Abstract
The ζ family includes ζ, η, and FcεRIγ (Fcγ). Dimers of the ζ family proteins function as signal transducing subunits of the T cell antigen receptor (TCR), the pre-TCR, and a subset of Fc receptors. In mice lacking ζ/η chains, T cell development is impaired, yet low numbers of CD4+ and CD8+ T cells develop. This finding suggests either that pre-TCR and TCR complexes lacking a ζ family dimer can promote T cell maturation, or that in the absence of ζ/η, Fcγ serves as a subunit in TCR complexes. To elucidate the role of ζ family dimers in T cell development, we generated mice lacking expression of all of these proteins and compared their phenotype to mice lacking only ζ/η or Fcγ. The data reveal that surface complexes that are expressed in the absence of ζ family dimers are capable of transducing signals required for α/β–T cell development. Strikingly, T cells generated in both ζ/η−/− and ζ/η−/−–Fcγ−/− mice exhibit a memory phenotype and elaborate interferon γ. Finally, examination of different T cell populations reveals that ζ/η and Fcγ have distinct expression patterns that correlate with their thymus dependency. A possible function for the differential expression of ζ family proteins may be to impart distinctive signaling properties to TCR complexes expressed on specific T cell populations.
The T cell antigen receptor (TCR) is a multimeric complex consisting of subunits that function primarily either in antigen recognition (α/β or γ/δ) or signal transduction (CD3-γ, -δ, and -ε, and a ζ family dimer) (1). The ζ family members constitute a group of structually and functionally related proteins that include ζ, η (an alternatively spliced form of ζ), and FcεRIγ (Fcγ; references 1, 2).
Thymocytes from mice lacking expression of both ζ and η chain (ζ/η−/−) are reduced in number and express extremely low levels of surface TCR relative to ζ/η+/+ mice (3–5). Nevertheless, T cell development is not completely arrested in ζ/η−/− mice as they contain both CD4+CD8+, or double positive (DP),1 and CD4+ CD8− and CD4− CD8+, or single positive (SP) thymocytes and peripheral SP T cells (3–5). In contrast, expression of the CD3 (γ/δ/ε) complex is absolutely required for thymocyte development, as mice lacking expression of CD3-ε subunits fail to develop beyond the the most immature CD4− CD8−, or double negative (DN), stage (6). The transition of DN thymocytes into DP thymocytes is regulated by the pre-TCR, a signaling complex composed of β chain, pre-Tα, and CD3 subunits, and which is also thought to include a ζ family dimer (7). The fact that low numbers of DP thymocytes (10–30% of normal) are generated in ζ/η−/− mice indicates that, though important, ζ and η are not essential for pre-TCR function. Likewise, the presence of low numbers of SP thymocytes and peripheral T cells in ζ/η−/− mice (3–5) demonstrates that ζ/η chains are not absolutely required for α/β-TCR expression or for promoting T cell development. Because of the extremely low levels of surface expression in ζ/η−/− mice, the subunit composition of surface pre-TCR and TCR complexes has not been accurately determined. One possibility is that the pre-TCR and TCR can be expressed in the absence of a ζ family dimer, and function, albeit inefficiently, to promote thymocyte development. Another possibility is that in ζ/η−/− mice the pre-TCR and/or α/β-TCR complexes associate with Fcγ chain homodimers, since Fcγ is reported to be expressed during early thymocyte development (8–10). In mice lacking Fcγ, thymocyte development is unaffected, and therefore Fcγ normally does not play a significant role in the development of thymus-dependent T cells (11). Nevertheless, Fcγ, together with ζ chain, functions as a component of the TCR complex expressed on restricted populations of T cells (“thymus-independent” T cells), and in both Fcγ−/− and ζ/η−/− mice these T cells express relatively high levels of surface TCR (4, 5, 10). In addition, overexpression of Fcγ chain (or η chain) in thymocytes restores TCR surface expression and α/β-T cell development in ζ/η−/− mice (12–14). Therefore, all of the ζ family proteins are capable of independently supporting α/β-TCR surface expression and promoting the development of α/ β-TCR+ thymocytes.
In this study, we have generated mice lacking all three ζ family proteins (ζ/η−/−–Fcγ−/− mice) and compared the T cell populations present in these animals to those found in mice lacking either ζ/η or Fcγ alone. The results provide direct evidence that pre-TCR and α/β-TCR complexes lacking a ζ family dimer are capable of supporting T cell development, positive selection, and T cell activation. Moreover, they reveal that, in the absence of specific stimuli, Fcγ is not normally expressed in thymus-dependent T cell populations, whereas both ζ/η and Fcγ are expressed in thymus-independent T cells. A possible function for the restricted expression of different ζ family proteins may be to modify the TCR signaling response in distinct populations of T cells.
Materials and Methods
Mice.
The generation of ζ/η−/− mice and Fcγ−/− mice has been previously described (3, 11). ζ/η−/− (Fcγ+/+) mice were mated to (ζ/η+/+) Fcγ−/− mice and F1 progeny from these matings (i.e., ζ/η+/−–Fcγ+/−) were then mated. Since the ζ/η and Fcγ loci map to the same region of mouse chromosome 1 (2), F2 progeny were screened for crossover events that resulted in either a ζ/η−/−–Fcγ+/− or ζ/η+/−–Fcγ−/− genotype. One ζ/η−/+– Fcγ−/− mouse, identified among the first 100 F2 progeny analyzed, served as a founder line for the generation of ζ/η−/−–Fcγ−/− mice. Genotypes were identified initially by Southern blotting as previously described (3, 11) and were subsequently screened by PCR. Screening of ζ/η was performed with oligonucleotides Z1: 5′-GAAGAGAGGAATATGACGTCTTGGAGAAGA-3′; Z2: 5′-AAGGACGATCTGAGTACTGAG-3′; and ZNEO: 5′-TTCTGGATTCATCGACTGTGG-3′. PCR parameters were: 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s × 35 cycles. Screening of Fcγ was performed with oligonucleotides 4081: 5′-CTCACGGCTGGCTATAGCTGCCTT-3′; 4087: 5′-ACCCTACTCTACTGTCGACTCAAG-3′; and 2969: 5′-CTCGTGCTTTACGGTATCGCC-3′. PCR parameters were: 94°C for 20 s, 55°C for 20 s, and 72°C for 30 s × 35 cycles. PCR products were resolved on a 2% agarose gel and visualized by staining with ethidium bromide.
Antibodies and Multicolor Flow Cytometry.
mAbs used for flow cytometric analysis were purchased from PharMingen (San Diego, CA) and included fluorochrome (FITC or PE) or biotin-conjugated anti-CD4 (RM4.5); anti–TCR-β (H57-597); anti–CD8-α (53-6.7); anti-CD8-β (53-5.8); anti–CD3-ε, (145-2C11); anti-CD25 (7D4); anti-CD44 (IM7); anti-CD69; anti-B220 (RA3-6B2); anti-CD24 (M1/69); anti-NK1.1 (PK136); anti–IL-2Rβ (TM-β1); and anti–γ/δ-TCR (GL3). Ascites from hybridoma 2.4G2 was a kind gift of J. Titus (National Institutes of Health) and was purified in our laboratories. Streptavidin red 670 (GIBCO BRL, Gaithersburg, MD) was used in conjunction with biotinylated antibodies. Cells were stained with specific antibodies and analyzed on a Becton Dickenson Immunocytometry Systems FACScan® using standard Cell Quest software as previously described (13).
