Abstract
Susceptibility to Fas-mediated apoptosis in nontolerant B cells is regulated in a receptor-specific fashion. To explore the regulation of Fas killing in tolerant, autoreactive B cells, mice doubly transgenic for hen egg lysozyme (HEL)–specific B cell receptors and soluble HEL were examined. Engagement of CD40 led to enhanced Fas expression and acquisition of sensitivity to Fas-mediated apoptosis in tolerant B cells, similar to that observed in nontolerant, receptor transgenic B cells. Engagement of surface immunoglobulin by specific (HEL) antigen failed to induce Fas resistance in tolerant B cells, in contrast to its effect on nontolerant B cells; however, cross-linking of biotinylated HEL with streptavidin induced similar levels of Fas resistance in tolerant and nontolerant B cells, which approximated the degree of Fas resistance produced by anti-Ig. Unlike surface Ig (sIg) engagement, physiological engagement of IL-4 receptors produced similar levels of Fas resistance in tolerant and nontolerant B cells. Thus, tolerant B cells differ from nontolerant B cells in the diminished capacity of surface immunoglobulin engagement to produce Fas resistance; however, tolerant B cells can be induced to become resistant to Fas-mediated apoptosis by IL-4 or by higher order cross-linking of sIg receptors.
Bcells can be targets for cytotoxicity mediated by CD4+ Th1 effector cells that express Fas ligand and induce apoptosis in a Fas (CD95)-dependent fashion (1–7). Susceptibility to Fas-mediated apoptosis is dependent on the state of B cell activation and is regulated in a receptor-specific fashion. CD40 engagement by soluble, chimeric CD40 ligand (CD40L)1 induces expression of cell surface Fas and renders primary B cells highly sensitive to Fas killing (8–10). In contrast, antigen receptor cross-linking actively opposes Th1 cell–mediated cytotoxicity (Th1-CMC), as shown by the observation that B cells treated with CD40L in conjunction with anti-IgM, or in conjunction with specific antigen, are resistant to Th1-CMC (8, 11, 12). In addition to antigen receptor engagement, B cells are also rendered Fas-resistant by IL-4 receptor engagement (13). Both anti-IgM and IL-4 induce protection against Fas killing that is not associated with a loss or alteration of Fas expression, and that is intrinsic to the B cell target itself (11, 13). Despite these similarities, the signaling pathways engaged by anti-IgM and IL-4 to produce Fas resistance are distinct as shown by several criteria, and suboptimal doses of these reagents act in synergy to bring about maximal levels of Fas resistance (11, 13, 14).
Autoreactive B cells express receptors with specificity for self-antigen, and several mechanisms provide for their exclusion from the immune repertoire. High affinity autoreactive B cells undergo clonal deletion or receptor editing in the bone marrow (15–18). Many of the remaining clones that recognize autoantigens migrate to the periphery where they exist in a state of tolerance characterized by impaired signaling via the antigen receptor, normal signaling via CD40 and IL-4R, and shortened life span (19–21). Several lines of evidence suggest that Fas (CD95) plays a role in regulating the disposition of activated, tolerant, autoreactive B cells: (a) Fas-deficient lpr/lpr mice and Fas knockout mice express excessively high titers of autoantibodies (22, 23); (b) the recovery of tolerant B cells after adoptive transfer is improved if the B cells are Fas-deficient or if the cotransferred T cells are deficient in Fas ligand (24, 25); and (c) transgenic mice that overexpress IL-4 produce autoantibodies (13).
To determine the potential role of inducible Fas resistance in determining the fate of tolerant B cells, we carried out experiments using anti–hen egg lysozyme (HEL)/HEL double transgenic (DTg) mice. We sought to determine whether the rules for receptor-specific induction of Fas resistance that we previously identified in nontolerant B cells would apply to tolerant B cells as well. This is particularly relevant with respect to the antigen receptor, where it is important to delineate the capacity of self-antigen to protect autoreactive B cells against Fas-mediated apoptosis.
Materials and Methods
Animals.
Male BALB/cByJ and C57BL/6J mice at 8–14 wk of age were obtained from The Jackson Laboratory (Bar Harbor, ME). MD4 × ML5 anti-HEL/soluble HEL (sHEL) DTg and MD4 anti-HEL single transgenic (STg) breeders were kindly provided by Dr. Michael C. Carroll (Harvard Medical School, Boston, MA) and Dr. Christopher C. Goodnow (Howard Hughes Medical Institute and Stanford University School of Medicine, Stanford, CA). DTg and STg mice were bred at Boston University Medical Center and offspring were screened as described below. DTg and STg mice were studied at 8–14 wk of age.
Genotyping.
