex4 × Hep3 × PEA2 × | ||||||||||
| 907.0 | 2724.0 | 2724.5 | KDO1 × P1 × LipidA-OH | |||||||
| 920.7 | 2765.1 | 2766.3 | ChoP × Hex4 × Hep3 × PEA1 × KDO1 × P1 × | |||||||
| LipidA-OH | ||||||||||
| 962.0 | 2889.0 | 2889.4 | ChoP × Hex4 × Hep3 × PEA1 × KDO1 × P1 × | |||||||
| LipidA-OH | ||||||||||
| Hex5 | 919.9 | 919.8 | 2762.4 | 2763.5 | Hex5 × Hep3 × PEA1 × KDO1 × P1 × LipidA-OH | |||||
| Hex5 × Hep3 × PEA1 × | ||||||||||
| 961.0 | 2886.0 | 2886.5 | KDO1 × P1 × LipidA-OH | |||||||
| 975.0 | 2928.0 | 2928.6 | ChoP × Hex5 × Hep3 × PEA1 × KDO1 × P1 × | |||||||
| LipidA-OH | ||||||||||
| 1016.0 | 3051.0 | 3051.5 | ChoP × Hex5 × Hep3 × PEA2 × KDO1 × P1 × | |||||||
| LipidA-OH | ||||||||||
| Hex6 | 974.1 | 974.0 | 2025.0 | 2925.6 | Hex6 × Hep3 PEA1 × KDO1 × P1 × LipidA-OH | |||||
| Hex6 × Hep3 × PEA2 | ||||||||||
| 1015.0 | 3048.0 | 3048.6 | KDO1 × P1 × LipidA-OH | |||||||
| 1029.0 | 3090.0 | 3090.7 | ChoP × Hex6 × Hep3 × PEA1 × KDO1 × P1 × LipidA-OH | |||||||
| Hex7 | 1027.8 | − | 3086.4 | 3087.8 | Hex7 × Hep3 × PEA1 KDO1 × P1 × LipidA-OH | |||||
| Hex8 | 1081.6 | − | 3247.8 | 3249.9 | Hex8 × Hep3 × PEA1 × KDO1 × P1 × LipidA-OH | |||||
| Hex9 | 1136.1 | − | 3411.3 | 3412.0 | Hex9 × Hep3 × PEA1 × KDO1 × P1 × LipidA-OH | |||||
The spectra were dominated by molecular peaks corresponding to triply deprotonated ions.
Average mass units used for calculation of molecular mass values based on proposed compositions as follows: Hex, 162.15; Hep, 192.17; KDO, 220.18; P, 79.98; PEA (phosphoethanolamine), 123.05; ChoP, 165.05.
Contribution of ChoP to Susceptibility to Serum Bactericidal Activity.
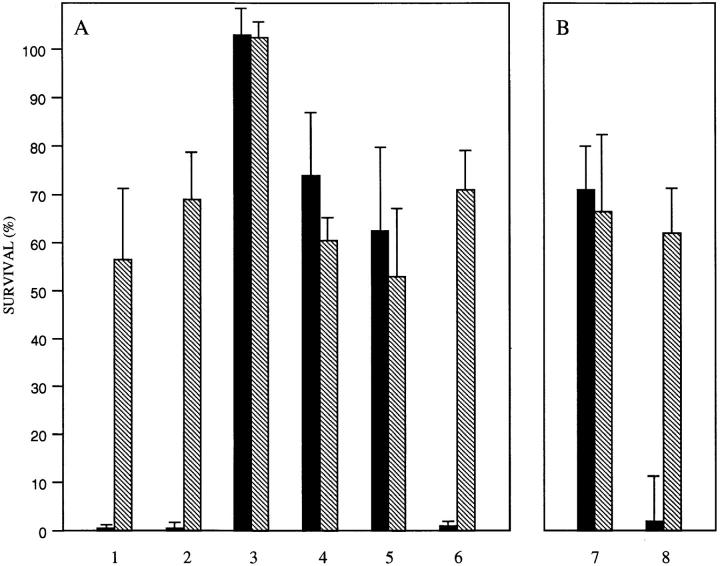
Phase variants of a nontypable clinical isolate differing in the expression of ChoP were compared for their ability to survive the bactericidal effect of human serum. Survival of the ChoP+ variant in 10% NHS for 60 min was <1% compared to the ChoP− variant of the same strain (Fig. 2 A). The difference in serum sensitivity did not appear to be caused by the presence of naturally acquired antibody against ChoP since removal of IgG had no effect on the increased susceptibility of the ChoP+ variant. However, killing of both H. influenzae variants appeared to be dependent on the classical rather than alternative pathway of complement activation as the addition of 0.05 M Mg2+ EGTA to chelate divalent cations completely eliminated serum killing of both variants (21).
