Abstract
Circulating leukocytes are thought to extravasate from venules through open interendothelial junctions. To test this paradigm, we injected N-formyl-methionyl-leucyl-phenylalanine (FMLP) intradermally in guinea pigs, harvesting tissue at 5–60 min. At FMLP-injected sites, venular endothelium developed increased surface wrinkling and variation in thickness. Marginating neutrophils formed contacts with endothelial cells and with other neutrophils, sometimes forming chains of linked leukocytes. Adherent neutrophils projected cytoplasmic processes into the underlying endothelium, especially at points of endothelial thinning. To determine the pathway by which neutrophils transmigrated endothelium, we prepared 27 sets of serial electron microscopic sections. Eleven of these encompassed in their entirety openings through which individual neutrophils traversed venular endothelium; in 10 of the 11 sets, neutrophils followed an entirely transendothelial cell course unrelated to interendothelial junctions, findings that were confirmed by computer-assisted three-dimensional reconstructions. Having crossed endothelium, neutrophils often paused before crossing the basal lamina and underlying pericytes that they also commonly traversed by a transcellular pathway. Thus, in response to FMLP, neutrophils emigrated from cutaneous venules by a transcellular route through both endothelial cells and pericytes. It remains to be determined whether these results can be extended to other inflammatory cells or stimuli or to other vascular beds.
Among the earliest and most important events in acute inflammation is the emigration of neutrophils and other inflammatory cells from the blood into the tissues by way of postcapillary venules and small veins (1, 2). This process, which has been the subject of intense investigation for more than a century, has been resolved into successive phases of leukocyte margination, rolling, adhesion, and finally, transmigration across endothelium (3). In recent years, much has been learned about the molecular events responsible for neutrophil attachment to venular endothelium. It is now clear that chemical mediators generated during inflammation induce, redistribute, or increase the binding avidity of complementary “adhesion” molecules present on both endothelial cells (ECs)1 and inflammatory cells; many such adhesion molecules have been identified including L-, P- and E-selectins, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), platelet endothelial cell adhesion molecule 1 (PECAM-1), and several integrins (3). It is thought that members of the selectin family are largely responsible for the early, loose tethering of rolling neutrophils to endothelium, and that subsequent firm adhesion requires activation of the β2 (CD18) integrin family for binding to EC counterreceptors such as ICAM-1.
In contrast to the numerous studies of leukocyte rolling and adhesion, very little attention has been paid in recent years to the pathway by which adherent inflammatory cells cross endothelium. It is widely believed that this issue was settled definitively in the 1960s. At that time, several groups of electron microscopists described adhesion of inflammatory cells, particularly neutrophils but also other granulocytes and monocytes, to venular endothelium at sites of inflammation (4–9). They noted that adherent inflammatory cells extended pseudopod-like processes into the underlying ECs. In some instances, emigrating leukocytes appeared to be present in large EC vacuoles, apparently at a distance from the nearest interendothelial junction (7). This finding raised the possibility that leukocytes had been engulfed or phagocytosed by ECs and that neutrophils emigrated through endothelium rather than passing between adjacent ECs. However, interpretation was appropriately cautious because it was recognized that leukocytes that appeared distant from junctions in single images might nonetheless have extended into and extravasated through intercellular junctions that would only have become evident at deeper levels of sectioning.
It was agreed that serial electron microscopic sections would be needed to determine if neutrophils or other leukocytes exited venules by passing between or through ECs. However, preparation of numerous consecutive thin sections is a formidible task even with modern equipment and was especially difficult with the technology available in the early 1960s. Nonetheless, at least two groups attempted this approach. In studies of leukocyte emigration in inflamed skin, Hurley and Xeros (6) performed limited numbers of serial sections that showed “penetration is occurring through, or immediately adjacent to, a junction between endothelial cells”. In a second study Marchesi prepared what Hurley (2) described as “semi-serial” sections of neutrophils emigrating from mesenteric venules and reported that “in most of them passage through an intercellular junction could be demonstrated” (5). After the publication of these papers, opinion, which had up until that time been divided, crystallized around the position that leukocytes emigrated from venules by passing through open inter-EC junctions. Thus, Hurley wrote in 1983, “It appears almost certain that all neutrophils, eosinophils and monocytes escape in this way [between endothelial cells] but the work necessary to produce and examine serial sections of large numbers of emigrating leucocytes has prevented conclusive proof of this hypothesis” (2). Since that time, the consensus view has only rarely been challenged (10–13).
Over the past 35 yr, there have been substantial improvements in all aspects of electron microscope technology. For this reason and because our own studies led us to doubt the consensus model of leukocyte transmigration, we reinvestigated the pathway by which neutrophils emigrated across venular endothelium in the setting of acute inflammation. To this end, we made extensive use of serial electron microscopic sections accompanied by computerized three-dimensional reconstructions. We report here that in response to the bacterial chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (FMLP), neutrophils emigrate from inflamed venules primarily by a transendothelial pathway and that, after crossing the endothelial barrier, they often traverse underlying pericytes, also by a transcellular route.
Materials and Methods
Experimental Design.
Acute inflammatory lesions were induced in the flank skin of female Hartley guinea pigs (700–995 g; Elm Hill, Chelmsford, MA) with FMLP. Animals were anesthetized intramuscularly with 10 mg/kg ketamine and 20 mg/kg xylazine. Hair was clipped from the flanks and various concentrations of test and control substances were injected intradermally in a randomized pattern at 1–60 min before killing. FMLP (Sigma Chemical Co., St. Louis, MO) was dissolved in DMSO to provide a stock solution of 4.38 mg/ml (10−2 M); this was diluted in HBSS such that the 0.2-ml injection volumes contained 8.76 ng– 87.6 μg FMLP (10−7–10−3 M).
