Abstract
CD8+ T lymphocytes recognize antigens as short, MHC class I-associated peptides derived by processing of cytoplasmic proteins. The transporter associated with antigen processing translocates peptides from the cytosol into the ER lumen, where they bind to the nascent class I molecules. To date, the precise location of the class I-TAP interaction site remains unclear. We provide evidence that this site is contained within the heavy chain α3 domain. Substitution of a 15 amino acid portion of the H-2Db α3 domain (aa 219-233) with the analogous MHC class II (H-2IAd) β2 domain region (aa 133-147) results in loss of surface expression which can be partially restored upon incubation at 26°C in the presence of excess peptide and β2-microglobulin. Mutant H-2Db (Db219-233) associates poorly with the TAP complex, and cannot present endogenously-derived antigenic peptides requiring TAP-dependent translocation to the ER. However, this presentation defect can be overcome through use of an ER targeting sequence which bypasses TAP-dependent peptide translocation. Thus, the α3 domain serves as an important site of interaction (directly or indirectly) with the TAP complex and is necessary for TAP-dependent peptide loading and class I surface expression.
The MHC class I molecule is a heterotrimeric complex comprised of a 44-kD heavy chain, β2-microglobulin (β2m; 12-kD light chain),1 and a peptide of 8–10 residues (1–4). This complex is recognized by CD8+ T cells when displayed on the surface of cells. Assembly of class I molecules occurs in the endoplasmic reticulum (ER) when the newly synthesized heavy chain associates with resident ER chaperone calnexin, which facilitates folding and disulfide bridge formation of the heavy chain and promotes its binding to β2m (5, 6). Class I–β2m dimers then associate with a heterodimeric, ER membrane protein called TAP (for transporter associated with antigen processing), which consists of TAP1 and TAP2. TAP transports peptides which are predominantly derived from cytosolic proteins into the ER lumen in an ATP-dependent manner (7, 8).
Physical association of class I heavy chain–β2m dimers with TAP as determined by coprecipitation studies (9–12) suggests a specific role of TAP in delivering peptides directly to the MHC class I. It is not clear at present whether TAP associates with MHC class I directly or via an adaptor molecule. A recently described protein, tapasin, is required for class I interaction with TAP (13–17) and has more recently been shown to be necessary for β2m association with TAP (18). Thus, tapasin can be described as a molecular bridge between class I and TAP molecules. Studies on the role of tapasin have been carried out using human cell lines and although tapasin seems to be required for proper class I assembly and subsequent expression in these cell lines, a murine counterpart for tapasin remains to be identified.
Peptide loading of MHC class I can also occur in a TAP-independent manner, as evidenced by the surface expression on TAP-deficient cells of class I molecules that are loaded with signal sequence-derived peptides (19, 20). However, this TAP-independent peptide loading seems to be a minor pathway as it is relevant for a limited set of MHC class I alleles that can bind signal sequence peptides, and the diversity of the bound peptides is very limited (19, 20). Once localized to the ER lumen, peptides can bind to and thereby stabilize nascent class I molecules. Peptide binding results in the release of the class I molecule from the ER (9, 10) and subsequent transport to the cell surface via the exocytic pathway. The majority of misfolded, incompletely assembled, or empty class I molecules are retained in the ER from where they are removed to the cytosol and degraded by the proteasome (21).
Thus, association of class I heavy chain–β2m with the TAP complex (TAP1, TAP2, and possibly tapasin) appears to be a critical event in MHC class I assembly. The location of the site of interaction on class I with TAP complex remains uncertain. Both the extracellular (22) and the transmembrane region/cytoplasmic tail (23) have been implicated in this interaction. Point mutations introduced in the α3 domains of both H-2Ld and H-2Dd resulted in the loss of TAP coprecipitation with the class I heavy chain (11, 22). However, these same point mutations do not affect the ability of these molecules to be expressed at the cell surface (24–26) and to present endogenous peptides (26), in contrast to mutations in either TAP or β2m that drastically affect both cell surface expression and antigen presentation of MHC class I (27–30). Evidence is presented here that physical association with the TAP complex, TAP-dependent peptide loading, and cell surface expression of class I is completely abolished by a 15–amino acid substitution made in the H-2Db α3 domain. Thus, this region could define an interaction site on the murine class I heavy chain with the TAP complex.
Materials and Methods
Generation of Chimeric H-2Db Constructs.
PCR overlap extension was used to create H-2Db cDNA with substitutions in the α3 domain. For the 62–amino acid replacement mutant, class I α3 domain base pairs 666–849 (cDNA) were replaced with class II β2 domain base pairs 2830–3013 (genomic DNA). The latter fragment was obtained using the genomic H-2IAβd as template and the following primers: sense = 5′-GAGAGATCTAACCACCACAAC-3′, antisense = 5′-GGTACACACGGCATGTGTAGACCTCTCCCTG-3′. The product of this amplification and the H-2Db cDNA were used as templates in a subsequent reaction where the H-2IAβd sequence was sewn to the base pairs 850–1097 portion of the H-2Db using the same sense primer as above and 5′-GGATCCACGCTTTACA-3′ as a reverse primer. For the 15–amino acid replacement mutant, class I α3 domain base pairs 736–780 were replaced with class II β2 domain base pairs 2900–2944 using H-2Db cDNA as template. The base pairs 751–1097 mutant portion of the gene was generated with the sense = 5′-TCATCCACACAGCTTAGGCCTGCAGGGGAT-3′, and antisense = 5′-GGATCCACGCTTTACA-3′. The amplification product was extended using sense 5′-GAGACAGTGGGGGTCTCATCCACACAGCTT-3′ and the same antisense primer to obtain the 736–1097 base pair gene fragment. The 1–750 mutant fragment was obtained using sense 5′-GGATCCCAGATGGGG-3′ and antisense 5′-GACCCCCACTGTCTCCTCCTGGCCATTCCTCTGCCAGGTCAGGGT-3′ primers. The 1–750 and 736–1097 base pair fragments were then sewn using sense = 5′-GGATCCCAGATGGGG-3′ and antisense = 5′-GGATCCACGCTTTACA-3′ primers.
