Abstract
V(D)J (V, variable; D, diversity; J, joining) combination of immunoglobulin (Ig) genes established in premature B cells has been thought to be conserved throughout differentiation at mature stages. However, germinal center (GC) B cells have been shown to reexpress recombination-activating gene (RAG)-1 and RAG-2 proteins in immunized mice. Here, we present several lines of evidence indicating that RAG proteins thus induced are functional as the V(D)J recombinase. DNA excision product reflecting Vλ1 to Jλ1 rearrangement was generated in parallel with the expression of RAG genes in mature mouse B cells that were activated in vitro with LPS and IL-4. Similar λ chain gene rearrangement was observed in the draining lymph node of immunized mice. Further, B cells that underwent λ gene rearrangement were shown by in situ PCR to be localized within GCs. Thus, secondary rearrangement of Ig genes (receptor editing) can occur in mature B cells.
Variable regions of Ig genes are generated from three classes of germline DNA segments, variable (V),1 diversity (D), and joining (J). These are assembled in a random fashion to generate a set of V(D)J in a given B cell clone during its development in the primary lymphoid organ, thus giving rise to large varieties of rearranged V(D)J genes in B cells (1). V(D)J rearrangement is mediated by the products of recombination activating genes, recombination-activating gene (RAG)-1 and RAG-2 (2). A V(D)J combination established in the primary lymphoid organ has been thought to be conserved throughout B cell differentiation thereafter, although the nucleotide sequences are modified by somatic hypermutations (3). One exception is the secondary revision of Ig genes termed receptor editing in which an original V(D)J rearrangement is replaced by a secondary rearrangement in the bone marrow (4). This phenomenon was first pointed out in mice transgenic for genes encoding autoantibodies to self H-2K (5, 6) or double-stranded DNA (7). In these transgenic mice, B cell clones that escaped deletion were found to lose the autoreactivity due to the replacement of the transgenic V(D)J by a new endogenous rearrangement of L and/or H chain genes (5–7). Further, bone marrow–derived IgM+ immature B cells have been shown to retain RAG expression and to undergo Vλ–Jλ recombination during culture (8) or when the immature B cells were stimulated by surface Ig engagement in vitro (9).
However, mature B cells in which RAG-1 and RAG-2 expression is downregulated (8, 10), have been believed to undergo no further Ig gene rearrangement. But we have recently found that RAG-1 and RAG-2 proteins are reexpressed in IgD+ mature mouse B cells that are stimulated in vitro with LPS or mAb to CD40 in the presence of IL-4 or in germinal center (GC) B cells in the draining LN of mice immunized in vivo (11, 12). Similar observations have been made by Han et al. (13). However, it has remained unclear whether the RAG proteins thus induced in mature B cells are as functional as those expressed in premature B cells. Recently, GC B cells were shown to have intermediate products of κ–L chain gene rearrangement (14, 15). Further, mature B cells from mice with targeted VB1-8DJH2 and V3-83Jκ2 replacement alleles were found to undergo rearrangement of endogenous H and κ–L chain genes in response to LPS plus IL-4 (15), which induce RAG gene expression in vitro (11). In this report, we describe that mature mouse B cells undergo de novo rearrangement of λ–L chain genes in vitro and in GCs of immunized wild-type mice in parallel with RAG gene expression.
Materials and Methods
Preparation and Culture of Mouse B Cells.
Spleen cells from male C3H/HeN mice (7–9-wk of age; Japan Charles River, Kanagawa, Japan) were treated with 1/1,000-diluted anti-Thy 1.2 mAb (SeroTec, Kidlington, UK) followed by incubation with low toxic rabbit complement (Cederlane, Wesbury, NY) as described previously (11). The B cells thus obtained (3 × 106/ml) were cultured for 2 d with 20 μg/ml LPS from Escherichia coli 055 B5 (Sigma Chemical Co., St. Louis, MO) and 10 ng/ml mouse recombinant IL-4 (PeproTech, Princeton, NJ) in 1 ml of RPMI-1640 medium containing 10% FCS, 10−5 M 2-ME, 100 U/ml penicillin G, and 50 μg/ml streptomycin.
