Abstract
C3H/HeJBir mice are a new substrain that spontaneously develop colitis early in life. This study was done to determine the T cell reactivity of C3H/HeJBir mice to candidate antigens that might be involved in their disease. C3H/HeJBir CD4+ T cells were strongly reactive to antigens of the enteric bacterial flora, but not to epithelial or food antigens. The stimulatory material in the enteric bacteria was trypsin sensitive and restricted by class II major histocompatibility complex molecules, but did not have the properties of a superantigen. The precursor frequency of interleuken (IL)-2–producing, bacterial-reactive CD4+ T cells in colitic mice was 1 out of 2,000 compared to 1 out of 20,000–25,000 in noncolitic control mice. These T cells produced predominately IL-2 and interferon γ, consistent with a T helper type 1 cell response and were present at 3–4 wk, the age of onset of the colitis. Adoptive transfer of bacterial-antigen–activated CD4+ T cells from colitic C3H/HeJBir but not from control C3H/HeJ mice into C3H/HeSnJ scid/scid recipients induced colitis. These data represent a direct demonstration that T cells reactive with conventional antigens of the enteric bacterial flora can mediate chronic inflammatory bowel disease.
The inflammatory bowel diseases (IBD)1, encompassing Crohn's disease and ulcerative colitis, are complex chronic inflammatory diseases of the intestine whose etiology and pathogenesis remain unknown. There are multiple etiologic theories, one of which is that a dysregulated CD4 T cell response to the abundant antigens in the lumen may be responsible (1, 2). This hypothesis is based on theoretical grounds and there is only limited supporting data in humans as yet (3, 4). However, support for this hypothesis has come from the results of studies done in a number of recently developed experimental models of IBD, some of which have been the unexpected result of gene deletions by selective gene targeting (5–9). In a number of such models, CD4+ T cells have been found to mediate colitis, and most commonly this has involved an exaggerated Th1 response manifested by excessive IFN-γ production in the lesions (10–12). The localization of inflammatory disease to the colon of mice that have global deficiencies of an immune molecule suggests that the bacterial flora is the major immune stimulant leading to chronic intestinal inflammation. Indeed, in some models, animals that are raised germ-free no longer develop colitis (5, 13), and in others rederivation with a defined flora (6, 12) or antibiotic treatment (14) ameliorates the disease. Moreover, reconstitution of intestinal bacteria into germ-free animals can restore intestinal inflammation (15). However, it has remained unclear how the bacterial flora generates chronic intestinal inflammation.
Humans with IBD do not have absolute deficiencies of the immune molecules whose deletion in mice has resulted in colitis. For this reason, we have derived and studied a new strain of mice which develop colitis spontaneously, namely the C3H/HeJBir strain (16). C3H/HeJBir mice develop a predominantly right-sided colitis early in life that largely resolves by 3 mo of age. Previous studies on the immunopathogenesis of disease in this mouse strain have found that C3H/HeJBir mice, but not mice of the parenteral C3H/HeJ strain, have high titer serum IgG antibodies to a selected subset of antigens of the enteric bacterial flora (17). These antibodies are mainly of the IgG2a subclass, compatible with a predominant Th1 response to these bacterial antigens. This study was undertaken to define the CD4+ T cell response of C3H/HeJBir mice to enteric bacterial antigens. Compatible with our earlier results analyzing antibody responses, strong CD4+ Th1 T cell reactivity to protein antigens of the enteric bacterial flora was identified. Moreover, disease could be induced by transfer of enteric bacterial antigen-activated C3H/HeJBir CD4+ T cells, but not by control C3H/HeJ T cells, into C3H/ HeSnJ scid/scid recipients. These results demonstrate for the first time that CD4+ T cells reactive with conventional antigens of the bacterial flora are able to mediate chronic intestinal inflammation.
Materials and Methods
Mice
Age-matched female C3H/HeJBir and C3H/HeJ mice from The Jackson Laboratory (Bar Harbor, ME) were used in each experiment. All studies were approved by the Animal Care and Use Committees of the University of Alabama at Birmingham.
Reagents and Materials
Con A was purchased from Sigma Chemical Co. (St. Louis, MO). RPMI 1640, fetal bovine serum, 2-ME, Hepes, l-glutamine, and sodium pyruvate were purchased from GIBCO BRL (Bethesda, MD). Anti-CD3 monoclonal antibody was purchased from PharMingen (San Diego, CA). Anti–I-Ab and anti–I-Ak were purchased from American Type Culture Collection (ATCC, Rockville, MD).
Preparation of Antigens
Enteric Bacterial Antigens.
C3H/HeJ or C3H/HeJBir mice were killed and their cecums were removed. The cecums were opened and placed in 1 ml of PBS. The cecal bacteria were expelled by mixing with a vortex, and residual cecal tissue was removed. After addition of DNAse (10 μg/ml), 1 ml of this bacterial suspension was added to 1 ml of glass beads. The cells were disrupted at 5,000 revolutions per minute in a Mini-Bead Beater (BioSpec Products, Bartlesville, OK) for 3 min and then iced. The glass beads and unlysed cells were removed by centrifuging at 5,000 g for 5 min. The lysates were filter sterilized by a 0.2 micron syringe filter. Cultured bacteria were processed in a similar manner. In most experiments, cecal bacteria were obtained from normal C3H/HeJ mice.
For some experiments, as indicated in text, the lysates of cecal bacteria were digested with trypsin. The trypsin was added into cecal bacterial protein preparation at a 1:50 (wt/wt) ratio and was incubated for 24 h. The mixture was then dialyzed in a 12,000-kD tube against PBS for 24 h, then used to pulse APCs for use in the T cell cultures.
Enteric Bacterial Isolates.
Multiple bacterial strains were obtained from three different institutions: Escherichia coli and Eubacterium species were purchased from ATCC; Bacteroides vulgatus was provided by Dr. T. Ohkusa (Tokyo Medical and Dental University, Tokyo, Japan); and Proteus mirabilis was isolated from a C3H/ HeJBir mouse by Dr. K. Waites (University of Alabama, Birmingham, AL). All of these strains were either previously well characterized (ATCC) or were identified by standard biochemical tests.
Epithelial Cell Proteins and Food Antigens.
Protein extracts were made from a murine C3H/He–derived intestinal epithelial cell line, Mode-K, which was provided by Dr. Dominique Kaiserlian (Institut Pasteur, Lyon, France) or from the MCA-38 murine colon adenocarcinoma cell line, which was the gift of Dr. Barbara Barna (Cleveland Clinic, Cleveland, OH). The cells were washed three times with PBS, and suspended in 1 ml of 5 mM MgCl2 with 2 mM PMSF and 10 mM Tris-Cl, and then lysed by freezing (in dry ice/ethanol) and thawing three times. The lysate generated by this treatment was centrifuged at 16,000 g for 30 min. The supernatant was filter sterilized and immediately used.
Murine chow (Agway Pro Lab RMH 1000, Agway, Inc., Syracuse, NY) was prepared in PBS with homogenization in the same manner as the cecal bacteria.