PCR products were inserted into the pGEM vector (Promega, Madison, WI) from which they were sequenced using the following primers: sense = 5′-ACCGAGGTGTCTATGGACTTCTTGCCC-3′, antisense = 5′-AAAAGCCACCACAGCTCCAATGATGGC-3′. The BglII, SacI fragment of pGEM (now containing cDNA for mutant H-2Db) was used to replace the BglII, SacI fragment from the wild-type (wt)H-2Db cDNA contained in the Bluescript vector. The NotI, SacI fragment from Bluescript-mutant H-2Db then replaced the corresponding portion of the wtDb in the pCMU-Db plasmid (31). The BamHI-digested 1.1-kb fragment from pCMU-Db was then inserted in the appropriate orientation into the BamHI cloning site of the pHβ APr-I-neo expression vector and transfected into P815 cells by electroporation as described previously for the wtH-2Db (32).
Reverse Transcription PCR.
Total RNA was isolated from 5 × 106 cells using TRIzol reagent (GIBCO BRL, Gaithersburg, MD) following the manufacturer's protocol. cDNA was synthesized using the Superscript preamplification system for first-strand cDNA synthesis (GIBCO BRL). PCR was carried out using Taq polymerase (Fisher Scientific, Fairlawn, NJ) and 20 μg/ml of each primer. Amplification was conducted for 30 cycles. Each cycle consisted of 60 s at 94°C, 60 s at 60°C, and 90 s at 72°C. The following primers were used: for β-actin, sense = 5′-GTGGGCGCCCCAGGCACCA-3′, antisense = 5′-CTCCTTATTGTCACGCACGATTTC-3′; for H-2Db, sense = 5′-TACCTGCAGTTCGCCTATGAA-3′, antisense = 5′-TGATGGCCATAGCTCCAAGGA-3′ (PCR products were sequenced using these same primers); for neomycin, sense = 5′-GCGGCGGCTGCATAC-3′, antisense = 5′-TCATAGAAGGCGGCGG-3′. One fifth of each PCR sample was loaded onto a 0.8% agarose gel and visualized by ethidium bromide staining.
Cell Lines.
P815 transfectants were maintained in RPMI 1640/10% FCS (RP10) supplemented with 500 μg/ml Geneticin (GIBCO BRL). The influenza A/PR8/34 nucleoprotein (NP) peptide 366-374–specific CTL line, PR8.2 (29) was maintained by weekly restimulations with irradiated C57/BL6 spleen cells pulsed with 10 μM influenza NP peptide (ASNENMETM) in RP10 containing 5% rat Con A supernatant.
To generate CTLs specific for endogenous influenza peptide, C57BL/6 mice were immunized with influenza strain A/PR8/34 (a gift from Dr. A. Garcia-Sastre, Mt. Sinai Medical Center, New York) by intraperitoneal injection and spleens were harvested after 10 d and stimulated in vitro for 5–6 d with virus-infected, autologous splenocytes. These CTLs were used in cytotoxicity assays using recombinant vaccinia constructs.
Cytotoxicity Assays.
Target cells were pulsed with [51Cr]sodium chromate in RP10 medium for 1 h at 37°C, washed twice with PBS, and plated at 104 cells/well of a 96-well round-bottomed plate. Influenza NP 366–374 peptide as well as effector cells (CTLs) were then added to the wells to a final volume of 200 μl/well. After a 4-h incubation at 37°C, 100 μl of the supernatants were harvested and 51Cr-release was measured. Where flu peptide concentrations range from 1 nM to 10 μM, the effector to target ratio was kept constant at 10:1.
For induction of class I expression, P815 transfectants (targets) were incubated overnight at 26°C in serum-free medium (Opti MEM I; GIBCO BRL) in the presence of 10 μM NP 366–374 peptide with or without human β2m (Sigma Chemical Co., St. Louis, MO) at 5 μg/ml. Cells were then pulsed with 51Cr, washed three times with PBS, and plated at 104/well. CTL assays were carried out as above with effector to target ratios starting at 100:1 with serial, threefold dilutions of effector cells.
Flow Cytometry.
2–5 × 105 P815 and P815 transfectants were washed once in PBS/2% FCS followed by incubation with a saturating amount of biotinylated anti–H-2Db antibody (KH95; PharMingen, San Diego, CA) for 30 min at 4°C. Cells were washed twice with PBS/2% FCS and then suspended in 100 μl of a 1:100 dilution of streptavidin-PE (Caltag Labs., South San Francisco, CA). Cells were washed twice and resuspended in 300 μl PBS/1% formaldehyde. All samples were analyzed using a FACScan® flow cytometer (Becton Dickinson, Mountain View, CA).
Generation of Vaccinia Constructs.
To produce the NP 366–374 recombinant vaccinia virus (VV), complementary oligonucleotides were designed and synthesized to insert into a modified pSC11 plasmid (33). The plus strand (+) was composed of the following bases: TCGACCACCATGGCTTCCAATGAAAATATGGAGACTATGTGATAGGTACCGC. This sequence encoded an insertional SalI site extension (TCGA), Kozak's sequence (CCACC), a methionine initiation triplet (ATG), nine triplet bases coding for the desired antigenic determinant (ASNENMETM), two stop codons (TGA and TAG), and an insertional NotI site (GC). The complementary minus strand (−) was composed of the following bases: GGCCGCGGTACCTATCACATAGTCTCCATATTTTCATTGGAAGCCATGGTGG. The plus and minus strand oligonucleotides were annealed to create double-stranded DNA with SalI and NotI cloning sites and inserted into the modified version of pSC11 downstream of the early/late VV p7.5 promoter.