The mice were genotyped by PCR carried out on tail-digest DNA (26). In brief, tail DNA was analyzed by two separate PCR reactions. The first reaction, to screen for the presence of endogenous B cell antigen receptor (BCR) and anti-HEL transgenic BCR, included three oligonucleotide primers: IgR1 5′ ACCACAGACCAGCAGGCAGA 3′, shared by both the endogenous and transgenic BCR; IgF1 5′ GCGACTCCATCACCAGCGAT 3′, unique to the endogenous BCR; and IgF2 5′ CTGGAGCCCTAGCCAAGGAT 3′, unique to the transgenic BCR. The second PCR reaction used an oligonucleotide primer pair to screen for the presence of sHEL: HELF1 5′ GAGCGTGAACTGCGCGAAGA 3′, and HELR1 5′ TCGGTACCCTTGCAGCGGTT 3′. After 32 cycles of amplification, the PCR products were size fractionated on 8% native polyacrylamide gels and visualized by ultraviolet transillumination after ethidium bromide staining. DTg anti-HEL/sHEL mice yield PCR products of 264 bp (endogenous Ig), 430 bp (Ig transgene), and 160 bp (lysozyme transgene).
B Cell Purification.
Splenic B cells were purified by depletion of T cells with antibody (13-4 anti-Thy 1.2, GK1.5 anti-CD4, and 3.1.55 anti-CD8), plus rabbit complement, and dead cells were removed by sedimentation on Lympholyte M (Cedarlane Labs., Ltd., Hornby, Ontario, Canada), as previously described (8). Viable cells were resuspended in RPMI 1640 medium (BioWhittaker, Walkersville, MD) supplemented with 5% heat-inactivated FCS (Sigma Chemical Co., St. Louis, MO), 50 μM 2-mE, 2 mM l-glutamine, 10 mM Hepes, pH 7.2, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Cell-mediated Cytotoxicity.
B cells (2 × 106) were cultured in 1.2 ml of supplemented RPMI 1640 in the wells of 24-well tissue culture plates (Costar Corp., Cambridge, MA) for a total of 16 h at 37°C and 6% CO2. B cells were stimulated with CD40L– CD8α fusion protein (CD40L) cross-linked with CD8-specific antibody (as 1:10 and 1:80 dilutions of dialyzed supernatant, respectively) to induce susceptibility to Fas-mediated apoptosis, as previously described, or with CD40L–CD8α/anti-CD8 in combination with sHEL, F(ab′)2 fragments of goat anti–mouse IgM antibody (anti-IgM), dextran-conjugated anti–mouse IgD antibody (anti-IgD), or recombinant mouse IL-4 (rIL-4). B cells were also treated with CD40L–CD8α/anti-CD8 in combination with biotinylated sHEL in the presence or absence of streptavidin (SA). B cells were then tested as targets for Fas-mediated apoptosis in standard lectin-dependent 4-h 51Cr–release assays, as previously described (11), using AE7 CD4+ Th1 effector cells.
Flow Cytometric Analysis.
B cells stimulated as described above were examined for Fas expression by staining with PE-conjugated Jo-2 anti-Fas Ab or anti-CD28 isotype-matched control Ab (PharMingen, San Diego, CA) as previously described (11, 13). Unstimulated DTg and STg B cells were examined for bound HEL by staining with biotin-conjugated HyHEL9 anti-HEL monoclonal antibody, followed by PE-conjugated SA secondary antibody (PharMingen). B cells were stained with HyHEL9 in the presence of 0.1% azide before and after incubation with sHEL (Sigma Chemical Co.) at 100 ng/ml for 1 h at 4°C. Cells were then washed and analyzed by flow cytometry on a FACScan® instrument (Becton-Dickinson, San Jose, CA).
Reagents.
Supernatant from transfected J588L cells that secrete a chimeric CD40L–CD8α fusion protein (27) was collected and dialyzed against 25,000 mol wt cutoff dialysis tubing, as previously described (28). A similarly dialyzed supernatant containing anti-CD8 antibody from the 53-6-72 hybridoma was used to cross-link the fusion protein. rIL-4 was obtained from Genzyme Corp. (Cambridge, MA). F(ab′)2 fragments of affinity purified goat anti–mouse IgM were obtained from Jackson Immunoresearch Labs. (West Grove, PA). Mitogenic monoclonal anti-IgD (Hδa/1) conjugated to dextran was provided by Dr. James J. Mond (Uniformed Health Services University, Bethesda, MD; reference 29). [51Cr]sodium chromate (0.5 mCi/mmol) was obtained from Dupont-NEN (Boston, MA). sHEL was obtained from Sigma Chemical Co. Biotin-conjugated HyHEL9 (anti-HEL) was provided by Dr. Goodnow. SA was obtained from PharMingen.
Results
Several previous reports suggest that Fas-mediated apoptosis may play a role in regulating autoreactive B cells, in which case inducible Fas resistance might be expected to influence this process (13, 22–25). To directly study the capacity of tolerant B cells to acquire resistance to Fas-mediated apoptosis in a receptor-specific fashion, mice expressing transgenes for B cell receptors that recognize HEL (anti-HEL), with or without transgenes for sHEL, were examined. B cells from DTg mice develop in the continual presence of soluble autoantigen and are rendered tolerant. B cells from STg mice are not exposed to cognate antigen during development and thus are not tolerized (30, 31).