Most experiments were performed with 10−5 M FMLP, although no morphological evidence of cellular injury was detected with concentrations >10-fold higher. To determine the effective concentration of FMLP present in skin at various times after intradermal injection in a volume of 0.2 ml, we performed clearance studies with [3H]FMLP (NET563, 80 Ci/mmol; DuPont New England Nuclear Research Products, Boston, MA) diluted 400-fold in cold 10−5 M FMLP. The blebs raised at injection sites were outlined individually with a magic marker and squares of full-thickness skin corresponding to the initial bleb were harvested at time 0 and at intervals up to 2 h. Harvested skin was weighed, minced, dissolved in tissue solubilizer (Solvable; Packard Instrument Co., Inc., Meriden, CT), and diluted 10-fold in formula 989 liquid scintillation counting solution (Packard Instrument Co.) for beta counting. Clearance data were fit to a two component exponential decay curve (KaleidaGraph, v. 3.0.8; Synergy Software, Reading, PA). Based on these data, and assuming that 1 g of tissue corresponds to a volume of 1 ml, we calculated the actual concentration of FMLP present in skin to be as follows: time 0, 2.5 × 10−6 M; 10 min, 1 × 10−6 M; 60 min, 2.5 × 10−7 M.
Control sites were injected with HBSS alone or with HBSS supplemented with DMSO in concentrations corresponding to those present in FMLP injections. All animal protocols were approved by the Beth Israel Hospital Institutional Animal Care and Use Committee (Boston, MA).
Tissue Processing.
Animals were killed by C02 narcosis. Skin test and control sites were excised and fixed by immersion for 4 h in freshly prepared 2.0% paraformaldehyde–2.5% glutaraldehyde– 0.025% calcium chloride in 0.1 M sodium cacodylate buffer, pH 7.4. Tissues were postfixed for 2 h in 1.5% sym-collidine–buffered osmium tetroxide, stained en bloc with uranyl acetate, dehydrated in a graded series of alcohols, and embedded in Spurr resin as previously described (14, 15)
Alternatively, tissues were fixed by perfusion. Animals were anesthetized with ketamine and xylazine. The thoracic cavity and pericardium were opened and a blunt-tipped 19- gauge needle with attached PE-50 tubing was inserted into the apex of the left ventricle. After incising the right atrium to permit outflow, 250 ml (equivalent to approximately three blood volumes) of fixative was injected while maintaining normal systemic arterial pressure of 80–94 mm Hg. The entire procedure was complete within 15 min. Experimental and control skin sites were then excised and fixed for an additional 4 h in fixative before processing as above.
Preparation of Serial Thin Sections.
To follow the pathway of leukocyte diapedesis, we prepared multiple sets of consecutive serial electron microscopic sections. Venules that exhibited emigrating leukocytes were identified by light microscopy in 1 μm Giemsa-stained sections. Serial 100-nm-thick sections were then cut, collected on carbon-Formvar–coated single-slot grids, and viewed in an elecron microscope (CM10; Philips, Eindhoven, The Netherlands). Consecutive sections of individual vessels were photographed, generally at a magnification of 10,000.
Three-dimensional Reconstructions.
Membrane outlines of the endothelial openings through which neutrophils transmigrated as well as ECs and neutrophil plasma membranes were traced onto transparent plastic sheets. Consecutive images were then scanned into a Power Macintosh (6100/60) computer (Apple Computer Inc., Cupertino, CA) using a scanner (Scanjet 4c scanner; Hewlett-Packard Co., Palo Alto, CA) and Deskscan II 2.3 (Hewlett-Packard Co.) software. Color images were converted into 8-bit grayscale PICT files using Adobe Photoshop (version 3.0.4). Consecutive grayscale images were subsequently converted into hierarchical data format (HDF) image files (Transform PPC version 3.3.0; Fortner Research LLC, Sterling, VA). Finally, the HDF files representing the consecutive serial electron microscopic sections were reconstructed in three dimensions using software (Slicer v.1.1b3; Fortner) by volume rendering.
Morphometric and Statistical Analysis.
The extent of “wrinkling” of luminal and abluminal plasma membranes of venular endothelium was quantitated by calculating a folding index (16). Statistical analyses were performed using the nonparametric Kruskal-Wallis ANOVA test, Dunn's multiple comparisons test or the Mann-Whitney U test.
Results
In accord with many previous reports, circulating leukocytes, mostly neutrophils, marginated and became attached to the luminal surfaces of venular endothelium at skin sites injected with 10−5 –10−7 M FMLP (1, 2, 4–9). Both large venules/small veins (30–80 μm luminal diam) and smaller venules (10–30 μm diam) of the deep and superficial dermal vascular plexuses were involved (Figs. 1 and 2). Leukocyte margination and attachment first became evident at ∼15 min after FMLP injection and persisted through at least 60 min. Higher concentrations of FMLP increased the number of venules exhibiting leukocyte diapedesis and overall reaction intensity, but did not alter the kinetics; even at the highest concentrations tested leukocyte attachment was not detected before 10–15 min.
Figure 1.
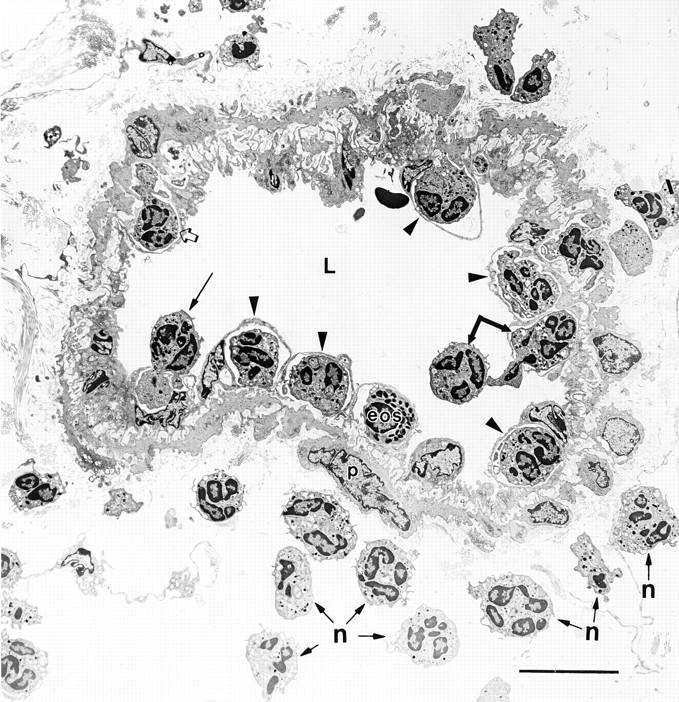
Large venule in guinea pig skin harvested 60 min after intradermal injection of 10−5 FMLP. Many neutrophils and a single eosinophil (eos) are captured at various stages of attachment to and extravasation across vascular endothelium and underlying pericytes (p). Two neutrophils (single joined arrow), one in the lumen and another partway across the endothelium, are tethered together. Another neutrophil (long arrow) has projected a cytoplasmic process into an underlying EC. Other neutrophils (arrowheads) and the eosinophil have crossed the EC barrier, but remain superficial to pericytes, forming dome-like structures that bulge into the vascular lumen. Still another neutrophil (open arrow) that has already crossed the endothelium has extended a process into the basal lamina and indents an underlying pericyte. Other neutrophils (some indicated by n) have crossed both the EC and pericyte barriers and have entered the surrounding connective tissues. L, lumen. Bar, 10 μm.
