Abstract
Human Fas ligand (L) (CD95L) and tumor necrosis factor (TNF)-α undergo metalloproteinase-mediated proteolytic processing in their extracellular domains resulting in the release of soluble trimeric ligands (soluble [s]FasL, sTNF-α) which, in the case of sFasL, is thought to be implicated in diseases such as hepatitis and AIDS. Here we show that the processing of sFasL occurs between Ser126 and Leu127. The apoptotic-inducing capacity of naturally processed sFasL was reduced by >1,000-fold compared with membrane-bound FasL, and injection of high doses of recombinant sFasL in mice did not induce liver failure. However, soluble FasL retained its capacity to interact with Fas, and restoration of its cytotoxic activity was achieved both in vitro and in vivo with the addition of cross-linking antibodies. Similarly, the marginal apoptotic activity of recombinant soluble TNF-related apoptosis-inducing ligand (sTRAIL), another member of the TNF ligand family, was greatly increased upon cross-linking. These results indicate that the mere trimerization of the Fas and TRAIL receptors may not be sufficient to trigger death signals. Thus, the observation that sFasL is less cytotoxic than membrane-bound FasL may explain why in certain types of cancer, systemic tissue damage is not detected, even though the levels of circulating sFasL are high.
Tumor necrosis factor (TNF)-α is a potent proinflammatory and immunomodulatory agent. However, elevated concentrations of circulating TNF-α seen during many host responses may be harmful or even fatal to the organism. Membrane-bound TNF-α is synthesized as a type II membrane protein that acts locally through cell-to-cell contact. Soluble TNF-α (s)TNF-α1 is released from the cell surface as the result of metalloproteinase cleavage (1, 2). Although both cell surface and secreted TNF-α appear to be biologically active, it is the sTNF-α released into circulation that appears to be primarily responsible for the deleterious physiological responses such as cachexia and endotoxic shock.
Fas ligand (L), a member of the TNF family, is also processed and shed from the surface of human cells. FasL has been implicated in one of the major cytolytic pathways of cytotoxic T cells (3–5), and is a key element in the elimination of activated T cells during the downregulation of the immune response in the periphery (6, 7). Moreover, expression of FasL in nonlymphoid and tumor cells contributes to the maintenance of immune privilege of tissues by preventing the infiltration of Fas-sensitive lymphocytes (8– 12). Presently, it is not clear whether the activities described for the ligand are primarily due to the cell surface form of FasL or whether sFasL is likewise implicated. As seen for TNF-α, naturally processed and recombinant sFasLs were found to be active, inducing apoptosis in mouse cells at a concentration of <10 ng/ml (13–16). Elevated levels (up to 10 ng/ml) of sFasL were found in sera from patients with large granular lymphocytic leukemias and natural killer cell lymphomas (17). Since the administration of recombinant FasL or agonistic Fas antibodies into mice leads to liver failure and to rapid death of the animals (18, 19), it has been proposed that sFasL is implicated in the pathogenesis of various diseases such as hepatitis or AIDS (20–22). The possible implication of sFasL in pathological processes prompted us to investigate the structure and activities of naturally processed sFasL in more detail. Here we report that, in contrast to membrane-bound FasL, the soluble ligand is only poorly active. A model is suggested whereby the apoptotic activity of the membrane-bound ligand is downregulated upon processing and release. This assures that circulating sFasL does not cause systemic tissue damage.
Materials and Methods
Materials.
The anti-Flag M2 monoclonal antibody and the anti-Flag M2 antibody coupled to agarose were purchased from Eastman Kodak Co. (Rochester, NY). Protein A–Sepharose and cyanogen bromide–activated Sepharose were purchased from Pharmacia (Uppsala, Sweden). Affinity-purified anti-FasL rabbit polyclonal antibody (anti-PE62) was obtained as described (23). Biotinylated anti-Fas antibody Jo-2 was purchased from PharMingen (San Diego, CA), and dichlorotriazinglaminofluorescein (DTAF)- labeled streptavidin and anti–mouse antibodies were purchased from Jackson ImmunoResearch (Milan, La Roche, Switzerland). Cell culture reagents were obtained from Life Sciences (Basel, Switzerland). Human (h) TNF-related ligand that weakly induces apoptosis (TWEAK) cDNA (24) and recombinant hIFN-γ were provided by J. Browning (Biogen, Cambridge, MA), and murine (mu)TNF-α cDNA was a gift from C.V. Jongeneel (Institut Suisse de Recherche Experimentale sur le Cancer, Epalinges, Switzerland). Soluble human TNF-related apoptosis-inducing ligand (TRAIL) and the fusion protein between the extracellular domain of hFas and the Fc portion of hIgG1 were produced as previously described (25, 26). muFasL-expressing Neuro-2a cells were described earlier (19).
