Abstract
Lymphotoxin (LT)α is expressed by activated T cells, especially CD4+ T helper type 1 cells, and by activated B and natural killer cells, but the functions of this molecule in vivo are incompletely defined. We have previously shown that follicular dendritic cell (FDC) clusters and germinal centers (GCs) are absent from the peripheral lymphoid tissues of LTα-deficient (LTα−/−) mice. LTα−/− mice produce high levels of antigen-specific immunoglobulin (Ig)M, but very low levels of IgG after immunization with sheep red blood cells. We show here that LTα-expressing B cells are essential for the recovery of primary, secondary, and memory humoral immune responses in LTα−/− mice. It is not necessary for T cells to express LTα to support these immune functions. Importantly, LTα-expressing B cells alone are essential and sufficient for the formation of FDC clusters. Once these clusters are formed by LTα-expressing B cells, then LTα-deficient T cells can interact with B cells to generate GCs and productive class-switched antibody responses. Thus, B cells themselves provide an essential signal that induces and maintains the lymphoid microenvironment essential for GC formation and class-switched Ig responses.
Lymphotoxin (LT)1α exists in two molecular forms, a soluble homotrimer that is structurally related to soluble TNF and that can bind and activate the two recognized TNF receptors TNFR-I and TNFR-II (1, 2), and a membrane-bound heterotrimer in association with the structurally related LTβ chain with stoichiometry LTα1LTβ2 (3, 4). This ligand interacts with the TNFR-related protein (TNFR-rp, also known as the LTβ receptor; references 4, 5), a receptor that is expressed widely on nonlymphoid cells. Several of the ligands and receptors of the TNF/LT family contribute to the formation of normal lymphoid tissue structure. For example, LTα (6–8), LTβ (9), and TNFR-I (10) are each required for the formation of normal Peyer's patch structure, and all of these plus TNF (11) are required for the formation of clusters of follicular dendritic cells (FDC) within the primary and secondary follicles of the spleen white pulp. In mice deficient in these cytokines or receptors, there was failure to form mature isotype-switched Ig responses after immunization with T cell–dependent antigens (such as sheep RBCs [SRBCs] or keyhole limpet hemocyanin) administered without adjuvants (7, 11–13). These results, then, suggest that both TNF- and LT-expressing cells may interact with FDCs or FDC precursors to support their development into organized clusters within primary spleen follicles. Bone marrow (BM)-derived LTα-expressing cells are able to restore the formation of clusters of FDCs after they are transferred into LTα-deficient (LTα−/−) mice (13). These restored FDC clusters can then support the formation of germinal centers (GCs) and mature isotype-switched Ig responses when the reconstituted mice are immunized with SRBCs and other antigens. Thus, LTα produced by BM-derived cells is essential for the formation of clusters of FDCs, which in turn contributes a permissive environment for the development of mature B cell responses.
Our initial experiments indicated that when wild-type BM cells were transferred into LTα−/− recipients, FDC clusters formed in the spleen white pulp between 2 and 4 wk after cell transfer, and effective IgG responses could be detected after that. In contrast, when reconstitution of LTα−/− mice was with mature wild-type spleen cells, no detectable FDC clusters or IgG to SRBCs could be identified 10 d after cell transfer and immunization with SRBCs (13). This suggested that several weeks are required for LTα-expressing BM-derived cells to restore the microenvironment, including the formation of FDC clusters, for effective IgG responses. With this in mind, it is likely that short-term reconstitution experiments in which LTα−/− mice receive either mixed splenocytes or purified lymphocyte subsets will not be suitable for analysis of the cellular elements that control the formation of functional FDC clusters.
It is known that both T and B cells are required for the generation of GCs and for the production of class-switched antibodies in response to T-dependent antigens. Prior studies of nude mice using partially purified preparations of mature T cells have shown that only a small fraction of T cells are required to cooperate with B cells in the formation of GCs and an isotype-switched IgG response (14, 15). To eliminate problems with contamination of one lymphocyte lineage with another, we elected to define the requirement for LTα-expressing cell lineages by transfer of BM from donor animals with genetic ablation of individual cell lineages rather than exclusively by transfer of purified populations of splenic LTα-expressing lymphoid cell subsets. We show here that LTα-expressing B cells are essential for the recovery of primary, secondary, and memory humoral immune responses in LTα−/− mice. Once proper FDC clusters are formed by B cells expressing LTα, then LTα-deficient T cells can interact with B cells to generate productive GCs and class-switched antibody responses.
Materials and Methods
Mice.
C57BL/6J, 129Sv, recombination activating gene (RAG)- 1−/− (catalog No. JR2216; C57BL/6J-Rag1tm1Mom), B cell receptor (BCR)−/− (catalog No. JR2288; C57BL/6J-Igh-6tm1Cgn), and TCR−/− (catalog No. JR2122; C57BL/6J-Tcrbtm1Mom Tcrdtm1Mom) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). LTα−/− mice (6) were maintained on a mixed 129Sv × C57BL/6 background and were bred under specific pathogen-free conditions.
Measurement of antigen-specific Ig.