Isolation of CD4+ T Cells and APCs
C3H/HeJBir or C3H/HeJ mouse spleen and mesenteric lymph nodes were removed and placed into cell suspensions by straining through a small mesh sieve. After two washes, the cells were passed through a nylon wool column as previously described (18). The column-passed cells were washed twice and treated with anti-CD8 antibody (TIB 211; ATCC) supernatant (1 ml supernatant/107 cells) for 30 min on ice. After washing three times, magnetic beads coated with anti-rat IgG were added to the cells (BioMag, Cambridge, MA) and incubated for 30 min on ice. After passing through a magnet, CD4+ T cells were collected and reconstituted at 4 × 106 cells per ml in complete RPMI media containing 10% FCS for use in cell culture.
For APCs, spleen cells from syngeneic mice were prepared and treated with appropriate concentration of antigens as indicated at 2 × 107 cells/5 ml in a 15-ml tube overnight at 37°C. After washing twice, the cells were reconstituted at 4 × 106 cells per ml in complete media containing RPMI 1640 10% FCS, 2 mM l-glutamine, 0.05 mM 2-ME, 100 U/ml penicillin, and 10 μg/ ml streptomycin for use in cell culture. These APCs were irradiated with 3,000 rads before being added to T cell cultures.
Assay of Antigen-specific Proliferation of T Cells
Spleen and mesenteric lymph node (MLN) CD4+ T cells were isolated as described above and 4 × 105 cells/well were incubated in triplicate in the presence of 4 × 105 antigen-pulsed APCs/well in wells of a 96-well flat-bottomed tissue culture plate (Falcon 3072, Lincoln Park, NJ) at 37°C in 5% CO2 humidified air. After different times of incubation as indicated in the text, 0.5 μCi of [3H]-thymidine (New England Nuclear, Boston, MA) was added to each culture in the last 18 h of the incubation period. The cells were harvested on glass fiber filters on a PHD cell harvester (Cambridge Technology, Inc., Watertown, MA), washed with distilled water, and dried. Proliferation was assessed as the amount of incorporation of 3H-thymidine into cell DNA, as measured by beta scintillation counting (Beckman Instruments, Palo Alto, CA) of the harvested samples and were expressed as cpm ± SD.
Cytokine Assays
Spleen and MLN CD4+ T cells were cultured in the presence of APCs pretreated with antigens as described above in complete media. The culture supernatants were collected at different times and pooled together for assay. The supernatants collected after 24 h of culture were used for IL-2 assay and the supernatants collected at 72 h of culture were used for IL-3, IL-4, and IFN-γ assays. IL-2 was assessed by using the IL-2–dependent cell line HT-2AB (19), IL-3 by using the IL-3–dependent cell line FDC-1 (20), IL-4 by using the IL-4–dependent cell line CT-4S (21), and IFN-γ by using the IFN-γ–dependent cell line WEHI 279 (22) as previously described (23).
Precursor Frequency Analysis by Limiting Dilution
A modification of a previously described method (24) was used to determine the frequency of IL-2–producing, cecal bacterial antigen–specific cells. In brief, CD4+ T cells were isolated from spleen and MLN of C3H/HeJ, C3H/HeJBir or C57BL/6 mice, and graded numbers of CD4+ cells were plated on 4 × 104 syngeneic, irradiated (3,000 rad) and cecal bacterial antigen–pulsed spleen cells in U-bottomed 96-well plates (Costar, Cambridge, MA) in a total volume of 50 μl. 24 wells were plated for each cell concentration; 12 control wells (no responder cells) were set up in each experimental plate. After incubation at 37°C for 5 d, the plates were irradiated (3,000 rad), 50 μl HT-2 AB IL-2–dependent cells were added to each well, and incubation was continued for another 24 h with [3H]TdR added to each well for the last 6 h of culture. Individual microcultures were scored as positive if their incorporation of [3H]thymidine was >2 SD above the incorporation of control wells. Regression curves were constructed by plotting the number of responder cells per well versus the log of the percentage of negative wells. Frequencies were calculated on the regression curve by interpolating the number of responder cells required to give 37% negative cultures (corresponding to one precursor per well according to Poisson statistics).
Cell Transfer
For the fresh CD4+ T cell transfer group, 2 × 106 freshly prepared CD4+ T cells from spleen and MLN of C3H/HeJ or C3H/HeJBir mice were transferred intravenously into C3H/ HeSnJ scid/scid recipients. Other CD4+ T cells isolated from spleen and MLN of C3H/HeJ or C3H/HeJBir mice were cultured with cecal bacterial antigen–pulsed and irradiated C3H/HeJ splenic cells in complete medium at 37°C for 4 d in 5% CO2 air before transfer as above. 3 mo later, the recipients were killed, and then cecum and proximal, medial, and distal portions of colon were fixed in formalin. Fixed tissues were embedded in paraffin, and sections were stained with hematoxylin and eosin for histologic examination. All slides were read by an experienced pathologist (A. Lazenby) without knowledge of their origin.
Statistics
The results were expressed as the mean ± SD. The significance of the difference in means was determined by Student's t test.
Results
C3H/HeJBir CD4+ T Cells Respond to Enteric Bacterial but not Food or Epithelial Cell Antigens.
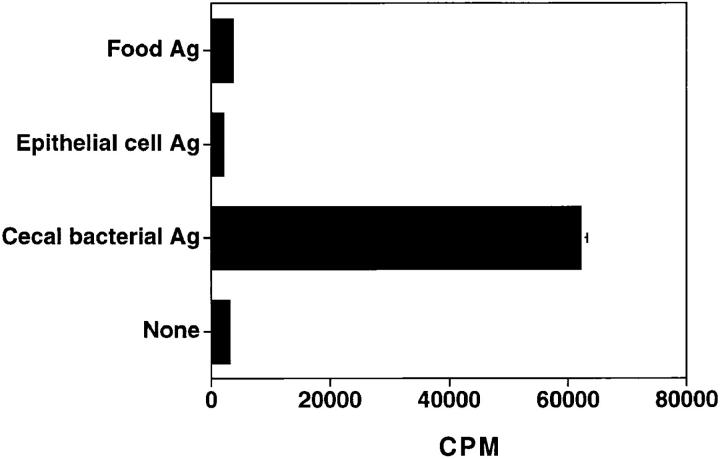
The focus of disease in C3H/HeJBir mice is the cecum and right colon, which contains a variety of antigens from bacteria, food, and shed epithelial cells. We tested whether C3H/HeJBir CD4+ T cells respond to antigens of any of these sources. Extracts of murine chow were used as food antigens and an extract of the Mode-K intestinal epithelial cell line was used as a source of epithelial cell antigens. As shown in Fig. 1, the C3H/HeJBir CD4+ T cells did not respond to stimulation of food antigens or epithelial cell antigens, but did respond strongly to stimulation by APCs pulsed with lysates of cecal bacteria (CBA). When various doses of food antigens or CBA epithelial antigens (from 0.2 to 1000 μg/ml) were used in the stimulation, C3H/HeJBir T cells still did not respond (data not shown). C3H/HeJBir T cells did not respond either to epithelial cell antigens derived from MCA-38 cells or from primary isolates of C3H/HeJBir small intestinal or colon epithelial cells (data not shown). C3H/HeJBir T cell reactivity was found to bulk cultures of cecal bacteria, although the level of stimulation was generally lower than to freshly obtained cecal bacteria (data not shown). Control T cells from normal C3H/HeJ mice did not respond at all to food or epithelial cell antigens (data not shown) and only weakly to CBA (see below).