The E3/19K leader/signal sequence (ES) NP 366–374 recombinant vaccinia virus was constructed by inserting synthetic oligonucleotides (StyI–NotI double-stranded DNA fragment) behind the ES cloned into pSC11 (33, 34). All oligonucleotide insertions into the pSC11 plasmids were confirmed by DNA sequencing. Finally, recombinant vaccinia viruses were generated in CV-1 cells by homologous recombination, plaque-purified at least three times, and propogated in thymidine kinase-deficient human 143B osteosarcoma cells as described (35).
Immunoprecipitations.
Metabolic labeling, immunoprecipitation, and 2D nonequilibrium pH-gradient gel electrophoresis (NEPHGE)–PAGE were performed in essence as previously described (36, 37), except that 1% digitonin was used instead of 0.5% NP-40. Antibodies used for precipitation were obtained as follows: the anticalnexin antiserum was purchased from Stressgen (Victoria, Canada), the anti–heavy chain serum (38) was obtained from H. Ploegh (Massachusetts Institute of Technology, Boston, MA), and anti-TAP antisera were produced by immunizing rabbits with purified recombinant mouse TAP1 or TAP2-GST fusion proteins, and will be described in detail elsewhere (Nandi, D., and J.J. Monaco, manuscript in preparation).
Results
Mutant H-2Db Molecules Are Not Expressed at the Cell Surface at Detectable Levels.
Sequences from the β2 domain of the mouse MHC class II H-2IAβd gene were substituted into the α3 domain of the class I H-2Db gene using a PCR overlap extension mutagenesis strategy. The class II β2 domain was chosen to substitute for the class I α3 domain due to its predicted structural homology to the α3 domain (39). Two such chimeric H-2Db constructs were created, one with an exchange of 15 amino acids and the other with a 62–amino acid replacement (Fig. 1 A). Due to sequence homology between these class I and II domains, the actual change in the number of amino acids is 11 and 42, respectively. However, we will refer to these molecules as 15– (Db219–233) and 62– (Db196–257) amino acid replacements in keeping with the total number of class II residues introduced.
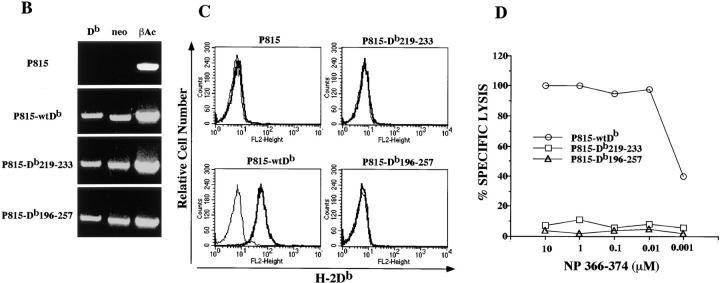
Figure 1.
Db219–233 and Db196–257 mutant heavy chains are not expressed at the cell surface. (A) Amino acid sequence (single letter code) of the portions of the α3 H-2Db heavy chain aligned to the homologous sequences of the β2 domain of H-2IAd. Db219–233 and Db196–257 mutant heavy chains were generated by substituting indicated portion of the H-2Db α3 domain with the corresponding region from the H-2IAd β2 domain. (B) Reverse transcription PCR analysis of the H-2Db, neomycin resistance, and β-actin expression in P815, P815-wtDb, P815-Db219–233, and P815-Db196– 257 cells. (C) Immunofluorescence analysis of P815, P815-wtDb, P815-Db219–233, and P815-Db196–257 cells cultured at 37°C. Cells were stained with biotinylated anti–H-2Db–specific monoclonal antibody followed by streptavidin-PE. Bold lines, cells stained with monoclonal antibody; plain lines, cells stained with secondary reagent alone. (D) Cytotoxicity assay using influenza NP 366-374–specific H-2Db–restricted CD81 cell line as effector and 51Cr-labeled P815-wtDb, P815-Db219–233, and P815-Db196–257 as target cells in the presence of indicated concentrations of the exogenous NP 366–374 peptide added directly to the assay.
The 15– and 62–amino acid mutant constructs as well as a wtH-2Db construct were transfected into the P815 murine mastocytoma cell line (H-2d) and screened for expression of the mutant molecules at the messenger RNA level using reverse transcription PCR. RNA was isolated and cDNA synthesized from each of the 15– and 62–amino acid mutant P815 transfectants (designated P815-Db219– 233 and P815-Db196–257, respectively) as well as from nontransfected P815 and wtDb P815 transfectants. The cDNA was amplified in a PCR using primers specific for the H-2Db molecule, but non–cross-reactive with H-2Ld, which is also expressed on P815 and shares 94% identity with H-2Db. The primers amplify a 558-bp fragment encompassing the region where the H-2IAd sequence is flanked by the H-2Db sequence. The products of this PCR amplification (Fig. 1 B) were verified by sequencing.
Despite expression of mutant H-2Db molecules at the messenger RNA level, immunofluorescence staining for H-2Db resulted in no detectable surface expression as compared with the wildtype control (Fig. 1 C). To test for potentially low levels of surface expression, transfectants were used as targets in a cytotoxicity assay that is generally more sensitive than FACS® analysis. The CD8 coreceptor-independent CTL line PR8.2, which is specific for the H-2Db– restricted influenza NP 366–374 peptide was used in a 51Cr–release assay where the level of killing of mutant transfectant targets pulsed with peptide was compared with that of P815-wtDb controls. Neither mutant molecule could sensitize P815 cells for lysis in the CTL assay (Fig. 1 D), demonstrating that the steady-state levels of mutant heavy chains available for peptide binding were below the detectable threshold for a CTL assay.
Mutant H-2Db Molecules Can Be Stabilized at the Cell Surface.