Specific antibodies were measured and analyzed as previously described (16). In brief, Immulon 4 plates (Dynatech Laboratories, Inc., Chantilly, VA) were coated with SRBCs (150 μl at 5 × 107/ml) suspended in 0.25% glutaraldehyde in PBS. Diluted mouse sera were then added and incubated at 4°C for 1 h. Alkaline phosphatase–conjugated goat anti–mouse isotype-specific antisera (Southern Biotechnology Assoc., Birmingham, AL) were diluted 1:500 for IgM and 1:2,000 for total IgG or IgG subclasses, and 100 μl were added and incubated at 4°C for 1 h, followed by washing and addition of the alkaline phosphatase substrate p-nitrophenyl phosphate (Sigma Chemical Co., St. Louis, MO) at 1 mg/ml. The mean OD at 405 nm from triplicate wells was compared to a standard curve of titrated serum to calculate the relative units using linear regression analysis. The results represent mean ± SEM.
Transfer of Lymphocytes.
Whole spleen cell suspensions were prepared from single mouse donor by mincing with scissors and teasing the spleen between two frosted microscope slides. T and B cells were enriched using a nylon wool column as described (17). B cells were further purified by treatment with anti-Thy1.2 plus complement as previously described (18). These preparations contained 92–95% B cells, defined as IgM+B220+ by flow cytometry. There were no detectable contaminating T cells. For T cell reconstitution, preparations were >80% T cells, and contained <10% B cells. The contaminating B cells are unlikely to be significant because the recipient TCR−/− mice contain large numbers of endogenous B cells. Where indicated, recipients were prepared by irradiation with 750 rads 3 h before cell transfer. Where indicated, SRBCs were mixed together with the spleen cell suspensions before intravenous injection. Each recipient received 107 purified cells.
BM Transplantation.
Bone marrow was harvested and recipients were prepared as described previously (13, 18). Recipient mice were lethally irradiated with 1,050 rads and reconstituted with 5 × 106 donor BM cells. 6 wk after transplantation, recipients were immunized intraperitoneally with 108 SRBCs, and serum samples were collected 10 d after primary or secondary immunization.
Evaluation of Spleen Follicle Structure.
Spleens were harvested, embedded in O.C.T. compound (Miles, Elkhart, IN), and frozen in liquid nitrogen. Frozen sections (6–10 μm thick) were fixed in cold acetone. Endogenous peroxidase was quenched with 0.2% H2O2 in methanol. After washing, the sections were stained by first incubating with FITC-conjugated B220 and biotinylated Thy1.2 (PharMingen, San Diego, CA), biotinylated anti–complement receptor (anti-CR)1 (8C12; PharMingen), or biotinylated peanut agglutinin (PNA; Vector, Burlingame, CA), all at 1:50–1:100 dilutions. Horseradish peroxidase–conjugated rabbit anti-FITC (diluted 1:20; Dako, Glostrup, Denmark) and alkaline phosphatase–conjugated streptavidin (diluted 1:20; Zymed, South San Francisco, CA) was added 1 h later. Color development for bound alkaline phosphatase and horseradish peroxidase was with an alkaline phosphatase reaction kit (Vector) and with diaminobenzidine.
Results and Discussion
LTα-expressing B Cells, but Not LTα-expressing T Cells Are Required for Primary, Secondary, and Memory Ig Responses.
LTα is expressed by activated T cells, especially CD4+ T helper type 1 cells, and by activated B and NK cells (2). Both T and B cells are required to induce formation of GCs and class-switched antibody responses to T cell–dependent antigens. The current experiments were designed to determine which BM-derived cellular elements, namely T, B, or NK cells, are required to deliver the LTα-dependent signal(s) for both formation of GCs and for production of a mature, isotype-switched Ig response. We used BM from wild-type mice as a source of LTα-expressing cells of all marrow- derived lineages. BM from Ig μ heavy chain–targeted mice (BCR−/−) provided all marrow-derived LTα-expressing lineages except B cells, and bone marrow from mice deficient in both TCR-β and TCR-δ (TCR−/−) provided all marrow-derived LTα-expressing lineages except T cells. BM from RAG-1−/− mice provided all marrow-derived LTα-expressing lineages except B and T cells. Compound BM chimeric animals were prepared by reconstituting lethally irradiated LTα−/− mice with a 1:1 mixture of BM from LTα−/− mice (to provide LTα−/− cells from all hematopoietic lineages) and wild-type, BCR−/−, TCR−/−, or RAG-1−/− mice. These reconstituted animals contained all hematopoietic lineages, but one or more of these lineages was incapable of expressing LTα.
Mice reconstituted with a mixture of LTα−/− and wild-type BM were able to generate a strong IgG anti-SRBC response when immunized intraperitoneally 6 wk after marrow transplant (Fig. 1 A). Thus, wild-type cells were dominant over LTα−/− cells for formation of an isotype-switched T cell–dependent antibody response. Similarly, mice reconstituted with a mixture of LTα−/− and TCR−/− BM were also able to generate a strong IgG anti-SRBC response. This indicates that LTα-expressing T cells are not required for the development of an isotype-switched antibody response. However, mice reconstituted with a mixture of LTα−/− and either BCR−/− or RAG-1−/− BM failed to generate an antigen-specific IgG response. Thus, LTα-expressing B lymphocytes are required for the recovery of an isotype-switched antibody response.
