Abstract
Chemokines and their receptors are important elements for the selective attraction of various subsets of leukocytes. To better understand the selective migration of functional subsets of T cells, chemokine receptor expression was analyzed using monoclonal antibodies, RNase protection assays, and the response to distinct chemokines. Naive T cells expressed only CXC chemokine receptor (CXCR)4, whereas the majority of memory/activated T cells expressed CXCR3, and a small proportion expressed CC chemokine receptor (CCR)3 and CCR5. When polarized T cell lines were analyzed, CXCR3 was found to be expressed at high levels on T helper cell (Th)0s and Th1s and at low levels on Th2s. In contrast, CCR3 and CCR4 were found on Th2s. This was confirmed by functional responses: only Th2s responded with an increase in [Ca2+]i to the CCR3 and CCR4 agonists eotaxin and thymus and activation regulated chemokine (TARC), whereas only Th0s and Th1s responded to low concentrations of the CXCR3 agonists IFN-γ–inducible protein 10 (IP-10) and monokine induced by IFN-γ (Mig). Although CCR5 was expressed on both Th1 and Th2 lines, it was absent in several Th2 clones and its expression was markedly influenced by interleukin 2. Chemokine receptor expression and association with Th1 and Th2 phenotypes was affected by other cytokines present during polarization. Transforming growth factor β inhibited CCR3, but enhanced CCR4 and CCR7 expression, whereas interferon α inhibited CCR3 but upregulated CXCR3 and CCR1. These results demonstrate that chemokine receptors are markers of naive and polarized T cell subsets and suggest that flexible programs of chemokine receptor gene expression may control tissue-specific migration of effector T cells.
It is well established that after activation, T lymphocytes acquire effector functions that are best exemplified by the pattern of cytokines produced (1, 2). Th1 effector cells produce IL-2 and IFN-γ and activate mononuclear phagocytes thus protecting against intracellular pathogens. In contrast, Th2 effector cells produce IL-4 and -5 and are involved in responses dominated by IgE, eosinophils and basophils.
In addition to effector function, activated T lymphocytes acquire different migratory capacities, a fact that is the key to an efficient regulation of the immune response (3). The regulation of leukocyte migration is an intricate process involving the participation of adhesion molecules such as selectins and integrins (4, 5), as well as chemokines and chemokine receptors (6). The combined action of adhesion molecules and chemokines is thought to provide an address code for leukocyte migration to different sites (7, 8). Indeed, early work has identified markers for T cell subsets that preferentially migrate to distinct anatomical sites. Thus, T cells that home to skin express the cutaneous lymphocyte–associated antigen (9), whereas those homing to the gut express the β7 integrins (10). In addition to adhesion molecules, chemokine receptor expression has been shown to be important for selective leukocyte migration. For instance, expression of the CC chemokine receptor (CCR)31 on eosinophils and the IL-8 receptors on neutrophils allows these cells to migrate to sites where agonists such as eotaxin or IL-8 are produced (11, 12).
Presumably, chemokines and chemokine receptors are also important for the selective migration of T cells, especially subsets of effector T cells. Indeed, a strong relationship between migratory capacity and cytokine production is emerging. It was shown recently that Th1s, but not Th2s express a functional ligand for E- and P-selectin that allows these cells to migrate to sites of inflammatory reactions (13, 14). In addition, we have shown recently that the eotaxin receptor CCR3 is expressed on Th2s and not Th1s (15). These results suggest that migration and effector function are coordinately acquired as part of a common developmental program.
In this study, we demonstrate that distinct profiles of chemokine receptors are acquired by T cells after polarization and are modulated by cytokines. CCR3 and CCR4 are expressed exclusively on Th2s, whereas CXC chemokine receptor (CXCR)3 and CCR5 are preferentially found on Th0s and Th1s. In addition, TGF-β inhibits CCR3, but enhances CCR4 and CCR7 expression, whereas IFN-α inhibits CCR3 and CCR4 but upregulates CXCR3 and CCR1. These results demonstrate flexible programs of chemokine receptor gene expression that best serve the complex requirements for tissue and microenvironment selective migration.
Materials and Methods
Media and Reagents.
The medium used throughout was RPMI 1640 supplemented with 2 mM L-glutamine, 1% nonessential amino acids, 1% pyruvate, 50 μg/ml kanamycin (GIBCO BRL, Gaithersburg, MD), 5 × 10−5 M 2-mercaptoethanol (Merk, Darmstadt, Germany), and 5% human serum (Swiss Red Cross, Bern, Switzerland) or 10% FCS (Hyclone Laboratories, Inc., Logan, UT). Human recombinant IL-2 and -4 were produced in our laboratory by PCR cloning and expression in the myeloma expression system (16). Eotaxin, IFN-γ–inducible protein 10 (IP-10), monokine induced by IFN-γ (Mig), macrophage inflammatory protein (MIP)-1β, and thymus and activation regulation chemokine (TARC) were purchased from Peprotech (London, UK).
Cells, T Cell Lines, and Clones.