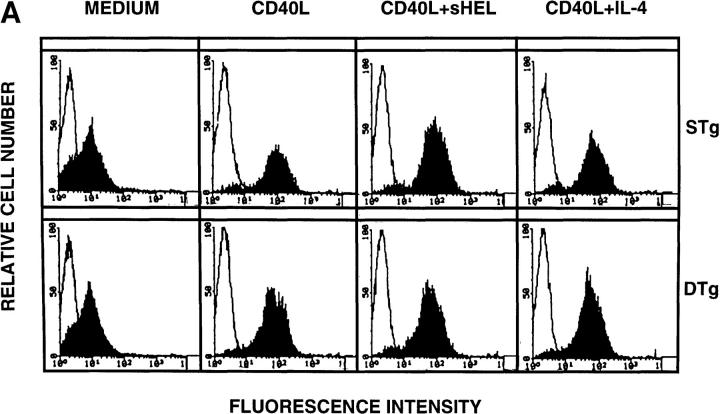
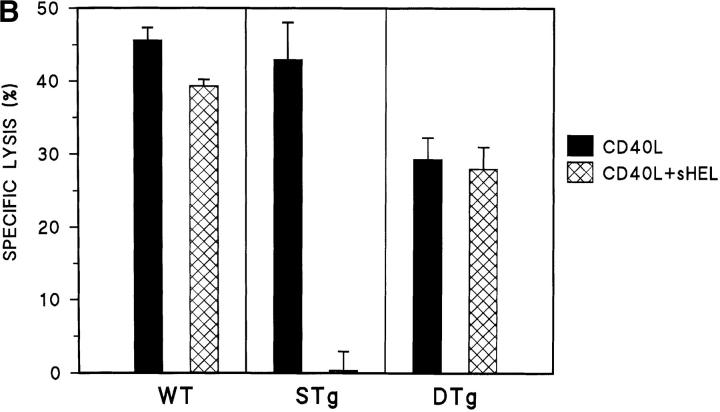
Primary B cells from DTg and STg mice were stimulated for 16 h with CD40L to assess relative levels of Fas sensitivity. As shown in Fig. 1 A, CD40L treatment resulted in substantial upregulation of Fas expression, which was similar for B cells from DTg and STg mice. B cells were then tested as targets for Fas-mediated apoptosis in lectin-dependent 4-h 51Cr–release assays using FasL-bearing AE7 CD4+ Th1 effector cells, as previously described (11). As shown in Fig. 1 B, CD40L-stimulated B cells from both DTg and STg mice were susceptible to Th1-CMC at a level that approximated the specific lysis of CD40L-stimulated wild-type C57BL/6J B cells. In 10 separate experiments, the specific lysis of DTg B cells was 39.1 ± 3.9% (mean ± SEM) and the specific lysis of STg B cells was 43.9 ± 3.4%, indicating little difference between the two populations.
Figure 1.
Fas expression, Fas sensitivity, and Fas resistance are induced in tolerant B cells by specific receptor engagement. (A) Primary B cells from STg or DTg mice were cultured in medium alone (medium) or were stimulated with either CD40L–CD8α fusion protein cross-linked with CD8-specific antibody (CD40L), CD40L plus soluble HEL at 100 ng/ml (CD40L+sHEL), or CD40L plus recombinant IL-4 at 100 U/ml (CD40L+IL-4) for 16 h as indicated. B cells were stained with PE-conjugated Jo-2 anti-Fas antibody (filled tracing), or with PE-conjugated anti-CD28 antibody, an isotype-matched control antibody (open tracing), in the presence of 2% rabbit serum. Stained cells were analyzed by flow cytometry; relative cell number is shown as a function of fluorescence intensity in arbitrary units. One of three comparable experiments is shown. (B) Primary B cells from wild-type (WT, C57BL/6J), STg, or DTg mice were stimulated with either CD40L–CD8α fusion protein cross-linked with CD8-specific antibody (black bars), or CD40L plus sHEL at 100 ng/ml (hatched bars), for 16 h as indicated. B cells were then radiolabeled and tested for susceptibility to Fas-dependent lysis in standard 4-h lectin-dependent 51Cr–release assays using AE7 CD4+ Th1 effector cells. Results for E/T cell ratios of 9:1 are presented. For each condition, the mean percentage of specific lysis of triplicate assays is displayed, along with a line indicating the SEM. One of three comparable experiments is shown.