Analysis of RAG-2 Transcripts.
Total RNA was extracted from 106 cells and reverse transcribed as described previously (16). The resultant cDNA was amplified by PCR using following sense and antisense primers (11, 13): RAG-2, 5′-CACATCCACAAGCAGGAAGTACAC-3′, 5′-GGTTCAGGGACATCTCCTACTAAG-3′; and GAPDH, 5′-CCATCACCATCTTCCAGGAG-3′, 5′-CCTGCTTCACCACCTTCCTTG-3′. Both primer pairs span an intron to avoid the amplification of contaminating genomic DNA. To heighten the specificity of the amplification, time release PCR was performed using AmpliTaq Gold (Perkin-Elmer Corp., Foster City, CA) as the polymerase according to the manufacturer's protocol. PCR was carried out as follows: for RAG-2, at 94°C for 12 min followed by 40 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min; for GAPDH, 26 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The amplified products were electrophoresed on 7.5% polyacrylamide gel and visualized by SYBER green I (FMC BioProducts, Rockland, ME) staining. RAG-1 and RAG-2 expression in LPS/IL-4–stimulated mature mouse B cells has been confirmed by reverse transcriptase–dependent PCR (RT-PCR) followed by Southern blotting as described previously (11).
Flow Cytometric Analysis.
Cultured B cells were incubated with 10 μg/ml biotinylated anti–mouse κ or λ chain mAb at 4°C for 30 min followed by staining with streptavidin-PerCP (Becton Dickinson, San Jose, CA). The anti-λ mAb used is reactive with λ1 and λ2 chains. Additionally, the cells were stained with phycoerythrin-labeled anti-B220 mAb (PharMingen, San Diego, CA) and FITC-labeled mAb to mouse IgG1 or IgM (PharMingen), respectively. Three-color analyses were carried out using FACSCalibur®.
Purification of κ+ or λ+ B Cells.
κ+ or λ+ B cells were purified from mouse spleen B cells using immunomagnetic beads. In brief, 108 spleen B cells were incubated with 5 μg/ml biotinylated mAb to mouse κ or λ chain in 1 ml of phosphate-buffered saline at 4°C for 30 min. After washing, the cells were mixed with 1 mg/ml streptavidin-coated magnetic beads (Dynal, Oslo, Norway) and incubated at 4°C for 30 min. Rosetted cells were separated by placing the tubes in magnetic particle concentrator (Dynal) for 2 min. This magnetic separation was repeated twice. Purity of each cell preparation was >99% as assessed by flow cytometry.
Proliferative Response of B Cells.
Unseparated, κ+ or λ+ B cells (each 105 cells) were cultured in 0.2 ml of the culture medium with or without 20 μg/ml LPS and 10 ng/ml IL-4 for 2 d. Then the cells were pulsed with 20 kBq [3H]thymidine (TdR; Daiichi Chemical, Tokyo, Japan) for 12 h. The incorporated radioactivity was measured by liquid scintillation counter (Aloka, Tokyo, Japan).
Detection of DNA Excision Product.
DNA excision product derived from λ chain gene rearrangement was extracted from cultured B cells or LN cells, and amplified by PCR. 1,000,000 cells were suspended in 200 μl of the lysis buffer, which consists of 10 mM Tris-HCl (pH 8.0), 10 mM KCl, 1.5 mM MgCl2, 0.5% Tween 20, and 0.1 mg/ml proteinase K. The suspension was incubated at 56°C for 45 min and 95°C for 10 min. Then the supernatants were directly subjected to the analysis by PCR with 28, 30, and 32 cycles, which were in the exponential phase of the amplification. AmpliTaq Gold was used as the polymerase. For amplification of Vλ1–Jλ1 excision product, the following primers described by Tiegs et al. (5) were used: 5′-GCTGCATACATCACAGATGC-3′ and 5′-CAATGATTCTATGTTGTGCC-3′. Each cycle consisted of 94°C for 30 s, 62°C for 30 s, and 72°C for 90 s. Amplified products were electrophoresed and visualized by Southern blotting using a 32P-labeled probe, which is the HindIII-NspI 183-bp fragment derived from a DNA segment spanning the Vλ1–Jλ1 signal joint of the excision product (provided by Dr. D. Nemazee, National Jewish Medical and Research Center, Denver, CO). Exon 4 of γ-actin gene was amplified as a control using a primer pair 5′-ATGTTTGAAACCTTCAATAC-3′ and 5′-GGAAGGAAGGCTGGAAGAGT-3′. The product was visualized by staining with SYBER green I.