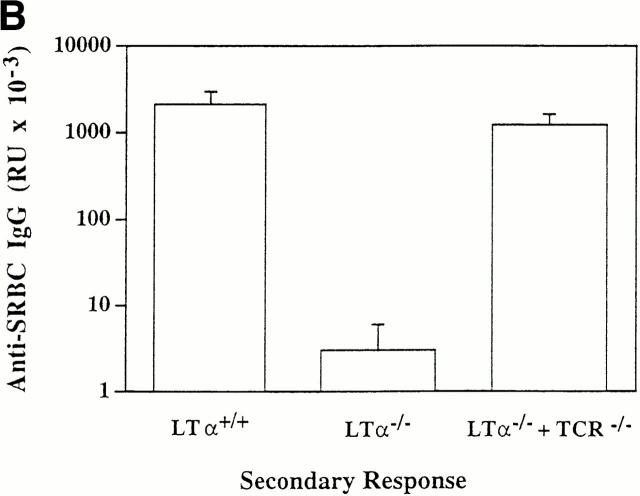
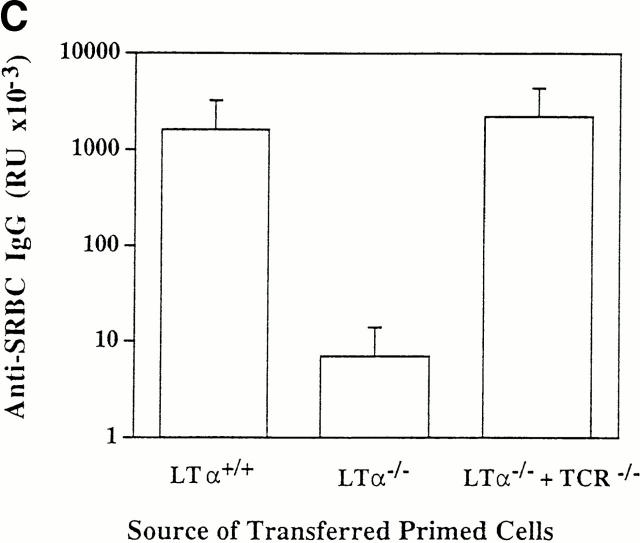
Figure 1.
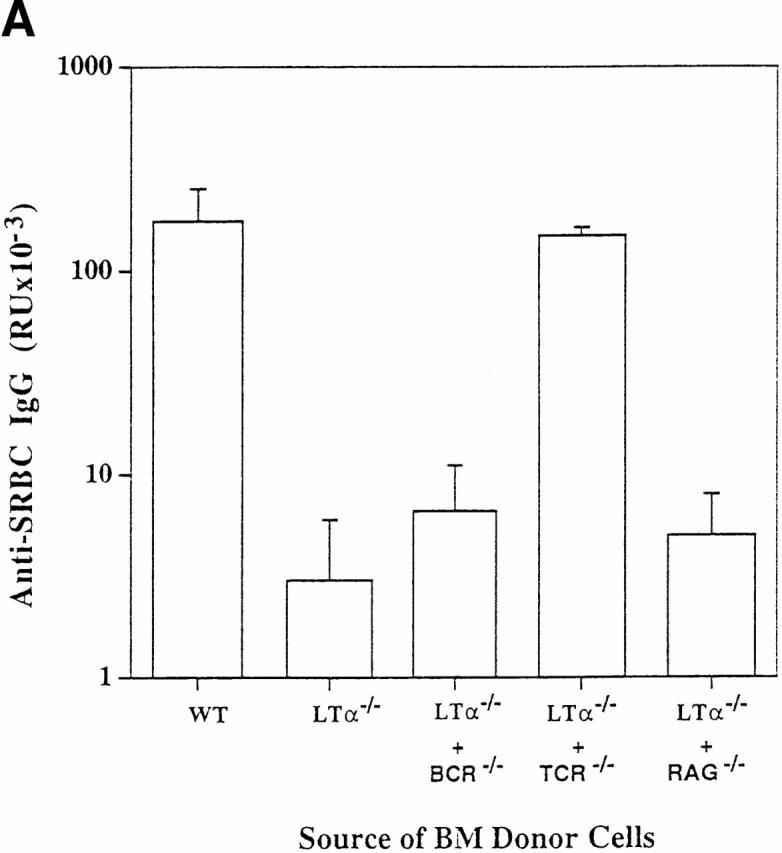
LTα-expressing B cells can restore primary, secondary, and memory anti-SRBC IgG responses in LTα−/− mice. Groups of three to five 8-wk-old mice were lethally irradiated and reconstituted with 1:1 mixtures of BM from LTα−/− mice and either BCR−/−, TCR−/−, or RAG-1−/− mice as indicated. Lethally irradiated wild-type mice reconstituted with BM from wild-type donors and LTα−/− mice reconstituted with BM from LTα−/− donors served as controls. 6 wk after BM reconstitution, all mice were immunized intravenously with 108 SRBCs in PBS. Primary anti-SRBC IgG responses (A) were measured 10 d later by ELISA (16). For measurement of secondary responses (B), mice received a booster immunization of 108 SRBCs in PBS and sera were collected 7 d later. For measurement of memory responses (C), mice that had been reconstituted with the indicated mixtures of BM were primed with a single dose of 108 SRBCs and 7 d later, 5 × 107 splenocytes were transferred to sublethally irradiated (750 rads) normal C57BL/6 mice and the recipients were immediately challenged with the same dose of SRBCs. 7 d later, serum anti-SRBC IgG responses were determined. Data shown represent the means ± SEM of triplicate determinations from three to five mice. RU, relative units. Each experiment was repeated at least twice with similar results.