Cell Preparations and Stimulation.
Thymus, spleen, and lymph nodes were excised from mice and single cell suspensions were prepared. Intestinal intraepithelial lymphocytes (i-IELs) were prepared from the small intestine as previously described (13). Dendritic epithelial T cells (DETCs) were obtained from trunk skin and were prepared as previously described (15). For thymocyte activation, DP thymocytes were isolated as previously described (16) and incubated at 37°C on plates that had been previously coated with anti–TCR-β (H57-597). After 12–16 h, the stimulated cells were assessed for CD69 or CD5 surface expression by flow cytometric analysis (FCM) as previously described (14). Lymph node T cells were purified from total lymph node cell suspensions by magnetic bead separation (Miltenyi Biotec, Auburn, CA) using biotinylated anti-CD4 and anti-CD8 and streptavidin microbeads. Purity of magnetically separated T cell populations assessed by FCM was ⩾90% for all samples.
Cytokine Assay
For cytokine assays, purified T cells (106) were either cultured with media alone, media containing PMA (10 ng/ml; Sigma Chemical Co., St. Louis, MO) and ionomycin (1 μM; Sigma Chemical Co.), or plate-bound anti-CD3ε (145-2C11) for 18 h. Supernatants were collected and elaboration of IFN-γ, IL-4, and IL-2 were measured by ELISA. Quantitation of IFN-γ and IL-4 were determined according to manufacturer's instructions (Endogen, Cambridge, MA). IL-2 ELISAs were performed using the reagents and protocol obtained from PharMingen.
For semiquantitative reverse transcription (RT) PCR, cells were pelleted and RNA was extracted with STAT-60 reagent (Tel-Test Inc., Friendswood, TX) and treated with DNase to remove contaminating genomic DNA. The quality of RNA preparations was assessed by gel electrophoresis, and RNA was reverse transcribed using the Superscript Preamplification system (GIBCO BRL). Serial dilutions (1:2 to 1:128) of the RT reaction were made and then amplified by PCR using oligonucleotides corresponding to cyclophilin A: 5′-GGGTGGTGACTTTACACGCCATAATG-3′ and 5′-TCAAAAGAAATTAGAGCTGTCCACAGTCGG-3′; and CD3ε: 5′-TACAAAGTCTCCATCTCAGG-3′ and 5′-TGGCCGCTCCTTGTTTTG-3′. PCR reactions were stopped after 25 cycles and again after 35 cycles then run on a 2% agarose gel. Bands were visualized by staining with ethidium bromide and quantitated by densitometry. Dilutions of the RT reaction that yielded equivalent control bands were then amplified for 35 cycles using primers specific to IL-2, IL-4, and IFN-γ (Clontech, Palo Alto, CA). For all PCR reactions, parameters were: 94°C for 3 min, then 35 cycles of 94°C for 45 s, 60°C for 45 s, 72°C for 2 min, then 72°C for 5 min.
Results
Thymocyte Development in Mice Lacking Expression of All ζ Family Proteins (ζ,η, and Fcγ).
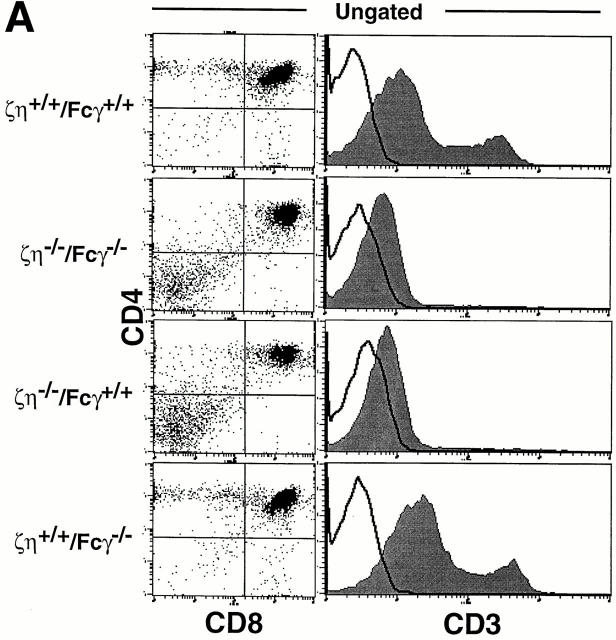
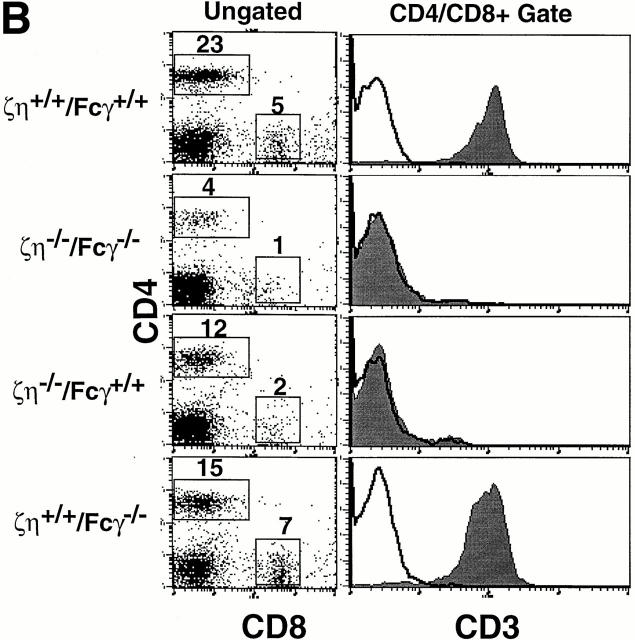
Mice lacking expression of all ζ family proteins (ζ/η−/−–Fcγ−/−) were generated as described in Materials and Methods. Examination of thymocytes from ζ/η−/−–Fcγ−/− mice by FCM revealed a phenotype essentially identical to that of ζ/η−/− (Fcγ+/+) mice (Fig. 1 A). In addition, ζ/η−/− and ζ/η−/−–Fcγ−/− mice contained similar total numbers of thymocytes (10– 30% of normal, data not shown), which consisted almost entirely of DN and DP cells. DP thymocytes from ζ/η−/− mice express extremely low but detectable levels of TCR as assessed by staining with anti–CD3-ε (3–5, 14, and Fig. 1 A) and anti–TCR-β mAbs (3, 14). A similarly low but discernable level of TCR surface expression was observed on DP thymocytes from ζ/η−/−–Fcγ−/− mice by FCM (Fig 1 A). Significantly, although CD4+CD8− and CD4−CD8+ SP cells were not readily detectable in the thymus, SP T cells were present in the lymph nodes (data not shown) and spleen (Fig. 1 B) of ζ/η−/−–Fcγ−/− mice in numbers similar to those observed in ζ/η−/− mice (data not shown). Together, these findings demonstrate that the low but detectable level of TCR expression on DP thymocytes from ζ/η−/− mice, and the ability to generate “mature” SP T cells are not dependent upon the expression of endogenous Fcγ chain.
Figure 1.