In Situ PCR Analysis.
For in situ PCR of LN sections, 8-μm-thick cryosections mounted on silane-coated slides were allowed to air dry for 1 h and fixed in ice-cold 4% paraformaldehyde for 2 h. Then the slides were washed, dehydrated by ethanol for 5 min, and air dried for 30 min. The thermal cycler designed for in situ PCR (GeneAmp 1000; Perkin-Elmer Corp.) and core PCR reagent kit (Perkin-Elmer Corp.) optimized for this apparatus were used according to the manufacturer's protocol. Digoxigenin (DIG)-labeled dUTP (Boehringer Mannheim GmbH, Mannheim, Germany) was added to the reaction mixture at 2.5 μM. DNA excision product derived from Vλ1–Jλ1 rearrangement was amplified using the primer pair described above. PCR conditions were 20 cycles of 1 min at 94°C, 1 min at 60°C, and 1 min 30 s at 72°C. The amplification was performed without primers or with primers for human IL-2 gene as a negative control. DIG-labeled amplified products were detected using DIG nucleic acid detection kit (Boehringer Mannheim GmbH). In brief, slides were treated with Fab fragments of sheep anti-DIG Ab conjugated with alkaline phosphatase, and visualized by the reaction with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium salt. Positive reaction gives blue/purple precipitates. The slides were finally counterstained with methyl green.
Results and Discussion
Enhanced Expression of λ L Chains on B Cells Stimulated with LPS Plus IL-4 In Vitro.
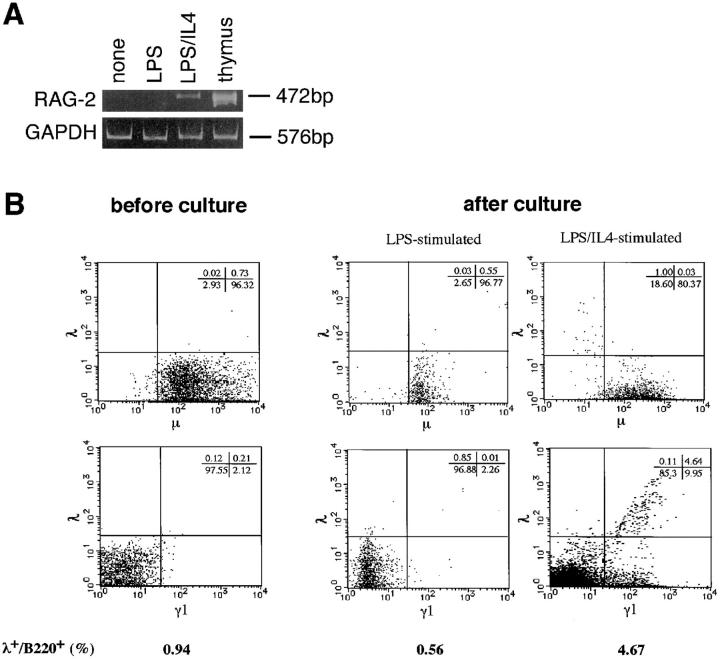
As reported previously (11), stimulation of mature mouse B cells with LPS/IL-4, but not with LPS alone resulted in the expression of RAG-2 in vitro (Fig. 1 A). It was then investigated whether LPS/ IL-4–induced RAG gene products can mediate receptor editing in mature mouse B cells. One of the criteria for the occurrence of receptor editing is the induction of λ chains in κ chain–bearing B cells since this process involves de novo rearrangement of λ genes (5, 17). Thus, mouse spleen B cells were stimulated in vitro with LPS/IL-4 and examined for the induction of λ chains after the culture. Before the culture, >95% of B220+ B cells were IgM+λ−, and λ+/B220+ cells were <1% of total cells (Fig. 1 B). IgG1+ (γ1+) cells were 2.3% before culture, but increased to 15.6% after culture with LPS/IL-4 due to IL-4–induced Ig class switching (16). Interestingly, LPS/IL-4 stimulation resulted in an increase of λ+/B220+ cells from 0.94 to 4.67%, a majority of which belonged to γ1+ population. Enhanced expression of λ chains was not observed in B cells stimulated with LPS alone that did not lead to RAG expression (Fig. 1, A and B). B cells cultured without stimuli showed similar flow cytometric patterns to those of the original cells (not shown). When purified κ+ cells (>99% pure) were stimulated with LPS/IL-4 for 4 d, 3–4% of the total cells became positive for λ chains (our unpublished result).