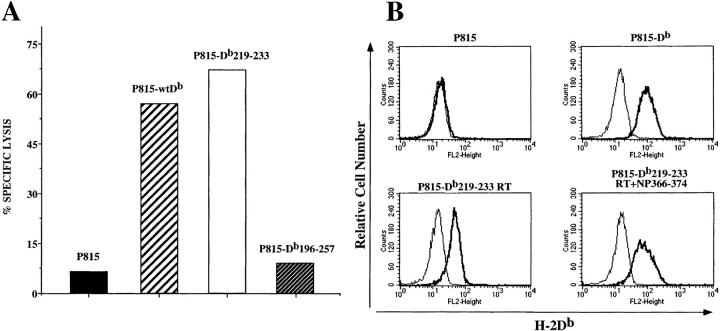
To test whether mutant heavy chains that may be reaching the cell surface in very limited quantities could be captured and stabilized at the cell surface, transfectants were incubated overnight at 26°C in the presence of excess influenza NP 366–374 peptide and β2m. Transfectants were then labeled with 51Cr and used in the influenza peptide– specific cytotoxicity assay. The results show that P815-Db219–233 was lysed comparably to P815-wtDb, but that P815-Db196–257 was not specifically lysed (Fig. 2 A). We conclude that the 15–amino acid mutant H-2Db molecules can be stabilized at the cell surface by addition of exogenous peptide and β2m and that the stabilized molecule can present antigenic peptide to CTLs, suggesting that it is not grossly misfolded. The phenotype of the Db196–257 is much more severe, however, perhaps due to misfolding of the molecule. Subsequent studies were carried out using only the P815-Db219–233 transfectant.
Figure 2.
Low temperature, peptide, and β2m upregulate the Db219–233 at the cell surface. (A) Cytotoxicity assay using influenza NP 366-374–specific H-2Db–restricted CD8+ cell line as effector and 51Cr-labeled P815, P815-wtDb, P815-Db219–233, and P815-Db196–257 as target cells. All targets were preincubated overnight at 26°C in the presence of 10 μM NP 366–374 and 5 μg/ml human β2m. Effector to target ratio was 10:1. (B) Immunofluorescence analysis of P815, P815-wtDb, and P815-Db219–233 cells. P815 and P815-wtDb were cultured at 37°C, whereas P815-Db219–233 cells were cultured at 26°C in the absence or presence of NP 366–374 plus β2m. Cells were stained with biotinylated anti–H-2Db–specific monoclonal antibody followed by streptavidin-PE. Bold lines, cells stained with monoclonal antibody; plain lines, cells stained with secondary reagent alone.
To determine whether incubation at 26°C and addition of exogenous peptide and β2m can upregulate surface expression enough to be detected by FACS® analysis, transfectants treated in this manner were stained with an antibody against the H-2Db molecule. The FACS® results indicate that surface expression of the 15–amino acid mutant molecule can be detected at a level comparable to that of P815-wtDb maintained at 37°C (Fig. 2 B). In fact, mere incubation at 26°C in the absence of peptide (but presence of β2m) results in significant upregulation of Db219–233 cell surface expression.
To exclude the possibility of a randomly linked mutation related to general antigen processing and/or class I assembly, we examined the surface expression of H-2Kd in P815, P815-wtDb, and P815-Db219–233. Comparable levels of H-2Kd were seen in these cells (data not shown) suggesting that the defect in proper class I assembly is restricted to the mutant heavy chain.
Upregulation of Surface Expression of Mutant H-2Db Molecules Requires β2m.
The 15–amino acid mutant contains substitutions within the class I α3 domain that could possibly affect the ability of β2m to bind to the heavy chain. Substituted amino acid positions 231 and 233 are thought to be 2 of the 13 contact sites between the α3 domain and β2m (40). However, the α1 and α2 domains contain 11 and 13 potential β2m interaction sites, respectively, so it seems unlikely that a change in only two β2m contact sites would abrogate its interaction with the heavy chain. Still, it is conceivable that substitutions made at these positions could negatively affect the overall interaction between the heavy chain and β2m to a degree such that proper class I assembly in the ER does not occur, resulting in intracellular retention of the molecule. However, the fact that mutant H-2Db molecules are stabilized by addition of peptide and β2m suggests that these molecules are capable of association with β2m. In fact, an appreciable upregulation of surface expression is seen only in the presence of exogenous β2m and cannot be seen by the addition of peptide alone (data not shown). Lack of upregulation of surface expression by peptide alone is also evident when cells treated in this manner are used as targets in a CTL assay (Fig. 3). These results suggest that the mutant H-2Db heavy chain is able to associate with β2m.
Figure 3.
Presentation of NP 366–374 to specific CD8+ cells by Db219–233 requires the presence of β2m. P815, P815-wtDb, and P815-Db219–233 cells were incubated overnight at 26°C in the absence of fetal calf serum. 10 μM NP 366–374 and/or 5 μg/ml human β2m were added as indicated. Cells were then labeled with 51Cr and used in a cytotoxicity assay with an NP 366-374–specific CD8+ cell line as effector cells.
Mutant H-2Db Molecules Are Deficient in TAP-dependent Peptide Loading.