Figure 2.
Serum bactericidal assay showing the contribution of immunoglobulin G and CRP to the survival of phase variants in the expression of ChoP in NHS. ChoP+ (solid bar) or ChoP− (hatched bar) variants of nontypable strain H233 were grown to midlog phase and treated for 60 min in 10% pooled NHS. The percentage of survival is the number of CFUs remaining compared to controls in which complement activity was inactivated. In A, determinations were carried out in the presence of untreated NHS (lane 1), NHS depleted of IgG (lane 2), NHS depleted of IgG with the addition of 0.05 M Mg2+ EGTA (lane 3), NHS depleted of IgG and CRP (lane 4), NHS depleted of CRP alone (lane 5), or NHS depleted of CRP with the addition of purified human CRP (5 μg/ml) (lane 6). In B, before use in the bactericidal assay with CRP-depleted NHS, phase variants were pretreated with purified human CRP in the presence of EDTA (lane 7) or Ca2+ (lane 8). Values are the geometric mean of at least three determinations in duplicate ± SD.
The Effect of CRP on Serum Killing of H. influenzae.
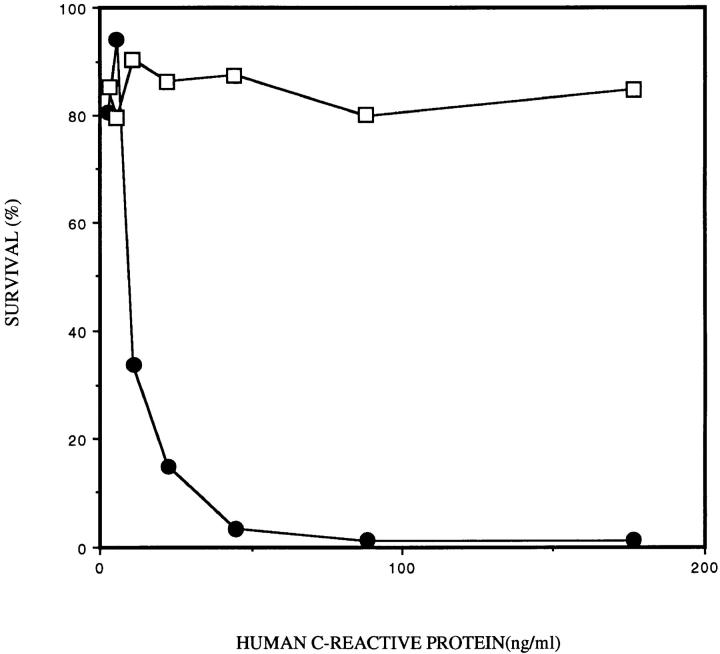
The role of CRP (which binds both to ChoP in the presence of calcium and to the first component of complement) in the antibody-independent complement activation by the classical pathway was examined (22, 23). Removal of CRP from the serum eliminated the difference in serum sensitivity between the ChoP variants of strain H233 (Fig. 2 A). The role of CRP in the bactericidal activity of serum was confirmed by the addition of purified CRP to the NHS depleted of CRP. The addition of CRP at a concentration of 5 μg/ml completely restored the increased serum killing of the ChoP+ variant but had no effect on the ChoP− variant. CRP (5 μg/ml) added to bacteria in the absence of serum or in serum pretreated at 56°C for 30 min to remove complement activity had no effect on viability. A dose response determination for the contribution of CRP to serum bactericidal activity showed that the half-maximal effect on killing of the ChoP+ variant was at a CRP concentration of 10 ng/ml (Fig. 3). The effect of CRP was shown to require the presence of calcium. There was no increased killing of the ChoP+ variant when bacteria were pretreated with CRP (5 μg/ml) in 2 mM EDTA in comparison to pretreatment in 10 mM Ca2+ (Fig. 2 B).
Figure 3.
The dose response to CRP in serum bactericidal assays. Survival of phase variants expressing (solid circles) or not expressing (open squares) ChoP in 10% NHS depleted of CRP with purified human CRP added at the concentration indicated was compared. The percentage of survival is the number of CFUs remaining compared to controls in which complement was inactivated.