Figure 1.
CD4+ T cells from C3H/HeJBir mice responded to cecal bacterial antigens but not food or epithelial cell antigens. Cecal bacterial antigens from C3H/HeJ mice, food antigens, and epithelial cell antigens were prepared as described in Materials and Methods. APCs from C3H/HeJ mice were incubated with 200 μg/ml of antigen for 18 h, then 4 × 105 antigen pulsed APCs were added to 4 × 105 splenic and MLN CD4+ T cells of C3H/HeJBir mice. 0.5 μCi of [3H]TdR was added to the wells for the last 18 h of a 5-d culture. Results are expressed as mean cpm ± SD of triplicate cultures and representative of three experiments with similar results.
Responses of C3H/HeJBir CD4+ T Cells to Cecal Bacterial Antigens.
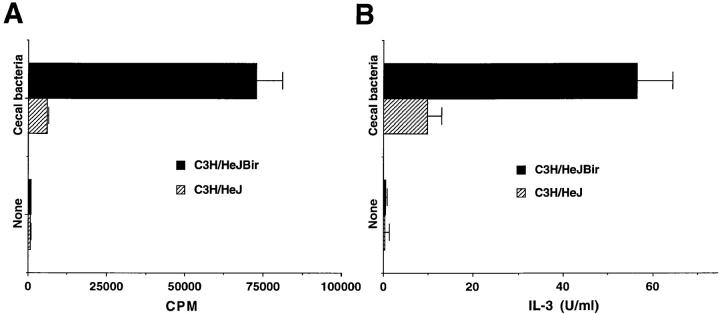
In further experiments, purified CD4+ T cells from spleen and MLN of C3H/HeJBir mice or from normal, noncolitic C3H/HeJ mice were stimulated with APCs previously pulsed with lysates of enteric bacteria. C3H/ HeJBir CD4+ T cells proliferated strongly to APCs pulsed with lysates of cecal bacteria, whereas spleen and MLN CD4+ T cells of normal C3H/HeJ mice had low proliferative response to the same APCs (Fig. 2 A). Similarly, a high level of IL-3 was produced by C3H/HeJBir CD4+ T cells stimulated with cecal bacterial antigens, whereas control C3H/HeJ CD4+ T cells produced low levels of IL-3 when cultured with cecal bacterial antigens (Fig. 2 B). There was no difference in the CD4+ T cell response whether the cecal bacterial antigens were derived from the colitic C3H/ HeJBir or from the normal C3H/HeJ strains (data not shown), thus, in the following experiments the cecal bacterial antigens were derived from normal C3H/HeJ mice.
Figure 2.
Responses of C3H/HeJBir and C3H/HeJ CD4+ T cells to enteric bacterial antigens. APCs from C3H/HeJ mice were prepared as in Fig. 1, then 4 × 105 CD4+ T cells from spleen and MLN of 12-wk-old mice were cultured with 4 × 105 of antigen pulsed APCs per well. (A) Proliferative response. 0.5 mCi of [3H]TdR was added to the wells for the last 18 h of a 5-d culture. Results are expressed as mean cpm ± SD of triplicate cultures and representative of three experiments with similar results. (B) IL-3 cytokine response. Supernatants were collected at day 3 of triplicate cultures and pooled together for IL-3 bioassay by using the IL-3–dependent cell line FDC-1 (see Materials and Methods). These results are representative of three experiments.
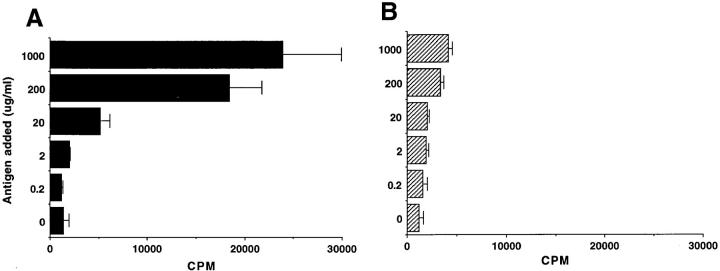
To determine whether the C3H/HeJBir CD4+ T cell response was dose dependent and whether the lower C3H/ HeJ CD4+ T cell response was due to a difference in the dose response, a broad range of cecal bacterial antigen doses, from 0.2 to 1,000 μg/ml, were used to pulse APCs that were then cocultured with CD4+ T cells. CD4+ T cells of C3H/HeJBir mice had a proliferative response to cecal bacterial antigens starting from a dose of 20 μg/ml, and the response increased with increasing cecal bacterial antigen concentrations (Fig. 3 A). The CD4+ T cells of C3H/HeJ mice had low proliferative responses to all tested concentrations of cecal bacterial antigens (Fig. 3 B). When the kinetics of proliferation of CD4+ T cells to cecal bacterial antigens was studied by pulsing with [3H]thymidine at different times of culture from day 3 to day 5, the optimal response of the C3H/HeJBir CD4+ T cells to cecal bacterial antigens was at day 5 of culture; CD4+ T cells of normal C3H/HeJ had low proliferative responses at each time point (data not shown).
Figure 3.
Dose–response of C3H/HeJBir CD4+ T cells (A) and C3H/ HeJ CD4+ T cells (B) to cecal bacterial antigens. 4 × 105 CD4+ T cells from spleen and MLN of 12-wk-old C3H/HeJBir mice were cultured with 4 × 105 per well of APCs pulsed overnight concentrations of antigen ranging between 0.2 and 1,000 μg/ml. 0.5 μCi of [3H]TdR was added to the wells for the last 18 h of a 5-d culture. Results are expressed as mean cpm ± SD of triplicate cultures and are representative of three experiments.
H-2 Restriction of CD4+ T Cell Response to Cecal Bacterial Antigens.
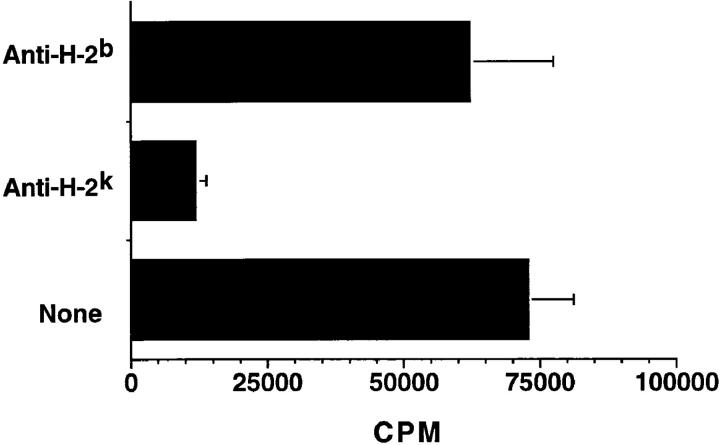
To determine whether the C3H/HeJBir CD4+ T cell response to cecal bacterial antigens was due to a mitogen or due to MHC–T cell receptor signaling as in a conventional antigen-specific T cell response, monoclonal antibodies to I-Ak and I-Ab MHC class II molecules were added to the cultures of CD4+ T cells plus cecal bacterial antigen-pulsed APCs (Fig. 4). The addition of the relevant anti–H-2k antibody to cultures blocked the T cell proliferative response, whereas the addition of the irrelevant control anti–H-2b antibody had no significant effect (Fig. 4). These data plus the kinetics of the response are not compatible with a mitogenic effect but instead indicate that C3H/HeJBir CD4+ T cell response to the cecal bacteria is due either to conventional antigen or possibly to a superantigen.