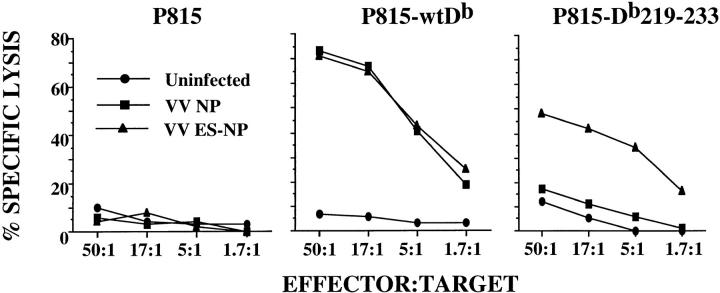
The phenotype of the mutant H-2Db transfectants is reminiscent of that of the cell line RMA-S as well as other TAP-deficient cell lines or cells lacking β2m (27–30). The low level of surface expression of class I on RMA-S is due to deficient peptide loading via the TAP complex. To determine whether a similar deficiency may be occurring in P815-Db219–233, these cells were infected with recombinant vaccinia virus containing a minigene construct for the H-2Db–restricted influenza epitope (NP 366–374), which was either linked COOH terminally to an ER insertion sequence (VV ES-NP) or not (VV NP). The linking of an ER insertion sequence to the peptide allows for TAP-independent peptide translocation to the ER (41, 42). If the inability of the mutant H-2Db molecule to be loaded with peptide is due to a disruption in its association with TAP, infection with the vaccinia construct containing the ER insertion signal linked to the influenza peptide minigene should bypass TAP-dependent peptide loading of the molecule. The use of these vaccinia-infected cells as targets in the flu-specific CTL assay shows that P815-Db219–233 targets infected with the VV ES-NP were specifically killed, but those infected with the VV NP were not (Fig. 4). Infection with either VV ES-NP or VV NP rendered P815-wtDb targets equally susceptible to lysis, whereas parental P815 were not lysed after infection with either of the vaccinia constructs (Fig. 4). These results demonstrate that TAP-dependent peptide transport to the mutant H-2Db molecule is specifically impaired. In addition, these results reconfirm the fact that the mutant heavy chain is capable of association with β2m. Thus, the α3 domain of class I must contain important sites of interaction either directly or indirectly with TAP that are critical for proper peptide loading and subsequent surface expression of class I molecules.
Figure 4.
TAP-independent, but not TAP-dependent, delivery of endogenous antigens to Db219–233 sensitizes P815-Db219–233 for lysis by NP 366-374–specific CTLs. Uninfected, VV NP–, or VV ES-NP–infected P815, P815-wtDb, or P815-Db219–233 cells were labeled with 51Cr and used as targets in a CTL assay. Spleen cells of C57BL/6 mice infected with influenza A/PR8/34, restimulated in vitro with influenza A/PR8/34, were used as effector cells.
TAP Does Not Associate with the 15–Amino Acid Mutant H-2Db Molecules.
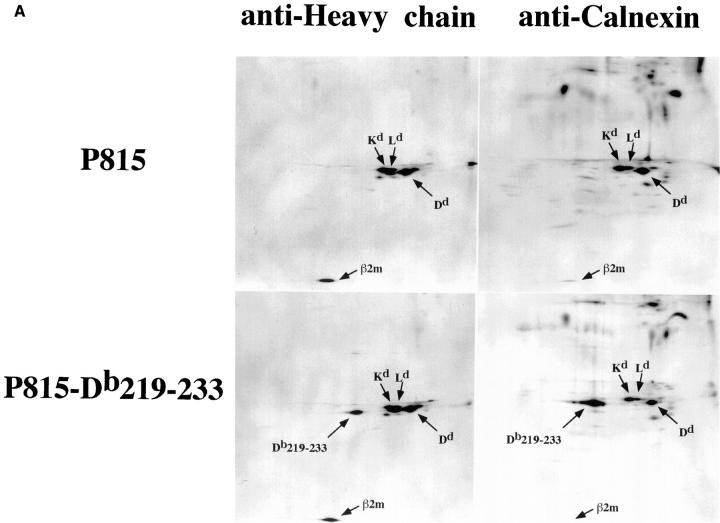
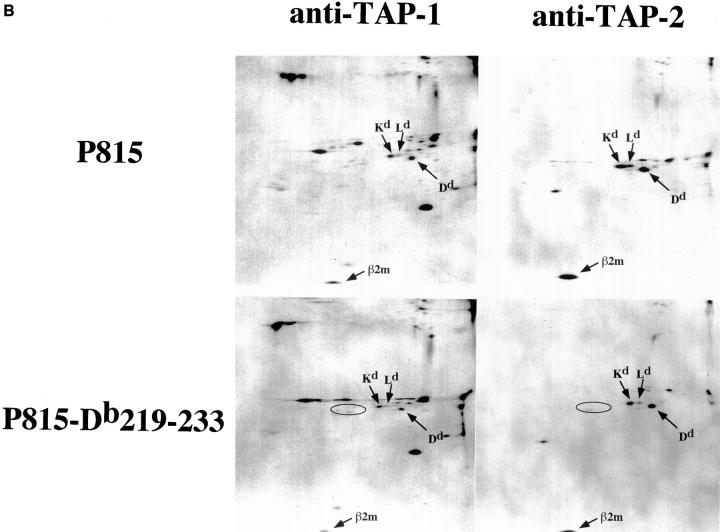
The above results, showing a functional defect in TAP-dependent peptide loading of the Db219– 233 molecule, suggest that the mutant heavy chain may be incapable of physical association with the TAP complex. To test this, immunoprecipitation of [35S]methionine-labeled P815, P815-wtDb, or P815-Db219–233 was carried out using antibodies specific for MHC class I heavy chain, calnexin, TAP1, or TAP2. Immunoprecipitates were resolved using 2D NEPHGE-PAGE. Immunoprecipitation of parental P815 cell lysate using heavy chain– and calnexin–specific antibodies revealed distinct spots that were identified based on their predicted mobility to correspond to the H-2Kd (mol wt = 39,368 daltons; pI = 6.43), H-2Dd (mol wt = 37,278 daltons; pI = 5.13), and H-2Ld (mol wt = 38,400 daltons; pI = 6.20) heavy chains (Fig. 5 A). The same heavy chain pattern was observed when TAP-1– or TAP-2–specific antibodies were used for immunoprecipitation (Fig. 5 B). In addition, β2m can be identified in all precipitates, as well as several spots that, based on their molecular weight, could correspond to tapasin. However, since the sequence of mouse tapasin is not yet published, we do not know which of these, if any, represent tapasin. Precipitation of P815-wtDb lysate with anti–heavy chain or anticalnexin antibodies did not reveal an additional distinct class I heavy chain, but resembled the pattern seen with P815 (data not shown). This is most likely due to the indistinguishable migration patterns of H-2Db and H-2Ld because of their extensive sequence homology. However, Db219–233 is predicted to migrate significantly differently (mol wt = 38,295 daltons; pI = 7.19), and should be observed as a distinct spot. Indeed, precipitation of P815-Db219–233 lysate using anti–heavy chain antibodies revealed a new spot with a migration pattern expected for the mutant H-2Db molecule (Fig. 5 A). Based on the intensity of this spot, we conclude that Db219–233 is synthesized at a level comparable to the three endogenous heavy chains (H-2Kd, H-2Ld, and H-2Dd). However, anti-TAP antibodies precipitated significantly lower amounts (if any) of Db219– 233 compared with the endogenous heavy chains (Fig. 5 B). In contrast to what is seen in the TAP immunoprecipitates, more Db219–233 relative to the endogenous heavy chains appears associated with calnexin (Fig. 5 A), consistent with the data in the previous figures indicating that this molecule fails to traffic efficiently to the cell surface and, hence, accumulates in the ER.