Blood samples were obtained from healthy volunteers and PBMCs were separated by the standard Ficoll-paque method. Cord blood T cells were stimulated with PHA (1 μg/ml; Murex Ltd., Dartford, UK) and rIL-2 (40 U/ml) under Th1-polarizing conditions (human rIL-12 [2 ng/ml; Hoffmann–La Roche, Nutley, NJ] plus neutralizing mAb to human IL-4 [200 ng/ml; PharMingen, San Diego, CA]) or Th2-polarizing conditions (human rIL-4 [200 U/ml] plus neutralizing mAb to human IL-12 [2 μg/ml; R&D Sys., Inc., Minneapolis, MN]). After 1 and 2 wk, the cultures were restimulated with PHA and irradiated allogeneic PBMCs (3,000 rad from a 137Cs source) in the same polarizing conditions and analyzed after a further 10 d. Cell lines were also generated from sorted CD4+ or CD8+ T cells. In some experiments, IFN-α (1,000 U/ml; Hoffmann–La Roche) or TGF-β1 (2 ng/ml; R&D Sys., Inc.) were added to Th1- or Th2-polarizing cytokines. T cell blasts were cloned by limiting dilution and maintained by periodic restimulation with PHA, irradiated allogeneic PBMCs, and rIL-2 as previously described (17).
FACS® Analysis.
Cell staining was performed using mAbs followed by FITC- or PE-conjugated, affinity-purified, isotype-specific goat anti–mouse antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL). For three-color analysis biotin-labeled goat anti–mouse antibodies were used followed by APC-conjugated streptavidin (Molecular Probes, Eugene, OR). The following mouse antibodies were used: anti-CXCR3 (1C6, IgG1; reference 18), anti-CCR3 (7B11, IgG2a; reference 19), anti-CCR5 (5C7, IgG2a; reference 20), anti-CXCR4 (12G5, IgG2a; R&D Sys., Inc.), anti-CD4 (OKT4, IgG2b and 6D10, IgG1; American Type Tissue Collection, Rockville, MD), anti– TCR-α/β (BM 031, IgG2b), anti-CD45RA (ALB11, IgG1), and anti-CD45RO (UCHL1, IgG2a) (all from Immunotech, Marseille, France). Because of the need for three antibodies of different isotypes in the indirect three-color staining, we used anti-CD45RO or -CD45RA mAbs to distinguish between naive and memory T cells. The samples were analyzed on a FACSCalibur® (Becton Dickinson, Mountain View, CA) using propidium iodide to exclude dead cells.
Cytokine Detection at the Single Cell Level.
T cells were stimulated with 10−7 M PMA plus 1 μg/ml ionomycin (Sigma Chemical Co., St. Louis, MO) for 4 h. Brefeldin A (10 μg/ml, Sigma Chemical Co.) was added during the last 2 h. Cells were fixed with 2% paraformaldehyde, permeabilized with phosphate buffered saline containing FCS (1%) and saponin (0.5%) and stained with FITC-labeled anti–IFN-γ (IgG1) and PE-labeled anti–IL-4 (IgG2b) mAbs (Becton Dickinson).
Ca2+ Flux Measurement.
T cells were loaded with Indo-1 (Sigma Chemical Co.) and their response to various concentrations of chemokines was analyzed on a FACS Vantage® flow cytometer (Becton Dickinson) as described (21). In some experiments, fluxing and nonfluxing cells were isolated by cell sorting over a 1-min period and further analyzed for cytokine production.
RNase Protection Assay.
Total RNA was extracted from T cells using RNAzol B (Tel-Test, Inc., Friendswood, TX). Multiprobe template sets hCR5 (containing DNA templates for CCR1, CCR3, CCR4, CCR5, CCR8, CCR2a+b, CCR2a, CCR2b, and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) and hCR6 (containing DNA template for CXCR1, CXCR2, CXCR3, CXCR4, Burkitt's lymphoma receptor (BLR)-1, BLR-2/CCR7, V28, and GAPDH) were purchased from PharMingen. The DNA templates were used to synthesize the α-[32P]UTP (3,000 Ci/mmol, 10 mCi/ml; Amersham Life Science, Buckinghamshire, UK) –labeled probes in the presence of a GACU pool using a T7 RNA polymerase (Promega, Madison, WI). Hybridization with 5–15 μg of each target RNA was performed overnight followed by digestion with RNase A (Boehringer Mannheim, Rotkreuz, Switzerland) and T1 (Calbiochem, La Jolla, CA) according to the PharMingen standard protocol. The samples were treated by proteinase K (Boehringer Mannheim) –SDS mixture and then extracted with chloropan and precipitated in the presence of ammonium acetate. The samples were loaded on an acrylamide-urea sequencing gel next to labeled DNA molecular weight markers and to the labeled probes, and run at 50 watts with 0.5× Tris-borate/EDTA electrophoresis buffer (TBE). The gel was adsorbed to filter paper, dried under vacuum, and exposed on film (X-AR; Kodak, Rochester, NY) with intensifying screens at −70°C.
Northern Blot Analysis.