Cells.
Murine B lymphoma A20 and A20R cells (11) were maintained in DMEM containing 5% heat-inactivated FCS. The human T lymphoblastoma Jurkat cells, colon adenocarcinoma HT-29 cells, BJAB Burkitt lymphoma, and murine fibrosarcoma WEHI-164 cells were grown in RPMI supplemented with 10% FCS. Human embryonic kidney cells 293 were cultured in DMEM–nutrient mix F12 (1:1) supplemented with 2% FCS. 293T cells were grown in DMEM, 10% FCS. All media contained antibiotics (penicillin and streptomycin at 5 μg/ml each and neomycin at 10 μg/ml). The IL-2–dependent murine cytotoxic T cell line CT6 (27), a gift of P. Vandenabeele (University of Ghent, Ghent, Belgium), was grown in RPMI supplemented with 10% FCS, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 10 mM Hepes, and 10% conditioned EL-4 cell supernatant.
Vector Construction and Establishment of Cell Lines Expressing Ligands of the TNF Family.
hFasL was amplified by PCR from activated peripheral blood lymphocyte cDNA using the following oligonucleotides: JT289 5′-CCTCTACAGGACTGAGAAGAAG-3′ and JT290 5′-CAACATTCTCGGTGCCTGTAAC-3′. muFasL was amplified by PCR from mouse testis cDNA with primers JT277 5′-AAGGCTGTGAGAAGGAAACC-3′ and JT271 5′-AAGGATCCTAGCTGACCTGTTGGACCTTGC-3′. The PCR products were subcloned into PCR-II TA-cloning vector and then resubcloned as EcoRI fragments into the PCR-III mammalian expression vector (Invitrogen, NV Leek, the Netherlands). Point mutations in FasL were obtained by two rounds of PCR using the following oligonucleotide pairs: for L126E, T7/ JT915 5′-GCTTCTCGAGCTCTGATGCTGTGTGCATC-3′ and JT914 5′-CAGAGCTCGAGAAGCAAATAGGC-3′/Sp6; for S127E, T7/JT917 5′-CAGAGCTCGCTGTGTGCATCTGG-3′ and JT916 5′-GCACACAGCGAGCTCTGAGGAGAAGCAAATAGGC-3′/Sp6; for SL126-127EE, T7/JT963 5′-GCTTCTCCTCCTCGCTAGCTGTGTGCATCTGG-3′ and JT962 5′-AGCGAGGAGGAGAAGCAAATAGG-3′/Sp6.
The extracellular domains of hTWEAK (amino acids [aa] 141–284), muTNF-α (aa 76–235), and hTNF-α (aa 85–233) were amplified by PCR using the oligonucleotide pairs JT560 5′-CTGCAGAAGGAGTGGCGATCGCAGCCCATTATG-3′/ JT561 5′-TCTAGATCAGTGAACCTGGAA-3′, JT408 5′-CCTGCAGACCCTCACACTC-3′/JT409 5′-CTCGAGTCACAGAGCAAGGAC-3′, and JT437 5′-TCTGCAGAGTGACAAGCCTGTAG-3′/JT423 5′-ACTAGTTCACAGGGCAATGAT-3′, respectively, followed by subcloning into the PCR-II vector. muTNF-α and TWEAK were subcloned into a modified version of the PCR-III vector (26) in frame with the hemagglutinin signal peptide and Flag epitope, using the PstI site and the suitable 3′ restriction site. muTNF-α and hTNF-α were subcloned into a modified pQE9 bacterial expression vector (Qiagen, Basel, Switzerland) containing the Flag sequence between the BamHI and PstI sites, using the PstI site and the suitable 3′ restriction site. The vector for Flag tagged, soluble secreted hFasL (residues 103– 281) was prepared as previously described (26). Stably transfected 293 cells were obtained by selection in 800 μg/ml G418 (60% active) for 2 wk and then cloned. Supernatants of stably transfected clones were harvested after 10–12 d in culture and screened by Western blotting for expression levels of the protein of interest. For the production of recombinant proteins, selected clones were grown for 14 d in DMEM–nutrient mix F12 (1:1) (Life Technologies, Basel, Switzerland) supplemented with 2% FCS, after which time conditioned supernatants were collected.
Purification of Recombinant Proteins.