Figure 5.
Reduced physical association of Db219–233 with TAP. [35S]methionine-labeled P815 or P815-Db219–233 lysates immunoprecipitated with MHC class I heavy chain–, calnexin–, TAP-1–, or TAP-2–specific antibodies. Immunoprecipitates were resolved using 2D NEPHGE-PAGE. Arrows, spots corresponding to H-2Kd, H-2Dd, H-2Ld, and H-2Db219–233 β2m. (B) Circles, the expected position of H-2Db219–233.
Discussion
We have shown that substitution of amino acids 219– 233 within the α3 domain of H-2Db results in the loss of its expression at the cell surface. Cell surface expression of Db219–233 can be rescued by incubation at 26°C with addition of excess peptide and β2m. The rescued molecule is functional in its ability to present exogenous peptide for recognition by CD8+ T cells, suggesting that substitutions introduced into the α3 domain do not grossly affect the conformation of the molecule. P815-Db219–233 exhibits a phenotype very similar to that of RMA-S cells, which led us to believe that the defect of Db219–233 expression is due to a lack of TAP-dependent peptide loading. This was demonstrated by the ability of Db219–233 to present endogenous influenza NP 366–374 peptide only when it is linked COOH terminally to an ER insertion sequence, thus allowing it to bypass the requirement for TAP-dependent peptide loading. Finally, the functional defect in TAP-mediated peptide translocation to Db219–233 correlates with the finding that physical association of Db219–233 with the TAP complex is drastically reduced. Together, these results argue that amino acids 219–233 of the α3 domain serve as an important docking site for the TAP complex during the assembly of MHC class I molecules.
Although human β2m is clearly binding to Db219–233, as evidenced by the requirement for human β2m to stabilize the Db219–233 at the cell surface (Fig. 3), this does not necessarily reflect the ability of mouse β2m to bind Db219– 233. Still, the fact that antigen presentation by Db219–233 occurs when peptides are targeted to the ER in a TAP-independent manner (Fig. 4) provides evidence that mouse β2m too is binding to the mutant heavy chain. If the lack of Db219–233 surface expression and antigen presentation were due to impaired β2m binding, the phenotype would remain consistent, even when peptides are targeted to the ER by linkage to an ER insertion sequence.
It has been previously suggested that TAP may associate with the α3 domain of the class I heavy chain. This was based on findings that substitution of a single amino acid within the α3 domain (H-2Ld227 or H-2Dd222) can result in the loss of class I association with TAP, as determined in coprecipitation studies (11, 22). However, these molecules are still present at the cell surface at levels detectable by FACS® analysis (24–26) and are able to present endogenous peptides (26), suggesting that a true loss of TAP-dependent peptide loading has not occurred. Still, the loss of class I–TAP association as detected in immunoprecipitations using H-2Ld 227 and H-2Dd 222 hints to the α3 domain as an important site of interaction with the TAP complex. It is known that the association between TAP and class I is very labile in most detergents other than digitonin (10). Perhaps the change of even one critical residue involved in TAP association renders this interaction even more labile, even in mild detergents. This change, however, must not abrogate the in vivo function of TAP in loading peptide onto class I. This could explain why H-2Ld227 and H-2Dd222 are still expressed and function normally at the cell surface, yet are shown by immunoprecipitation not to associate with TAP. Perhaps caution must be taken when interpreting the results of immunoprecipitations that indicate a lack of TAP association with class I molecules. This is further supported by the findings of allelic variations in the ability of human class I heavy chains to associate with TAP, as HLA-B35 alleles do not coprecipitate with TAP (43) and yet are expressed at the cell surface and present antigenic peptides efficiently (44, 45).
Point mutations of the α2 domain of the human class I molecule HLA-A0201 (position 134) results in ∼80% reduced surface expression and diminished ability to present endogenous antigens (46, 47), implicating the α2 domain of the heavy chain in binding to TAP. However, the same mutant molecule is rapidly transported to the cell surface without bound peptides. Apparently, this molecule escapes degradation that normally happens to the majority of partially assembled class I molecules (21). It has therefore been suggested that mutation at position 134 disrupts interaction with an accessory molecule (such as calreticulin) responsible for sorting the peptide-free class I molecules to the degradative pathway and/or ER retention of unloaded molecules (48). Our results do not exclude the role of the α2 domain in contributing to class I association with TAP. In fact, an α2 domain contact with the TAP complex could enhance the association necessary for peptide transfer onto the class I molecule. We do show, however, that a net change of 11 amino acids within the α3 domain is sufficient to dissociate class I from TAP function.