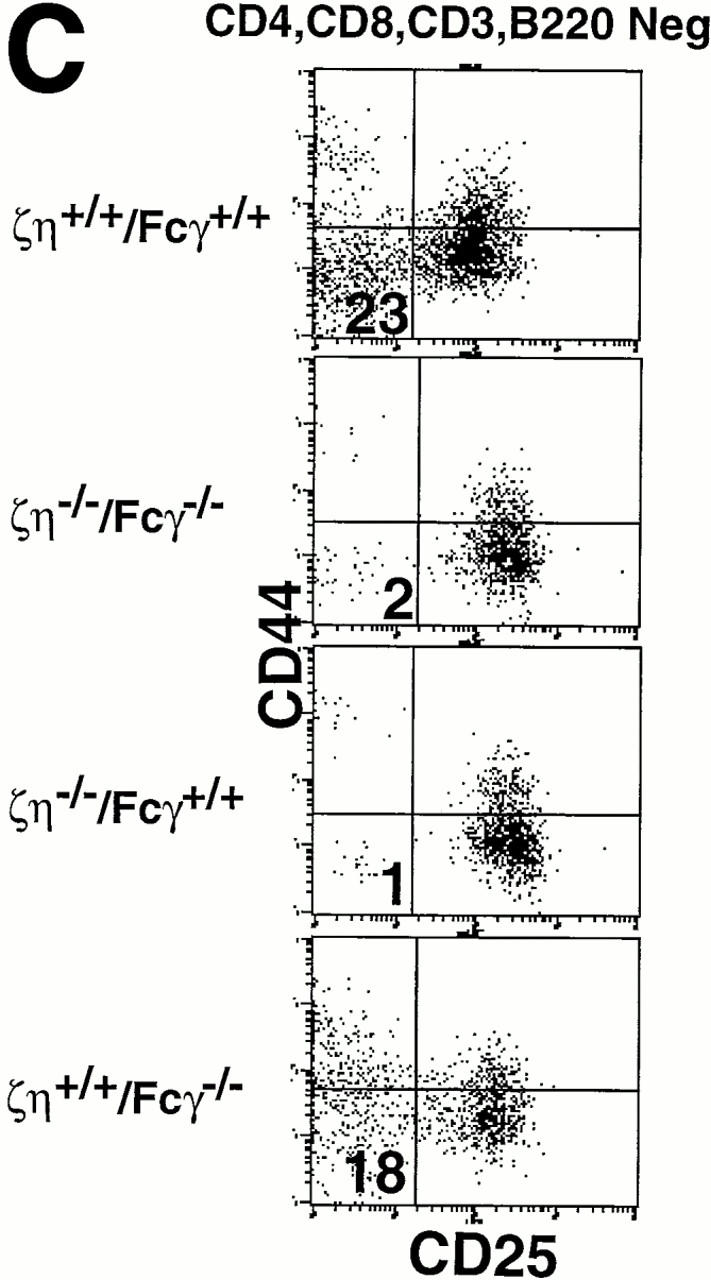
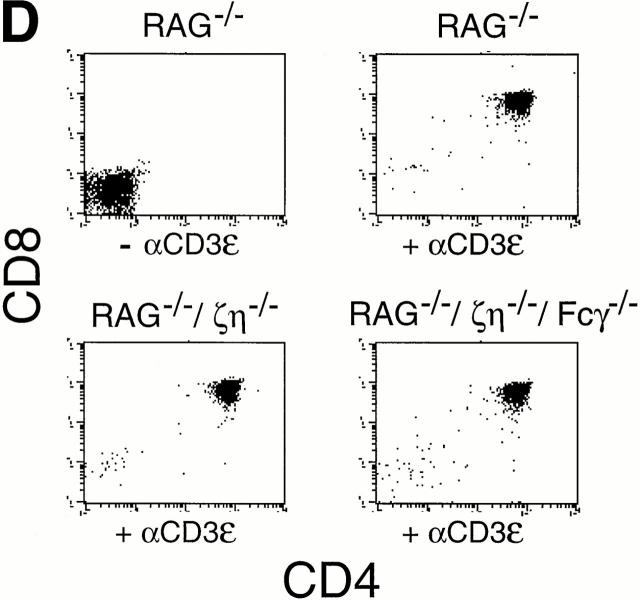
Phenotypic analysis of thymocytes and T cells from ζ/η+/+–Fcγ+/+, ζ/η−/−–Fcγ−/− , ζ/η−/−–Fcγ+/+, and ζ/η+/+–Fcγ−/− mice. (A) CD4, CD8, and CD3 expression on total thymocytes. (B) CD4 versus CD8 expression on ungated splenocytes. CD3 expression is shown on CD4+ and CD8+ gated splenocytes (CD4+/CD8+). (C) CD25 versus CD44 expression on gated (CD4−CD8−CD3−B220−) thymocytes. Immunofluorescence and flow cytometric analysis was performed with thymocytes and splenocytes from young adult (4–8 wk-old) mice. Shaded areas represent staining with experimental antibodies and solid lines represent staining with negative control antibody. Numbers in quadrants of dual parameter histograms represent the percentage of cells contained within that quadrant. (D) Induction of CD4+CD8+ thymocytes in RAG1−/−, ζ/η−/− and in RAG1−/−, ζ/η−/−–Fcγ−/− mice by anti-CD3-ε antibodies. 6–12-wk-old mice were injected intraperitoneally with 200 μl PBS containing 75 μg of affinity purified CD3-ε specific antibody 145-2C11. Control animals were injected with 200 μl PBS. 6 d after injection, thymocytes were counted and analyzed by flow cytometry for expression of CD4 and CD8. Results shown are representative of three separate experiments for each genotype.
Thymocyte development has been shown to be severely compromised at the DN stage in ζ/η−/− mice (17). Whereas the most mature subset of DN thymocytes (CD44−CD25−) constitutes 10–20% of the DN population in normal adults and in mice lacking only Fcγ (Fig. 1 C), these cells are nearly absent in ζ/η−/− mice (reference 17 and Fig. 1 C). Examination of DN thymocyte subsets from both ζ/η−/− and ζ/η−/−–Fcγ−/− mice revealed a block at the identical (CD44−/loCD25+ → CD44−CD25−) stage of maturation (Fig. 1 C), indicating that neither Fcγ nor ζ/η chain is required for thymocyte development before the CD44−/loCD25+ stage. Since the generation and subsequent expansion of CD44−CD25− DN thymocytes is thought to be controlled by signaling through the pre-TCR complex (7, 18), the paucity of CD44−CD25− DN thymocytes in ζ/η−/− mice implies a function for ζ (and/or η) as components of the pre-TCR. However, the fact that some DP thymocytes are generated in both ζ/η−/− and ζ/η−/−–Fcγ−/− mice indicates that the pre-TCR is capable, albeit inefficiently, of transducing signals that promote the development of DN thymocytes to the DP stage in the absence of all ζ family proteins. To further evaluate the ability of surface complexes expressed on DN thymocytes in ζ/η−/−–Fcγ−/− mice to transduce signals that promote the formation of DP thymocytes, we generated ζ/η−/−–Fcγ−/− RAG-1−/− mice. Thymocytes from mice deficient in RAG-1 or RAG-2 are blocked in their development at the DN stage but can be induced to differentiate to the DP stage upon stimulation with anti-CD3 mAb (19). Injection of anti–CD3-ε mAb into both ζ/η−/−–Fcγ+/+ RAG-1−/− (Fig. 1 D and reference 20) and ζ/η−/−–Fcγ−/− RAG-1−/− mice (Fig. 1 D) resulted in increased thymic cellularity (5–20× control) and the generation of large numbers of DP thymocytes. Together these data demonstrate that both early and late stages of thymocyte development are not absolutely dependent on the expression of ζ family proteins.