10–15 μg of total RNA were loaded on a RNA formaldehyde denaturing gel and run with 3-[N-morpholino]propane sulfonic acid (MOPS)/EDTA buffer for 3 h, followed by an overnight transfer to Genescreen membrane (New England Nuclear, Boston, MA) with 10× standard saline citrate (SSC). RNA was covalently fixed to the membrane by UV cross-linking using Stratalinker (Stratagene, La Jolla, CA). The membranes were hybridized with either full-length IL-12Rβ2 or with β-actin. The probes were labeled with α-[32P]dCTP (Amersham) by random labeling (random primed DNA labeling kit; Boehringer Mannheim), purified on Biospin 30 spun column (BioRad Labs., Hercules, CA), heat inactivated, and quenched on ice before adding to the hybridization mixture. The hybridization mix was composed by 50% of deionized formamide, 5× Denhardt's, 5× SSC, 10 mM EDTA, pH 8.0, 1% SDS buffered with 25 mM NaH2PO4, and 25 mM Na2HPO4, and supplemented with 200 μg/ml heat denaturated salmon sperm DNA (Sigma Chemical Co.).
Results
Chemokine Receptor Expression on Naive and Memory T Cells in Peripheral Blood.
Using specific monoclonal antibodies, we analyzed the expression of chemokine receptors in T cells expressing different CD45 isoforms that identify naive or memory T cells (22; Fig. 1). CXCR4 was present at high levels on all cord blood T cells and on CD45RA+ adult naive T cells, whereas it was present at lower levels and only on a fraction (15–40%) of CD45RA− memory T cells. In contrast, CCR3, CCR5, and CXCR3 were absent in cord blood T cells and were expressed preferentially in adult peripheral blood T cells with memory phenotype. CCR3 was expressed on a small fraction (1–10%) of CD45RA− T cells that has been previously identified to comprise Th2s (15). CCR5 was expressed on 18–32% of CD45RA− T cells, whereas CXCR3 was expressed at high levels on a much larger fraction (58–73%) of CD45R0+ memory T cells. Interestingly, CXCR3 was also present on a population of CD3+ CD45R0− cells present in both cord blood and adult blood. In conclusion, CXCR4 is a major receptor in naive T cells, whereas CCR3, CCR5, and CXCR3 are selectively expressed on memory CD45R0+ RA− T cells.
Figure 1.
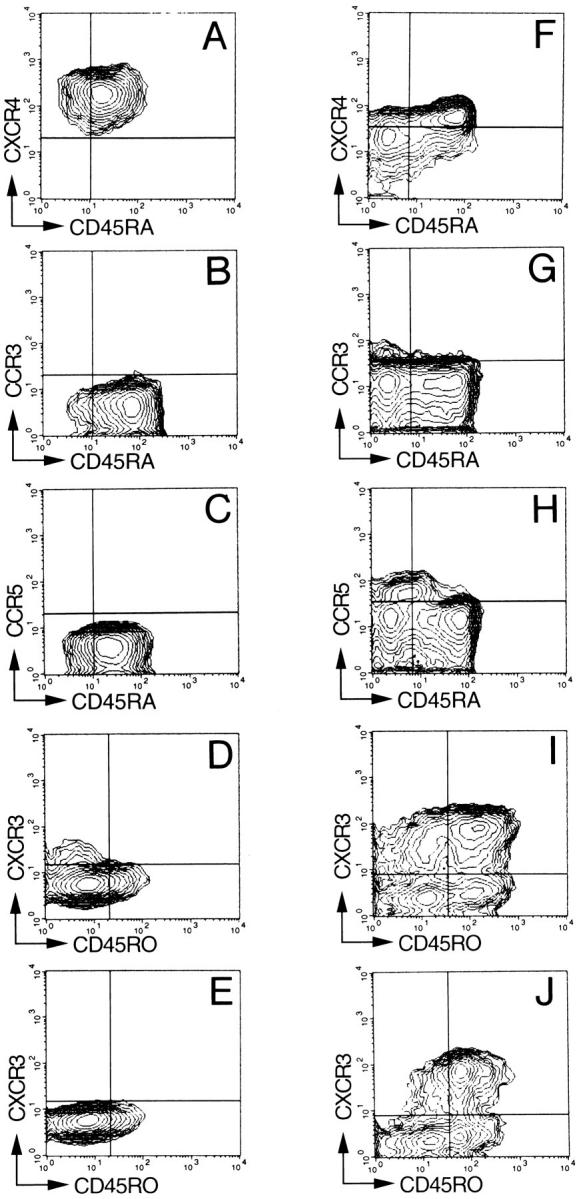
Chemokine receptor expression on naive and memory peripheral blood T cells. Three-color immunofluorescent staining of mononuclear cells from cord blood (A–E) or adult blood (F–J). Contour plots represent live cells gated for expression of CD3 (A–D and F–I) or CD4 (E and J). In different panels, memory T cells are identified as CD45RA− or CD45RO+ because of constraints in the choice of antibody isotypes (see Materials and Methods). Quadrants were set according to the staining of control mAbs. The CD3+ CD45R0− CXCR3+ cells were identified as CD8+ cells in separate experiments. Comparable results were obtained with three additional cord blood and six adult blood samples.
Expression of CXCR3, CCR5, and CCR3 after T Cell Polarization In Vitro.