Figure 2.
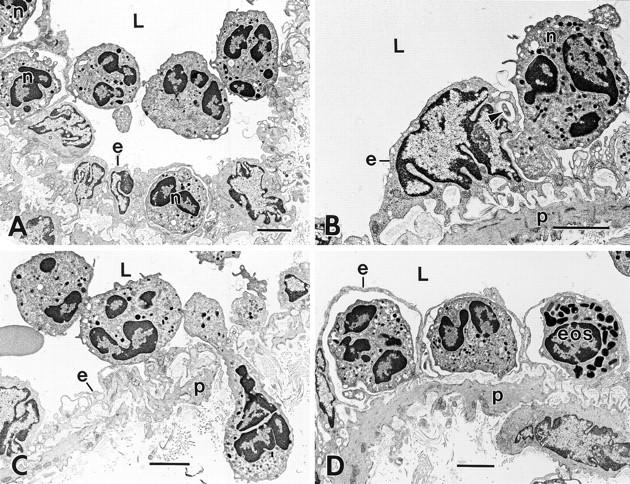
Progression of neutrophil migration across venular endothelium at 60 min after intradermal injection of 10−5 M FMLP. (A) Three neutrophils in the vascular lumen (L) are tethered together at point contacts; two of these also attach to the endothelium (e) at disparate sites. Three additional neutrophils (n) have already crossed the endothelial barrier. (B) An adherent neutrophil (n) projects two separate pseudopods into an EC (e). As confirmed in deeper serial sections, the smaller of these (arrowhead) projects into the EC at a point adjacent to the nucleus, whereas the larger (dominant) pseudopod forms a blunt projection into a thinned region of EC cytoplasm nearby. The EC shows extensive wrinkling of its abluminal surface, except over the nucleus, which is rounded with pinched folds and bulges into the lumen. (C) A chain of three interconnected neutrophils. One of these (elongate cell) has traversed both endothelium (e), basal lamina, and pericyte (p) layers and extends into the underlying connective tissue while its trailing edge remains within the vascular lumen. The second neutrophil has formed attachments to endothelium at two discontinuous sites and the third remains in the vascular lumen with no attachments to endothelium. (D) Higher magnification of a portion of Fig. 1, illustrating two neutrophils and an eosinophil (eos) that have crossed the endothelium but have not penetrated the pericyte (p) layer; each is covered over with a thin overlay of flattened, relatively smooth endothelium that, together with the leukocytes, form dome-like structures that bulge into the vascular lumen. L, lumen. Bar, 3 μm.
Alterations in Venular Endothelium at FMLP-injected Skin Sites.
Beginning as early as 5 min after injection of FMLP, the profile of venular endothelium changed significantly from a “coast of California” to a “coast of Maine” contour (Table 1). Both the luminal and abluminal plasma membranes were thrown into thin folds that projected for varying distances into the vascular lumen and abluminally into the underlying basal lamina (Figs. 1 and 2). Surface wrinkling developed before leukocyte adhesion and persisted through the latest times studied (i.e., 60 min). However, portions of ECs in immediate contact with attached or transmigrating leukocytes generally exhibited less or no wrinkling (Table 1). In addition, EC nuclei became rounded with accentuated nuclear folds and projected into the vascular lumen beneath a thin covering of cytoplasm. Finally, EC thickness became less uniform after FMLP injection; mean EC thickness changed little, but there was a significant decrease in median cell thickness and a significant increase in the range of thickness (Table 2). As a result, zones of extreme EC thinning alternated with zones of increased thickness, the latter being especially prominent over rounded nuclei that projected into the vascular lumen. Changes were similar in tissues that were fixed by immersion or perfusion.
Table 1.
Folding Index as a Measure of Venule EC Membrane Wrinkling after Intradermal Injection of FMLP or HBSS
| Chemoattractant | Folding index | |||
|---|---|---|---|---|
| LM/d | AbLM/d | |||
| HBSS ± DMSO (n = 48) | 1.19 ± 0.02 | 1.17 ± 0.01 | ||
| FMLP, 10 min (n = 15) | 2.39 ± 0.15‡ | 1.77 ± 0.09‡ | ||
| FMLP, 15–60 min | ||||
| Membranes with attached | ||||
| neutrophils (n = 16) | 1.16 ± 0.02 | 1.16 ± 0.02 | ||
| Membranes without attached | ||||
| neutrophils (n = 16) | 2.41 ± 0.25‡ | 2.96 ± 0.17‡ | ||
To calculate the folding index, multiple segments of luminal (LM) and (AbLM) membrane perimeters were traced and these actual lengths (nm) were divided by the lengths (nm) of smoothed lines (d) connecting the same points. A completely smooth surface has a ratio of 1.0, whereas values >1.0 measure the extent of membrane wrinkling. There was no difference between HBSS injection sites with or without added DMSO and so these data were pooled.
Ratios significantly different from HBSS control (P <0.001) as calculated using Kruskal-Wallis ANOVA and Dunn's multiple comparison test.
Table 2.