TCR Complexes Expressed on DP Thymocytes and T Cells from ζ/η− /−–Fcγ− /− Mice Transduce Activating Signals.
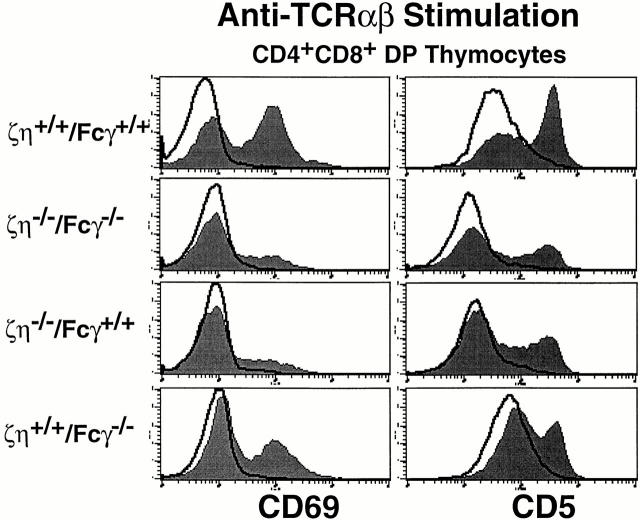
Maturation of DP thymocytes into SP T cells is controlled by TCR-mediated signals that are received during positive selection in the thymus (21). Although DP thymocytes from ζ/η−/− mice express barely detectable levels of surface TCR, these TCR complexes are nevertheless capable of transducing signals that result in the positive selection of low numbers of thymocytes (3–5). Cross-linking of surface TCR complexes on DP thymocytes from ζ/η−/− mice results in the upregulation of CD69 and CD5 (reference 14 and Fig. 2), events which are associated with positive selection in vivo (22). To determine if a similar TCR-mediated signaling response could be elicited in thymocytes from ζ/η−/−– Fcγ−/− mice, DP thymocytes were stimulated in vitro with antibodies directed against either CD3-ε or TCR-β. Significantly, in vitro cross-linking of the TCR complexes on DP thymocytes from ζ/η−/−–Fcγ−/− mice with anti–TCR-β (Fig. 3) or anti–CD3-ε (data not shown) induced upregulation of both CD69 and CD5. Moreover, the extent of CD5 and CD69 upregulation was similar in thymocytes from ζ/ η−/−–Fcγ−/− and ζ/η−/−mice after stimulation (Fig. 2).
Figure 2.
CD69 and CD5 upregulation on DP thymocytes in response to TCR engagement. DP thymocytes were purified and stimulated for 12–16 h on plates coated with either PBS or anti–TCR-β. Cells were then stained with anti-CD69 or anti-CD5 and analyzed by FCM. Shaded areas depict cells stained with anti-CD69 or anti-CD5 after anti–TCR-β stimulation. Solid lines depict cells stained with anti-CD69 or anti-CD5 after incubation in media without antibody stimulation.
Figure 3.
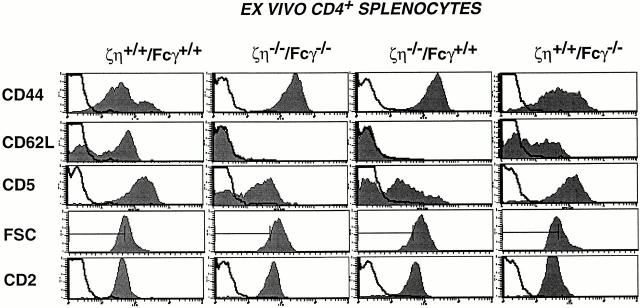
Surface phenotype of peripheral T cells from ζ/η−/−– Fcγ−/− mice. Activation-memory phenotype of splenic T cells. Cells were stained with anti-CD4-PE versus anti-CD44-FITC, anti-CD62L-FITC, anti-CD2-FITC, or anti-CD5-FITC. Single color histograms show staining on CD4+ T cells. Data were collected on 5 × 103 gated cells. Shaded areas represent staining with experimental antibodies and solid lines depict staining with negative control antibodies.
To determine if the TCR complexes expressed on SP T cells from ζ/η−/−–Fcγ−/− mice were also capable of transducing activating signals, we examined lymph node T cells for expression of cell surface molecules associated with activation and memory. Surprisingly, although T cells from ζ/η−/−– Fcγ−/− mice express extremely low levels of surface TCR, a high percentage of these cells appeared to have an activated or memory phenotype (i.e., CD44hi, CD62Llo; Fig. 3). A high percentage of SP T cells from ζ/η−/− were also CD44hi, CD62Llo, whereas the majority of T cells from both control (ζ/η+/+–Fcγ+/+) and Fcγ−/− mice displayed a naive phenotype (CD44lo, CD62Lhi; Fig. 3). Nevertheless, T cells from both ζ/η−/−–Fcγ−/− and ζ/η−/− mice were largely refractory to direct TCR stimulation in vitro as they did not appreciably increase levels of CD69 or IL-2Rα and proliferated poorly in response to treatment with cross-linking anti-TCR antibodies or anti-TCR plus anti-CD28 (data not shown).
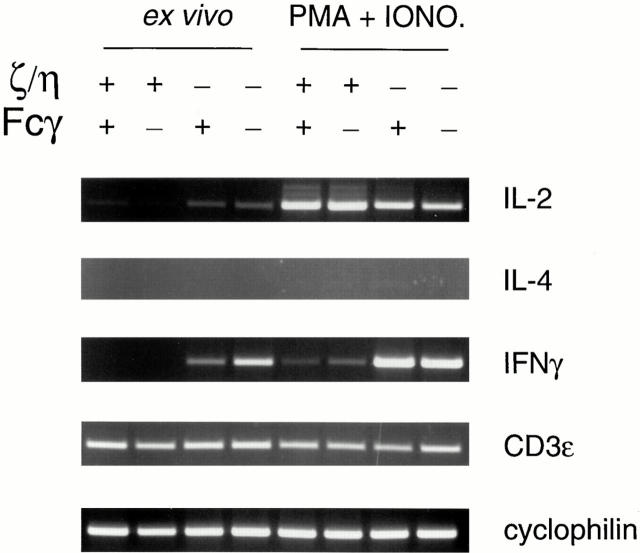
We next examined the ability of T cells from ζ/η−/− and ζ/η−/−–Fcγ−/− mice to produce cytokines after stimulation for 18 h with either PMA and ionomycin or anti– CD3-ε mAb. Stimulation of purified T cells from ζ/η−/− and ζ/η−/−–Fcγ−/− mice (as well as from Fcγ−/− and ζ/ η+/+–Fcγ+/+ mice) resulted in production of IL-2 (Table 1), but in all samples IL-4 and IL-10 remained undetectable (data not shown). However, although T cells from ζ/η+/+– Fcγ+/+ and Fcγ−/− mice produced only low levels of IFN-γ after stimulation, T cells from both ζ/η−/− and ζ/η−/−– Fcγ−/− mice produced large quantities of IFN-γ after stimulation (Table 1). A similar cytokine profile was observed when cytokine production was assessed by RT-PCR (Fig. 4). IFN-γ mRNA was also detectable in freshly isolated ex vivo (unstimulated) T cells from ζ/η−/−–Fcγ−/− mice and ζ/η−/− mice by RT-PCR (Fig. 4). Since purified populations of T cells were used for these experiments, it is unlikely that IFN-γ was derived from contaminating cell populations, such as NK cells. Together, these findings indicate that despite their low levels of surface TCR, a high percentage of T cells from both ζ/η−/− and ζ/η−/−–Fcγ−/− mice appear to be endogenously activated and exhibit a Th1 memory cell phenotype.