We have shown that CCR3 is acquired after Th2 polarization in vitro (15). We therefore investigated whether CXCR3 and CCR5 could be regulated in a similar fashion (Fig. 2). When cord blood T cells were polarized by two cycles of stimulation in the presence of the appropriate cytokines, CXCR3, CCR5, and CCR3 were progressively acquired. CXCR3 was expressed at high levels and on all cells in Th1 lines, whereas it was expressed at lower levels and with an heterogeneous pattern in Th2 lines. In contrast, CCR5 expression was variable, since no consistent preferential expression was evident from the analysis of several Th1 and Th2 lines. Finally, CCR3 was expressed on a high proportion of cells in Th2 lines, but on only a few cells in Th1 lines, which indeed represent contaminating Th2 polarized cells (15). These results suggest that while CCR3 is a marker of Th2s, CXCR3 is preferentially expressed on Th0s/Th1s.
Figure 2.
Acquisition of CXCR3, CCR5, and CCR3 after T cell polarization in vitro. Cord blood T cells were activated for two consecutive cycles under Th1- (IL-12 + α–IL-4) or Th2- (IL-4 + α–IL-12) polarizing conditions. The cells were analyzed for IFN-γ and IL-4 production by intracellular staining (dot plots in a four-decade logarithmic scale) and for expression of chemokine receptors (histograms in a four-decade logarithmic scale) 10 d after the first polarization (A) and 10 d after the second polarization (B). Comparable results were obtained in three additional experiments.
Reciprocal Modulation of CXCR3 and CCR3 by IFN-α and TGF-β.
We investigated whether IFN-α and TGF-β, which have been shown to affect T lymphocyte polarization (23, 24), may modulate chemokine receptor expression when added to Th1- or Th2-polarizing conditions (Fig. 3).
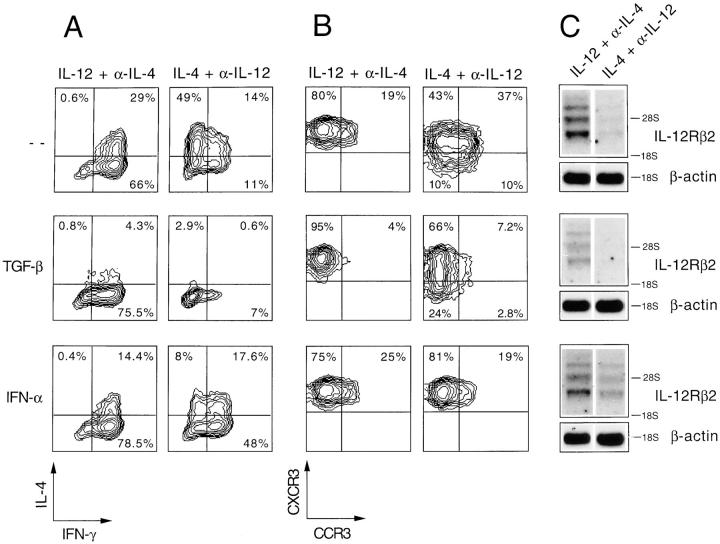
Figure 3.
Modulation of IFN-γ and IL-4 production and CCR3 and CXCR3 expression in polarized T cell lines by TGF-β and IFN-α. Cord blood T cells were polarized by two consecutive cycles of stimulation in the presence of IL-12 + α-IL-4 (Th1) or IL-4 + α-IL-12 (Th2) alone or together with TGF-β or IFN-α. (A) IFN-γ and IL-4 production at the single cell level measured by intracellular staining (four-decade logarithmic scale); (B) CCR3 and CXCR3 expression (four-decade logarithmic scale); (C) IL-12Rβ2 mRNA expression as determined by Northern blot on total RNA using as probe the full-length human IL-12Rβ2 subunit cDNA. Exposure time was 7 d using an intensifying screen at −70°C. Two major messages were found as described (42). As a loading control, the blot was stripped and rehybridized for β-actin.
IFN-α did not show any effect on Th1 polarization. However, when added to Th2-polarizing conditions, IFN-α induced a shift from Th2 to Th1 as shown by IFN-γ production and by upregulation of the IL-12Rβ2 subunit, which is a marker of Th1s (23). Interestingly, this treatment also resulted in an upregulation of CXCR3 and inhibition of CCR3 expression. These results are compatible with the notion that IFN-α is dominant over IL-4 and induces a complete switch to a Th1 phenotype characterized by expression of higher levels of CXCR3 and lower levels of CCR3.
T cells polarized under Th2 conditions in the presence of TGF-β completely lacked both IL-4 production and CCR3 expression in keeping with the described effect of this cytokine on Th2 development (24). However, this was not accompanied by a switch to Th1, since the cells generated did not produce IFN-γ, expressed low levels of CXCR3, and lacked the IL-12Rβ2 subunit.
Differential Response to Chemokines by Th1 and Th2 Lines.