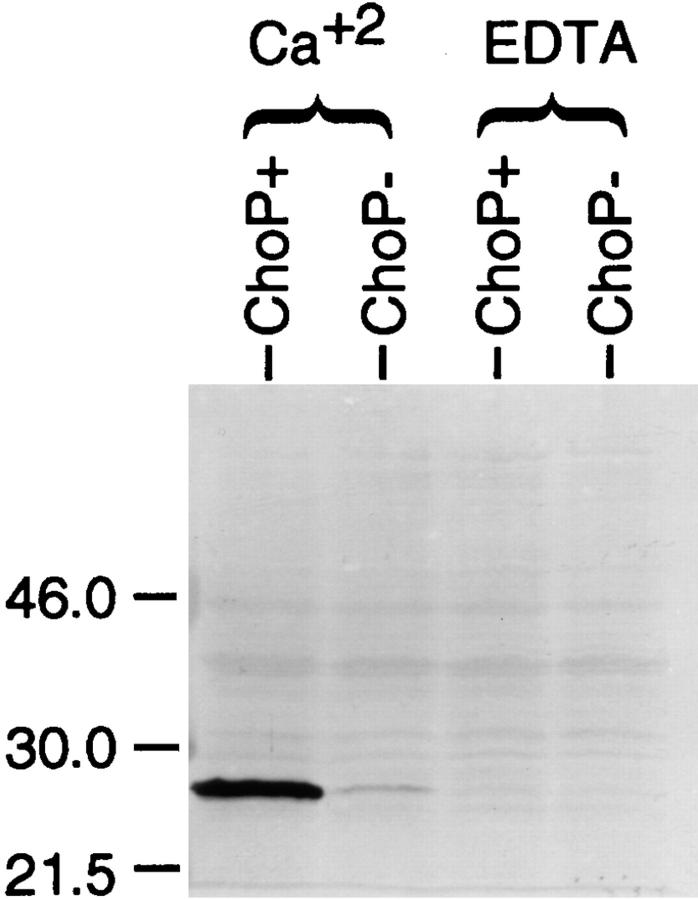
The direct interaction of purified CRP with strain H233 expressing ChoP and the requirement for calcium were demonstrated by incubating both variants in CRP (5 μg/ ml) in the presence of Ca2+ (10 mM) or EDTA (2 mM) (Fig. 4). After washing the cells to remove the unbound CRP, the binding of CRP to whole bacteria was detected on Western blot analysis using an mAb to human CRP. Binding of CRP required Ca2+ and only the ChoP-containing variant bound significant amounts of CRP. The faint binding to the ChoP-variant in the presence of Ca2+ probably represents the <1% of ChoP+ revertants in this population of organisms. The requirement of Ca2+ raised the possibility that bactericidal assays in the presence of 0.05 M Mg2+ EGTA inhibited CRP binding rather than the classical pathway of complement activation. However, in bactericidal assays where bacteria were preincubated with CRP in 10 mM Ca2+, chelation of divalent cations did not remove bound CRP but eliminated killing by complement. This confirmed that CRP-mediated killing requires complement activation by the classical pathway.
Figure 4.
The binding of human CRP to phase variants expressing ChoP in the presence of calcium. Equivalent numbers of phase variants with or without ChoP were incubated in purified CRP in the presence of Ca2+ or EDTA. After removing the unbound CRP, the bound CRP was detected on Western blot analysis using an mAb that recognizes human CRP.
The Contribution of ChoP in Nasopharyngeal Colonization of Infant Rats.
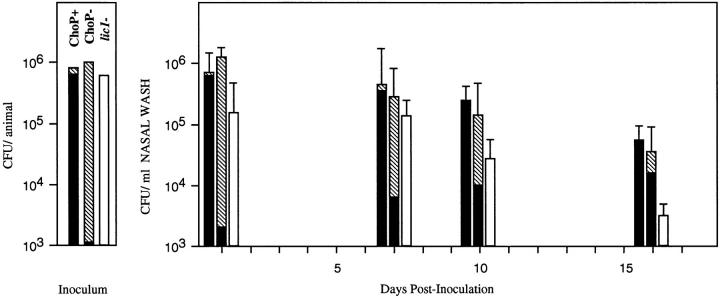
The results of the bactericidal assays suggested that the ChoP− phenotype may be important in evading humoral clearance mechanisms, but left unclear the contribution of the ChoP+ phenotype to the biology of H. influenzae. This question was addressed by comparing ChoP+ and ChoP− variants in the infant rat model of nasopharyngeal colonization (Fig. 5). H. influenzae type b (strain Eagan) was used in these experiments, since nontypable isolates colonize poorly in animal models of carriage. After an intranasal inoculum with 106 CFU of ChoP+ or − variants of a population >97% the desired phenotype, the number of organisms obtained from nasal washes remained relatively constant for up to 16 d. Animals receiving the ChoP+ variant remained colonized with organisms of this phenotype (>99%) when assessed by colony immunoblotting. Those animals receiving the ChoP− variant, in contrast, showed a gradual shift in the phenotype of organisms cultured from the nasopharynx; at the final time point, >73% of colonies had switched to the ChoP+ phenotype. This finding suggests that decoration of the LPS with ChoP was associated with an enhanced ability to persist within the nasopharynx. This result was confirmed by showing the diminished ability of a lic1 mutant of strain Eagan with a constitutively ChoP− phenotype to persist in the infant rat nasopharynx. By day 10 after the inoculation the lic1 mutant was present in significantly fewer numbers compared to the ChoP+ variant. At day 16 after the inoculation, 8 out of 25 pups receiving the mutant had no detectable organisms in nasal washes versus 0 out of 22 receiving the ChoP+ variant (P = 0.0034, Fisher's exact test).