Table 1.
Cytokine Elaboration (106 Cells)
| Mouse | Stimuli | IFN-γ | IL-2 | |||
|---|---|---|---|---|---|---|
| ng/ml | pg/ml | |||||
| ζ/η+/+–Fcγ+/+ | PMA+I | 79 | 3,228 | |||
| anti-CD3 | 31 | 22 | ||||
| ζ/η−/−–Fcγ−/− | PMA+I | 3,403 | 490 | |||
| anti-CD3 | 1,596 | 272 | ||||
| ζ/η−/−–Fcγ+/+ | PMA+I | 3,330 | 1,562 | |||
| anti-CD3 | 1,552 | 846 | ||||
| ζ/η+/+–Fcγ−/− | PMA+I | 224 | 2,477 | |||
| anti-CD3 | 175 | 60 |
Results from a representative experiment are shown. Splenic T cells were purified and 106 cells were stimulated in culture for 18 h with PMA (10 ng/ml) and ionomycin (1 μM) (PMA+I) or plate-bound and anti–CD3-ε. Supernatants were serially diluted and tested by ELISA as described in Materials and Methods. Concentrations were determined by comparison with known concentrations of cytokines provided by the manufacturers of the monoclonal antibodies used for ELISA.
Figure 4.
Semiquantitative RT-PCR for detection of IFN-γ, IL-2, or IL-4 mRNA. RNA obtained from unstimulated purified splenic CD4+ and CD8+ T cells (ex vivo) or after 18 h of stimulation with PMA + ionomycin was reverse transcribed and amplified with oligonucleotide primers specific for IL-2, IL-4, and IFN-γ. Reactions were standarized by performing PCR with oligonucleotides corresponding to cyclophilin and CD3-ε, whose mRNAs should be equivalent in purified T cell populations. +, indicates homozygosity for the wild-type ζ/η or Fcγ alleles; − indicates homozygosity for the mutant ζ/η or Fcγ alleles, as indicated.
i-IEL Populations in ζ/η− /−–Fcγ+ /+, ζ/η+ /+–Fcγ− /−, and ζ/η− /−–Fcγ− /− Mice.
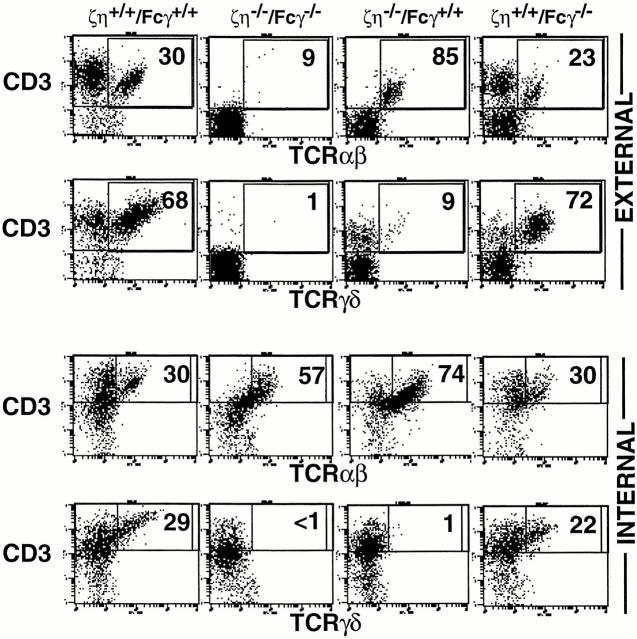
Distinct populations of lymphocytes have been defined within the intestinal epithelium (i-IELs, references 23, 24). Athough some of these cells (CD4+ and/or CD8-α/β+) appear to be dependent on the thymus for their generation, those expressing a homodimer of CD8-α (CD8-α/α) are thought to arise through a thymus-independent developmental pathway (24). Similar to peripheral (lymph node and spleen) CD4+ and CD8-α/β+ α/β–T cells, thymus-dependent i-IEL populations are unaffected in Fcγ−/− mice (11) but are reduced in number and are TCRlo/− in both ζ/η−/− mice (4, 5, 25) and ζ/η−/−–Fcγ−/− mice (data not shown). These results are consistent with the idea that thymus-dependent i-IELs express ζ/η but not Fcγ during their development. On the other hand, thymus-independent i-IELs have been shown to express both ζ and Fcγ chains (8), and mice lacking either ζ/η or Fcγ contain α/β-TCR+ CD8-α/α+ and γ/δ-TCR+ i-IELs that express only moderately reduced levels of surface TCR when compared with similar populations of i-IELs from ζ/η+/+–Fcγ+/+ mice (references 4, 5, and Fig. 5). Significantly, in the absence of ζ, η, and Fcγ chains, all population of i-IELs (thymus-dependent and -independent) are TCRlo/− (Fig. 5).
Figure 5.
i-IEL development in ζ/η−/−–Fcγ−/− mice i-IELs were prepared from mice as described (13) and three-color FCM was performed. For internal staining, cells were first stained with anti-CD4 and anti– CD8-β externally, then treated with intracellular staining buffer followed by staining with anti-CD3, anti–TCR-β, or anti–TCR-δ mAbs. Data depict two-color analysis of CD3 versus TCR-β or CD3 versus TCR-δ on software-gated CD4− CD8-β− cells. Numbers reflect the percentage of gated CD4−CD8-β− cells in that quadrant.
To examine the lineage of TCR− i-IELs in ζ/η−/−– Fcγ−/− mice, cells were analyzed for the presence of intracellular TCR-β and TCR-δ chains. Interestingly, intracellular staining for TCR-δ chains revealed that TCR-γ/δ lineage T cells are virtually absent in ζ/η−/−–Fcγ−/− mice whereas TCR-α/β lineage T cells are readily detected in the same animals (Fig. 5, bottom). Notably, TCR-γ/δ+ cells are also markedly reduced in number in ζ/η−/− mice, but not Fcγ−/− mice, despite the fact that i-iELs from these animals express comparable levels of surface TCR (references 4, 5 and Fig. 5, 4th and 5th columns). Together, these findings indicate that either (a) the generation and/or survival of TCR-γ/δ+ T cells is particularly dependent on expression of ζ/η chain, or (b) that Fcγ is poorly expressed in developing γ/δ lineage T cells. Finally, these results demonstrate that expression of a ζ family dimer is required for efficient TCR surface expression on all T cell populations including both thymus-dependent and thymus-independent TCR-α/β+ and TCR-γ/δ+ cells.