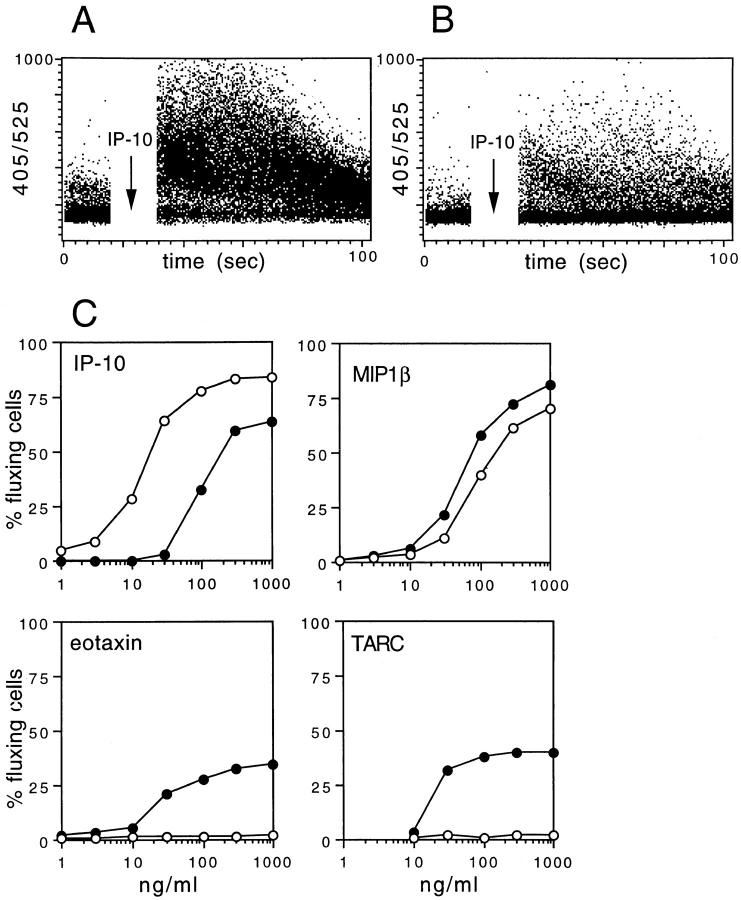
Polarized T cell lines were tested for their capacity to respond to chemokines that selectively engage distinct receptors. Fig. 4 shows the percentage of T cells that undergo [Ca2+]i increase in response to various concentrations of chemokines. Low concentrations of the CXCR3 agonist IP-10 (25) triggered [Ca2+]i increase in Th1 lines, whereas they were ineffective on Th2 lines. Indeed, Th2 lines responded only to 10-fold higher concentrations of IP-10, with a lower percentage of fluxing cells and with a lower [Ca2+]i increase. These results indicate that Th1s have a higher capacity to respond to IP-10 (and Mig, data not shown) than Th2s, demonstrating that the level of receptor expression determines the sensitivity to the agonist. On the other hand, the CCR5-specific agonist MIP-1β (26, 27) showed the same effect on Th1s and Th2s, in keeping with the expression of comparable levels of this receptor on both cell lines. Finally, the CCR3 agonist eotaxin (28) triggered [Ca2+]i increase in Th2, but not Th1 lines. Interestingly, TARC, which has been reported to be a selective agonist for CCR4 (29), efficiently induced a [Ca2+]i increase in Th2, but not Th1 lines, suggesting that the cognate receptor might be selectively expressed on Th2s.
Figure 4.
Differential response to chemokines of Th1 and Th2 lines. (A and B) Time course of [Ca2+]i increase in Th1 (A) and Th2 (B) T cell lines stimulated with 100 ng/ml IP-10. (C) Percent of fluxing cells in Th1 (open circles) and Th2 (closed circles) lines stimulated with various concentrations of the indicated chemokines. The percentage of fluxing cells was calculated cumulatively over a 1-min period after the addition of chemokine. Comparable results were obtained with two additional cell lines as well as with several T cell clones.
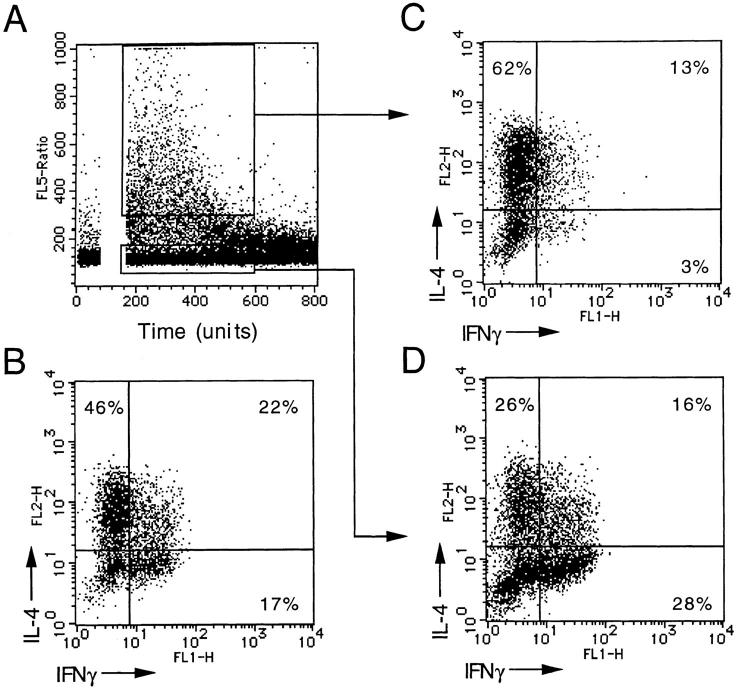
To investigate the relationship between response to TARC and cytokine production, we stimulated a polyclonal Th2 line with TARC, sorted the fluxing cells, and measured cytokine production after stimulation with PMA and ionomycin. As shown in Fig. 5, the TARC-responsive population was highly enriched in Th2s and depleted in Th1s and Th0s, demonstrating that the TARC receptor is expressed on Th2s.