To examine the capacity of surface Ig (sIg) to mediate Fas resistance in tolerant B cells, cytotoxicity assays were carried out following concurrent treatment of B cell targets with CD40L plus sHEL for 16 h. As shown in Fig. 1 B, the addition of sHEL to nontolerant B cells from STg mice expressing lysozyme-specific BCR produced virtually complete resistance to Th1-CMC (and this could not be attributed to a change in the level of Fas expression; compare with Fig. 1 A). In direct contrast, sHEL completely failed to modulate the Fas sensitivity of CD40L-stimulated tolerant B cells from DTg mice. Similar results were obtained using soluble recombinant FasL (provided by Dr. Andreas Hohlbaum, Boston University School of Medicine) to effect cytolysis (data not shown). HEL had no effect on the Fas sensitivity of wild-type B cells, in keeping with the expectation that normal mice would have no or few B cells with chicken lysozyme specificity. Thus, engagement of sIg by specific antigen did not alter the susceptibility to Fas-mediated apoptosis of tolerant B cells, yet induced a high level of Fas resistance in nontolerant, HEL-specific B cells.
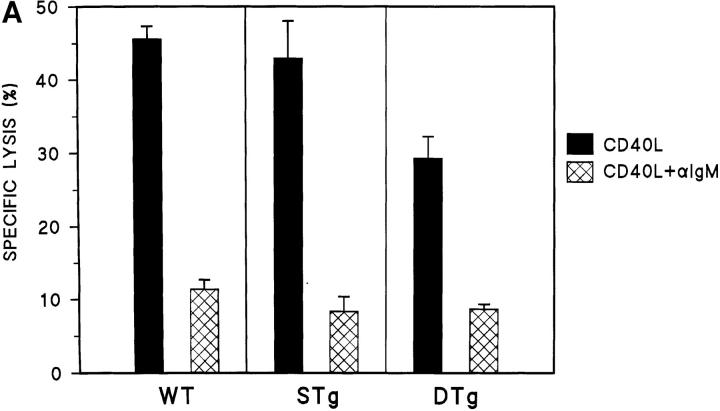
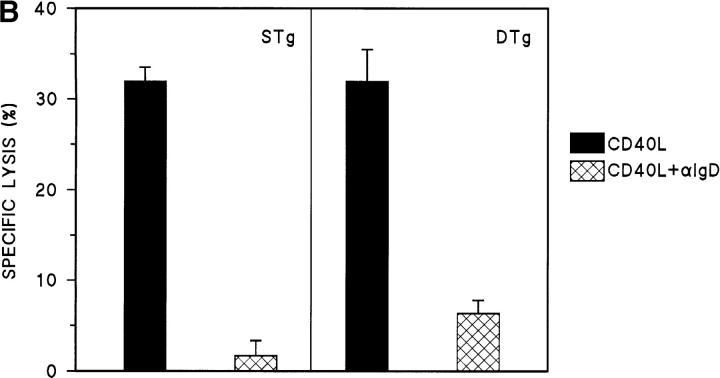
Cytotoxicity assays were also carried out to examine the effect of cross-linking B cell receptors with anti-Ig. Primary B cells from wild-type, STg, and DTg mice were treated with CD40L alone or were treated with the combination of CD40L plus anti-IgM for 16 h. As shown in Fig. 2 A, the addition of anti-IgM to CD40L produced similar levels of resistance to Th1-CMC in all three B cell populations. Further, STg and Dtg B cell populations differed little in the levels of Fas resistance produced by anti-IgD (Fig. 2 B). Thus, although sIg engagement by specific antigen failed to induce Fas resistance in tolerant B cells, sIg cross-linking by either anti-IgM or anti-IgD did so. These results indicate that the variant effect of sHEL on tolerant and nontolerant B cells cannot be explained by the different levels of sIgM and sIgD expressed.
Figure 2.
Antigen receptor cross-linking induces Fas resistance in tolerant B cells. (A) Primary B cells from wild-type (WT, C57BL/6J), STg, or DTg mice were stimulated with either CD40L–CD8α fusion protein cross-linked with CD8-specific antibody (black bars), or CD40L plus F(ab′)2 fragments of goat anti–mouse IgM at 10 μg/ml (hatched bars), for 16 h as indicated. B cells were then radiolabeled and tested for susceptibility to Fas-dependent lysis in standard 4-h lectin-dependent 51Cr–release assays using AE7 CD4+ Th1 effector cells. Results for E/T cell ratios of 9:1 are presented. For each condition, the mean percentage of specific lysis of triplicate assays is displayed, along with a line indicating the SEM. One of three comparable experiments is shown. (B) Primary B cells from STg or DTg mice were stimulated with either CD40L (black bars) or CD40L plus anti-IgD at 100 ng/ml (hatched bars) for 16 h as indicated. B cells were then radiolabeled and tested for susceptibility to Fas-dependent lysis as above. Results for E/T cell ratios of 9:1 are presented as described above.