γ/δ+-TCR DETCs and NK1.1+ T Cells Use ζ Chain for TCR Surface Expression.
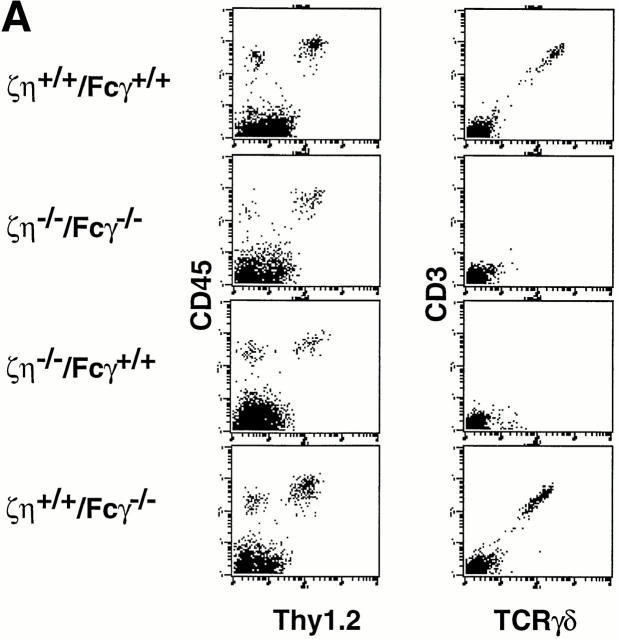
We next examined mice lacking expression of ζ/η, Fcγ, or all ζ family proteins for the presence of DETCs that express γ/δ-TCR and are thymically-derived (26). We observed that though present, DETCs from both ζ/η−/− mice and ζ/η−/−–Fcγ−/− mice express extremely low or undetectable levels of surface TCR, whereas DETCs from Fcγ−/− mice express high levels of γ/δ-TCR (Fig. 6 A). These results were unexpected as it had been previously reported that ζ−/− mice contain DETCs that express relatively high levels of γ/δ-TCR (27–29).
Figure 6.
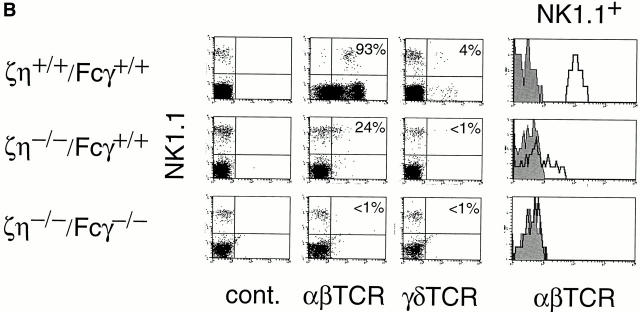
Phenotype of DETC and DN NK1.1+ thymocytes from ζ/η+/+–Fcγ+/+, ζ/η−/−–Fcγ+/+, ζ/η+/+–Fcγ−/−, and ζ/η−/−–Fcγ−/− mice. (A) DETCs were purified as described in Materials and Methods and stained with antibodies directed against TCR-γδ and CD3-ε or Thy 1 and CD45. (B) Expression of surface TCR on NK1.1+ thymocytes from ζ/η+/+–Fcγ+/+, ζ/η−/−–Fcγ+/+ and ζ/η−/−–Fcγ−/− mice. Shown are two-color plots of NK1.1 versus. α/β-TCR or γ/δ-TCR on gated (CD24−) thymocytes. Numbers in quadrants refer to percent of NK1.1+ thymocytes that express TCR (α/β-TCR or γ/δ-TCR). Single-color plots show expression of α/β-TCR on gated NK1.1+ thymocytes. Shaded areas in single color histograms represent staining with negative control antibodies.
We also examined thymocytes from ζ/η−/− and ζ/η−/−– Fcγ−/− for the presence of NK1.1+ T cells that are also thymically derived but not necessarily thymus-dependent (30). Although both ζ/η−/− and ζ/η−/−–Fcγ−/− mice contained thymocytes of the expected “activation-NK” phenotype (i.e., NK1.1+, IL-2Rβ+, CD44+, MEL-14−) TCR+ cells were detectable only in ζ/η−/− mice and these cells were exclusively α/β-TCRlo (Fig. 6 B). Significantly, although an earlier study had reported the presence of large numbers of NK1.1+ γ/δ-TCR+ thymocytes in ζ−/− mice (31), we found that NK1.1+ γ/δ-TCR+ T cells were virtually undetectable in both ζ/η−/− and ζ/η−/−–Fcγ−/− mice (Fig. 6 B) . The most likely explanation for the striking variance between our results and those of previous studies is that the latter analyzed ζ−/− mice in which η chain is expressed (28); thus η chain most likely contributed to the TCR surface expression observed on DETCs and NK1.1+ thymocytes from these mice.
Discussion
The results of this study demonstrate that “partial” TCR complexes that lack ζ family proteins (ζ, η, and Fcγ) can promote the maturation of at least some thymocytes. Indeed, thymocytes from ζ/η−/−–Fcγ−/− mice appear to undergo a relatively normal developmental program; originating from precursor DN thymocytes they develop to the DP stage, undergo positive selection, and emerge as SP T cells. T cells generated in ζ/η−/−–Fcγ−/− mice express a functionally active TCR such that stimulation of these complexes by direct engagement results in the production of specific cytokines. Collectively, these observations indicate that pre-TCR and TCR complexes that contain CD3 subunits but not a ζ family dimer can transduce signals normally associated with fully assembled TCR complexes.
Although some thymocytes are capable of developing in ζ/η−/− and ζ/η−/−–Fcγ−/− mice, the partial arrest that occurs at two specific checkpoints is revealing as to the in vivo function of the ζ family dimers. Early DN thymocytes from ζ/η−/− mice can give rise to DP thymocytes; however, DP thymocytes are reduced in number as are their immediate precursors (CD4−CD8− CD44−CD25− thymocytes). Since Fcγ is reported to be expressed in early thymocytes (8–10), and because transgenic Fcγ can restore development of DN CD44−CD25− thymocytes in ζ/η−/− mice (13), it was possible that endogenous Fcγ enabled the development of some thymocytes in the absence of ζ/η by functioning as a component of the pre-TCR. However, the observation that ζ/η−/− and ζ/η−/−–Fcγ−/− mice contain similar numbers of DP thymocytes and show partial arrest at the same stage of development demonstrates that ζ family members are not absolutely required for pre-TCR function. Notwithstanding, the markedly reduced number of both DN CD24−CD44− thymocytes and DP thymocytes in both ζ/η−/− and ζ/η−/−–Fcγ−/− mice argues that in the presence of a ζ family dimer, the pre-TCR is much more efficient at promoting this transition. The other partial developmental arrest observed in both ζ/η−/− and ζ/η−/−–Fcγ−/− occurs during the transition of cells from the DP to SP stage of development. SP thymocytes are nearly undectable in ζ/η−/− and ζ/η−/−–Fcγ−/− mice, yet both mice contain significant numbers of peripheral SP T cells that accumulate with age. Thus ζ family proteins are also not absolutely required for late stages of T cell development. Nevertheless, ζ chain is required for efficient TCR surface expression and ζ immunoreceptor tyrosine-based activation motif (ITAM)-meditated signals have been shown to play an important role in positive and negative selection of the T cell repertoire (32).