Figure 5.
The contribution of ChoP expression to H. influenzae carriage in an infant rat model. ChoP variants of strain Eagan and a lic1 mutant of this strain were compared for their ability to colonize the infant rat nasopharynx. At each time point the number and phenotype of organism recovered in nasal washes were determined. The vertical axis represents the average number of organisms per animal in the inoculum or nasal washes on the day after intranasal inoculation indicated (first bar at each time point, ChoP+ inoculum; middle bar at each time point, ChoP− inoculum; last bar at each time point, lic1- mutant inoculum). Values are the geometric mean ± SD for at least 22 pups per variant or mutant in three separate experiments. The proportion of variants of the ChoP+ or ChoP− phenotype in the inoculum or recovered in nasal washes at each time point were determined by colony immunoblotting and is indicated (ChoP + solid portion; ChoP − hatched portion). Colony immunoblotting was not performed for the constitutive lic1 mutant (open bars).
Factors Affecting the Expression of ChoP.
A number of growth conditions were compared to determine whether an environmental condition other than the availability of choline might account for the increase in the proportion of organism of the ChoP+ phenotype in the nasopharynx. The frequency of phase variation between phenotypes and the incorporation of choline were compared after growth to midlog phase in a chemically defined medium under various conditions including temperature (30–39°C), pH (6.8–8.0), supplemental NaCl (0–1 M), Ca2+ (0.07–1.4 mM), Mg2+ (0.49–4.9 mM), or glucose (0.17–20 mM). Alternative sources of carbon (glycerol, pyruvate, lactate, maltose, lactose, and galactose) at a concentration of 5 mM in lieu of glucose were also examined. There was no significant difference for the ChoP+ variant in [3H]choline incorporation/milligrams of total cellular protein or in the rate of reversion to the ChoP− phenotype in colony immunoblots after growth under these conditions.
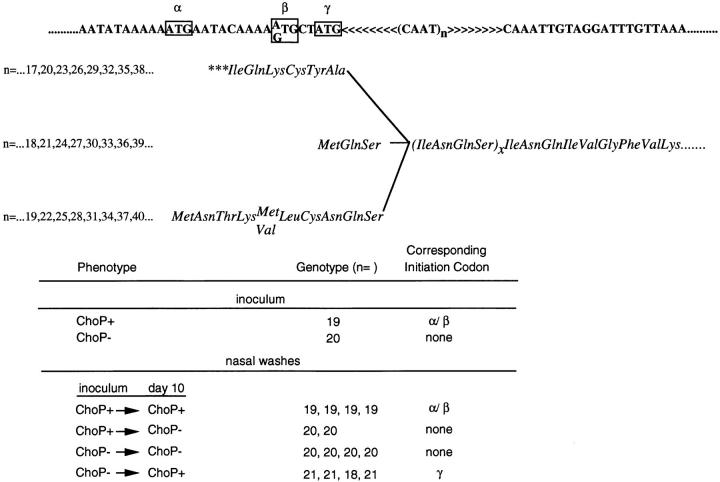
Chromosomal DNA extracted from organisms used to inoculate rat pups was used in the PCR reaction to isolate the 5′-end of the lic1 locus to determine the number of repeats of 5′-CAAT-3′ within licA. As predicted the ChoP− variants had a number of repeats (n = 20) in which there was no full-length translation product because no upstream initiation codon was in frame with the licA gene (Fig. 6). The ChoP+ variant, in contrast, had one fewer repeat (n = 19) which would place two potential initiation codons (labeled α and β) that are in the same reading frame, in the correct orientation for translation of the full-length licA gene product. Colonies obtained from nasal washes at day 10 after the inoculation were immunoblotted and used as a source of DNA for amplification of this same region. In each instance those colonies retaining the phenotype of the inoculum had the same number of repeats as those in the inoculum. Organisms that switched from ChoP+ in the inoculum to ChoP− at day 10 acquired an additional repeat as predicted (n = 19 varied to n = 20). Organisms that switched from ChoP− in the inoculum to ChoP+ at day 10 either gained one (n = 21) or lost two repeats (n = 18). Thus in four out of four colonies with organisms reverting to the ChoP+ phenotype, the number of repeats indicated that licA was in frame with an initiation codon, although in each case this was the γ and not the α/β initiation codons. It was concluded that the only identifiable factor contributing to variation in expression of ChoP on the LPS was the number of repeats of 5′-CAAT-3′ in licA.