Figure 1.
(A) Induction of RAG gene expression in activated mouse mature B cells in vitro. Mouse spleen B cells were cultured with LPS or LPS plus IL-4 for 2 d, and assessed for RAG-2 expression by RT-PCR. (B) Enhanced expression of λ chains in mouse spleen B cells stimulated with LPS/IL-4. After stimulation with LPS or LPS/IL-4 for 4 d, B220+ population of the cultured B cells was analyzed for the expression of λ, μ, and γ1 chains by flow cytometry.
Detection of DNA Excision Product Derived from Vλ1–Jλ1 Rearrangement.
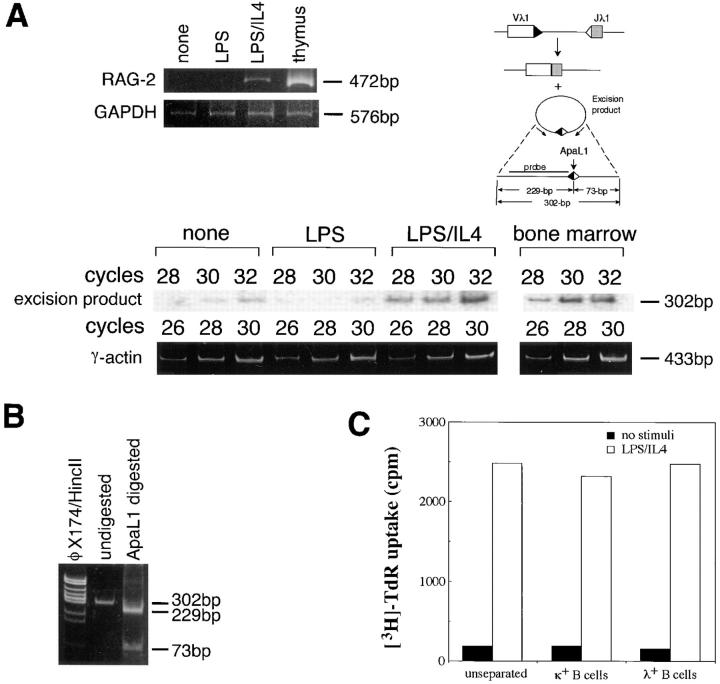
If the expressed λ chains are produced by newly rearranged λ genes, circular DNA excision product derived from Vλ–Jλ rearrangement might be detected (5). Fig. 2 A shows that this is the case. PCR amplification of the extracted DNA followed by Southern blotting with the probe depicted in Fig. 2 B revealed that LPS/IL-4–stimulated B cells in which RAG-2 was expressed, were shown to contain a definitely higher level of DNA excision product reflecting Vλ1–Jλ1 rearrangement compared with unstimulated or LPS-stimulated B cells. It has been reported that the signal joint formation generates a restriction site cleavable with ApaLI (18). The amplification product (302 bp) was cleaved by ApaLI into two fragments with expected lengths of 229 and 73 bp, respectively (Fig. 2 B), thus confirming the identity of the amplified product. The initial stage of V(D)J recombination is the generation of broken signal ends and hairpin coding ends. It has been reported that the joining of signal ends is slower than the formation of coding joints, and may require the cell cycle progression (19). Vλ1–Jλ1 excision product detected in LPS/IL-4–stimulated B cells, however, may not be due to the mere joining of the preexisting signal breaks since a much lower level of the circular excision product was detected in B cells stimulated with LPS alone that progresses the cell cycle, but does not induce RAG expression (11).