Acknowledgments
The authors thank Sean Uiterwyk (New York University Medical School, New York) for assistance in generating mutant fragments, Adolfo Garcia-Sastre (Mount Sinai Medical School, New York) for the influenza A/PR8/34 virus, John Hirst (New York University Medical School, New York) for the FACS® analysis, Moriya Tsuji (New York University Medical School, New York) for help with the vaccinia experiments, and David Ginsburg (University of Cincinnati, OH) for the technical assistance with 2D NEPHGE-PAGE.
This work was supported by the Markey Charitable Trust Junior Investigator Award, National Cancer Institute core support grant 5P30 CA-16087, and National Institutes of Health grant AI-33605.
Footnotes
1 Abbreviations used in this paper: β2m, β2-microglobulin; ER, endoplasmic reticulum; ES, E3/19K leader/signal sequence; NEPHGE, nonequilibrium pH-gradient gel electrophoresis; NP, nucleoprotein; RP10, RPMI 1640/10% FCS; TAP, transporter associated with antigen processing; VV, vaccinia virus; wt, wild type.
References
- 1.Schumacher TNM, Heemels M-T, Neefjes JJ, Kast WM, Melief CJM, Ploegh HL. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990;62:563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- 2.Townsend A, Ohlen C, Bastin J, Ljungren H-G, Foster L, Karre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989;340:443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 3.Van Bleek GM, Nathenson SG. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kbmolecule. Nature. 1990;348:213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- 4.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee H-G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 5.Tector M, Salter RD. Calnexin influences folding of human class I histocompatibility proteins but not their assembly with beta 2-microglobulin. J Biol Chem. 1995;270:19638–19642. doi: 10.1074/jbc.270.33.19638. [DOI] [PubMed] [Google Scholar]
- 6.Vasillakos A, Cohen-Doyle M, Peterson PA, Jackson MR, Williams DB. The molecular chaperone calnexin facilitates folding and assembly of class I histocompatibility molecules. EMBO (Eur Mol Biol Organ) J. 1996;15:1495–1506. [PMC free article] [PubMed] [Google Scholar]
- 7.Shepherd JC, Schumacher TNM, Ashton-Rickardt PG, Imaeda S, Ploegh HL, Janeway CA, Tonegawa S. TAP1-dependent peptide translocation in vitro is ATP-dependent and peptide selective. Cell. 1993;74:577–584. doi: 10.1016/0092-8674(93)80058-m. [DOI] [PubMed] [Google Scholar]
- 8.Neefjes JJ, Momburg F, Hammerling GJ. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993;261:769–771. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- 9.Suh W-K, Cohen-Doyle MF, Fruh K, Wang K, Peterson PA, Williams DB. Interaction of MHC class I molecules with the transporter associated with antigen processing. Science. 1994;264:1322–1326. doi: 10.1126/science.8191286. [DOI] [PubMed] [Google Scholar]
- 10.Ortmann B, Androlewicz M, Cresswell P. MHC class I/β2-microglobulin complexes associate with TAP transporters before peptide binding. Nature. 1994;368:864–867. doi: 10.1038/368864a0. [DOI] [PubMed] [Google Scholar]
- 11.Carreno BM, Solheim JC, Harris M, Stroynowski I, Connolly JM, Hansen TH. TAP associates with a unique class I conformation, whereas calnexin associates with multiple class I forms in mouse and man. J Immunol. 1995;155:4726–4733. [PubMed] [Google Scholar]
- 12.Androlewicz MJ, Ortmann B, van Endert PM, Spies T, Cresswell P. Characteristics of peptide and major histocompatibility complex class I/β2-microglobulin binding to the transporters associated with antigen processing (TAP1 and TAP2) Proc Natl Acad Sci USA. 1994;91:12716–12720. doi: 10.1073/pnas.91.26.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu Y, Demars R. Production of human cells expressing individual transferred HLA-A, -B, -C genes using and HLA-A, -B, -C null human cell line. J Immunol. 1989;142:3320–3328. [PubMed] [Google Scholar]
- 14.Grandea AGI, Androlewic MJ, Athwal RS, Geraghty DE, Spies T. Dependence of peptide binding by MHC class I molecules on their interaction with TAP. Science. 1995;270:105–108. doi: 10.1126/science.270.5233.105. [DOI] [PubMed] [Google Scholar]
- 15.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles of calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Sjogren H-O, Hellman U, Pettersson RF, Wang P. Cloning and functional characterization of a subunit of the transporter associated with antigen processing. Proc Natl Acad Sci USA. 1997;94:8708–8713. doi: 10.1073/pnas.94.16.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AGI, Riddell SR, Tampe R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I–TAP complexes. Science. 1997;277:1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- 18.Solheim JC, Harris MR, Kindle CS, Hansen TH. Prominence of β2-microglobulin, class I heavy chain conformation, and tapasin in the interactions of class I heavy chain with calreticulin and the transporter associated with antigen processing. J Immunol. 1997;158:2236–2241. [PubMed] [Google Scholar]
- 19.Wei M, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 20.Henderson RA, Michel H, Sakaguchi K, Shabanowitz J, Appella E, Hunt DF, Engelhard VH. HLA-A2.1– associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992;255:1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- 21.Hughes E, Hammond C, Cresswell P. Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Natl Acad Sci USA. 1997;94:1896–1901. doi: 10.1073/pnas.94.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh W-K, Mitchell EK, Yang Y, Peterson PA, Waneck GL, Williams DB. MHC class I molecules form ternary complexes with calnexin and TAP and undergo peptide-regulated interaction with TAP via their extracellular domains. J Exp Med. 1996;184:337–348. doi: 10.1084/jem.184.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee N, Malacko AR, Ishitani A, Chen M-C, Bajorath J, Marquardt H, Geraghty DE. The membrane-bound and soluble forms of HLA-G bind identical sets of endogenous peptides but differ with respect to TAP association. Immunity. 1995;3:591–600. doi: 10.1016/1074-7613(95)90130-2. [DOI] [PubMed] [Google Scholar]