Variation in Thickness of Venule EC in Guinea Pig Skin Measured 60 min after Intradermal Injection of 10− 5 M FMLP or HBSS
| Endothelial cell thickness* | Intradermal injection | |||
|---|---|---|---|---|
| FMLP | HBSS | |||
| μm | μm | |||
| Mean ± SE | 1.10 ± 0.16 | 0.97 ± 0.05 | ||
| Median | 0.32 | 0.88 | ||
| Range | 0.11–8.26 | 0.33–2.60 | ||
EC cross-sectional thickness was measured in multiple randomly selected sites in venules that were cut at right angles to the direction of blood flow. For FMLP injection sites, n = 106 and for HBSS control sites, n = 87. Mean EC thickness at FMLP- or HBSS-injected sites was not significantly different; however, the difference in medians was highly significant (P <0.0001, Mann-Whitney test) and the range of EC thickness at FMLP-injected sites was greatly increased.
Leukocyte Adhesion and Diapedesis.
Initial contacts between circulating neutrophils and venular endothelium were focal and often multiple with intervening skip areas (Fig. 1 and Fig. 2, A and C). Neutrophils also developed contacts with other neutrophils, forming chains of tethered cells; often more than one of these was in contact with vascular endothelium (Fig. 1 and Fig. 2, A and C).
In agreement with earlier descriptions (4–6, 8), adherent neutrophils extended one or more cytoplasmic processes into the underlying endothelium (Fig. 2 B). These initial projections showed no particular relation to inter-EC junctions, which remained normally closed, or to zones of cytoplasm that were thick or thin. However, projections that became dominant and that led to neutrophil transmigration generally involved zones of thinned endothelium.
Having breached the endothelium, neutrophils sometimes proceeded to cross the underlying basal lamina immediately, and in such instances, it was possible to observe a single neutrophil whose leading front had advanced into the interstitium before its trailing edge had left the vascular lumen (Fig. 2 C). More commonly, however, neutrophils that had crossed the endothelial barrier were held up for a time at the level of the basal lamina. Such neutrophils were covered with a thin rim of overlying EC cytoplasm and formed dome-like structures that bulged into the vascular lumen (Fig. 1, and Fig. 2 D). At sites of neutrophil–EC contact, both the luminal and abluminal EC surfaces lost their wrinkled appearance and exhibited relatively smooth contours (Fig. 2, A–D; Table 1).
Neutrophil attachment and transmigration did not noticeably impair the patency of larger venules and small veins (Figs. 1 and 2). However, in smaller venules, marginating and transmigrating neutrophils sometimes accumulated in such numbers that their lumens were compromised with resulting vessel distension and a relative smoothing of EC contours (not shown).
Pathway of Neutrophil Transmigration across Endothelium as Determined by Serial Thin Sections and Three-dimensional Reconstructions.
To ascertain the pathway of neutrophil extravasation across venular endothelium, we analyzed 27 sets of serial sections prepared from skin sites injected with 10−5 M FMLP (effective starting concentration in skin was 2.5 × 10−6 M) at intervals of 15, 30, or 60 min (Table 3). In 11 of these sets, serial sections encompassed the entire pore through which individual neutrophils emigrated, and included uninvolved surrounding endothelium on all sides.
Table 3.
Sets of Serial Thin Sections Performed on Guinea Pig Skin that Encompassed the Pores through which Neutrophils Emigrated across Venular Endothelium or Pericytes in Response to Locally Injected FMLP
| Venular endothelium | Pericytes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Set | Time after FMLP | W/P | No. serial sections | Set | Time after FMLP | W/P | No. serial sections | |||||||
| min | min | |||||||||||||
| 1 | 60 | W | 83 | |||||||||||
| 2 | 60 | W | 65 | 1 | 60 | W | 25 | |||||||
| 3 | 60 | W | 45 | 2* | 60 | W | 27 | |||||||
| 4 | 60 | W | 95 | 3* | 60 | W | 57 | |||||||
| 5 | 60 | W | 53 | 4 | 60 | W | 90 | |||||||
| 6 | 60 | W | 74 | 5 | 60 | W | 35 | |||||||
| 7 | 60 | W | 33 | 6 | 60 | W | 33 | |||||||
| 8 | 60 | W | 45 | 7 | 60 | W | 46 | |||||||
| 9 | 60 | W | 7 | 8 | 60 | W | 40 | |||||||
| 10 | 60 | W | 35 | 9* | 60 | W | 55 | |||||||
| 11* | 60 | W | 26 | 10* | 60 | W | 77 | |||||||
| 12 | 15 | P | 15 | 11 | 60 | W | 75 | |||||||
| 13 | 15 | P | 7 | 12 | 60 | W | 49 | |||||||
| 14 | 15 | P | 41 | 13 | 60 | P | 45 | |||||||
| 15 | 15 | P | 32 | 14 | 60 | P | 85 | |||||||
| 16 | 15 | P | 16 | 15 | 60 | P | 31 | |||||||
| 17 | 15 | P | 42 | 16 | 60 | P | 32 | |||||||
| 18 | 15 | P | 41 | 17 | 60 | P | 26 | |||||||
| 19 | 15 | P | 8 | 18 | 60 | P | 29 | |||||||
| 20 | 15 | P | 29 | 19 | 60 | P | 33 | |||||||
| 21 | 15 | P | 41 | 20 | 60 | P | 38 | |||||||
| 22 | 15 | P | 10 | |||||||||||
| 23 | 30 | P | 44 | |||||||||||
| 24 | 30 | P | 12 | |||||||||||
| 25 | 30 | P | 23 | |||||||||||
| 26 | 60 | P | 45 | |||||||||||
| 27 | 60 | P | 38 | |||||||||||
W, whole; P, part.
Indicates that interpretation was not definitive as to whether neutrophil passed through endothelium or pericyte entirely by a transcellular route.
Representative serial sections illustrating two such pores are illustrated in Fig. 3–5, along with a three-dimensional reconstruction of one. (Journal space limitations precluded additional illustrations of serial sections and reconstructions, which are available from the authors.) These demonstrated definitively that at least 10 of the 11 openings were trans-EC pores, not inter-EC gaps. Certain properties of these 10 pores are summarized in Table 4. In none of the 10 did the pore involve or abut upon an inter-EC junction. In only one case (No.11 in Table 3) did a pore extend sufficiently close to an intercellular junction that we could not determine whether it actually extended into that junction.
Figure 3.