LTα−/− mice manifest impaired secondary (Fig. 1 B) and memory (Fig. 1 C) responses after challenge with SRBCs. To investigate whether LTα-expressing B cells were able to reconstitute these responses, the compound BM chimeric animals were evaluated further. Strong secondary and memory IgG responses were detected in mice reconstituted with a mixture of BM from LTα−/− and TCR−/− mice (Fig. 1, B and C). As expected, secondary and memory IgG responses were absent in mice that had been reconstituted with mixtures of LTα−/− and either BCR−/− or RAG-1−/− marrow (data not shown). These data indicate both that LTα-expressing B cells support the development of secondary and memory responses, and also that T cells do not require LTα expression to provide help for the responses. Although signals from T cells by membrane proteins such as CD40 ligand (CD40L) are essential for generating memory B cells, LTα from T cells is not essential to provide B cell help for these responses. Consistent with these data, we found in other experiments that LTα−/− mice could not generate memory B cells, but could generate memory T cells (data not shown).
LTα-expressing B Cells, but Not LTα-expressing T Cells Induce the Formation of the FDC Microenvironment.
Clusters of FDCs are prominent components of lymphoid follicles that are thought to support the formation of GCs that are required for the development of robust primary and secondary IgG responses in an essential fashion (19, 20). We have recently shown that the reconstitution of antigen-specific IgG responses in LTα−/− mice by transplantation of wild-type BM is closely associated with the recovery of FDC clusters (13). These prior studies demonstrated that BM-derived cells produced a population of LTα-expressing cells that was capable of supporting the development of clusters of FDCs within the splenic white pulp nodules. To define the essential LTα-expressing lineage responsible for delivering this signal, we analyzed the structures of spleen follicles from LTα−/− mice that had been lethally irradiated and reconstituted with a mixture of BM from LTα−/− mice and either BCR−/− or TCR−/− mice. FDC clusters were detected by immunohistochemistry using the anti-CR1 monoclonal antibody 8C12 (21), and GCs were detected by binding PNA (Fig. 2). Both FDC clusters and GCs were restored in LTα−/− mice that had received a mixture of BM cells from LTα−/− mice and TCR−/− mice. This demonstrated that it was not necessary for cells of the T lymphocyte lineage to express LTα for FDC clusters and GCs to form. In contrast, neither FDC clusters nor GCs were detected in mice that had received a mixture of BM from LTα−/− and BCR−/− mice. Thus, LTα-expressing B cells are required for formation of splenic primary follicles including clusters of FDCs. Although both T and B cells are required for GC formation and for production of IgG in response to T cell–dependent antigens like SRBCs, the signals provided by each of these cell populations have been incompletely defined. Our data demonstrate that interactions between LTα-expressing B cells from TCR−/− mice and LTα-deficient T cells from LTα−/− mice are capable of supporting GC formation, isotype class switching, and B cell memory in the reconstituted environment. Of particular interest, our data demonstrate that B cells in an important way use LTα to condition their lymphoid tissue microenvironment in a way that supports the development of effective IgG responses.
Figure 2.
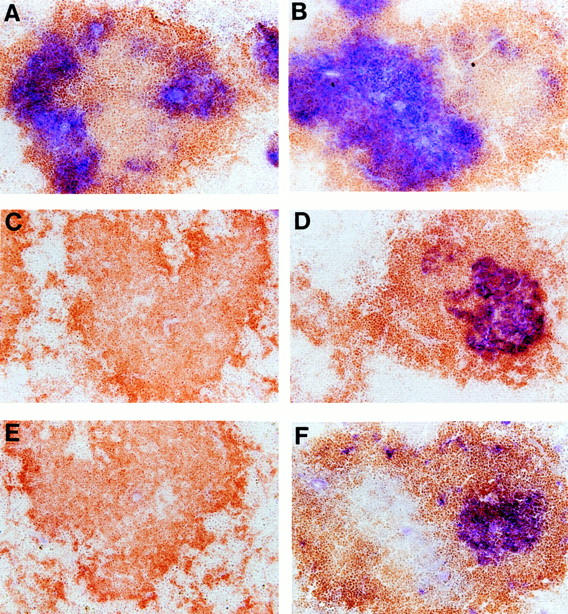
Structure of spleen follicles in compound BM chimeric mice. After sera were collected from the mice shown in Fig. 1 A, the spleens were harvested and frozen sections were stained with anti-Thy1.2 (blue) and anti-B220 (brown) to visualize the T and B cell zones (A and B), anti-CR1 monoclonal antibody 8C12 (blue) and anti-B220 (brown) to visualize clusters of FDC (C and D), or PNA (blue) and anti-IgD (brown) to visualize the GC reaction (E and F). A, C, and E show spleen sections from LTα−/− mice reconstituted with mixtures of bone marrow from LTα−/− and BCR−/− mice; B, D, and F show spleen sections from LTα−/− mice reconstituted with mixtures of BM from LTα−/− and TCR−/− mice. Proper segregation of B and T cell zones was not restored in either compound BM chimera; however, both formation of FDC clusters and GC were restored in mice reconstituted with LTα-expressing B cells.