Figure 2.
(A) Detection of circular DNA excision product derived from Vλ1–Jλ1 rearrangement in mouse spleen B cells cultured with LPS/ IL-4. After the B cells were stimulated with LPS or LPS/IL-4 for 2 d, the excision product derived from 106 cultured cells was amplified by PCR with 28, 30, and 32 cycles. The amplified products were visualized by Southern blotting using the probe depicted. The same cells were assessed for RAG-2 expression by RT-PCR. Thymocytes or bone marrow cells were used as a positive control. (B) Confirmation of an ApaLI restriction site in the PCR-amplified segment of Vλ1–Jλ1 excision product. (C) Comparison of proliferative response to LPS/IL-4 between purified λ+ B cells and κ+ B cells. Purified λ+ B cells, κ+ B cells, or unseparated B cells were cultured with or without LPS/IL-4 for 2 d followed by incubation with [3H]TdR for an additional 12 h. Data were presented as the mean of triplicate assays.
As shown in Fig. 2 C, purified λ+ B cells and κ+ B cells showed a comparable [3H]TdR uptake either in the presence or absence of LPS/IL-4. Thus, LPS/IL-4–induced increase of λ+ B cells is not considered to be due to the preferential expansion or survival of a small number of λ+ cells present in the original B cell preparation during the culture. Taken together, λ chains expressed in response to LPS/IL-4 are thought to be generated from newly rearranged λ chain genes.
As shown in Fig. 1 B, λ chains were predominantly expressed on γ1+ B cells. It remains unclear whether λ gene rearrangement is undertaken more efficiently after isotype switching, or whether these two events apparently proceed in a synchronous manner due to their similar IL-4 dependence. It is interesting to note that Ku, a DNA-dependent protein kinase activator protein complex that is involved both in isotype switching (20) and in V(D)J recombination (21), was induced in mouse spleen B cells stimulated by surface Ig engagement and IL-4 (22).
λ Chain Gene Rearrangement in GC B Cells In Vivo.
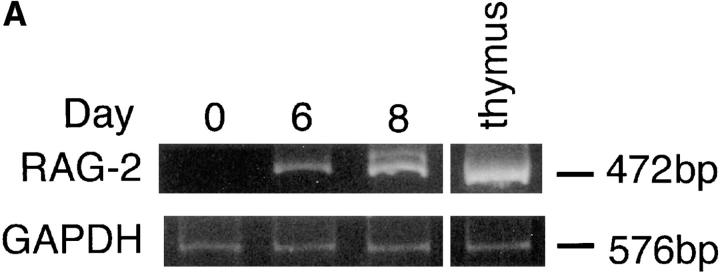
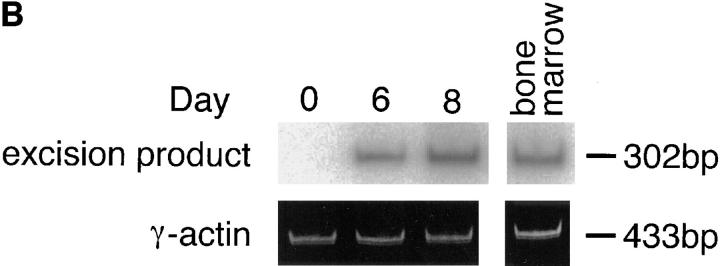
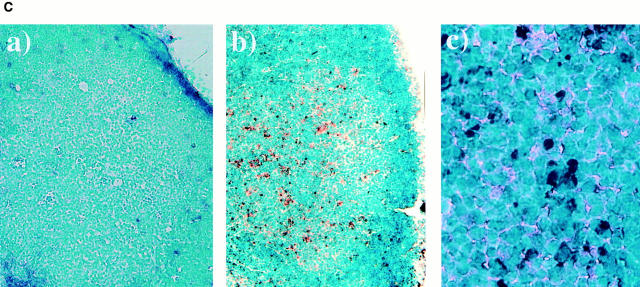
Do mature B cells also undergo λ chain gene rearrangement in vivo? We have reported that RAG-1 and RAG-2 are induced in the draining LN cells of immunized mice (11, 12). It was confirmed that popliteal LN cells expressed RAG-2 transcripts on days 6 and 8 after immunization when mice were immunized in the footpads with TNP-KLH and alum (Fig. 3 A). Very interestingly, DNA excision product derived from Vλ1–Jλ1 rearrangement was detected by PCR in the same LN cells as those in Fig. 3 A on days 6 and 8 after immunization, but not on day 0 (Fig. 3 B), suggesting that the induced RAG proteins are functional and able to mediate λ chain gene rearrangement in vivo. Further, the localization in the LN of the cells that underwent Vλ1–Jλ1 rearrangement was examined in the LN section by in situ PCR. On day 8 after immunization, well-developed GCs were observed in LN sections (Fig. 3 C, a and b). A majority of the cells possessing DNA excision product of Vλ1–Jλ1 