T cell receptors expressed on mature T cells from ζ/η−/− mice exhibit high affinity for self-ligand, a finding not observed in TCRs derived from normal mice (33). According to signal strength models of selection, it is likely that only those T cells with relatively high affinity for self-ligands are positively selected and survive in ζ/η−/− and ζ/η−/−– Fcγ−/− mice. The absence of ζ-mediated signaling during thymic selection and the consequent selection of T cells with high affinity receptors for self has a critical impact on the phenotype and responsiveness of the mature T cells that are generated. Indeed, despite their low levels of surface TCR, a high percentage of T cells from both ζ/η−/− and ζ/η−/−–Fcγ−/− mice appear to be endogenously activated and exhibit a Th1 memory cell phenotype. The high-affinity TCRs expressed by these cells could contribute to this phenotype, as high affinity interactions, with a long association rate, could promote differentiation of cells towards Th1 type memory cells by enabling coreceptor molecules to be effectively recruited into the receptor complex. Indeed, recent studies have suggested that individual cytokine responses may be regulated in a hierarchical manner that depends on the particular signaling threshold and the recruitment of costimulatory molecules (34). Although these findings (34) are based on data obtained by varying the concentration of ligand, our results would suggest that both ligand density and TCR affinity influence the biochemical response made by a particular T cell. Since T cells from ζ/ η−/− and ζ/η−/−–Fcγ−/− mice express extremely low levels of TCR, the affinity of TCR-ligand interactions, rather than the absolute number of TCR complexes that are engaged, could be critical for dictating the type of cytokine response generated by T cells in our model. Alternatively, the skewing toward a Th1-like response in these mice may also be reflective of the genetic background (C57BL/6) or the functional alteration of NK1.1+ T cell populations that are known to produce IL-4 (28). Whatever the foundation for the Th1 phenotype, the generation of Th1 type cytokines appears to have important physiologic consequences as a similar cytokine profile is exhibited by intestinal lamina propria T cells in both ζ/η−/− and ζ/η−/−–Fcγ−/− mice and these mice develop inflammatory bowel disease at extremely high frequency (Ono, M., T. Kawabe, E.W. Shores, P.E. Love, and J. Ravetch, manuscript in preparation).
This and previous (4, 5, 8, 29) studies have served to reveal distinct patterns of expression for ζ/η and Fcγ during T cell ontogeny that correlate with the thymus dependency of T cell populations. Most, if not all, thymus-dependent T cells belonging to both the γ/δ-TCR+ and α/β-TCR+ lineages, including peripheral CD4+ α/β-TCR+ and CD8-α/β+ α/β-TCR+ T cells, peripheral γ/δ-TCR+ T cells, and dendritic epidermal γ/δ-TCR+ T cells express very low or undetectable levels of surface TCR in the absence of ζ/η. On the other hand, “thymus-independent” T cells such as CD8-α/α+ i-IELs express only moderately reduced levels of surface TCR in the absence of either ζ/η or Fcγ and thus normally express both proteins. Several recent studies have demonstrated that Fcγ is also expressed in other T cell populations, such as NK1.1+ thymocytes and T cells (35, 36) and lymphokine-activated γ/δ-TCR+ T cells (37). However, these cells are clearly distinguishable from CD8-α/α+ i-IELs as they express extremely low levels of surface TCR in the absence of ζ/η. Since a common property of these T cells is their responsiveness to cytokines, these observations suggest that expression of Fcγ can be induced by lymphokines. However, this property is not universally shared by all T cell populations, as peripheral CD4+ α/β-TCR+ and CD8-α/β+ α/β-TCR+ T cells do not induce synthesis of Fcγ after cytokine stimulation (reference 8 and our unpublished data).
The restricted expression of ζ/η and Fcγ in different T cell populations also suggests that these proteins may perform specific functions, perhaps by quantitatively or qualitatively influencing the TCR signaling response. ζ chain, which contains 3 ITAM signaling motifs appears optimized to facilitate the development of thymus-dependent, self-MHC–restricted T cells. Indeed, thymocyte positive selection is markedly impaired in transgenic mice in which Fcγ chain, which contains only a single ITAM, is substituted for the ζ chain (13). The ability of ζ chain to amplify signals resulting from low avidity TCR-ligand interactions is therefore particularly critical for thymocyte selection (32). On the other hand, the reduced signaling potential of Fcγ chain may be important for limiting the responsiveness of i-IELs and lymphokine-activated T cells to antigen. Thus, the restricted potential of different T cell populations to express and use specific members of the ζ family as components of the TCR could be a mechanism for regulating the T cell response to antigen.
Acknowledgments
We thank Amy Rosenberg, Ronald Schwartz, Dorothy Scott, and Melanie Vacchio for reading the manuscript and for helpful discussion.
Footnotes
Abbreviations used in this paper: DETC, dendritic epidermal T cell; DN, double negative; DP, double positive; Fcγ, FcεRIγ; FCM, flow cytometric analysis; i-IEL, intestinal-intraepithelial lymphocytes; ITAM, immune-recptor tyrosine-based activation motif; SP, single positive.