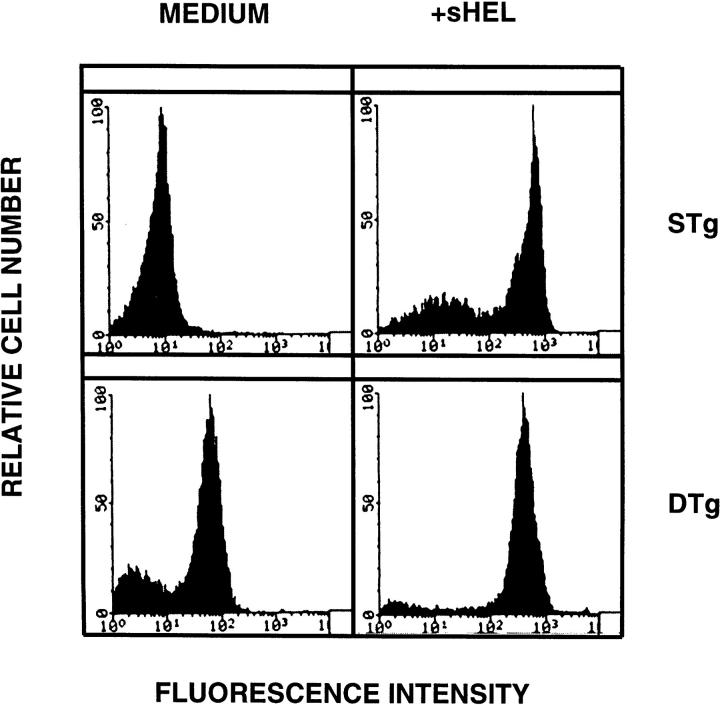
In the anti-HEL/sHEL system, tolerant B cells develop in the presence of specific antigen, raising the possibility that constitutive occupation of transgenic BCR by HEL might block additional antigen binding (31). To address this issue, the degree of BCR occupancy was evaluated by flow cytometric analysis after staining of B cells with HyHEL9. DTg and STg B cells were examined before and after addition of sHEL in vitro. As shown in Fig. 3, addition of HEL to the STg BCR produced a marked increase in HEL binding detected by HyHEL9. Addition of HEL to the DTg BCR also produced an increase in HEL binding although the increment was not as great as that observed with STg B cells, because DTg B cells contain some bound HEL at baseline, derived from endogenous sources. Thus, DTg BCR can be effectively engaged by added sHEL, albeit to a lesser extent than the STg BCR. The identification of unoccupied receptors in DTg B cells suggests that some mechanism other than simple receptor blockade is responsible for deficiencies in sIg signaling unless there is an incremental threshold required for induction of Fas-resistance.
Figure 3.
HEL is bound by transgenic receptors in tolerant B cells. Primary B cells from STg or DTg mice were examined before and after addition of sHEL at 100 ng/ml for 1 h at 4°C in the presence of 0.1% sodium azide. B cells were stained with biotin-conjugated anti-HEL monoclonal antibody HyHEL9, followed by PE-conjugated SA, in the presence of 2% rabbit serum. Stained cells were analyzed by flow cytometry; relative cell number is shown as a function of fluorescence intensity. One of three comparable experiments is shown.
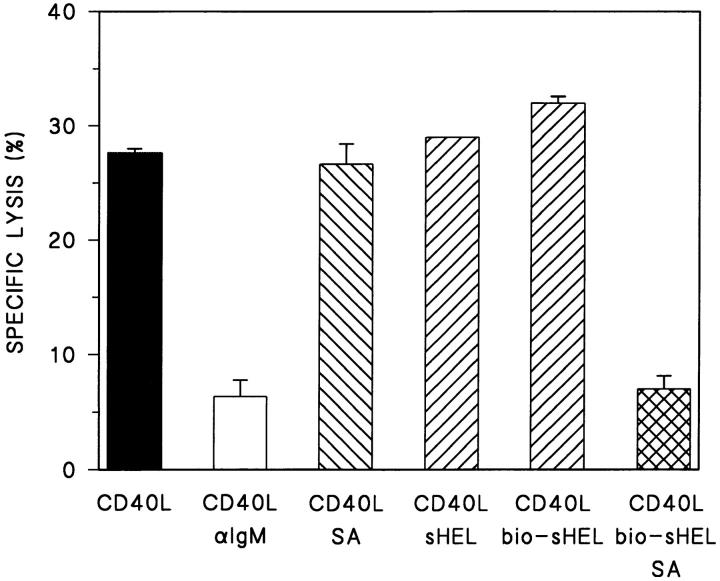
The disparity between sHEL and anti-Ig in the induction of Fas resistance in tolerant B cells could relate to the relative degree of sIg cross-linking produced by these agents. To address this possibility, the outcome of cross-linking sHEL bound to the DTg BCR was evaluated. DTg B cells were treated with CD40L alone or with CD40L in conjunction with biotin-conjugated sHEL in the presence or absence of SA for 16 h, and then tested as targets for Th1-CMC. As shown in Fig. 4, SA-induced cross-linking of biotinylated sHEL produced Fas resistance in DTg B cells that were unaffected by treatment with sHEL alone (native or biotinylated). Furthermore, the level of Fas resistance produced by cross-linked sHEL approximated that produced by anti-IgM–induced BCR cross-linking. These findings suggest that the sIg-triggered pathways responsible for bringing about resistance to Th1-CMC remain intact in tolerant B cells. However, it appears that a higher order of BCR engagement is required for induction of Fas resistance in tolerant as opposed to nontolerant B cells.