rearrangement were found within GCs in which RAG-expressing B cells have been reported to be localized (11–13; Fig. 3 C, b and c). Intracellular deposition of the amplified DIG-labeled DNA fragments was observed when in situ PCR was performed in the presence of primers, but not in their absence. The primers for human IL-2 gene did not generate the DIG-labeled product either (not shown), thus indicating the specificity of the method. Thus, data presented here strongly suggest that GC B cells undergo receptor editing in parallel with RAG gene expression.
Figure 3.
Rearrangement of λ chain genes in the draining LN of immunized mice. (A) Mice were immunized in the hind footpads with 20 μg of TNP-KLH and 0.45 mg of alum. On days 0, 6, and 8 after immunization, popliteal LN cells were assessed for the expression of RAG-2 by RT-PCR. (B) The same LN cells were examined for the occurrence of DNA excision product reflecting Vλ1–Jλ1 rearrangement by PCR followed by Southern blotting. (C) Localization of B cells that underwent λ chain gene rearrangement in GCs of popliteal LNs. The DNA excision product derived from Vλ1–Jλ1 rearrangement was detected in cryosections prepared from the LNs on day 8 after immunization by in situ PCR, which was performed with (b and c) or without (a) primers. GCs that stained dully with methyl green are observed in a and b. Dark blue/purple DIG-labeled deposits indicate the presence of the excision product. Original magnification: a and b, 100; c, 400.
These findings and recent observations made by other investigators (14, 15) provide a new aspect in immunology that V(D)J combination established in a given premature B cell clone can be revised even at mature stages. What is a biological role of RAG gene products expressed in GC B cells? GC is a microenvironment in which somatic hypermutations, isotype switching, and clonal selection for affinity maturation of antibodies are undertaken (23, 24). Somatic hypermutations in GCs may produce not only high affinity antibodies, but also generate autoreactive B cell clones. The high affinity clones will be selected positively through interaction with follicular dendritic cells retaining immune complexes on their surface, thus leading to affinity maturation of antibodies (25). On the other hand, autoreactive clones must be either deleted or rendered anergic to maintain self tolerance. Receptor editing in mature B cells suggests another possible way to extinguish autoreactivity in GCs. We have observed that a majority of RAG-expressing B cells in GCs are apoptotic and present in tingible bodies (12). This may reflect, at least in part, a result of RAG- dependent revision of antigen receptors and their subsequent selection in GCs. Further elucidation of the role of RAG gene products in GCs will provide valuable clues to understanding the onset of autoimmune diseases, lymphomas, and other immune disorders.
Footnotes
This work was supported by a grant-in-aid from The Ministry of Education, Science and Culture of Japan and the grant from Nagase Science and Technology Foundation.