Figure 4.
C3H/HeJBir CD4+ T cell response to enteric bacterial antigens is MHC class II– dependent. 4 × 105 C3H/HeJBir spleen and MLN CD4+ T cells from 12-wk-old C3H/HeJBir mice were cultured with 4 × 105 cecal bacterial antigen pulsed APCs from C3H/HeJ mice per well prepared as in Fig. 1, in the presence or absence of 10 μg/ml monoclonal anti–I-Ab or anti–I-Ak. 0.5 μCi of [3H]TdR was added to the wells for the last 18 h of a 5-d culture. Results are expressed as mean cpm ± SD of triplicate cultures and are representative of three experiments.
The Cecal Bacterial Lysate Component that Stimulates C3H/ HeJBir CD4+ T Cells Is a Protein but Not a Superantigen.
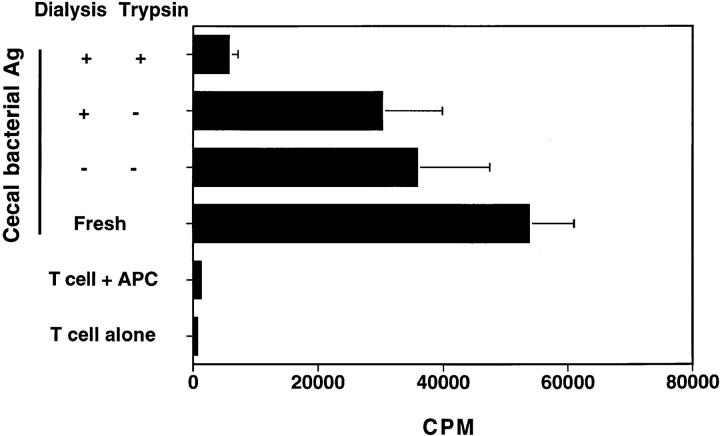
To further characterize the stimulatory component, lysates of cecal bacteria were treated with trypsin and then dialyzed, were dialyzed but not trypsin-treated, or were stored in a tube for an equivalent amount of time. Trypsin treatment significantly decreased the ability of cecal bacterial lysates to stimulate C3H/HeJBir CD4+ T cells, whereas the cecal bacterial lysates that were dialyzed only or were stored still strongly stimulated C3H/HeJBir CD4+ T cells to proliferate (Fig. 5). These results indicate that the stimulatory material in cecal bacteria is a trypsin-sensitive protein.
Figure 5.
The cecal bacterial lysate component that stimulates C3H/ HeJBir CD4+ T cells is a protein. APCs from C3H/HeJ mice were pulsed with cecal bacterial antigens from C3H/HeJ mice that had been pretreated with trypsin and then dialyzed, or dialyzed but not trypsin-treated, or stored in a tube for an equivalent amount of time. Control APCs were pulsed overnight with freshly obtained cecal bacterial antigens. Each of these antigen-pulsed APCs was added to aliquots of the same pool of C3H/HeJBir CD4+ T cells and cultured as described in the legend to Fig. 1. 0.5 μCi of [3H]TdR was added to the wells for the last 18 h of a 5-d culture. Results are expressed as mean cpm ± SD of triplicate cultures and are representative of three experiments.
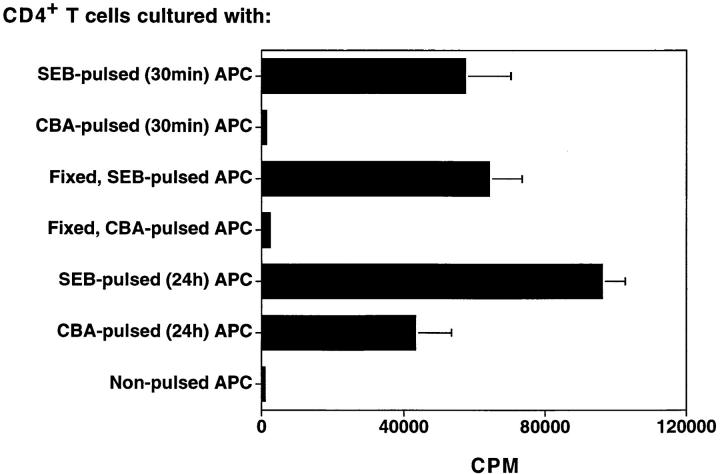
Many superantigens are derived from enteric bacteria and have been implicated in various immune-mediated diseases (25). To test whether the protein in cecal bacterial lysates that stimulated C3H/HeJBir CD4+ T cells was a superantigen, APCs were pulsed in different ways either with cecal bacterial antigens or with Staphylococcal enterotoxin B (SEB), a known superantigen, before their addition to C3H/HeJBir CD4+ T cells. When APCs were pulsed with SEB for 30 min, and then cultured with C3H/HeJBir CD4+ T cells, the T cells responded strongly (Fig. 6). However, when APCs were pulsed with cecal bacterial lysates for 30 min and then added to C3H/HeJBir CD4+ T cells, there was no significant response. When APCs were fixed with paraformaldehyde and then pulsed with SEB overnight, CD4+ T cells still responded well. In contrast, when fixed APCs were pulsed with cecal bacterial lysates overnight, they failed to stimulate a significant C3H/ HeJBir T cell response (Fig. 6). These results indicate that the proteins in the cecal bacterial preparation that stimulated C3H/HeJBir CD4+ T cells are not superantigens but rather conventional protein antigens.
Figure 6.
The cecal bacterial protein that stimulates C3H/HeJBir CD4+ T cells is not a superantigen. C3H/HeJBir CD4+ T cells were cultured with APCs from C3H/HeJ mice that were pulsed with SEB or CBA from C3H/HeJ mice for 30 min, or fixed with paraformadehyde and then pulsed with SEB or CBA overnight, or pulsed with SEB or CBA overnight. 0.5 μCi of [3H]TdR was added to the wells for the last 18 h of a 5-d culture. Results are expressed as mean cpm ± SD of triplicate cultures and are representative of three experiments.
Frequency of IL-2–producing CD4+ T Cells Specific to Cecal Bacterial Antigen.
To further confirm that the stimulation of C3H/HeJBir CD4+ T cells by cecal bacterial lysates was not due to a superantigen, the precursor frequency of IL-2– producing C3H/HeJBir CD4+ T cells specific for cecal bacterial antigens was determined and compared to that of normal C3H/HeJ mice and C57BL/6J mice. The precursor frequency of IL-2–producing CD4+ T cells specific for cecal bacterial antigens in C3H/HeJBir mice was 1 in 2000 CD4+ T cells, whereas that in normal C3H/HeJ or C57BL/6J mice was 10 times lower, i.e., 1 in 21,250 in C3H/HeJ and 1 in 25,000 in C57BL/6 mice.