B Cells Can Induce the Formation of FDC clusters in the Absence of T Cells.
As observed in LTα−/− mice, the peripheral lymphoid tissues of scid mice are devoid of FDC clusters (21), suggesting that mature B and/or T lymphocytes are required for FDC cluster formation to occur. Using scid recipients, Kapasi et al. suggested that either T or B cells could partially reconstitute FDC cluster formation in lymph nodes, but that both cell populations were required for full restoration of FDCs (21). A special role for B cells inducing development of FDCs had been previously postulated by Cerny et al. (22). Additionally, Yoshida et al. showed, using an allogeneic system, that when graft versus host disease was suppressed by treatment of recipients with anti–T cell antibodies, that splenocytes (presumably with T cell function blocked) could restore endogenous FDC cluster formation in scid recipients (23). However, the ability of B or T cells alone to induce FDC clusters is difficult to assess because of the difficulties in defining pure cell populations in these studies. In addition, T lymphocytes (even in very small numbers) have been unequivocally demonstrated to be essential for the formation of antigen-driven GCs (14, 15). Experimental interference with T cell function either by selective T cell depletion or by blocking costimulatory signals such as those delivered by CD40–CD40L or B7– CD28 prevents the formation of GCs and the development of specific IgG responses (20, 24–29). It is of interest, therefore, that FDCs express CD40 (30), which has the potential to interact in a productive fashion with CD40L on T cells, and also that patients with a congenital deficiency of CD40L have lymph nodes with severe depletion of FDC clusters (25). These observations suggest that some CD40–CD40L interaction may be essential for the development of FDC clusters. It was, therefore, unexpected that we found that mice with targeted deficiency of CD40L (31), although they showed no formation of GC after intraperitoneal immunization with SRBCs, nevertheless manifested splenic primary follicles with clusters of FDC similar to those seen in normal mice (data not shown). This suggests that the development and/or maintenance of FDC clusters and segregated T and B cell zones may be independent of a CD40–CD40L interaction.
Because FDC clusters were preserved in CD40L−/− mice that have profound impairment of B–T cell interaction, we investigated whether, in fact, any B–T cell interaction was required for the formation of splenic FDC clusters. Sections from the spleens of TCR−/− and BCR−/− mice were stained with the anti-CR1 monoclonal antibody 8C12 to detect the presence of FDC clusters (Fig. 3). Strikingly, the spleens of TCR−/− mice manifest FDC clusters similar to those seen in wild-type mice. Spleen sections from BCR−/− mice showed no detectable clusters of FDCs. These data indicated both that LTα-expressing B cells are required for the formation of FDC clusters and that these B cells can support the formation of FDC clusters in the absence of T cells. That B cells are the only lymphoid cell type necessary for the induction of FDC clusters is suggested by our preliminary analysis of CD3ε transgenic mice. These mice lack NK and mature T cell activities (32, 33). Interestingly, immunohistochemical analysis of sections from their spleens shows clusters of FDCs within B cell zones, similar to those seen in the TCR−/− mice (data not shown), suggesting that neither NK or T cells are required for the development of FDC clusters.
Figure 3.
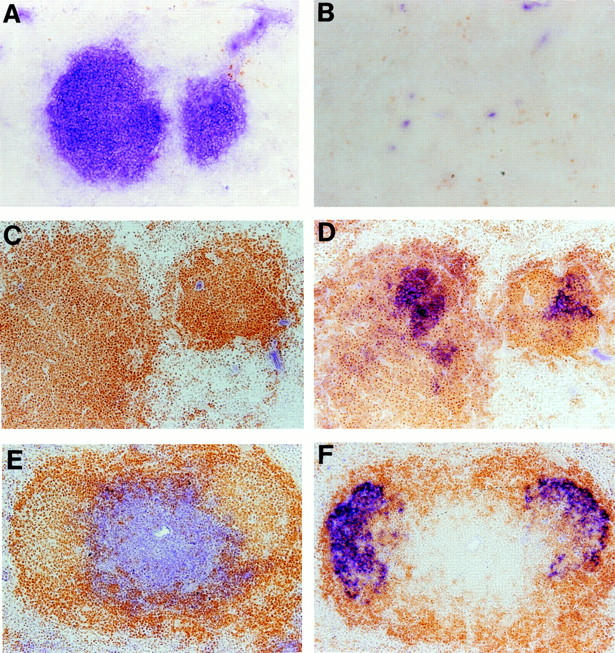
Presence of FDC clusters in TCR−/− but not BCR−/− mice. BCR−/− (A and B), TCR−/− (C and D), and wild-type (E and F) mice were immunized intraperitoneally with 108 SRBCs, and spleens were harvested 10 d later. Frozen sections were stained with anti-Thy-1.2 (blue) and anti-B220 (brown) to visualize the T and B cell zones (A, C, and E). FDC clusters were observed by staining with the anti-CR1/2 monoclonal antibody 8C12 (blue; B, D, and E).