Figure 5.
TARC-responsive cells are Th2. (A) A polyclonal Th2-polarized cell line was loaded with Indo-1 and challenged with 100 ng/ml TARC. Fluxing and nonfluxing cells were sorted over a 1-min period. Unsorted (B), and sorted fluxing (C) and nonfluxing (D) T cells were stimulated with PMA + ionomycin for 4 h and IFN-γ and IL-4 were measured by intracellular staining.
CCR4 Is Preferentially Expressed on Th2s, whereas CCR1 and CCR7 Are Upregulated by IFN-α and TGF-β, Respectively.
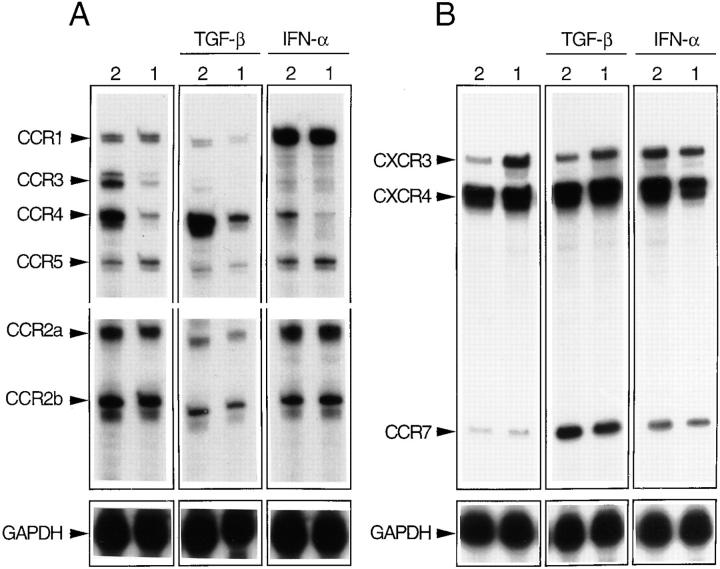
To corroborate the above results on CCR4 expression and to extend the analysis to chemokine receptors for which antibodies were not available, we performed RNase protection assays on Th1 and Th2 lines generated in the absence or in the presence of TGF-β or IFN-α (Fig. 6).
Figure 6.
CCR4 is preferentially expressed on Th2s and CCR1 and CCR7 are upregulated by IFN-α and TGF-β. Chemokine receptor message was determined by RNase protection assay using the multiprobe template sets hCR5 (A) and hCR6 (B, see Materials and Methods). T cell lines generated under Th2- or Th1-polarizing conditions in the absence or in the presence of TGF-β or IFN-α were analyzed. The results are representative of one out of three experiments with consistent results. The upregulation of CCR7 in cultures supplemented with IFN-α was not reproduced in other experiments.
As expected from the above results, CXCR4 and CCR5 messenger RNAs (mRNAs) were present at comparable levels in Th1 and Th2 lines, whereas CXCR3 mRNA was more abundant in Th1 and CCR3 mRNA was present at much higher levels in Th2 lines. In addition, lines obtained in the presence of TGF-β or IFN-α showed changes in CCR3 and CXCR3 mRNAs that were consistent with those observed using mAbs.
Remarkably, CCR4 mRNA was expressed at high levels in Th2 lines and was virtually absent on Th1 lines. In addition, CCR4 was upregulated by TGF-β and downregulated by IFN-α. Taken together with the selective response of Th2s to TARC, these data indicate that CCR4, like CCR3, is selectively expressed on Th2s. However, CCR4 is regulated differently from CCR3 in response to TGF-β.
Two receptors were expressed similarly on Th1s and Th2s, but were differentially modulated by IFN-α and TGF-β. CCR1 was expressed at low levels in Th1s and Th2s polarized under standard conditions. Addition of IFN-α resulted in a dramatic increase in CCR1 message, whereas addition of TGF-β resulted in an almost complete inhibition. In contrast, CCR7 was absent in Th1 and Th2 lines, but its expression was strongly upregulated by TGF-β.
Taken together, the above results indicate that IFN-α and TGF-β can add further functionality to polarized T cells by modulating the expression of chemokine receptors.
Chemokine Receptor Expression on T Cell Clones.
A large panel of T cell clones were analyzed for IL-4 and IFN-γ production and for chemokine receptor expression. Three examples are shown in Fig. 7, A–C. In general, clonal populations showed a considerable heterogeneity in the pattern of cytokine production, since they comprised cells that did not make any cytokine as well as cells that made all the possible combinations of IFN-γ and IL-4. Indeed, even typical Th1 or Th2 clones contained a few cells that produced the reciprocal cytokine combination. The expression of CXCR3 and CCR3 on individual clones reflected their expression on polyclonal lines. CCR3 was expressed only by Th2 clones, whereas CXCR3 was expressed at higher levels on Th1 than on Th2 clones, with few exceptions (Fig. 7 D). In contrast, CCR5 expression on T cell clones was variable since the receptor was absent on several (but not all) Th2 clones (Fig. 7 E). In addition, CCR5 expression was influenced by the activation state and by the presence of IL-2. Indeed, IL-2 withdrawal for 4 d resulted in a complete loss of CCR5 expression, whereas CXCR3 or CCR3 were retained (data not shown).