In Vivo Kinetics of the C3H/HeJBir CD4+ T Cell Response to Cecal Bacterial Antigens.
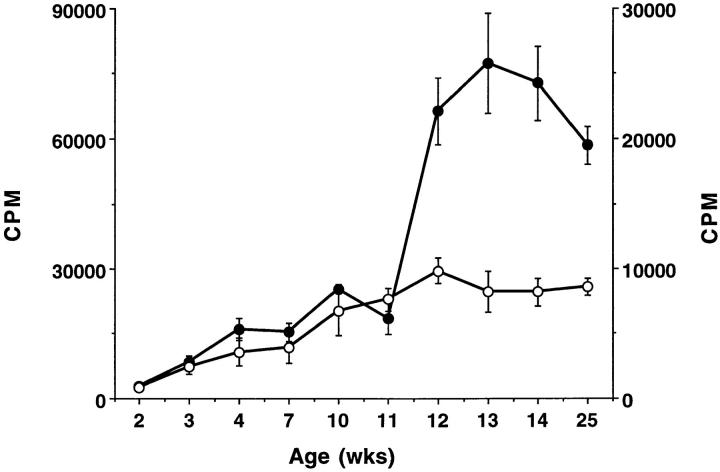
Because colitis in C3H/HeJBir mice peaks at 4–8 wk of age (16), the time course of the CD4+ T cell response to enteric bacteria was investigated. Spleen and MLN CD4+ T cells of C3H/HeJBir mice of age from 2 wk to 6 mo were stimulated with APCs pulsed with lysates of cecal bacteria. The C3H/HeJBir CD4+ T cells responded to cecal bacterial antigens beginning at 3 wk of age and this response increased with age up to 12 wk when it plateaued (Fig. 7). These data were derived using a mixture of MLN and spleen CD4+ T cells. When MLN and spleen CD4+ T cells were isolated and tested separately for reactivity to enteric bacterial antigens at 4, 5, 11, and 14 wk, the proliferative response of the MLN CD4+ T cells was greater than that of spleen at each time point (data not shown).
Figure 7.
Kinetics of the C3H/HeJBir CD4+ T cell response to enteric bacterial antigens. 4 × 105 spleen and MLN CD4+ T cells from C3H/ HeJBir mice of various ages from 2 to 25 wk were cultured with 4 × 105 cecal bacterial (closed circles) or E. coli (open circles) antigen pulsed APCs from C3H/HeJ mice per well, prepared as in Fig. 1. 0.5 μCi of [3H]TdR was added to the wells for the last 18 h of a 5-d culture. Results are expressed as mean cpm ± SD of triplicate cultures.
Production of High Levels of IL-2 and IFN-γ but Low Levels of IL-4 by C3H/HeJBir CD4+ T Cells Exposed to Cecal Bacterial Antigens.
To define the CD4 T cell subset response, the cytokines produced upon stimulation by cecal bacterial antigen were measured. As shown in Table 1, CD4+ T cells from colitic C3H/HeJBir mice produce substantial amounts of IL-2 and IFN-γ upon stimulation with cecal bacterial antigens, whereas CD4+ T cells from normal, noncolitic C3H/HeJ mice produced only minimal amounts of cytokines. Of particular interest is that in contrast to IL-2 and IFN-γ, CD4+ T cells from C3H/HeJBir colitic mice produced only low levels of IL-4, indicating a Th1-predominant response to enteric bacterial antigens.
Table 1.
Cytokine Production of CD4+ T Cells in Response to Cecal Bacteria and E. coli Antigens*
| CD4+ T cells | Stimulant | IL-2‡ | IFN-γ§ | IL-4§ | ||||
|---|---|---|---|---|---|---|---|---|
| U/ml | U/ml | U/ml | ||||||
| C3H/HeJBir | None | 1.5 ± 0.3 | 0.2 ± 0.1 | 0.4 ± 0.1 | ||||
| Cecal bacteria | 64.4 ± 6.8 | 78.8 ± 7.3 | 3.3 ± 1.1 | |||||
| E. coli | 80.2 ± 12.9 | 112.3 ± 11.5 | 4.5 ± 2.0 | |||||
| C3H/HeJ | None | 2.0 ± 0.3 | 0.4 ± 0.1 | 0.3 ± 0.1 | ||||
| Cecal bacteria | 7.2 ± 1.3 | 4.3 ± 0.5 | 0.8 ± 0.2 | |||||
| E. coli | 4.0 ± 1.2 | 2.1 ± 0.6 | 0.5 ± 0.2 |
4 × 105 CD4+ T cells from spleen and MLN of 12-wk-old mice were cultured with 4 × 105 antigen-pulsed APCs per well per legend to Fig. 1. Data represent the mean ± SD of three experiments.
Supernatants were collected at 24 h of culture for IL-2 bioassay using the cell line HT-2AB.
Supernatants were collected at 72 h of culture for IL-4 and IFN-γ bioassay using the IL-4–dependent cell line, CT.4S, or IFN-γ–dependent cell line, WEHI 279.
Phenotype of C3H/HeJBir CD4+ T Cells Stimulated by Cecal Bacterial Antigens.
The surface phenotype of the C3H/ HeJBir CD4+ T cells activated by cecal bacterial antigens was determined by flow cytometry. CD4+ T cells freshly isolated or stimulated with cecal bacterial antigens for 4 d were stained with antibodies to CD45RB, CD44, CD69, IL-2R, L-selectin, integrin β7, and antibodies to TCR Vβ families. Fresh CD4+ T cells expressed low levels of CD44, CD69, and IL-2R, and high levels of L-selectin. Upon stimulation with cecal bacterial antigens, the expression increased for CD44 (from 65 to 91%), CD69 (from 5.3 to 69%), and IL-2R (from 3.5 to 77%), whereas L-selectin expression decreased (from 83 to 59%). Approximately 85% of fresh CD4+ T cells expressed integrin β7, compared to 40% after stimulation with cecal bacterial antigens, and there was variable expression of CD45RB molecules. The pattern of TCR Vβ usage by CD4+ T cells cultured in vitro with cecal bacterial antigen-pulsed APCs was altered in favor of certain TCR Vβ families, particularly TCR Vβ 6, 8, and 10 (data not shown).
C3H/HeJBir CD4+ T Cell Reactivity Toward Different Enteric Bacterial Species.