Figure 4.
Cross-linking sHEL induces Fas resistance in tolerant B cells. Primary B cells from DTg mice were stimulated with either CD40L–CD8α fusion protein cross-linked with CD8-specific antibody (CD40L), CD40L plus F(ab′)2 fragments of goat anti–mouse IgM antibody at 10 μg/ml (CD40L/αIgM), CD40L plus SA at 10 μg/ml (CD40L/SA), CD40L plus soluble HEL at 100 ng/ml (CD40L/sHEL), CD40L plus biotin-conjugated sHEL at 100 ng/ml (CD40L/bio-sHEL), and CD40L plus bio-sHEL followed by SA (CD40L/bio-HEL/SA), as indicated. B cells were then radiolabeled and tested for susceptibility to Fas-dependent lysis in standard 4-h lectin-dependent 51Cr–release assays using AE7 CD4+ Th1 effector cells. Results for E/T cell ratios of 9:1 are presented. For each condition, the mean percentage of specific lysis of triplicate assays is displayed, along with a line indicating the SEM. One of three comparable experiments is shown.
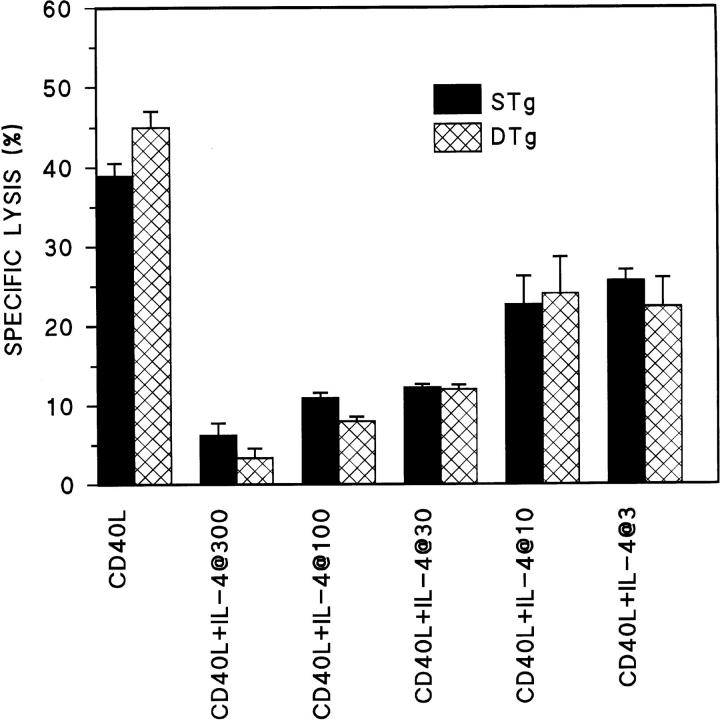
Beyond the antigen receptor, engagement of the IL-4 receptor has been shown to induce Fas resistance in nontolerant B cells (13). To examine the capacity of IL-4 to mediate Fas resistance in tolerant B cells, cytotoxicity assays were carried out after concurrent treatment of B cell targets with CD40L plus IL-4 for 16 h. As shown in Fig. 5, the addition of IL-4 to CD40L over a range of doses produced similar levels of resistance to Th1-CMC both in tolerant B cells from DTg mice and nontolerant B cells from STg mice. This is consistent with previous reports that IL-4R signaling in tolerant B cells is unimpaired (19, 20). In neither case could IL-4–mediated Fas resistance be attributed to a change in the level of Fas expression (compare with Fig. 1 A). Thus, physiologic engagement of IL-4R in tolerant B cells produces Fas resistance, whereas BCR engagement by specific antigen does not.
Figure 5.
IL-4 induces Fas resistance in tolerant B cells. Primary B cells from STg (black bars) or DTg (hatched bars) mice were stimulated with either CD40L–CD8α fusion protein cross-linked with CD8-specific antibody (CD40L), or CD40L plus IL-4 at the doses indicated in U/ml (CD40L+IL-4), for 16 h, as indicated. B cells were then radiolabeled and tested for susceptibility to Fas-dependent lysis in standard 4-h lectin-dependent 51Cr–release assays using AE7 CD4+ Th1 effector cells. Results for E/T cell ratios of 3:1 are presented. For each condition, the mean percentage of specific lysis of triplicate assays is displayed, along with a line indicating the SEM. One of three comparable experiments is shown.