Figure 6.
The correlation between ChoP expression and the genotype of the lic1 locus. Shown above is the molecular mechanism controlling phase variation in expression of ChoP. The nucleotide sequence at the 5′ end of lic1 contains a variable number (n) of tandem repeats of CAAT. Variation in the number of repeats creates a translational switch with possible translation products in phase with the open reading frame of licA indicated below the nucleotide sequence. Three potential initiation codons (boxed) labeled α, β and γ are present in only two of the three possible reading frames since α and β are positioned in the same phase. (In some strains the β codon is GTG rather than ATG as shown.) Only when an initiation codon is in frame with the remainder of the open reading frame of licA is there expression of ChoP. The table on the bottom shows that ChoP expression correlates with number of CAAT repeats (n). Colonies obtained from nasal washes of infant rats 10 d after the inoculation showed a variant number of repeats only when the phenotype in colony immunoblots was different from that of the corresponding inoculum.
The Expression of ChoP in H. influenzae in the Human Respiratory Tract.
In the animal model there was an absolute correlation between the phenotype of organisms with respect to ChoP and the number of repeats of CAAT in licA. Based on this observation, the genotype of H. influenzae in respiratory tract secretions from humans was determined in order to deduce the in vivo phenotype (ChoP+ or −) of organisms within the natural host. Purulent or nonpurulent respiratory tract secretions were used as a source of DNA for the PCR reaction to obtain the 5′ end of lic1 for sequencing to determine the number of CAAT repeats. For 13 out of 14 specimens the number of repeats indicated that the phenotype within the human respiratory tract was ChoP+ (Table 2). As was demonstrated in the animal model in instances of reversion to the ChoP+ phenotype, there was a preference for a number of repeats that would position the γ initiation codon in frame with licA (12 out of 14 for γ, 1 out of 14 for α/β). The only specimens in which the number of repeats would not predict licA translation from the γ initiation codon were from purulent sputum that fulfilled the case definition of nontypable H. influenzae pneumonia (20).
Table 2.
Genotypic Analysis of the Number of CAAT Repeats (n) in licA in Nontypable H. influenzae (NTHI) in Human Respiratory Tract Secretions*
| Genotype (n =) | NTHI pneumonia‡ | Predicted phenotype ChoP+/ChoP− | Corresponding initiation codon(s) | |||
|---|---|---|---|---|---|---|
| 6 | no | + | γ | |||
| 24 | no | + | γ | |||
| 24 | no | + | γ | |||
| 24 | no | + | γ | |||
| 18 | no | + | γ | |||
| 25 | yes | + | α/β | |||
| 30 | yes | + | γ | |||
| 33 | no | + | γ | |||
| 20 | yes | − | none | |||
| 18 | yes | + | γ | |||
| 21 | yes | + | γ | |||
| 18 | no | + | γ | |||
| 18 | no | + | γ | |||
| 18 | no | + | γ |
The relationship between genotype, predicted phenotype, and the designation of corresponding initiation codons is described in Fig. 6.
Clinical criteria for NTHI pneumonia are described in methods section.
Discussion
We previously reported that one of the phase-variable structures on the H. influenzae LPS included the ChoP epitope. Structural analysis in this study confirms that variation in the display of this epitope corresponds to the presence or absence of the ChoP structure on the oligosaccharide of the LPS. Phase variants were then separated based on the expression of ChoP as determined by reactivity with mAbs recognizing this structure to address how ChoP contributes to the pathogenesis of infection caused by H. influenzae.
Since ChoP appears to be a common feature on the cell surface of mucosal pathogens, particularly those residing in the human respiratory tract, observations on H. influenzae may be of relevance to the other species expressing the ChoP structure/epitope (2, 12). This study was carried out using H. influenzae because both the genetic and structural basis of choline incorporation have now been defined in this species (1, 5). We concentrated on nontypable isolates; these organisms have been a predominant cause of disease in adults for many years, and have become increasingly important in young children as H. influenzae type b disease has begun to disappear due to widespread vaccination. Phase variation in ChoP expression made it possible to examine bacteria with and without this characteristic in an identical genetic background. An additional feature favoring the use of H. influenzae in these experiments was that, unlike the pneumococcus, H. influenzae does not appear to have multiple surface proteins anchored to ChoP (25). The absence of such proteins in H. influenzae allowed us to examine the direct contribution of ChoP to pathogenesis.