The Microenvironment Established in the Absence of T Cells Can Support the Interactions of T and B Cells in the Formation of GCs and the Class-switched IgG Response.
As expected, when TCR−/− mice were immunized with SRBCs, although there was a substantial IgM anti-SRBC response, there was no development of morphologically defined GCs and no detectable production of IgG anti-SRBC antibody (data not shown). In contrast, when BM from TCR−/− was adoptively transferred into LTα−/− mice, there was full recovery of GC formation and ability to produce antigen-specific IgG (Figs. 1 and 2). These data indicated that LTα−/− T cells from the recipient animal could act together with the LTα-expressing B cells from the donor to form GCs and to support Ig class switching. Additional evidence that T cells can support the formation of GCs in an LTα-independent fashion was obtained by transfer of purified T cells from either wild-type or LTα−/− mice into FDC clusters containing TCR−/− recipients. Unirradiated TCR−/− mice were treated by infusion of 107 purified splenic T cells from either wild-type or LTα−/− donors together with an infusion of 108 SRBCs. 10 d later, both GCs (Fig. 4) and high levels of IgG anti-SRBC antibodies (data not shown) were detected in the TCR−/− recipients, irrespective of whether they received wild-type or LTα−/− T cells. This indicates that the FDC clusters that are found in TCR−/− mice are competent to support the formation of GCs and a productive isotype-switched Ig response once supplemental T cells are provided. In contrast to T cells from CD40L−/− mice, LTα-deficient T cells can activate B cells for the GC reaction and can support isotype switching once the proper microenvironment has been established by LTα-expressing B cells.
Figure 4.
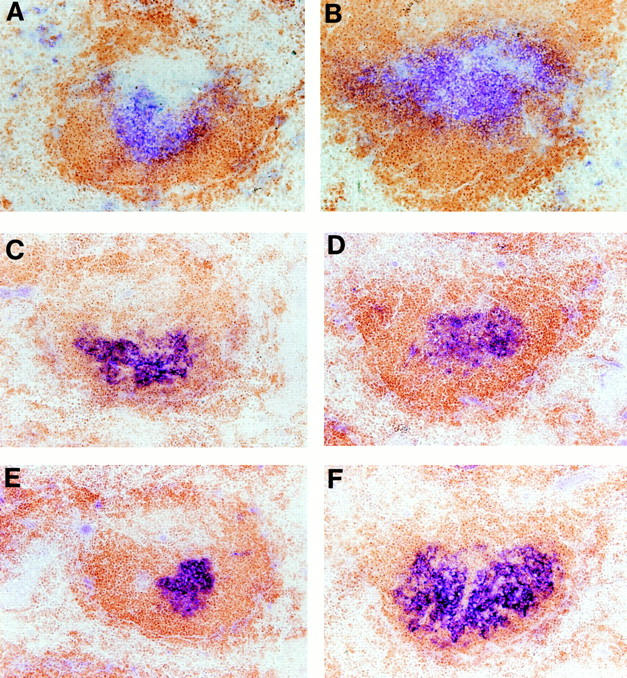
Purified LTα-expressing and LTα-deficient T cells both restore GC formation in TCR−/− mice. Unirradiated TCR−/− mice were treated with an infusion of 107 purified T cells from the spleens of LTα−/− (B, D, and F) or wild-type mice (A, C, and E). At the same time, the mice were immunized with 108 SRBCs administered intravenously. The spleens of the recipients were harvested 10 d later, and frozen sections were stained as described for Fig. 2. A and B are stained for B (brown) and T (blue) cells; C and D are stained for B cells (brown) and FDC (blue); E and F are stained with B220 (brown) and PNA (blue).
Mature B Cells Are Sufficient for the Restoration of FDC Clusters.