Figure 7.
Chemokine receptor expression on T cell clones. (A–C) IFN-γ and IL-4 production and chemokine receptor expression (both on four-decade logarithmic scale) on three representative Th0, Th1, and Th2 clones; (D) CXCR3 expression level and (E) percent of CCR5+ cells in Th0, Th1, and Th2 clones. The mean and standard deviation for each group is also shown. No direct correlation was found in the expression of CXCR3 and CCR5. T cell clones were defined according to cytokine profile (Th0, IFN-γ and IL-4; Th1, IFN-γ and no IL-4; Th2, IL-4 and no IFN-γ).
Discussion
We have shown that after activation, T cells acquire new chemokine receptors as they develop into memory/effector cells. In particular, Th1s and Th2s (generated under the influence of IL-12 and -4, respectively) express distinct patterns of chemokine receptors, which can be further modulated by IFN-α and TGF-β.
CXCR4 is expressed at high levels on cord blood T cells and on adult peripheral blood CD45RA+ naive T cells, but is expressed at lower levels on adult CD45RA− memory T cells, and is even absent on some of them. In addition, while CXCR4 is expressed on short-term T cell lines and is even upregulated by stimulation with IL-2 and PHA (20), it was absent on most long-term T cell clones (data not shown). Taken together, these results suggest that CXCR4 is progressively lost after repeated antigenic stimulations.
We have previously shown that CCR3 is selectively expressed on Th2s (15), and in this work, we identify CCR4 as an additional Th2-specific marker. Th2 lines express high levels of CCR4 message and undergo [Ca2+]i increase in response to TARC, a selective ligand for CCR4 (29). In addition, T cell responsiveness to TARC can be used to directly isolate an almost pure population of Th2s from cell lines that contain mixed populations of Th1s, Th0s, and Th2s. Interestingly, CCR4 is also expressed on basophils, while CCR3 is expressed on both eosinophils and basophils (19, 30, 31). The sharing of chemokine receptors between Th2 and the effector cells of allergy is likely to represent a key mechanism for the generation of allergic reactions. CCR3 and CCR4 ligands may selectively attract not only eosinophils and basophils, but also Th2s that, upon activation by antigen, could provide a source of IL-4 and IL-5 required for activation and survival of the effector cells (15).
CXCR3 is selectively expressed on lymphocytes and binds the IFN-γ–induced chemokines IP-10 and Mig (25). We have shown that CXCR3 is present on most peripheral blood CD45R0+ T cells and is expressed at higher levels on Th0s and Th1s and at low levels on Th2s. The responsiveness to IP-10 and Mig, together with the expression of ligands for E- and P-selectin (13), may favor the migration of Th1 and Th0 cells to sites of inflammatory reaction dominated by IFN-γ production (32).
CCR5 is a receptor for regulated on activation normal T cell expressed and secreted (RANTES), MIP-1α and MIP-1β (27) and was expressed at comparable levels on Th1 and Th2 lines. However, when clones were analyzed, CCR5 appeared to be expressed at higher levels on Th1, whereas many Th2 clones were negative. One should note that CCR5 expression is influenced by the activation state of the T cells and, as other receptors (33), is upregulated by IL-2 (20). Thus, while CXCR3 is expressed as a stable marker of memory Th1s and Th0s, CCR5 reflects the activation state of the cells. This may be the reason why CCR5 is present on fewer peripheral blood memory T cells than CXCR3.
IFN-α and TGF-β have a major influence on Th1/Th2 polarization and also affect the expression of chemokine receptors. It has been shown that in humans, IFN-α, and similarly IL-12, induce signal transducer and activator of transcription 4 (STAT4) phosphorylation (34) and consequently dominantly induce Th1 differentiation (23). We have shown that when added to Th2-polarizing conditions (IL-4 plus anti–IL-12 antibodies) IFN-α not only reverts the cytokine profile from Th2 to Th1, but also the chemokine receptor profile, since it prevents the acquisition of CCR3 and CCR4 and allows expression of high levels of CXCR3. In addition, IFN-α upregulates CCR1 expression in all polarizing conditions, thus conferring additional properties to Th1s besides those induced by IL-12.
TGF-β has been reported to inhibit Th2 development and maintain T cells in a state close to naivety (24). We have shown that TGF-β indeed prevents the development of the IL-4–producing phenotype, as well as CCR3 expression. However, the expression of CCR4 is not inhibited, but rather enhanced by TGF-β, indicating that CCR3 and CCR4, although both expressed on Th2s, are differentially regulated. Thus, only CCR3 represents a reliable marker for Th2s, since it correlates precisely with the acquisition of the IL-4–producing phenotype. On the other hand, CCR4, although expressed on Th2s, does not appear to be a selective Th2 marker since it is also expressed on cells that are not capable of producing IL-4.
In addition to upregulating CCR4, TGF-β also upregulates CCR7, a receptor specific for EBV-induced gene 1 (EBI1) ligand chemokine (ELC)/MIP-3β (35). Furthermore, CCR7 was also found on CD45RA+ naive T cells by RNase protection (F. Sallusto, unpublished data). It is interesting to speculate that T cells activated under the aegis of TGF-β may be in a particular “seminaive” state characterized by low effector function combined with the capacity to migrate to secondary lymphoid organs in response to the cognate chemokines ELC/MIP-3β to complete their education.