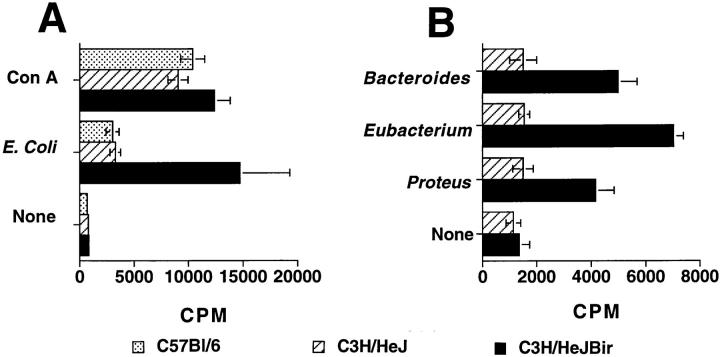
The reactivity of CD4+ T cells from C3H/HeJBir mice to specific, cultured bacterial strains, was assessed by their proliferative response and IL-3 production. CD4+ T cells from C3H/HeJBir mice proliferated significantly when cultured in the presence of E. coli, Bacteroides species, and Eubacteria species antigen-pulsed APCs (Fig. 8), and also had a high level of IL-3 production (data not shown), whereas CD4+ T cells of C3H/HeJ control mice had low responses to the same antigen-pulsed APCs when measured by either proliferation (Fig. 8) or IL-3 production (data not shown). Notably, the proliferative response of C3H/HeJBir mice CD4+ T cells to cultured bacterial antigens of these strains was significantly lower than that observed to freshly obtained cecal bacteria. There was no difference in CD4+ T cell proliferative responses to polyclonal Con A stimulation between C3H/HeJBir and C3H/HeJ mice (data not shown). When the CD4+ T cell cytokine production was measured upon stimulation with APCs pulsed with lysates of E. coli, Bacteroides species, or Eubacteria species, C3H/HeJBir CD4+ T cells produced a high level of IL-2 and IFN-γ but a low level of IL-4 (Table 1 and data not shown), again indicating a Th1-predominant response.
Figure 8.
C3H/HeJBir CD4+ T cell responses to different enteric bacterial species. 4 × 105 spleen and MLN CD4+ T cells from 12-wk-old C3H/HeJBir, C3H/HeJ or C57Bl/6 mice were cultured with 4 × 105 syngeneic APCs that were pulsed with lysates of E. coli (A), or with Bacteroides vulgatus, Eubacterium species, or Proteus mirabilis (B). C3H/HeJ APCs were used for both C3H/HeJBir and C3H/HeJ cultures. 0.5 μCi of [3H]TdR was added to the wells for the last 18 h of a 5-d culture. Results are expressed as mean cpm ± SD of triplicate cultures.
Transfer of Colitis by Bacterial Antigen-activated CD4+ T Cells into C3H/HeSnJ scid/scid Recipients.
To determine whether this CD4+ T cell response is a cause or a secondary effect of the colitis, CD4+ T cells isolated from spleen and MLN of normal C3H/HeJ mice or of colitic C3H/HeJBir mice were transferred separately into groups of four C3H/HeSnJ scid/scid recipients. 3 mo later, the recipients were killed and the histopathology of cecum and the proximal, medial, and distal portions of colon were examined. These freshly isolated CD4+ T cells did not transfer disease although both reconstituted gut lymphoid tissue histologically. However, when C3H/HeJBir CD4+ T cells were activated with cecal bacterial antigens in vitro for 4 d and then transferred to scid/scid mice, three out of four recipients developed colitis (Fig. 9). The lesions in the recipients were focal, similar to those that occur in the donor C3H/HeJBir mice. In contrast, none of the four recipients that received C3H/HeJ CD4+ T cells that were activated with cecal bacterial antigens in vitro for 4 d developed colitis. Lastly, transfer of C3H/ HeJBir CD4+ T cells activated in vitro with the polyclonal activator, monoclonal anti-CD3, to four scid/scid mice did not result in colitis in any of the four recipients.
Figure 9.
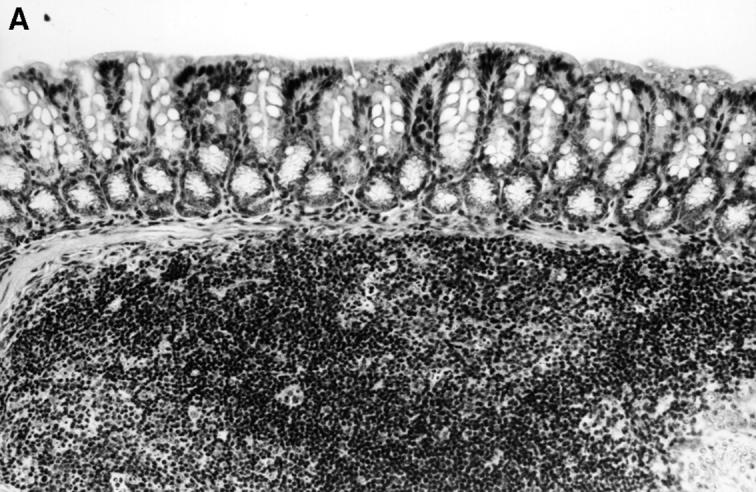
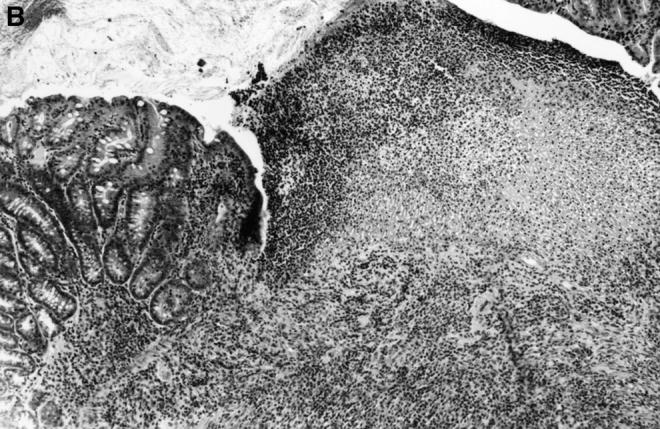
Histopathology of the colon of C3H/HeSnJ scid/scid mice that had been injected with CD4+ T cells from either C3H/HeJBir or C3H/HeJ mice 3 mo before. Before the transfer, CD4+ T cells from both sources were activated for 4 d with cecal bacterial antigen-pulsed APCs. Three months after transfer, the colon of the recipient of the activated C3H/HeJ T cells (left panel) shows normal mucosa overlying a lymphoid follicle, whereas the colon of the recipient of activated C3H/HeJBir T cells (right panel) shows inflammation and a focal ulcer.
Discussion
C3H/HeJBir mice represent a new strain that develops colitis spontaneously (16). The cecum and right colon are most affected; no inflammation is seen in the small intestine or other organs. Disease occurs early in life at 3–4 wk (a time corresponding to bacterial colonization), peaks 2–4 wk later, and heals by 10–12 wk. This strain was generated by a program of selective breeding to capture a trait, namely perianal ulceration and colitis, that had been occurring sporadically in the C3H/HeJ strain at the Jackson Laboratory. Subsequently it has been found that C3H/HeJ mice are quite susceptible in a number of models of chronic intestinal inflammation, and there is preliminary evidence that there are a number of susceptibility genes present in the parental C3H/HeJ strain that makes this strain prone to chronic intestinal inflammation (26).
C3H/HeJBir mice have demonstrated surprisingly selective antibody reactivity to antigens of the bacterial flora in a previous study (17). In these studies, Western blot analysis was performed using sera from C3H/HeJBir mice to detect possible stimulatory antigens of epithelial cells, food, or enteric bacteria. Although no reactivity was found to the former two, a number of trypsin-sensitive bacterial antigens were detected by serum antibody. This antibody response was highly selective when one compared the number of proteins present to those detected by colitic sera. For the most abundant anaerobic bacterial species, only one to three proteins were recognized out of the thousands present. These data indicate that even in inflamed mucosa the responsiveness of the immune system toward enteric bacterial antigens is restricted. The mechanism of this selectivity is unknown.