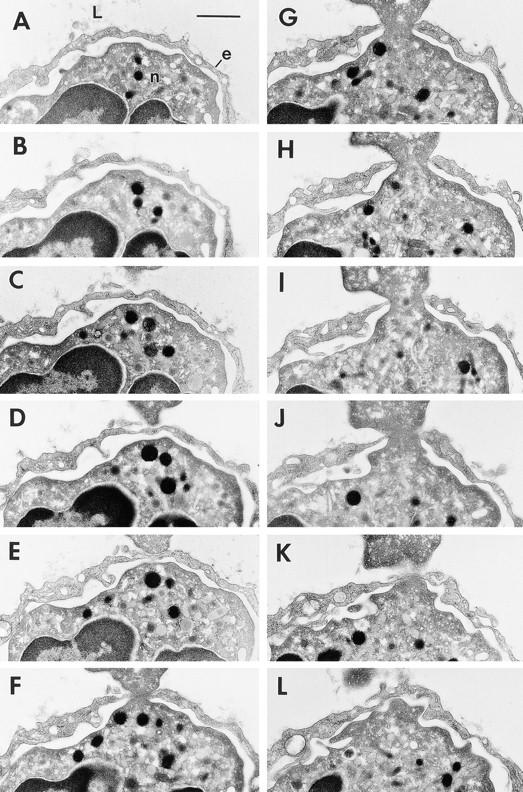
Transmigration of a neutrophil (n) across a thinned portion of venular endothelium (e) at a skin site injected 60 min earlier with 10−5 M FMLP. 12 of a series of 45 consecutive serial sections (sections 10–19 and 21–22) are illustrated and together encompass in its entirety the transendothelial pore (F–J) through which the neutrophil is migrating. Maximum pore diameter was 0.75 μm. L, lumen. Bar, 1 μm.
Table 4.
Some Properties of the 10 Definitive Trans-EC Pores through which Neutrophils Migrated in Response to FMLP
| Properties of pores | Range | Mean ± SE | Median | |||
|---|---|---|---|---|---|---|
| μm | μm | μm | ||||
| Distance, pore margin to | ||||||
| nearest EC junction | 0.2 –3.6 | 2.2 ± 0.4 | 2.5 | |||
| Maximum pore diameter | 0.2–2.9 | 1.2 ± 0.3 | 1.1 | |||
| EC thickness immediately | ||||||
| adjacent to the pore | 0.11–0.35 | 0.17 ± 0.02 | 0.15 |
In 16 sets (Table 3), serial sections began at a point somewhere within the pore and extended distally to include endothelium beyond the pore, thereby sampling large portions of an EC opening, but not encompassing it completely. In none of these sets did the pore involve or abut on an inter-EC junction.
Neutrophil Passage through Pericytes Underlying Venular Endothelium.
Pericytes provide an extensive, though incomplete, covering around venular ECs, which emigrating neutrophils commonly encountered after traversing venular endothelium and basal lamina (17). Unlike venular ECs, pericytes showed no evidence of plasma membrane wrinkling or other changes in contour in response to FMLP. Nonetheless, neutrophils passed through pericytes in a manner analogous to their passage across vascular ECs by extending processes into and eventually through the pericyte cytoplasm (Figs. 6 and 7). Pericyte cytoplasm adjacent to sites of neutrophil penetration demonstrated striking cytoskeletal organization such that microfilaments were oriented in a direction perpendicular to the invaginating neutrophil process. Serial sections confirmed these findings. Of 20 sets of serial sections studied, 12 entirely encompassed the pore through which neutrophils crossed pericytes (Table 3) and in at least 8 of these, 12 neutrophils traversed pericytes by a transcellular route.
Figure 6.
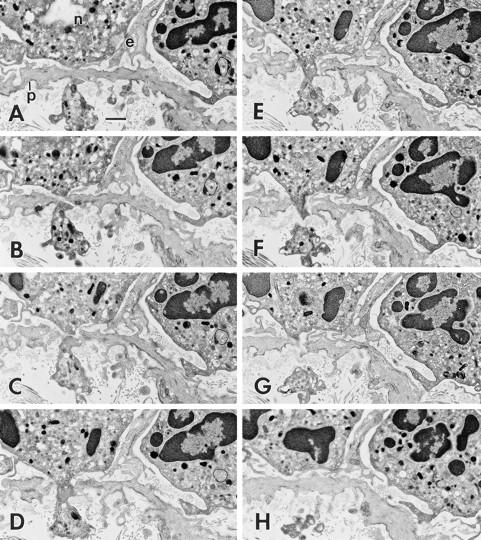
Neutrophil (n) that has already crossed endothelium (e) is at an early stage of transmigration through a subendothelial pericyte (p) 60 min after intradermal injection of 10−5 M FMLP. 8 of a series of 90 consecutive serial sections (sections 60–66 and 68) are illustrated. B–G illustrate the transpericyte pore through which the neutrophil has advanced a cytoplasmic projection. Maximum pore diameter was 0.83 μm. Bar, 1 μm.
Figure 7.
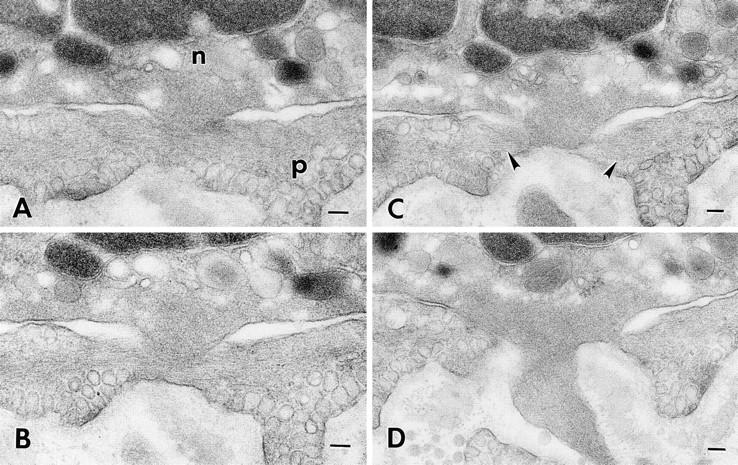
Neutrophil (n) migrating through a subendothelial pericyte (p) 60 min after intradermal injection of 10−5 M FMLP. Four (sections 12–14 and 16) of a series of 46 consecutive serial sections illustrate a transcytoplasmic pore in the pericyte through which the neutrophil has extended a blunt cytoplasmic projection. The projecting neutrophil pseudopod is filled with a feltwork of microfilaments. Note also the prominent, horizontally arrayed microfilaments in the immediately adjacent pericyte cytoplasm (C, arrowheads). Bar, 0.1 μm.