Our studies suggest that chemokine expression will be a central factor controlling the type of T cell immune response in a tissue, for instance, Th1 or Th2. Expression of the CXCR3 ligands IP-10 or Mig would lead to a preferential Th1 recruitment, whereas expression of CCR3 or CCR4 ligands such as eotaxin and TARC would lead to preferential Th2 recruitment. The regulation of chemokine expression is still poorly understood. IP-10 and Mig are induced by IFN-γ (36) and IP-10 is found at sites of Th1 type immune responses and delayed-type hypersensitivity reactions (32). Eotaxin is induced in Th2 type immune responses, such as in allergic airway disease, but is largely absent from arthritic lesions (12; and Mackay, C.R., unpublished data).
Besides their role in leukocyte migration, some chemokine receptors such as CXCR4, CCR5, and CCR3 behave as coreceptors for HIV entry into target cells (37–40). The differential expression of these receptors on naive and polarized T cells will determine their susceptibility to be infected by viruses carrying different surface glycoproteins. It is interesting that the CC chemokine macrophage-derived chemokine (MDC) has been recently shown to be a powerful and broad spectrum inhibitor of HIV infection, raising the possibility that the cognate receptor, CCR4, plays a major role as a coreceptor for viral entry (41). We have shown that both CCR3 and CCR4 are expressed on Th2s and that CCR4 is upregulated on all cells by TGF-β. In addition, CCR4 is expressed also on peripheral blood memory T cells at high levels (F. Sallusto, unpublished data).
In conclusion, our data show that chemokine receptor expression on T cells is controlled by activation and cytokines present in the milieu and correlates with distinct effector function. The expression of chemokine receptors is not only a useful marker of polarized T cells and a tool to study T cell differentiation, but also a possible target to interfere with an immune response by inhibiting the recruitment of distinct functional subsets.
Acknowledgments
We thank M. Dessing and A. Pickert for help with FACS® analysis and cell sorting and M. Cella and M. Colonna for critical reading and comments.
Abbreviations used in this paper
- CCR
CC chemokine receptor
- CXCR
CXC chemokine receptor
- IP-10
IFN-γ–inducible protein 10
- Mig
monokine induced by IFN-γ
- MIP
macrophage inflammatory protein
- mRNA
messenger RNA
- TARC
thymus and activation regulated chemokine
Footnotes
The Basel Institute for Immunology was founded and is supported by F. Hoffmann–La Roche Ltd., Basel, Switzerland.
The present address of Charles R. Mackay is Millenium Biotherapeutics Inc., Cambridge, MA 02139.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 3.Mackay CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 4.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 5.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 6.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 7.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 8.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 9.Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell– leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 11.Baggiolini M, Dewald B, Moser B. IL-8 and related chemotactic cytokines–CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 12.Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, Truong O, Hsuan JJ, Williams TJ. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 14.Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hamann A, Vestweber D. P-selectin glycoprotein ligand–1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185:573–578. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 16.Traunecker A, Oliveri F, Karjalainen K. Myeloma based expression system for production of large mammalian proteins. Trends Biotechnol. 1991;9:109–113. doi: 10.1016/0167-7799(91)90038-j. [DOI] [PubMed] [Google Scholar]
- 17.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 18.Qin S, Rottman JB, Myers P, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath PD, Mackay CR. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roosnek E, Demotz S, Corradin G, Lanzavecchia A. Kinetics of MHC–antigen complex formation on antigen-presenting cells. J Immunol. 1988;140:4079–4082. [PubMed] [Google Scholar]
- 22.Beverley PC. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992;4:35–41. [PubMed] [Google Scholar]
- 23.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sad S, Mosmann TR. Single IL-2–secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J Immunol. 1994;153:3514–3522. [PubMed] [Google Scholar]
- 25.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 27.Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β, and MIP-1α. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 28.Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell–directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 30.Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, LaRosa GJ, Rao P, Clark-Lewis I, Baggiolini M, Dahinden CA. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4 and other chemokines. J Clin Invest. 1997;100:1137–1143. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Power CA, Meyer A, Nemeth K, Bacon KB, Hoogewerf AJ, Proudfoot AE, Wells TN. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan G, Luster AD, Hancock G, Cohn ZA. The expression of a γ interferon–induced protein (IP-10) in delayed immune responses in human skin. J Exp Med. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho SS, Bacon CM, Sudarshan C, Rees RC, Finbloom D, Pine R, O'Shea JJ. Activation of STAT4 by IL-12 and IFN-α: evidence for the involvement of ligand-induced tyrosine and serine phosphorylation. J Immunol. 1996;157:4781–4789. [PubMed] [Google Scholar]
- 35.Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–13809. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- 36.Luster AD, Ravetch JV. Biochemical characterization of a γ-interferon–induced protein (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 38.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 39.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein–coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 40.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 41.Pal R, Garzino-Demo A, Markham PD, Burns J, Brown M, Gallo RC, DeVico AL. Inhibition of HIV-1 infection by the β-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 42.Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, Gately MK, Gubler U. A functional interleukin 12 receptor complex is composed of two β-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]