Our studies were initiated to determine the reactivity of T cells to epithelial, food, or bacterial antigens. Again, no reactivity was found to either epithelial or food antigens, whereas there was striking reactivity to enteric bacterial antigens. These studies used the strategy of isolating fresh cecal bacteria from C3H/HeJ donors, because these are the organisms that are present at the site of disease, not all organisms of the enteric flora can be cultured, and culture conditions can change gene and thus antigen expression. This may explain why T cell responses to lysates of bulk anaerobic cultures of cecal bacteria were generally lower that those obtained with freshly isolated bacteria. The kinetics of the in vitro response to these cecal bacterial antigens, the dose response and the inhibition by antibodies to class II MHC all indicate an antigenic rather than a mitogenic effect. Increased T cell responses could be detected to multiple specific enteric bacterial strains including both anaerobes and aerobes such as E. coli; however, the level of proliferative response was consistently lower than that found among the crude, freshly isolated cecal bacterial antigen preparation. Although the specific bacterial antigens stimulating T cells were not identified here, C3H/HeJBir T cells appear to be responding to many of the same antigens as do their B cells, because solid-phase absorption of cecal bacterial antigens with C3H/HeJBir serum antibody before pulsing APCs for the T cell cultures, substantially reduced the subsequent proliferative response (data not shown). In a previous study (17), serum antibody from C3H/HeJBir mice detected an increasing number of bacterial antigens on Western blots over time, compatible with epitope spreading. The same may occur in the CD4+ T cell compartment because there is a progressive increase in the T cell proliferative responses over the first 12 wk of life (Fig. 7). Lastly, certain bacterial lipids are known to be presented to T cells via CD1 molecules (27); however, the stimulatory material in cecal bacteria was trypsin-sensitive, indicating that it was a protein or glycoprotein and not lipid.
Superantigens derived from enteric bacteria have been implicated in various immune-mediated diseases (25). Thus, the possibility that the cecal protein stimulating C3H/ HeJBir T cells might be a superantigen was explored. The cecal bacterial extracts were directly compared to a known superantigen, SEB, by using short antigen pulses of APCs or pulsing paraformaldehyde-fixed APCs. Under both conditions, SEB was quite stimulatory, whereas the cecal bacterial extract did not stimulate. In addition, we measured the precursor frequency of CBA-reactive T cells in C3H/ HeJBir mice and compared it to that of the C3H/HeJ parent strain and the C57BL/6 strain. The frequency of antigen-reactive CD4 T cells producing IL-2 when stimulated with CBA-pulsed APCs was not consistent with a superantigen, but was in a range consistent with a response to conventional antigen. There was a 10-fold increase in C3H/ HeJBir mice T cells compared to those from either C3H/HeJ or C57BL/6. The latter both had high frequencies compared to the T cell response of naive mice toward a conventional exogenous protein antigen, consistent with low level in vivo priming of T cells by enteric bacteria.
To assess the phenotypic subset of the CD4+ T cells reactive to cecal bacterial antigen, the cytokines produced by CD4 T cells from either C3H/HeJBir or C3H/HeJ mice stimulated in vitro with antigen-pulsed APCs were measured. The production of IL-2 and IFN-γ by C3H/HeJBir CD4+ T cells was 10–20-fold higher than that of the noncolitic parental strain CD4+ T cells. Although there was a significant increase in IL-4 as well, the overall pattern was predominantly Th1. The cytokine pattern when E. coli antigens were used was also predominantly Th1. Interestingly, although the cytokine response of C3H/HeJBir CD4+ T cells to cecal bacterial and E. coli antigens was comparable, the CD4+ T cell proliferative response was substantially lower to E. coli than to cecal bacteria. We postulate that this might be due to an antigen dosage effect in vivo in that E. coli is a minor constituent of the bacterial flora. Thus, the Th1 subset is implicated in the C3H/HeJBir mice just as it has been in a number of other models (10–12).
The question remained as to the significance of the T cell reactivity to these bacterial antigens, because it seemed possible that the CD4+ T cell responses might be a result rather than a cause of colitis. Thus the kinetics of appearance of this T cell reactivity was defined. Increased T cell reactivity to cecal bacterial antigens could be demonstrated as early as 3–4 wks of age, which is the time of onset of colitis in these mice. In addition, adoptive transfer studies showed that when C3H/HeJBir CD4+ T cells were activated with CBA-pulsed APCs in vitro and then transferred into C3H/ HeSnJ scid/scid recipients, three out of four developed colitis. In contrast, none of the four recipients of similarly treated CD4+ T cells from C3H/HeJ strain developed disease, nor did any of the recipients of anti–CD3-activated C3H/ HeJBir CD4+ T cells, indicating that the induction of disease needs CBA-specific activation of C3H/HeJBir CD4+ T cells, and that nonspecific activation is not sufficient. The activation by antigen may disturb the balance between effector and regulator cells, allowing the former to expand more rapidly in vivo. Notably, the lesions in the scid/scid recipients were focal as are the lesions in the C3H/HeJBir donors. These data demonstrate that the CD4+ T cells reactive to enteric bacterial antigens in C3H/HeJBir mice are pathogenic. Although this possibility has been postulated for a number of years as a possible pathogenic mechanism of inflammatory bowel disease (1), this is a direct demonstration that this does occur.
In recent years a number of transgenic or knockout mice, collectively termed induced mutants, have developed colitis in the absence of any further manipulation. These strains represent a small minority of the induced mutants of immune-related genes that have been generated, arguing that they affect pathways critical to maintenance of normal intestinal homeostasis. The histopathology varies among these strains but often they have hyperproliferative crypts and rectal prolapse, neither of which is a feature of the spontaneously colitic C3H/HeJBir mouse. The genetic background of the inbred strain on which the mutation is placed is a key determinant of the disease and, as mentioned above, the C3H/HeJ strain seems highly susceptible in a number of these models. Abnormal mucosal CD4+ T cell reactivity has been implicated in a number of models, particularly abnormal Th1 responses manifested by excessive IFN-γ in the lesions. The restriction of the inflammation to the colon in these mutants implicates the enteric bacterial flora as the stimulant of the inflammation and indeed, in several models, germ-free animals do not get disease (5, 13) and in one, the disease is considerably lessened by specific pathogen-free conditions (6, 11). The mechanism by which the enteric bacterial flora stimulates disease in such models is unknown. The results of this study suggest that one possible mechanism is CD4+ T cell reactivity to conventional antigens of the commensal bacteria, similar to what has been found in C3H/HeJBir mice; but it remains possible that the lesions in these models are due to other bacterial effects such as mitogens, superantigens, or other immunomodulatory molecules.