- 24.Potter TA, Rajan TV, Dick RF, II, Bluestone JA. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989;337:73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- 25.Connoly J, Hansen TH, Ingold AL, Potter TA. Recognition by CD8 on cytotoxic T lymphocytes is ablated by several substitutions in the class I α3 domain: CD8 and the T-cell receptor recognize the same class I molecule. Proc Natl Acad Sci USA. 1990;87:2137–2141. doi: 10.1073/pnas.87.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killeen N, Moriarty A, Teh H-S, Littman DR. Requirement for CD8-major histocompatibility complex class I interaction in positive and negative selection of developing T cells. J Exp Med. 1992;176:89–97. doi: 10.1084/jem.176.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams DB, Barber BH, Flavell RA, Allen H. Role of β2-microglobulin in the intracellular transport and surface expression of murine class I histocompatibility molecules. J Immunol. 1989;142:2796–2806. [PubMed] [Google Scholar]
- 28.Vitiello A, Potter TA, Sherman LA. The role of β2-microglobulin in peptide binding by class I molecules. Science. 1990;250:1423–1426. doi: 10.1126/science.2124002. [DOI] [PubMed] [Google Scholar]
- 29.Attaya M, Jameson S, Martinez CK, Hermel E, Aldrich C, Forman J, Fischer-Lindahl K, Bevan MJ, Monaco JJ. Ham-2corrects the class I antigen processing– defect in RMA-S cells. Nature. 1992;355:647–649. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- 30.Spies T, Cerundolo V, Collona M, Cresswell P, Townsend A, DeMars R. Presentation of viral antigen by MHC class I molecules is dependent on a putative peptide transporter heterodimer. Nature. 1992;355:644–646. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- 31.Joly E, Oldstone MBA. Manufacture of a functional cDNA for the H-2Dbmolecule using a retroviral shuttle vector. Immunogenetics. 1991;34:62–65. doi: 10.1007/BF00212315. [DOI] [PubMed] [Google Scholar]
- 32.Jhaver KG, Rao TD, Frey AB, Vukmanović S. Apparent split tolerance of CD8+T cells from β2-microglobulin–deficient (β2m−/−) mice to syngeneic β2m+/+ cells. J Immunol. 1995;154:6252–6261. [PubMed] [Google Scholar]
- 33.Eisenlohr LC, Bačík I, Bennink JP, Bernstein K, Yewdell JW. Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I– restricted T lymphocytes. Cell. 1992;71:963–972. doi: 10.1016/0092-8674(92)90392-p. [DOI] [PubMed] [Google Scholar]
- 34.Bačík I, Cox JH, Anderson R, Yewdell JW, Bennink JR. TAP-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences when located at the amino but not carboxy terminus of the peptide. J Immunol. 1994;152:381–387. [PubMed] [Google Scholar]
- 35.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown MG, Driscoll J, Monaco JJ. Structural and serological similarity of MHC-linked LMP and proteasome (multicatalytic proteinase) complexes. Nature. 1991;353:355–357. doi: 10.1038/353355a0. [DOI] [PubMed] [Google Scholar]
- 37.Nandi D, Woodward E, Ginsburg DB, Monaco JJ. Intermediates in the formation of mouse 20S proteasomes: implications for the assembly of precursor β subunits. EMBO (Eur Mol Biol Organ) J. 1997;16:5363–5375. doi: 10.1093/emboj/16.17.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machold RP, Andree S, Van Kaer L, Ljunggren H-G, Ploegh HL. Peptide influences the folding and intracellular transport of free major histocompatibility complex class I heavy chains. J Exp Med. 1995;181:1111–1122. doi: 10.1084/jem.181.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JH, Jardetzky TS, Gorga JC, Stern JC, Urban RG, Strominger JL, Wiley DC. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364:33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 40.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 41.Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweerink H. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I–restricted cell-mediated lysis. J Exp Med. 1991;174:489–492. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenlohr LC, Yewdell JW, Bennink JR. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992;175:481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neisig A, Wubbolts R, Zang X, Melief C, Neefjes J. Allele-specific differences in the interaction of MHC class I molecules with transporters associated with antigen processing. J Immunol. 1996;156:3196–3206. [PubMed] [Google Scholar]
- 44.Ooba T, Hayashi H, Karaki S, Tanabe M, Kano K, Takiguchi M. The structure of HLA-B35 suggests that it is derived from HLA-Bw58 by two genetic mechanisms. Immunogenetics. 1989;30:76–80. doi: 10.1007/BF02421534. [DOI] [PubMed] [Google Scholar]
- 45.Koziel MJ, Dudley D, Wong JT, Dienstag J, Houghton M, Ralston R, Walker BD. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992;149:3339–3344. [PubMed] [Google Scholar]
- 46.Lewis JW, Neisig A, Neefjes J, Elliott T. Point mutations in the a2 domain of HLA-A2.1 define a functionally relevant interaction with TAP. Curr Biol. 1996;6:873–883. doi: 10.1016/s0960-9822(02)00611-5. [DOI] [PubMed] [Google Scholar]
- 47.Peace-Brewer AL, Tussey LG, Matsui M, Li G, Quinn DG, Frelinger JA. A point mutation in HLA-A*0201 results in failure to bind the TAP complex and to present virus-derived peptides to CTL. Immunity. 1996;4:505–514. doi: 10.1016/s1074-7613(00)80416-1. [DOI] [PubMed] [Google Scholar]
- 48.Elliott T. How does TAP associate with MHC class I molecules. Immunol Today. 1997;18:375–379. doi: 10.1016/s0167-5699(97)01097-9. [DOI] [PubMed] [Google Scholar]