Discussion
The data presented here and summarized diagramatically in Fig. 8 provide strong evidence that in response to FMLP, neutrophils extravasate from dermal venules predominantly by a trans-EC route rather than by emigrating through inter-EC junctions. These data challenge the long-dominant paradigm that neutrophils emigrate from venules exclusively by passing between EC, i.e., through EC junctions. It remains to be determined whether these results can be extended to other inflammatory cells, to other inflammatory stimuli, or to other vascular beds. Nevertheless, our findings establish the important principle that leukocytes can extravasate by a trans-EC route. Our data also imply that venular ECs are joined together by junctions whose attachments are unexpectedly durable and remain intact, even in acute inflammation. This is consistent with studies reporting that openings in vascular endothelium induced by vasoactive agents, cytokines, or by supraphysiological intraluminal pressure are frequently trans-EC rather than inter-EC (18, 19).
Figure 8.
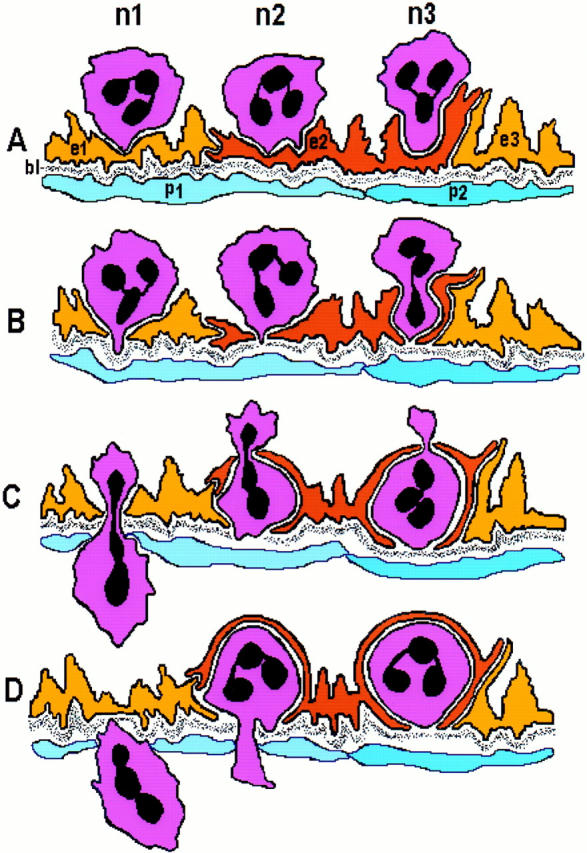
Schematic diagram summarizing different aspects of neutrophil diapedesis in response to FMLP. In A, neutrophil n1 extends a cytoplasmic process that forms contact with a thinned portion of EC e1 as in Fig. 2, A and B; in B–D n1 has progressed through e1, the underlying basal lamina (bl) and a pericyte (p1). This pattern of near-simultaneous trans-migration through endothelium as well as other components of the vascular wall is illustrated in Fig. 2 C, but was less common than that exhibited by neutrophils n2 and n3 that, after crossing the EC barrier, paused for a time before progressing farther. In A, n2 initially makes contact with the endothelial surface with two processes, as in Fig. 2 B; one of these becomes dominant and leads the way as the neutrophil begins to traverse EC e2 in B, again as in Fig. 2 B. Having crossed the endothelial cell barrier, n2 does not immediately progress through the basal lamina; instead it remains covered over for a time (C and D) by a thin overlying rim of flattened EC cytoplasm (as in Fig. 1, arrowheads) and Fig. 2 D) before extending a pseudopod through the basal lamina and underlying pericyte in D. As it progresses through e2 (C and D), it is embraced by cytoplasmic processes that extend from abluminal portions of both e2 and an adjacent EC, e1 (as in Fig. 5). Transmigration of n3 is similar to that of n1 and n2, except that n3 persists for a longer time in the subendothelial space superficial to the basal lamina. n3 in A corresponds to the neutrophil marked by a long arrow in Fig. 1; n3 in C and D models neutrophils illustrated in Fig. 1, arrowheads and Fig. 2 A and D. Extravasation of all three neutrophils is transendothelial and inter-EC junctions remain closed.
Having traversed the endothelium, some neutrophils proceeded to cross the underlying basal lamina immediately. More commonly, however, neutrophils paused for a time between endothelium and basal lamina before proceeding farther. After crossing the basal lamina, extravasating neutrophils encountered pericytes that they frequently crossed by a transcellular route. This finding is remarkable in that pericytes do not provide a continuous covering around venules (17). Presumably, therefore, neutrophils could have migrated around pericytes; that they instead commonly migrated through pericytes affirms their proclivity for transcellular migration.
It is possible that differences in experimental design contributed to the differences between our results and those of investigators who formulated the consensus paradigm. Our work was performed in a single species (guinea pig), in a single tissue (skin), with a single inflammatory agent (FMLP), and applies primarily to a single type of inflammatory cell, the neutrophil (a single eosinophil was also encompassed in our serial sections; Fig. 1, and Fig. 2 D). In contrast, the reports emanating from the early 1960s that proposed an inter-EC route of inflammatory cell transmigration were performed in the rat (mesentery, skin), rabbit (ear skin, mesentery), or dog (pancreas) and used different agents (mechanical trauma, homologous serum, UV light, ligation of pancreas, heat, nitrogen mustard, or turpentine) to induce leukocyte diapedesis (4–9). The possibility remains, therefore, that neutrophils traverse venular endothelium by different routes in different species, in different tissues, and in response to different inciting agents. On the other hand, there is support in these early studies for the mechanism we have proposed in that all of the authors cited reported that neutrophils projected pseudopod-like processes into ECs (4–9). More recently, studies of monocyte and neutrophil chemotaxis across an endothelial cell monolayer have added support for a trans-EC pathway for inflammatory cell migration. Thus, Migliorisi et al. (20) reported that both adherent monocytes and neutrophils frequently extended pseudopods into the apical plasma membranes of ECs and only rarely projected such processes into the junctional region between cells.