Although the bacterial flora drives the development of the mucosal immune system and to a lesser extent the systemic immune system, little is known about how the normal response to enteric bacterial antigens is regulated. Experiments in which germ-free mice have been monocontaminated with specific bacterial strains indicate that there is a wide spectrum in the resulting response, with some commensal enteric bacteria failing to elicit any immune response, even when injected parenterally (28, 29). This result has given rise to the concept of an “autochthonous flora”, i.e., bacterial strains that do not stimulate the immune system due to an extended coevolution with the host (30). Recently, T cells isolated from the intestinal lamina propria have been found to be tolerant to an individual's own enteric bacteria, but reactive to the enteric bacteria of other individuals (31, 32). This relative unresponsiveness to the abundant antigens of the intestinal flora may be related to the limited T cell receptor repertoire found in intestinal intraepithelial T cells as compared to that found in peripheral blood, lymph node, or spleen (33–36). The TCR repertoire of mouse lamina propria lymphocytes is unknown. Although relative hyporesponsiveness to enteric bacterial antigens appears to exist, the mechanisms that initiate and maintain such relative hyporesponsiveness remain unknown. Some insight into these mechanisms will probably result from studies of models such as C3H/HeJBir mice, in which these mechanisms appear to be impaired and in which IBD results.
Acknowledgments
This work was supported by the National Institutes of Health grants DK-44240 and T32 AI-07051.
Abbreviations used in this paper
- CBA
cecal bacteria
- IBD
inflammatory bowel disease
- MLN
mesenteric lymph node
- SEB
staphylococcal enterotoxin B
References
- 1.Elson, C.O., and R.P. McCabe. 1995. The immunology of inflammatory bowel disease. In Inflammatory Bowel Disease, 4th ed. J.B. Kirsner and R.G. Shorter, editors. Williams & Wilkins, Baltimore, MD. 203–251.
- 2.Sartor, R. 1995. Microbial factors in the pathogenesis of Crohn's disease, ulcerative colitis, and experimental intestinal inflammation. In Inflammatory Bowel Disease, 4 ed. J. Kirsner and R. Shorter, editors. Williams and Wilkins, Baltimore, MD. 96–124.
- 3.Pirzer U, Schonhaar A, Fleischer B, Hermann E, Meyer zum Buschenfelde KH. Reactivity of infiltrating T lymphocytes with microbial antigens in Crohn's disease. Lancet. 1991;338:1238–1239. doi: 10.1016/0140-6736(91)92104-a. [DOI] [PubMed] [Google Scholar]
- 4.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–375. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10–deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 7.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 8.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 10.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 11.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10–deficient mice are associated with aberrant cytokine production and CD4+Th1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrhardt RO, Ludviksson BR, Gray B, Neurath M, Strober W. Induction and prevention of colonic inflammation in IL-2–deficient mice. J Immunol. 1997;158:566–573. [PubMed] [Google Scholar]
- 13.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrissey, P., and K. Charrier. 1995. Induction of colitis in SCID mice by the transfer of normal CD4+/CD45RBhi T cells. In Inflammatory Bowel Disease, vol. Falk Symposium 85. G. Tytgat, J. Bartelsman and S. van Deventer, editors. Kluwer Academic Publishers, Boston, MA. 418–425.
- 15.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Jr, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially Bacteroidesspecies, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human β2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundberg JP, Elson CO, Bedigian H, Birkenmeier EH. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology. 1994;107:1726–1735. doi: 10.1016/0016-5085(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 17.Brandwein SL, McCabe RP, Cong Y, Waites KB, Ridwan BU, Dean PA, Ohkusa T, Birkenmeier EH, Sundberg JP, Elson CO. Spontaneously colitic C3H/HeJBir mice demonstrate selective antibody reactivity to antigens of the enteric bacterial flora. J Immunol. 1997;159:44–52. [PubMed] [Google Scholar]
- 18.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds DS, Boom WH, Abbas AK. Inhibition of B lymphocyte activation by interferon-gamma. J Immunol. 1987;139:767–773. [PubMed] [Google Scholar]
- 20.De Magistris MT, Romano M, Nuti S, Rappuoli R, Tagliabue A. Dissecting human T cell responses against Bordetella species. J Exp Med. 1988;168:1351–1362. doi: 10.1084/jem.168.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu-Li J, Ohara J, Watson C, Tsang W, Paul WE. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S) J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- 22.Lanier LL, Lynes M, Haughton G, Wettstein PJ. Novel type of murine B-cell lymphoma. Nature. 1978;271:554–555. doi: 10.1038/271554a0. [DOI] [PubMed] [Google Scholar]
- 23.Davis, L.S., P.E. Lipsky, and K. Bottomly. 1995. Measurement of human and murine interleukin 2 and interleukin 4. In Current Protocols in Immunology, vol. 1. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. John Wiley & Sons, Inc., Philadelphia, PA. 6.3.1–6.8. [DOI] [PubMed]
- 24.Eichmann K, Falk I, Melchers I, Simon MM. Quantitative studies on T cell diversity. I. Determination of the precursor frequencies for two types of streptococcus A–specific helper cells in nonimmune, polyclonally activated splenic T cells. J Exp Med. 1980;152:477–492. doi: 10.1084/jem.152.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin MJ, Gascoigne NR. Interplay between superantigens and the immune system. J Leukocyte Biol. 1993;54:495–503. doi: 10.1002/jlb.54.5.495. [DOI] [PubMed] [Google Scholar]
- 26.Mähler M, Sundberg J, Birkenmeier EH, Bristol IJ, Elson CO, Leiter EH. Chromosomal location of genes determining susceptibility of mice to dextran sulfate sodium (DSS)-induced colitis. Gastroenterology. 1997;112:A1031. . (Abstr.) [Google Scholar]
- 27.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 28.Pollard M, Sharon N. Responses of the Peyer's patches in germ-free mice to antigenic stimulation. Infect Immun. 1970;2:96–100. doi: 10.1128/iai.2.1.96-100.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg RD, Savage DC. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect Immun. 1975;11:320–329. doi: 10.1128/iai.11.2.320-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savage DC, Dubos R, Schaedler RW. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968;127:67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer K-H Zum Büschenfelde. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duchmann R, Schmitt E, Knolle E, Meyer K-H Zum Büschenfelde, and M. Neurath. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 33.Balk SP, Ebert EC, Blumenthal RL, McDermott FV, Wucherpfennig KW, Landau SB, Blumberg RS. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991;253:1411–1415. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- 34.Blumberg RS, Yockey CE, Gross GG, Ebert EC, Balk SP. Human intestinal intraepithelial lymphocytes are derived from a limited number of T cell clones that utilize multiple Vβ T cell receptor genes. J Immunol. 1993;150:5144–5153. [PubMed] [Google Scholar]
- 35.Regnault A, Cumano A, Vassalli P, Guy-Grand D, Kourilsky P. Oligoclonal repertoire of the CD8αα and the CD8αβ TCR-α/β murine intestinal intraepithelial T lymphocytes: evidence for the random emergence of T cells. J Exp Med. 1994;180:1345–1358. doi: 10.1084/jem.180.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regnault A, Levraud J-P, Lim A, Six A, Moreau D, Cumano A, Kourilsky P. The expansion and selection of T cell receptor αβ intestinal intraepithelial T cell clones. Eur J Immunol. 1996;26:914–921. doi: 10.1002/eji.1830260429. [DOI] [PubMed] [Google Scholar]