H. influenzae is found exclusively in the human nasopharynx, where it is able to persist for extended periods. In this study, an animal model of H. influenzae carriage was used to demonstrate a gradual selection for ChoP+ phase variants during prolonged colonization of the nasopharynx. As predicted from this comparison of phase variants, a constitutively ChoP− mutant was cleared from the infant rat nasopharynx significantly more rapidly than from the wild-type controls. The capacity of ChoP+ variants to evade clearance may help to explain why this structure may be widely conserved among species that occupy a similar environment but are otherwise highly distinct. However, this study does not address the specific mechanism by which decoration of the LPS with ChoP allows H. influenzae to persist in the nasopharynx. ChoP, which is also found on eukaryotic membrane lipids, may provide a selective advantage on the mucosal surface through molecular mimicry of the host. In tissue explants from the human nasopharynx, H. influenzae displays a tropism for the mucus layer (26). Phospholipids related to phosphatidylcholine, which encompass the ChoP structure, are present in mucus. No contribution of the lic1 genes to the interaction of H. influenzae with human nasal turbinate tissue in culture was demonstrated (26). However, these studies did not determine whether controls were phase on (ChoP+) or phase off (ChoP−). In addition, it has been suggested that ChoP contributes to adherence of the pneumococcus to host cells by binding to the receptor for platelet activating factor, whose natural ligand also contains ChoP (27). If ChoP is functioning as an adhesin in H. influenzae, we would predict that its contribution to colonization would be apparent at an earlier stage after the intranasal challenge than was observed in the animal studies.
H. influenzae variants can be stratified into serum-resistant and serum-sensitive phenotypes based on the presence of ChoP. The increased serum sensitivity of the ChoP+ variants has been previously shown to correlate with the presence of anti-ChoP antibody in human serum (1). This study demonstrates that the difference in serum sensitivity is independent of antibody yet involves the classical pathway of complement activation. LPS structure is known to affect the deposition of complement, but in previous reports this involved alterations of the O-antigen and was mediated by the alternative pathway (28). The activation of the classical pathway in the absence of antibody was shown to occur by the calcium dependent binding of CRP exclusively to the ChoP+ variant. CRP is capable of substituting for attached antibody in activating the complement cascade by the classical pathway through binding to C1q (29, 30). Therefore, it appears that CRP, an acute phase reactant, contributes to innate immunity to bacterial pathogens other than the pneumococcus in which its role in host protection has long been postulated (31). In the case of the pneumococcus, CRP is thought to promote clearance by opsonization, whereas for H. influenzae CRP contributes to killing through the bactericidal activity of complement. The effect of CRP on serum bactericidal activity against H. influenzae strain H233 occurred at concentrations as low as 10 ng/ml. This is well below the level of CRP in normal, unstimulated human serum (<200 ng/ml). A further implication of these results is that the innate immunity mediated by CRP may be particularly important in protection against certain invasive mucosal pathogens, such as the pneumococcus, meningococcus, and some H. influenzae strains, in the time preceding emergence of antibody-mediated opsonizing and/or bactericidal activity.
We have noted that for H. influenzae, as well as for the pathogenic Neisseria and the pneumococcus, there is variation between phenotypes with greater or lesser amounts of the ChoP structure/epitope (references 1 and 14 and Weiser, J.N., J. Goldberg, N. Pan, and M. Virgi, manuscript submitted for publication). This suggests that there may be a role for both these phenotypes in the interaction of ChoP-expressing pathogens and their host. H. influenzae has the ability to switch off expression of ChoP, which eliminates binding of CRP and the subsequent activation of complement through the classical pathway. This suggests that the biological role of the ChoP− phenotype might be evasion of CRP-mediated clearance. It is predicted that there would be a selection for the ChoP− variants from among the predominantly ChoP+ population in the commensal state whenever the concentration of CRP and complement is sufficient. This may occur during invasive infection, localized inflammation, or systemic inflammatory states when serum levels of CRP rise precipitously. It was not possible to demonstrate a selection for ChoP− variants among the infant rats developing bacteremia after the intranasal inoculation. Unlike humans, CRP expression in rats is at constitutively low levels for the first 15 d after gestation (32). The 30-fold increase in CRP levels in normal adult rats may also account for the age-related sensitivity of this model host to invasive infection by H. influenzae.