References
- 1.Klausner RD, Lippincott-Schwartz J, Bonifacino JS. The T cell antigen receptor. Annu Rev Cell Biol. 1990;6:403–431. doi: 10.1146/annurev.cb.06.110190.002155. [DOI] [PubMed] [Google Scholar]
- 2.Küster H, Thompson H, Kinet J-P. Characterization and expression of the gene for the human Fc receptor γ subunit. Definition of a new gene family. J Biol Chem. 1990;265:6448–6452. [PubMed] [Google Scholar]
- 3.Love PE, Shores EW, Johnson MD, Tremblay M, Lee EJ, Grinberg A, Huang SP, Singer A, Westphal H. T cell development in mice that lack the ζ chain of the T cell antigen receptor complex. Science. 1993;261:918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- 4.Malissen M, Gillet A, Rocha B, Trucy J, Vivier E, Boyer C, Kontgen F, Brun N, Mazza G, Spanopoulou E, et al. T cell development in mice lacking the CD3-ζ/η gene. EMBO (Eur Mol Biol Organ) J. 1993;12:4347–4355. doi: 10.1002/j.1460-2075.1993.tb06119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C-P, Ueda R, She J, Sancho J, Wang B, Weddell G, Loring J, Kurahara C, Dudley EC, Hayday A, et al. Abnormal T cell development in CD3-ζ−/−mutant mice and identification of a novel T cell population in the intestine. EMBO (Eur Mol Biol Organ) J. 1993;12:4863–4875. doi: 10.1002/j.1460-2075.1993.tb06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B. Altered T cell development in mice with a targeted mutation of the CD3ε gene. EMBO (Eur Mol Biol Organ) J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Boehmer H, Fehling HJ. Structure and function of the pre-T cell receptor. Annu Rev Immunol. 1997;15:432–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- 8.Guy-Grand D, Rocha B, Mintz P, Malassis-Seris M, Selz F, Malissen B, Vassalli P. Different use of T cell receptor transducing modules in two populations of gut intraepithelial lymphocytes are related to distinct pathways of T cell differentiation. J Exp Med. 1994;180:673–679. doi: 10.1084/jem.180.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flamand V, Shores EW, Tran T, Huang K, Lee E, Grinberg A, Kinet J-P, Love PE. Delayed maturation of CD4−CD8− FcγRII/III+T and NK cell precursors in FcεRIγ transgenic mice. J Exp Med. 1996;184:1725–1735. doi: 10.1084/jem.184.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiken H, Schulz R-J, Ravetch JV, Reinherz E, Koyasu S. T lymphocyte development in the absence of FcεRIγ subunit: analysis of thymus-dependent and independent αβ and γδ pathways. Eur J Immunol. 1996;26:1935–1943. doi: 10.1002/eji.1830260839. [DOI] [PubMed] [Google Scholar]
- 11.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcRγ chain deletion results in pleiotropic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 12.Liu C-P, Lin W-J, Huang M, Kappler JW, Marrack P. Development and function of T cells in T cell antigen receptor/CD3ζ knockout mice reconstituted with FcεRIγ. Proc Natl Acad Sci USA. 1997;94:616–621. doi: 10.1073/pnas.94.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shores E, Flamand V, Tran T, Grinberg A, Kinet J-P, Love PE. FcεRIγ can support T cell development and function in mice lacking endogenous TCR ζ chain. J Immunol. 1997;159:222–230. [PubMed] [Google Scholar]
- 14.Shores EW, Huang K, Tran T, Lee E, Grinberg A, Love PE. Role of TCR-ζ chain in T cell development and selection. Science. 1994;266:1047–1050. doi: 10.1126/science.7526464. [DOI] [PubMed] [Google Scholar]
- 15.Borkowski TA, Letterio JJ, Farr AG, Udey M C. A role for endogenous transforming growth factor β1 in Langerhans cell biology: the skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama T, Singer A, Hsi ED, Samelson LE. Intrathymic signalling in immature CD4+CD8+thymocytes results in tyrosine phosphorylation of the T cell receptor zeta chain. Nature. 1989;341:651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- 17.Crompton T, Moore M, MacDonald HR, Malissen B. Double-negative thymocyte subset in CD3ζ chain–deficient mice: absence of HSA+CD44−CD25−cells. Eur J Immunol. 1994;24:1903–1907. doi: 10.1002/eji.1830240828. [DOI] [PubMed] [Google Scholar]
- 18.Godfrey DI, Zlotnik A. Control points in early T-cell development. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- 19.Levelt CN, Mombaerts P, Iglesias A, Tonegawa S, Eichmann K. Restoration of early thymocyte differentiation in T-cell receptor beta-chain–deficient mutant mice by transmembrane signaling through CD3-ε. Proc Natl Acad Sci USA. 1993;90:11401–11405. doi: 10.1073/pnas.90.23.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levelt CN, Mombaerts P, Wang B, Kohler H, Tonegawa S, Eichmann K, Terhorst C. Regulation of thymocyte development through CD3: functional dissociation between p56lck and CD3 sigma in early thymic selection. Immunity. 1995;3:215–222. doi: 10.1016/1074-7613(95)90091-8. [DOI] [PubMed] [Google Scholar]
- 21.Robey E, Fowlkes BJ. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 22.Guidos CJ. Positive selection of CD4+ and CD8+T cells. Curr Opin Immunol. 1996;8:225–232. doi: 10.1016/s0952-7915(96)80061-6. [DOI] [PubMed] [Google Scholar]
- 23.Lefrançois L. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J Immunol. 1991;147:1746–1751. [PubMed] [Google Scholar]
- 24.Rocha B, Vassalli P, Guy-Grand D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J Exp Med. 1994;180:681–686. doi: 10.1084/jem.180.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson S, Hollander G, She J, Levelt C, Huang M, Terhorst C. Selection of peripheral and intestinal T lymphocytes lacking CD3. Int Immunol. 1995;7:287–293. doi: 10.1093/intimm/7.2.287. [DOI] [PubMed] [Google Scholar]
- 26.Allison JP, Havran W. The immunobiology of T cells with invariant γδ antigen receptors. Annu Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- 27.Shimada S. T cell receptor expression by dendritic epidermal T cells. J Dermatol (Tokyo) 1994;21:829–832. doi: 10.1111/j.1346-8138.1994.tb03297.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohno H, Aoe T, Taki S, Kitamura D, Ishida Y, Rajewsky K, Saito T. Development and functional impairment of T cells in mice lacking CD3ζ chains. EMBO (Eur Mol Biol Organ) J. 1993;12:4357–4366. doi: 10.1002/j.1460-2075.1993.tb06120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno H, Ono S, Hirayama N, Shimada S, Saito T. Preferential usage of the Fc receptor complex by γ/δ T cells localized in epithelia. J Exp Med. 1994;179:365–369. doi: 10.1084/jem.179.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald HR. NK1.1+ T cell receptor-αβ+cells: new clues to their orgin, specificity, and function. J Exp Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arase H, Ono S, Arase N, Park SY, Wakizaka K, Watanabe H, Ohno H, Saito T. Developmental arrest of NK1.1+ T cell antigen receptor (TCR)-αβ+ T cells and expansion of NK1.1+ TCR-γδ+T cell development in CD3ζ-deficient mice. J Exp Med. 1995;182:891–895. doi: 10.1084/jem.182.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shores EW, Tran T, Grinberg A, Sommers CL, Shen H, Love PE. Role of the multiple T cell receptor (TCR)-ζ chain signaling motifs in selection of the T cell repertoire. J Exp Med. 1997;185:893–900. doi: 10.1084/jem.185.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S-Y, Ardouin L, Gillet A, Malissen M, Malissen B. The single positive T cells found in CD3-ζ/η−/−mice overtly react with self-major histocompatabiltiy complex molecules upon restoration of normal surface density of T cell receptor–CD3 complex. J Exp Med. 1997;185:707–715. doi: 10.1084/jem.185.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh Y, Germain RN. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cytokine responses of CD4+T cells. J Exp Med. 1997;186:757–766. doi: 10.1084/jem.186.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curnow SJ, Boyer C, Buferne M, Schmitt-Verhulst A-M. TCR-associated ζ-FcεRIγ heterodimers on CD4−CD8− NK1.1+T cells selected by specific class I MHC antigen. Immunity. 1995;3:427–438. doi: 10.1016/1074-7613(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 36.Koyasu S, D'Adamio L, Arulanandam ARN, Abraham S, Clayton LK, Reinherz EL. T cell receptor complexes containing FcεRIγ heterodimers in lieu of CD3ζ and CD3η components: a novel isoform expressed in large granular lymphocytes. J Exp Med. 1992;175:203–209. doi: 10.1084/jem.175.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian D, Sperling AI, Lancki DW, Tatsumi Y, Barrett TA, Bluestone JA, Fitch FW. The gamma chain of the high-affinity receptor for IgE is a major functional subunit of the T-cell antigen receptor complex in gamma delta lymphocytes. Proc Natl Acad Sci USA. 1993;90:11875–11879. doi: 10.1073/pnas.90.24.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]