Other data presented here also have implications for the pathogenesis of acute inflammation. Among these is the finding that FMLP induced wrinkling of the EC plasma membrane as early as 5 min after injection and therefore before leukocyte attachment (Table 1). These results are consistent with studies from a number of different laboratories, indicating that ECs as well as other noninflammatory cells, express functional FMLP receptors that, depending on context, may be stimulated by FMLP to induce cell contraction, relaxation, and migration (21–23). Membrane wrinkling was accompanied by increased variation in EC thickness with areas of extreme thinning alternating with areas of increased thickness. Focal EC thinning may have facilitated leukocyte emigration by shortening the transmigration pathway.
Another finding of interest is the observation that emigrating neutrophils formed attachments not only to venular endothelium, but also to other neutrophils. These contacts were frequently maintained as neutrophils crossed endothelium in tandem (Figs. 1, 2). Consistent with this finding, Alon et al. (24) recently reported that in an in vitro system, neutrophils and other leukocytes formed “strings of rolling cells” that were linked together by L-selectin, and that such bridging favored cell attachment to endothelium. The linked chains of neutrophils observed in our study may have resulted from L-selectin bridges.
Finally, our findings have implications for an understanding of the molecular mechanisms that underlie leukocyte emigration from venules. Holding to the long-standing consensus paradigm of leukocyte diapedesis, most investigators have presumed that leukocytes crossed endothelium through opened EC junctions. Thus, they have postulated mechanisms that might be expected to cause EC junctions to open, e.g., leukocyte proteases that cleave cell adhesion molecules, mechanical forces exerted by leukocytes on EC junctions, and signaling events that cause EC to pull apart. If, however, neutrophils exit venules by passing through, rather than between, ECs, very different mechanisms may be imagined. These might include rearrangements of cell cytoskeleton that allow neutrophils to thrust processes into and through ECs and pericytes as well as molecular events that generate and reseal pores in ECs and pericyte plasma membranes.
Figure 5.
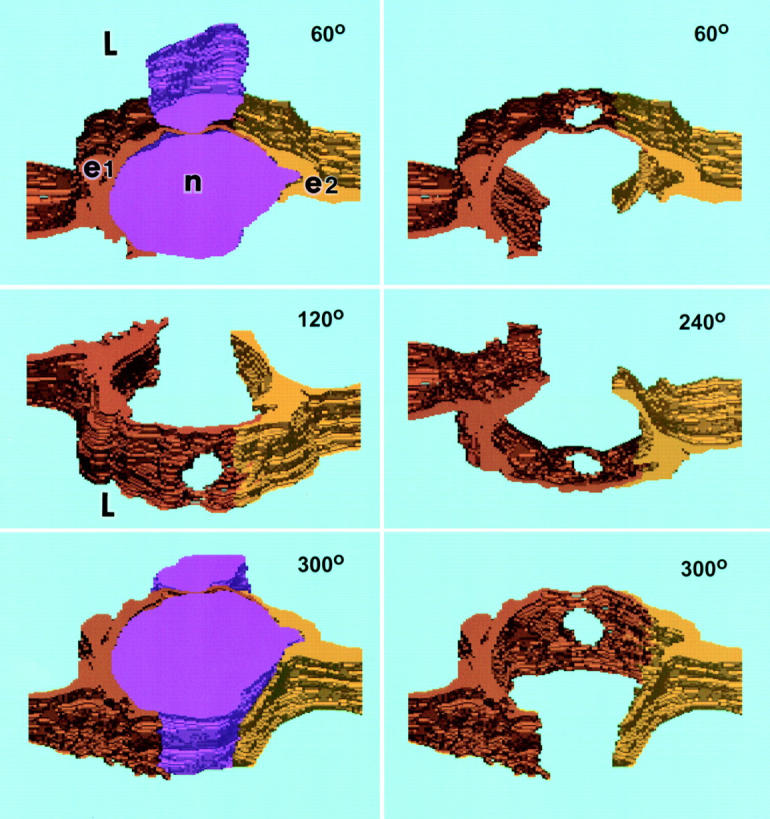
Computer-generated three-dimensional reconstruction of the transmigrating neutrophil illustrated in Fig. 4. The panels portray successive rotations toward the viewer around a horizontal axis at angles of 60°, 120°, 240°, and 300° as indicated. 0° (not shown) would represent a vascular cross section at right angles to the direction of blood flow and 90° (also not shown) would represent a view looking directly down on the luminal surface. Emigrating neutrophil (n), purple, upper and lower left; in the other panels, the neutrophil was subtracted electronically to visualize the pore that passes cleanly through the cytoplasm of EC e1 (orange-brown) distinctly apart from the junction of e1 with e2 (yellow). Cytoplasmic arms of both e1 and e2 embrace the neutrophil luminally and, to a lesser extent, abluminally. L, lumen.
Figure 4.
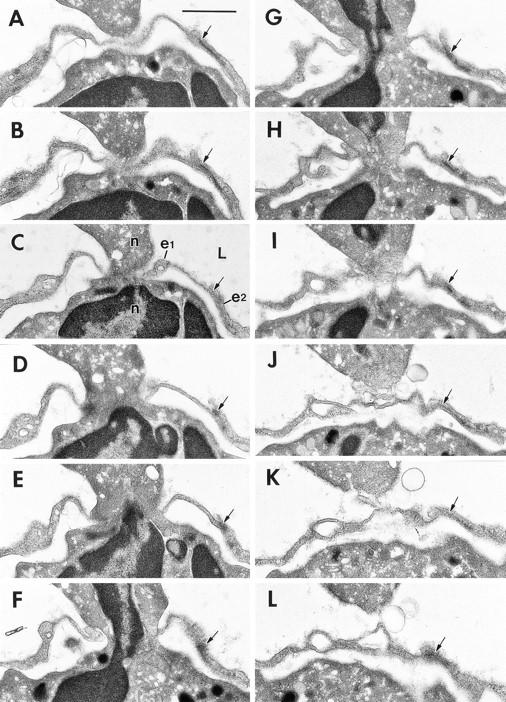
Transmigration of a neutrophil (n) across a thinned portion of venular endothelium at 60 min. after local intradermal injection of 10−5 M FMLP. 12 sections (numbers 13, 14, 16, 19, 21, 24, 25, 27, 29, and 30–32) of a series of 74 consecutive serial sections are illustrated. Portions of the neutrophil's nucleus (E–G) are included within the pore. The pore passes through a single EC (e1), but a junction of e1 with a second EC, e2, is indicated (arrow); this junction is intact and maintained a distance of >1 μm from the pore margin at all levels of sectioning. L, lumen. Bar, 1 μm.