The mechanism-controlling phase variation in ChoP expression provided a means of determining the phenotype of organisms without the need for in vitro culture (11). Avoiding in vitro growth was desirable because of the high rate of phenotypic switching. In preliminary experiments, we demonstrated that the only factor which affected phase variation was the number of repeats of CAAT within the lic1. In each of eight phenotypic revertants obtained from nasal washes of infant rats, there was a corresponding shift in the number of repeat units. The phenotype of organisms in the respiratory tract of the natural host was then deduced by determining the genotype with respect to lic1. If ChoP contributes to persistence in the nasopharynx, organisms in respiratory tract secretions at any point in time would tend to have ChoP that contains LPS. As expected, the overwhelming majority (92.9%) of patients with H. influenzae identified in their respiratory secretions had a number of repeats which would predict translation of the full length licA gene and expression of the ChoP+ phenotype. This finding supports the hypothesis that the ChoP decoration of the LPS contributes to the ability of H. influenzae to colonize and persist within the human respiratory tract, the initial step in the pathogenesis of infection.
Footnotes
The authors thank Dr. Alexander Szalai (University of Alabama at Birmingham, Birmingham, AL) for his expert advice, Douglas W. Griffith for large-scale production of cells used in structural studies, and Don Krajcarski for ESI-MS analyses.
J.N. Weiser is a Lucille P. Markey Charitable Trust Scholar. This work was supported by a grant from the Lucille P. Markey Charitable Trust (J.N. Weiser) and grant AI-38436 from the Public Health Service (J.N. Weiser).
Address correspondence to Jeffrey N. Weiser, 301B Johnson Pavilion, Department of Microbiology, University of Pennsylvania, Philadelphia, PA 19104-6076. Phone: 215-573-3511; Fax: 215-898-9557; E-mail: weiser@mail.med.upenn.edu
Abbreviations used in this paper: BHI, brain heart infusion; ChoP, phosphorylcholine; CRP, C-reactive protein; ESI-MS, electrospray ionization-mass spectrometry; NHS, normal human serum; NMR, nuclear magnetic resonance.
References
- 1.Weiser JN, Shchepetov M, Chong STH. Decoration of lipopolysaccaride with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. . Infect Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosser JL, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 3.Risberg A, Schweda EKH, Jansson P-E. Structural studies of the cell-envelope oligosaccharide from lipopolysaccharide of Haemophilus influenzaestrain RM 118-28. Eur J Biochem. 1997;243:701–707. doi: 10.1111/j.1432-1033.1997.00701.x. [DOI] [PubMed] [Google Scholar]
- 4.Leon MA, Young NM. Specificity for phosphorylcholine of six murine myeloma proteins reactive with pneumococcus C polysaccharide and beta-lipoprotein. Biochemistry. 1971;10:1424–1429. doi: 10.1021/bi00784a024. [DOI] [PubMed] [Google Scholar]
- 5.Schweda, E.K.H., H. Masoud, A. Martin, A. Risberg, D.W. Hood, E.R. Moxon, J.N. Weiser, and J.C. Richards. 1997. Phase variable expression and characterization of phosphorylcholine oligosaccharide epitopes in Haemophilus influenzae lipopolysaccharides. Glycoconj. J. 14:S23(Suppl.).
- 6.Zamze SE, Moxon ER. Composition of the lipopolysaccharide from different capsular serotype strains of Haemophilus influenzae. . J Gen Microbiol. 1987;133:1443–1451. doi: 10.1099/00221287-133-6-1443. [DOI] [PubMed] [Google Scholar]
- 7.Inzana TJ. Electrophoretic heterogeneity and inter strain variation of the lipopolysaccharide of Haemophilus influenzae. . J Infect Dis. 1983;148:492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- 8.Masoud H, Moxon ER, Martin A, Krajcarski D, Richards JC. Structure of the variable and conserved lipopolysaccharide oligosaccharide epitopes expressed by Haemophilus influenzaeserotype b strain Eagan. Biochemistry. 1997;36:2091–2103. doi: 10.1021/bi961989y. [DOI] [PubMed] [Google Scholar]
- 9.Weiser JN, Lindberg AA, Manning EJ, Hansen EJ, Moxon ER. Identification of a chromosomal locus for expression of lipopolysaccharide epitopes in Haemophilus influenzae. . Infect Immun. 1989;57:3045–3052. doi: 10.1128/iai.57.10.3045-3052.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiser JN, Maskell DJ, Butler PD, Lindberg AA, Moxon ER. Characterization of repetitive sequences controlling phase variation of Haemophilus influenzaelipopolysaccharide. J Bacteriol. 1990;172:3304–3309. doi: 10.1128/jb.172.6.3304-3309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiser JN, Love JM, Moxon ER. The molecular mechanism of phase variation of H. influenzaelipopolysaccharide. Cell. 1989;59:657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 12.Deutsch J, Salman M, Rottem S. An unusual polar lipid from the cell membrane of Mycoplasma fermentans. . Eur J Biochem. 1995;227:897–902. doi: 10.1111/j.1432-1033.1995.tb20216.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim JO, Shchepetov M, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. . Clin Infect Dis. 1997;25:427. doi: 10.1086/514205. . (Abstr.) [DOI] [PubMed] [Google Scholar]