The BM transfer studies described above indicate that LTα-expressing cells of the B cell lineage are required to induce formation of spleen FDC clusters. To investigate whether the mature B cell component of this lineage is sufficient to provide the LTα-dependent signal for FDC formation, we reconstituted unirradiated RAG-1−/− mice with an intravenous infusion of either 107 purified splenic B cells or 2.5 × 106 BM cells from TCR−/− donors. 3 wk after transfer, both groups of mice show similar splenic FDC clusters (Fig. 5). These data indicate that LTα-expressing mature B cells alone are sufficient to reconstitute formation of endogenous FDC clusters in the primary follicles. Interestingly, resting virgin B cells are thought to be negative for expression of LTα (29, 34). This implies, then, that the B cell must participate in a dialogue in which it is induced, presumably in the proper area of the white pulp, to express LTα, which then can deliver the signal for formation of FDC clusters. The nature of the signal that induces LTα expression, and the immediate target cell receiving the LTα signal, remain to be defined. The LT-dependent signal appears to be required not only for the induction, but also for the maintenance of FDC clusters. This is suggested by our prior data showing that FDC clusters disappear over the course of 2–3 wk after replacement of normal BM by LTα−/− BM (13). This, then, is a unique example of B cells providing a signal that serves to induce and maintain structural elements that support the further maturation of B cell functional responses.
Figure 5.
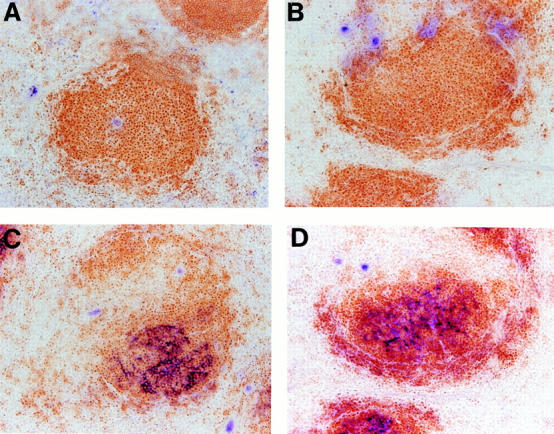
Similar reconstitution of FDC clusters in RAG-1−/− mice by transfer of BM or purified B cells. Unirradiated RAG-1−/− mice were treated with an infusion of 2.5 × 106 BM cells (A and C) or 107 purified spleen B cells (B and D) from TCR−/− donors. 3 wk later, the spleens were harvested and frozen sections were stained for B/T cell zones and FDC clusters as in Fig. 2, A–D.
Of particular interest in relation to the ability of B cells to induce FDC clusters within the lymphoid tissue compartment of the spleen are the prior descriptions of ectopic development of FDC-containing lymphoid follicles and GCs in certain chronic inflammatory conditions and autoimmune diseases. For example, patients with myasthenia gravis can manifest lymphoid follicles and GCs within the thymus (35). Similar follicles and GCs are found within the inflamed synovial tissues of patients with rheumatoid arthritis (36). These GCs contain clusters of FDCs similar to those found in normal lymphoid tissue GCs. We speculate that expression of LTα by B cells infiltrating these tissues may provide the initial signals for formation of FDC clusters that support the formation of these ectopic GCs. FDC clusters, however, do not colocalize with B cells throughout the entire B cell zones of peripheral lymphoid tissues. Rather, they form clusters near the centers of the primary B cell follicles and spare the periphery of these follicles. One possible explanation for this positioning is that local signals may regulate the expression of LTα within these B cell areas. Alternatively, this restricted positioning may imply that there are additional signals besides B cell–derived LTα that are required to induce the development and maintenance of FDC clusters. More complete understanding of the signals that regulate the formation and maintenance of FDC clusters may provide new opportunities for manipulation of the quality and magnitude of immune responses.
Acknowledgments
The authors thank David Randolph (Washington University School of Medicine, St. Louis, MO), Hector Molina (Washington University School of Medicine, St. Louis, MO), and Mitsuru Matsumoto (Ehime University School of Medicine, Ehime, Japan) for helpful discussions.
This work was supported by grant AI01431 (Y.-X. Fu) from the National Institutes of Health. D. Chaplin is an investigator of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used in this paper: anti-CR, anti–complement receptor; BCR, B cell receptor; BM, bone marrow; CD40L, CD40 ligand; FDC, follicular dendritic cell; GC, germinal center; LT, lymphotoxin; PNA, peanut agglutinin; RAG, recombination activity gene; SRBC, sheep RBC.