Discussion
Our results delineate the regulation of Fas-mediated apoptosis in tolerant B cells. In several respects tolerant B cells behave like nontolerant B cells: susceptibility to Fas killing is produced by CD40 engagement and is opposed by higher order cross-linking of surface immunoglobulin and by engagement of IL-4 receptors by physiological ligand. However, tolerant B cells differed markedly from nontolerant B cells in the response to BCR triggering by cognate antigen, in that Fas-resistance is not induced in tolerant B cells but is induced in nontolerant B cells. These results show for the first time that specific antigen fails to elicit Fas-resistance in tolerant B cells, but that tolerant B cells may be protected against Fas killing by higher order BCR engagement and by engagement of IL-4R.
Tolerant B cells can be lost through a Fas-dependent mechanism (references 24 and 25 and our results), and Fas deficiency is associated with substantial autoreactive antibody formation (22, 23), suggesting that interference with the susceptibility of tolerant B cells to Fas-mediated apoptosis may play a role in autoimmunity. This work suggests two mechanisms by which tolerant autoreactive B cells may escape Fas-dependent deletion. One involves high order cross-linking of surface immunoglobulin such as might be produced by immune complexes or by elevated levels of multivalent antigen. The other involves Th2-derived IL-4. Notably, sepsis may result in conditions fostering Fas-resistance in tolerant B cells in view of the association between overwhelming microbial infection, circulating microbial antigen, and elevated levels of cytokines (32). Considering the observation that autoantibodies in some cases recognize microbial antigens (33–35), it may be hypothesized that Fas resistance in tolerant B cells could be responsible for returning autoreactive B cells to the immunocompetent pool, resulting in the production of antimicrobial antibodies that cross-react with self, at the risk of initiating autoimmunity. This would be consistent with our earlier finding that IL-4 overexpressing transgenic mice express serological autoreactivity (13).
Tolerant B cells were relatively restricted in the nature of sIg cross-linking capable of inducing Fas resistance, in contrast to nontolerant B cells, which were more permissive. This suggests that a threshold exists in the level of BCR stimulation required to complete signaling for Fas resistance, and that the threshold is higher for tolerant as opposed to nontolerant B cells. Consistent with this, we found that B7.2 (CD86) expression, although effectively induced in tolerant B cells by anti-IgM, was poorly stimulated by sHEL, whereas both agents induced B7.2 in nontolerant B cells (data not shown).
It is worth noting that induction of Fas resistance by soluble antigen in nontolerant, BCR transgenic B cells extends our earlier observation that Fas resistance is induced in normal, wild-type B cells by cognate antigen that is membrane bound (8), and provides a direct explanation for the improved recovery of nontolerant HEL-specific B cells after adoptive transfer with sHEL and HEL-specific T cells (24, 25).
In sum, these results indicate that tolerant B cells are relatively insulated from self-antigen–induced modulation of the Fas death pathway, but, through the influence of IL-4 or super-threshold levels of BCR stimulation, may acquire Fas resistance and thereby contribute to autoantibody formation.
Acknowledgments
This work was supported by United States Public Health Service grant AI-40181 awarded by the National Institutes of Health.
Footnotes
1 Abbreviations used in this paper: BCR, B cell antigen receptor; CD40L, soluble, chimeric CD40 ligand; DTg, double transgenic (anti-HEL/ HEL); HEL, hen egg lysozyme; rIL-4, recombinant mouse interleukin 4; sHEL, soluble hen egg lysozyme; sIg, surface Ig; STg, single transgenic (anti-HEL); Th1-CMC, Th1 cell-mediated cytotoxicity.
Linda Foote's current address is Department of Biology, Merrimack College, North Andover, MA 01834.