Acknowledgments
This work was supported by United States Public Health Service National Institutes of Health grants CA50453, CA58845, HL54465, and AI33372 and by salary support from the Beth Israel Hospital Pathology Foundation, Inc.
Footnotes
Abbreviations used in this paper: EC, endothelial cell; FMLP, N-formyl-methionyl-leucyl-phenylalanine.
References
- 1.Grant, L. 1973. The sticking and emigration of white blood cells in inflammation. In The Inflammatory Process. Vol. II. B. Zweifach, L. Grant, and R. McCluskey, editors. Academic Press, New York. 205–249.
- 2.Hurley, J. 1983. Acute Inflammation. Churchill Livingstone, Edinburgh. 157pp.
- 3.Muller W. Migration of leukocytes across the vascular intima. Molecules and mechanisms. Trends Cardiovasc Med. 1995;5:15–20. doi: 10.1016/1050-1738(94)00028-T. [DOI] [PubMed] [Google Scholar]
- 4.Marchesi V, Florey H. Electron microscopic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- 5.Marchesi V. The site of leucocyte emigration during inflammation. Q J Exp Physiol Cogn Med Sci. 1961;46:115–118. doi: 10.1113/expphysiol.1961.sp001522. [DOI] [PubMed] [Google Scholar]
- 6.Hurley J, Xeros N. Electron microscopic observations on the emigration of leucocytes. Austral J Exp Biol. 1961;39:609–624. doi: 10.1038/icb.1961.60. [DOI] [PubMed] [Google Scholar]
- 7.Williamson J, Grisham J. Electron microscopy of leukocytic margination and emigration in acute inflammation in dog pancreas. Am J Pathol. 1961;39:239–256. [PMC free article] [PubMed] [Google Scholar]
- 8.Florey H, Grant L. Leucocyte migration from small blood vessels stimulated with ultraviolet light: an electron-microscopic study. J Pathaol Bacteriol. 1961;82:13–17. doi: 10.1002/path.1700820103. [DOI] [PubMed] [Google Scholar]
- 9.Movat H, Fernando N. Acute inflammation. The earliest fine structural changes at the blood–tissue barrier. Lab Invest. 1963;12:895–910. [PubMed] [Google Scholar]
- 10.Welsch U, Caesar R. Transendotheliale granulocytenemigration in der zunge des frosches bei der entzündung. Beitr Pathol Anat Allg Pathol. 1967;135:235–249. [PubMed] [Google Scholar]
- 11.Hammersen F, Hammersen E. The ultrastructure of endothelial gap-formation and leukocyte emigration. Prog Appl Microcirc. 1987;12:1–34. [Google Scholar]
- 12.Walker D, Chu F, MacKenzie A. Pathway of leukocyte emigration from the pulmonary vasculature during pneumonia in rabbits. Am Rev Respir Dis. 1994;143:A541. [Google Scholar]
- 13.Bamforth S, Lightman S, Greenwood J. Ultrastructural analysis of interleukin-1β–induced leukocyte recruitment to the rat retina. Investig Ophthalmol Vis Sci. 1997;38:25–35. [PubMed] [Google Scholar]
- 14.Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukocyte Biol. 1996;59:100–115. [PubMed] [Google Scholar]
- 15.Feng D, Nagy J, Hipp J, Dvorak H, Dvorak A. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine and serotonin. J Exp Med. 1996;183:1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elias H, Hyde D. An elementary introduction to stereology (quantitative microscopy) Am J Anat. 1980;159:411–446. doi: 10.1002/aja.1001590407. [DOI] [PubMed] [Google Scholar]
- 17.Braverman I, Sibley J, Keh-Yen A. A study of the veil cells around normal, diabetic, and aged cutaneous microvessels. J Invest Dermatol. 1986;86:57–62. doi: 10.1111/1523-1747.ep12283816. [DOI] [PubMed] [Google Scholar]
- 18.Neal C, Michel C. Openings in frog microvascular endothelium induced by high intravascular pressure. J Physiol (Lond) 1996;492:39–52. doi: 10.1113/jphysiol.1996.sp021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng D, Nagy J, Hipp J, Pyne K, Dvorak H, Dvorak A. Reinterpretation of endothelial cell gaps induced by vasoactive mediators: many are transcellular pores. J Physiol (Lond) 1997;504:747–761. doi: 10.1111/j.1469-7793.1997.747bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migliorisi G, Folkes E, Pawlowski N, Cramer EB. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and f-Met-Leu-Phe. Am J Pathol. 1987;127:157–167. [PMC free article] [PubMed] [Google Scholar]
- 21.Crowell RE, Van Epps DE, Reed WP. Responses of isolated pulmonary arteries to synthetic peptide F-Met-Leu-Phe. Am J Physiol. 1989;257:H107–112. doi: 10.1152/ajpheart.1989.257.1.H107. [DOI] [PubMed] [Google Scholar]
- 22.Dunkel CG, Saffitz JE, Evers AS. f-Met-Leu-Phe receptor expression by an interstitial cell in rabbit right atrium following left ventricular myocardial infarction. Circ Res. 1989;65:215–223. doi: 10.1161/01.res.65.1.215. [DOI] [PubMed] [Google Scholar]
- 23.Keitoku M, Kohzuki M, Katoh H, Funakoshi M, Suzuki S, Takeuchi M, Karibe A, Horiguchi S, Watanabe J, Satoh S, et al. FMLP actions and its binding sites in isolated human coronary arteries. J Mol Cell Cardiol. 1997;29:881–894. doi: 10.1006/jmcc.1996.0291. [DOI] [PubMed] [Google Scholar]
- 24.Alon R, Fuhlbrigge RC, Finger EB, Springer TA. Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J Cell Biol. 1996;135:849–865. doi: 10.1083/jcb.135.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]