References
- 1.Ruddle N. Tumor necrosis factor (TNFα) and lymphotoxin (TNFβ) Curr Opin Immunol. 1992;4:327–332. doi: 10.1016/0952-7915(92)90084-r. [DOI] [PubMed] [Google Scholar]
- 2.Ware CF, VanArsdale TL, Crowe PD, Browning JL. The ligands and receptors of the lymphotoxin system. Curr Top Microbiol Immunol. 1995;198:175–218. doi: 10.1007/978-3-642-79414-8_11. [DOI] [PubMed] [Google Scholar]
- 3.Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O'Brine-Greco B, Foley SF, Ware CF. Lymphotoxin β, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 4.Browning JL, Dougas I, Ngam-ek A, Bourdon PR, Ehrenfels BN, Miatkowski K, Zafari M, Yampaglia AM, Lawton P, Meier W, Benjamin CP, Hession C. Characterization of surface lymphotoxin forms. Use of specific monoclonal antibodies and soluble receptors. J Immunol. 1995;154:33–46. [PubMed] [Google Scholar]
- 5.Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, Browning JL, Din WS, Goodwin RG, Smith CA. A lymphotoxin-β–specific receptor. Science. 1994;264:707–710. [PubMed] [Google Scholar]
- 6.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 7.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-α–deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 8.Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. The role of lymphotoxin and type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 9.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β–deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 10.Neumann B, Luz A, Pfeffer K, Holzmann B. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J Exp Med. 1996;184:259–264. doi: 10.1084/jem.184.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eugster H-P, Muller M, Karrer U, Car BD, Schnyder B, Eng VM, Woerly G, Le Hir M, di Padova F, Aguet M, et al. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-α double-deficient mice. Int Immunol. 1996;8:23–36. doi: 10.1093/intimm/8.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y-X, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin-α supports development of splenic follicular structure that is required for IgG response. J Exp Med. 1997;185:2111–2120. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stedra J, Cerny J. Distinct pathways of B cell differentiation. I. Residual T cells in athymic mice support the development of splenic germinal centers and B cell memory without an induction of antibody. J Immunol. 1994;152:1718–1726. [PubMed] [Google Scholar]
- 15.Miller C, Stedra J, Kelsoe G, Cerny J. Facultative role of germinal centers and T cells in the somatic diversification of IgVH genes. J Exp Med. 1995;181:1319–1331. doi: 10.1084/jem.181.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina H, Holers VM, Li B, Fang Y-F, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trizio D, Cudcowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974;113:1093–1099. [PubMed] [Google Scholar]
- 18.Mariathasan S, Matsumoto M, Baranyay F, Nahm MH, Kanagawa O, Chaplin DD. Absence of lymph nodes in lymphotoxin-α (LTα)-deficient mice is due to abnormal organ development, not defective lymphocyte migration. J Inflamm. 1995;45:72–78. [PubMed] [Google Scholar]
- 19.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 20.Kelsoe G. Life and death in germinal centers (redux) Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- 21.Kapasi ZF, Burton GF, Shultz LD, Tew JG, Szakal AK. Induction of functional follicular dendritic cell development in severe combined immunodeficiency mice. Influence of B and T cells. J Immunol. 1993;150:2648–2658. [PubMed] [Google Scholar]
- 22.Cerny A, Zinkernagel RM, Groscurth P. Development of follicular dendritic cells in lymph nodes of B-cell–depleted mice. Cell Tissue Res. 1988;254:449–454. doi: 10.1007/BF00225818. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida K, van den Berg T, Dijkstra CD. The functional state of follicular dendritic cells in severe combined immunodeficient (SCID) mice: role of the lymphocytes. Eur J Immunol. 1994;24:464–468. doi: 10.1002/eji.1830240230. [DOI] [PubMed] [Google Scholar]
- 24.Borriello F, Settna MP, Boyd SD, Schweizer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 25.Facchetti F, Appiani C, Salvi L, Levy J, Notarangelo LD. Immunohistologic analysis of ineffective CD40–CD40 ligand interaction in lymphoid tissues from patients with X-linked immunodeficiency with hyper-IgM. Abortive germinal center cell reaction and severe depletion of follicular dendritic cells. J Immunol. 1995;154:6624–6633. [PubMed] [Google Scholar]
- 26.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 27.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 28.Van den Eertwegh AJ, Noelle RJ, Roy M, Shepherd DM, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J Exp Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worm M, Geha RS. CD40 ligation induces lymphotoxin α gene expression in human B cells. Int Immunol. 1994;6:1883–1890. doi: 10.1093/intimm/6.12.1883. [DOI] [PubMed] [Google Scholar]
- 30.Ogata T, Yamakawa M, Imai Y, Takahashi T. Follicular dendritic cells adhere to fibronectin and laminin fibers via their respective receptors. Blood. 1996;88:2995–3003. [PubMed] [Google Scholar]
- 31.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 32.Wang B, Biron C, She J, Higgins K, Sunshine MJ, Lacy E, Lonberg N, Terhorst C. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc Natl Acad Sci USA. 1994;91:9402–9406. doi: 10.1073/pnas.91.20.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, Hollander GA, Nichogiannopoulou A, Simpson SJ, Orange JS, Gutierrez-Ramos JC, Burakoff SJ, Biron CA, Terhorst C. Natural killer cell development is blocked in the context of aberrant T lymphocyte ontogeny. Int Immunol. 1996;8:939–949. doi: 10.1093/intimm/8.6.939. [DOI] [PubMed] [Google Scholar]
- 34.Hanissian SH, Geha RS. Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity. 1997;6:379–387. doi: 10.1016/s1074-7613(00)80281-2. [DOI] [PubMed] [Google Scholar]
- 35.Sloan HEJ. The thymus in myasthenial gravis, with observations on the normal anatomy and histology of the thymus. Surgery. 1943;13:154–174. [Google Scholar]
- 36.Randen I, Mellbye OJ, Forre O, Natvig JB. The identification of germinal centres and follicular dendritic cell networks in rheumatoid synovial tissue. Scand J Immunol. 1995;41:481–486. doi: 10.1111/j.1365-3083.1995.tb03596.x. [DOI] [PubMed] [Google Scholar]