References
- 1.Owen-Schaub LB, Yonehara S, Crump WL, Grimm EA. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992;140:197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- 2.Rouvier E, Luciani M-F, Golstein PJ. Fas involvement in Ca2+-independent T cell–mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell–mediated cytotoxicity. J Immunol. 1994;152:1127–1133. [PubMed] [Google Scholar]
- 4.Ju S-T, Cui H, Panka DJ, Ettinger R, Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA. 1994;91:4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanabuchi S, Koyanagi M, Kawasaki A, Shinohara N, Matsuzawa A, Nishimura Y, Kobayashi S, Yonehara S, Yagita H, Okumura K. Fas and its ligand in a general mechanism of T cell–mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:4930–4934. doi: 10.1073/pnas.91.11.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vignaux F, Vivier E, Malissen B, Depraetere V, Nagata S, Golstein P. TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med. 1995;181:781–786. doi: 10.1084/jem.181.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henkart PA. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 8.Rothstein TL, Wang JKM, Panka DJ, Foote LC, Wang Z, Stanger B, Cui H, Ju S-T, Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- 9.Garrone P, Neihardt E-M, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligand induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–1273. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schattner EJ, Elkon KB, Yoo D-H, Tumang J, Krammer PH, Crow MC, Friedman SM. CD40 ligation induces APO-1/Fas expression on human B lymphocytes and facilitates apoptosis through the APO-1/Fas pathway. J Exp Med. 1995;182:1557–1565. doi: 10.1084/jem.182.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foote LC, Schneider TJ, Fischer GM, Wang JKM, Rasmussen B, Campbell KM, Lynch DH, Ju S-T, Marshak-Rothstein A, Rothstein TL. Intracellular signaling for inducible antigen receptor–mediated Fas resistance in B cells. J Immunol. 1996;157:1878–1885. [PubMed] [Google Scholar]
- 12.Lagresle C, Mondiere C, Bella C, Krammer PH, Defrance T. Concurrent engagement of CD40 and the antigen receptor protects naive and memory human B cells from APO-1/Fas-mediated apoptosis. J Exp Med. 1996;183:1377–1388. doi: 10.1084/jem.183.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foote LC, Howard RG, Marshak-Rothstein A, Rothstein TL. IL-4 induces Fas resistance in B cells. J Immunol. 1996;157:2749–2753. [PubMed] [Google Scholar]
- 14.Schneider TJ, Foote LC, Grillot D, Nunez G, Rothstein TL. Bcl-xLprotects primary B cells against Fas-mediated apoptosis. J Immunol. 1997;159:4834–4839. [PubMed] [Google Scholar]
- 15.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 16.Hartley SB, Crosbie J, Brink R, Kantor AA, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 17.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prak EL, Weigert M. Light chain replacement: a new model for antibody gene rearrangement. J Exp Med. 1995;182:541–548. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eris JM, Basten A, Brink R, Doherty K, Kehry MR, Hodgkin PD. Anergic self-reactive B cells present self antigen and respond normally to CD40-dependent T cell signals but are defective in antigen-receptor–mediated functions. Proc Natl Acad Sci USA. 1994;92:99–105. doi: 10.1073/pnas.91.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke MP, Heath AW, Shokat KM, Zeng Y, Finkleman FD, Linsley PS, Howard M, Goodnow CC. Immunoglobulin signal transduction guides the specificity of B cell–T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izui S, Kelley VE, Masuda K, Yoshida H, Roths JB, Murphy ED. Induction of varioous autoantibodies by mutant gene lprin several strains of mice. J Immunol. 1984;133:227–233. [PubMed] [Google Scholar]
- 23.Adachi M, Suematsu S, Suda T, Watanabe D, Fukuyama H, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Enhanced and accelerated lymphoproliferation in Fas-null mice. Proc Natl Acad Sci USA. 1996;93:2131–2136. doi: 10.1073/pnas.93.5.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathmell JC, Cooke MP, Ho WY, Grein J, Townsend SE, Davis MM, Goodnow CC. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 25.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Evans GA. A simple screening method for transgenic mice using the polymerase chain reaction. Biotechniques. 1990;8:32–33. [PubMed] [Google Scholar]
- 27.Lane P, Brocker T, Hubele S, Padovan E, Lanzavecchia A, McConnell F. Soluble CD40 ligand can replace the normal T cell–derived CD40 ligand signal to B cells in T cell–dependent activation. J Exp Med. 1993;177:1209–1213. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis DA, Karras JG, Ke X-Y, Sen R, Rothstein TL. Induction of the transcription factor NF-κB, AP-1, and NF-AT during B cell stimulation through the CD40 receptor. Int Immunol. 1995;7:151–161. doi: 10.1093/intimm/7.2.151. [DOI] [PubMed] [Google Scholar]
- 29.Brunswick M, Finkelman FD, Highet PF, Inman JK, Dintzis HM, Mond JJ. Picogram quantities of anti-Ig antibodies coupled to dextran induce B cell proliferation. J Immunol. 1988;140:3364–3372. [PubMed] [Google Scholar]
- 30.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 31.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 32.DiPiro JT, Howdieshell TR, Goddard JK, Callaway DB, Hamilton RG, Mansberger AR., Jr Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130:1159–1162. doi: 10.1001/archsurg.1995.01430110017004. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson PM, Lampman GW, Furie BC, Naparstek Y, Schwartz RS, Stollar BD, Furie B. Homology of the NH2-terminal amino acid sequences of the heavy and light chains of human monoclonal lupus autoantibodies containing the dominant 16/6 idiotype. J Clin Invest. 1985;75:1138–1143. doi: 10.1172/JCI111808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll P, Stafford D, Schwartz RS, Stollar BD. Murine monoclonal anti-DNA autoantibodies bind to endogenous bacteria. J Immunol. 1985;135:1086–1090. [PubMed] [Google Scholar]
- 35.Duggan DB, Mackworth-Young C, Kari-Lefvert A, Andre-Schwartz J, Mudd D, McAdam KP, Schwartz RS. Polyspecificity of human monoclonal antibodies reactive with Mycobacterium leprae, mitochondria, ssDNA, cytoskeletal proteins, amd the acetylcholine receptor. Clin Immunol Immunopathol. 1988;49:327–340. doi: 10.1016/0090-1229(88)90123-7. [DOI] [PubMed] [Google Scholar]