Flag tagged recombinant proteins were purified on M2-agarose and eluted in 50 mM citrate-NaOH, pH 2.5, followed by neutralization with 0.2 volumes of 1 M Tris-HCl, pH 8. The buffer was exchanged to PBS in concentrators (Centrikon-30; Amicon Corp., Easton, TX). Soluble hFasL released from cells transfected with full-length FasL was subjected to phase separation in Triton X-114 (28) and the aqueous phase was then purified using human Fas with human immunoglobulin Fc (hFas/Fc)–Sepharose. hFas/Fc–Sepharose was prepared by coupling 4 mg of Fas/Fc to 1 ml cyanogen bromide–activated Sepharose according to the manufacturer's instructions. Bound FasL was eluted as described above for M2-agarose. The concentration of purified proteins was determined by the bicinchoninic acid method (Pierce Chemical Co., Rockford, IL) using bovine serum albumin as standard, and the purity of the samples was assessed by SDS-PAGE and Coomassie blue staining. The concentration of FasL in Neuro-2a cells supernatants was estimated by Western blotting with anti-FasL antibodies using known amounts of recombinant sFasL as standard.
Protein Sequencing.
Purified soluble FasL was blotted onto polyvinylidene difluoride membrane (BioRad Labs., Hercules, CA) as previously described (29), and then sequenced using a gas phase sequencer (ABI 120A; Perkin Elmer, Foster City, CA) coupled to an analyzer (ABI 120A; Perkin Elmer) equipped with a phenylthiohydantoin C18 2.1 × 250 mm column. Data was analyzed using software (ABI 610; Perkin Elmer).
Triton X-114 Extraction of Cells and Phase Separation.
293T cells transiently transfected with full-length hFasL (wild type and mutants), muFasL, or empty plasmid were transferred in Opti-MEM medium for 5 d. Supernatants were harvested, and the cells were lysed in 2% precondensed Triton X-114 in PBS (28) containing a protease inhibitor cocktail (Cømplete™; Boehringer Mannheim GmbH, Mannheim, Germany) and subjected to phase separation. Proteins were recovered free of detergent by precipitation with 1.25 volume CHCl3/MeOH (1:4, vol/vol) before Western blotting analysis.
Western Blotting.
FasL blotted onto nitrocellulose membranes was detected with anti-PE62 (2 μg/ml, in blocking buffer: PBS, 0.5% Tween 20, and 4% skimmed milk), followed by goat anti– rabbit immunoglobulins coupled to horseradish peroxidase. Peroxidase activity was detected by enhanced chemiluminescence.
Gel Permeation Chromatography.
The supernatant of Neuro-2a cells expressing FasL was concentrated 5 times, and then 200 μl was loaded onto a column (Superdex-200 HR10/30; Pharmacia). Proteins were eluted in PBS at 0.5 ml/min and fractions were analyzed by Western blotting and cytotoxic assays. The column was calibrated with standard proteins ferritin (440 kD), bovine serum albumin (67 kD), and ribonuclease A (13.7 kD).
Cytotoxic Assay.
The cytotoxic assay was performed essentially as described previously (26). In brief, 50,000 cells were incubated for 16 h in 100 μl medium containing the indicated concentrations of ligands in the presence or absence of 1 μg/ml M2 antibody (2 μg/ml for TRAIL). For HT-29 cells, incubation was carried out for 40 h in the presence of 50 ng/ml of hIFN-γ. Cell viabilities were determined using PMS/MTS (phenazine methosulfate 3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]- 2-[4-sulfophenyl]-2H-tetrazolium, inner salt) reagents (Promega Corp., Madison, WI), and color was allowed to develop as necessary (typically 1–3 h). The absorbance was measured at 490 nm. Chromium release assay was performed according to standard protocols.
Cell Proliferation Assay.
CT6 cells were grown for 4 d in the presence of a reduced concentration of EL-4 supernatant (2.5%) before the proliferation experiment. Cells were incubated in 96-well plates (40,000 cells/well) for 16 h with the indicated concentrations of muTNF-α or hTNF-α, in the presence or in the absence of 2 μg/ml of M2 mAb, and in the absence of EL-4 supernatant. Cells were pulsed for an additional 6 h with [3H]thymidine (0.5 μCi/well), exposed to three cycles of freezing and thawing, and harvested. [3H]thymidine incorporation was monitored by liquid scintillation counting.
In Vitro Fas–FasL Binding Assay.
ELISAs were performed as previously described (26) except that FasL incubation was done in the presence or in the absence of 1 μg/ml of M2 antibody.
FACS® Staining.
Cells expressing Fas (A20, Jurkat) or not expressing Fas (A20R) were stained in 25 μl FACS® buffer (PBS, 10% FCS, 0.02% NaN3) with 2.5 μg of Flag tagged soluble hFasL for 20 min at 4°C, followed by anti-Flag M2 (1.5 μg) and DTAF-labeled anti–mouse antibody. A20 cells were also stained with 1 μg biotinylated Jo-2 antibody followed by streptavidin-DTAF.