Address correspondence to Hitoshi Ohmori, Department of Biotechnology, Faculty of Engineering, Okayama University, Tsushima-Naka, Okayama 700, Japan. Phone: 81-86-251-8197; Fax: 81-86-251-8197; E-mail: hit2224@biotech.okayama-u.ac.jp
References
- 1.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 2.Schatz DG, Oettinger MA, Schlissel MS. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- 3.Wagner SD, Neuberger MS. Somatic hypermutation of immunoglobulin genes. Annu Rev Immunol. 1996;14:441–457. doi: 10.1146/annurev.immunol.14.1.441. [DOI] [PubMed] [Google Scholar]
- 4.Radic MZ, Zouali M. Receptor editing, immune diversification, and self-tolerance. Immunity. 1996;5:505–511. doi: 10.1016/s1074-7613(00)80266-6. [DOI] [PubMed] [Google Scholar]
- 5.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Prak EL, Weigert M. Editing disease-associated autoantibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- 8.Ghia P, Gratwohl A, Singer E, Winkler TH, Melchers F, Rolink AG. Immature B cells from human and mouse bone marrow can change their surface light chain expression. Eur J Immunol. 1995;25:3108–3114. doi: 10.1002/eji.1830251118. [DOI] [PubMed] [Google Scholar]
- 9.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+, IgD−bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 10.Grawunder U, Leu TMJ, Shatz DG, Werner A, Rolink AG, Melchers F, Winkler TH. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 11.Hikida M, Mori M, Takai T, Tomochika K, Hamatani K, Ohmori H. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–2094. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- 12.Hikida M, Mori M, Kawabata T, Takai T, Ohmori H. Characterization of B cells expressing recombination activating genes in germinal centers of immunized mouse lymph nodes. J Immunol. 1997;158:2509–2512. [PubMed] [Google Scholar]
- 13.Han S, Zheng B, Schatz DG, Spanopolou E, Kelsoe G. Neoteny in lymphocytes: RAG1 and RAG2 expression in germinal center B cells. Science. 1996;274:2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 14.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 15.Papavasiliou F, Casellas R, Suh H, Qin X-F, Besmer E, Pelanda R, Nemazee D, Rajewski K, Nussenzweig MC. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997;278:298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- 16.Hikida M, Takai T, Ohmori H. Requirements of a costimulus for IL-4–induced IgE class switching in murine B cells activated via antigen receptors. Effectiveness of 8-mercaptoguanosine. J Immunol. 1996;156:2730–2736. [PubMed] [Google Scholar]
- 17.Chen C, Radic MZ, Erikson J, Camper SA, Litwin S, Hardy RR, Weigert M. Deletion and editing of B cells that express antibodies to DNA. J Immunol. 1994;152:1970–1982. [PubMed] [Google Scholar]
- 18.van Gent DC, McBlane JF, Ramsden DA, Sadofsky MJ, Hesse JE, Gellert M. Initiation of V(D)J recombination in a cell-free system. Cell. 1995;81:925–934. doi: 10.1016/0092-8674(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 19.Ramsden DA, Gellert M. Formation and resolution of double-strand break intermediates in V(D)J rearrangement. Genes Dev. 1995;9:2409–2420. doi: 10.1101/gad.9.19.2409. [DOI] [PubMed] [Google Scholar]
- 20.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for Sμ–Sε heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 21.Bogue MA, Wang C, Zhu C, Roth DB. V(D)J recombination in Ku86-deficient mice: distinct effects on coding, signal, and hybrid joint formation. Immunity. 1997;7:37–47. doi: 10.1016/s1074-7613(00)80508-7. [DOI] [PubMed] [Google Scholar]
- 22.Zelazowski P, Max EE, Kehry MR, Snapper CM. Regulation of Ku expression in normal murine B cells by stimuli that promote switch recombination. J Immunol. 1997;159:2559–2562. [PubMed] [Google Scholar]
- 23.Pulendran B, van Driel R, Nossal GJV. Immunological tolerance in germinal centers. Immunol Today. 1997;18:27–32. doi: 10.1016/s0167-5699(97)80011-4. [DOI] [PubMed] [Google Scholar]
- 24.Kelsoe G. Life and death in germinal centers (redux) Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- 25.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]