- 14.Kim, J.O., and J.N. Weiser. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. In press. [DOI] [PubMed]
- 15.Weiser JN, Williams A, Moxon ER. Phase-variable lipopolysaccharide structures enhance the invasive capacity of Haemophilus influenzae. . Infect Immun. 1990;58:3455–3457. doi: 10.1128/iai.58.10.3455-3457.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiser JN, Chong STH, Greenberg D, Fong W. Identification and characterization of a cell envelope protein of Haemophilus influenzaecontributing to phase variation in colony opacity and nasopharyngeal colonization. Mol Microbiol. 1995;17:555–564. doi: 10.1111/j.1365-2958.1995.mmi_17030555.x. [DOI] [PubMed] [Google Scholar]
- 17.Michalka J, Goodgal S. Genetic and physical map of the chromosome of Haemophilus influenzae. . J Mol Biol. 1969;45:407–421. doi: 10.1016/0022-2836(69)90115-6. [DOI] [PubMed] [Google Scholar]
- 18.Masoud H, Altman E, Richards JC, Lam JS. General strategy for structural analysis of the oligosaccharide region of lipopolysaccharides. Structure of the the oligosaccharide component of Pseudomonas aeruginosaIATS serotype O6 mutant R5 rough-type lipopolysaccharide. Biochemistry. 1994;33:10568–10578. doi: 10.1021/bi00201a002. [DOI] [PubMed] [Google Scholar]
- 19.Hoiseth SK, Connelly CJ, Moxon ER. Genetics of spontaneous, high-frequency loss of b capsule expression in Haemophilus influenzae. . Infect Immun. 1985;49:389–395. doi: 10.1128/iai.49.2.389-395.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musher DM, Kubitschek KR, Crennan J, Baughn RE. Pneumonia and acute febrile tracheobronchitis due to Haemophilus influenzae. . Ann Intern Med. 1983;99:444–450. doi: 10.7326/0003-4819-99-4-444. [DOI] [PubMed] [Google Scholar]
- 21.Forsgren A, Mclean RH, Michael AF, Quie PG. Studies of the alternative pathway in chelated serum. J Lab Clin Med. 1975;85:904–912. [PubMed] [Google Scholar]
- 22.Volanakis JE, Kaplan MH. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971;136:612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan MH, Volankis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of CRP with pneumococcal C-polysaccharide and with choline phosphates, lecthin and sphingomyelin. J Immunol. 1974;112:2135–2147. [PubMed] [Google Scholar]
- 24.Schweda E, Hegedus O, Borrelli S, Lindberg A, Weiser J, Maskell D, Moxon E. Structural studies of the saccharide part of the cell envelope lipopolysaccharide from Haemophilus influenzae strain AH1-3 (lic3 +) Carbohydr Res. 1993;246:319–330. doi: 10.1016/0008-6215(93)84043-6. [DOI] [PubMed] [Google Scholar]
- 25.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. . Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 26.Jackson AD, Maskell D, Moxon ER, Wilson R. The effect of mutations in genes required for lipopolysaccharide synthesis on Haemophilus influenzaetype b colonization of human nasopharyngeal tissue. Microbiol Path. 1996;21:463–470. doi: 10.1006/mpat.1996.0076. [DOI] [PubMed] [Google Scholar]
- 27.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniaeanchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 28.Grossman N, Leive L. Complement activation via the alternative pathway by purified Salmonellalipopolysaccharide is affected by its structure but not its O-antigen length. J Immunol. 1984;132:376–385. [PubMed] [Google Scholar]
- 29.Volanakis JE, Kaplan MH. Interaction of C-reactive protein complexes with the complement system. II. Consumption of guinea pig complement by CRP complexes. Requirement for human C1q. J Immunol. 1974;113:9–17. [PubMed] [Google Scholar]
- 30.Szalai AJ, Agrawal A, Greenhough TJ, Volanakis JE. C-reactive protein. Immunol Res. 1997;16:127–136. doi: 10.1007/BF02786357. [DOI] [PubMed] [Google Scholar]
- 31.Szalai AJ, Briles DE, Volanakis JE. Role of complement in C-reactive-protein–mediated protection of mice from Streptococcus pneumoniae. . Infect Immun. 1996;64:4850–4853. doi: 10.1128/iai.64.11.4850-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Numomura W. C-reactive protein in rat: in development, pregnancy and effect of sex hormones. Comp Biochem Physiol. 1990;96:489–493. doi: 10.1016/0300-9629(90)90667-h. [DOI] [PubMed] [Google Scholar]