Treatment of Mice with sFasL, sTRAIL, sTNF-α, and α-Flag M2 Antibody.
Female BALB/c mice (8 wk old) were injected intravenously with 12.5 μg sFasL, sTNF-α, or sTRAIL diluted in 200 μl Ringer's solution. 10 min later, mice were treated intravenously with antibodies (50 μg, anti-Flag M2, or isotype Ab in 200 μl Ringer's solution). Mice were autopsied 24 h later or immediately after death. Fragments of tissues were fixed with 4% paraformaldehyde in PBS, embedded in paraffin, and 4-μm sections were stained with hematoxylin and eosin. DNA fragmentation in paraffin sections was visualized by terminal uridyltransferase nicked ends labeling (TUNEL) reaction according to the manufacturer's instructions (Apop Tag Peroxidase kit; Oncor Appligene, Basel, Switzerland).
Results and Discussion
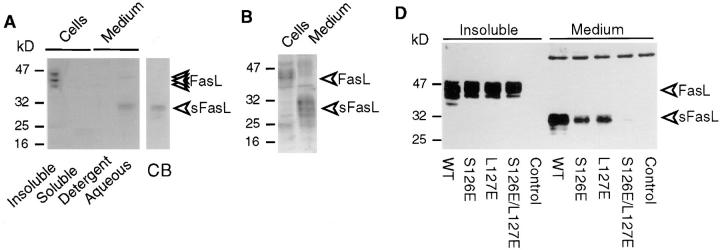
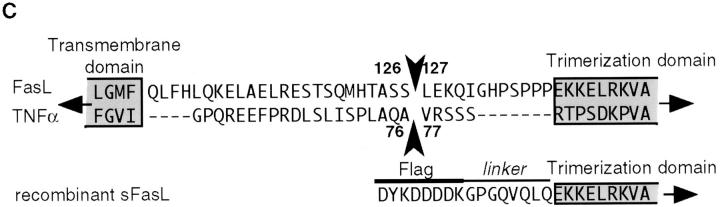
An uncharacterized metalloproteinase cleaves the 40-kD membrane-bound FasL to generate the 26–29 kD-soluble fragment (13, 14, 17). In agreement with these published results, we found that processed sFasL was detectable in supernatants of 293 cells transiently or stably transfected with the full-length human FasL (Fig. 1 A) and that this release was inhibited upon addition of metalloproteinase inhibitors (data not shown). Murine FasL underwent a similar conversion to soluble FasL (Fig. 1 B). By gel filtration chromatography, native sFasL of both species had an apparent molecular size of ∼70 kD, indicating that the released ligand had a homotrimeric structure (Fig. 2 B, and data not shown). Subsequent isolation of sFasL by Fas/Fc affinity chromatography and NH2-terminal sequence analysis revealed that sFasL begins with Leu127, suggesting that the cleavage occurs between Ser126 and Leu127 (Fig. 1 C). Cleavage in TNF-α occurs in the equivalent region between Ala76 and Val77, and is mediated by at least two zinc metalloproteases (TNF-α converting enzyme [TACE], and a disintegrin and metalloprotease 10 [AD10 or ADAM10]; references 30–32). Interestingly, the processing activity of TNF-α is only marginally affected by mutations Ala76Ser and Val77Leu (32), suggesting that the Ser–Leu site of FasL may also be a substrate of the TNF-α–converting proteases, in agreement with the observation that inhibitors known to block TNF-α conversion also inhibit FasL release (14, 15, 17). In contrast, mutations of the flanking amino acids Ala76 and Val77 to acidic residues influences the processing of TNF-α. We performed similar mutation experiments with FasL (Fig. 1 D). Mutations at the P1 (Ser126Glu) and P1′ (Leu127Glu) sites partially prevented the release of sFasL, whereas the double mutation (Ser126Glu, Leu127Glu) resulted in the loss of detectable sFasL, indicating that the metalloproteinase present in 293 cells uses this cleavage site. While this manuscript was under revision, Tanaka et al. reported on the identification of the cleavage of FasL transfected into WR19L cells (33). Their analysis revealed that membrane-bound human FasL expressed in the WR19L murine T cell line was cleaved between Lys129 and Gln130, three amino acid residues downstream of the cleavage site determined in this paper. Surprisingly, the mutation of their P1 residue Lys130 into Ala had no effect on the processing of FasL, and FasL resistant to cleavage was only generated after deletions of several amino acid residues, which probably affect or even delete the Ser126–Leu127 processing site. This suggests either that the murine metalloproteinase present in WR19L cells has a distinct specificity from its human homologue present in 293 cells, or that the sFasL found in WR19L cell supernatants has undergone further proteolytic processing by a trypsin-like protease.
Figure 1.
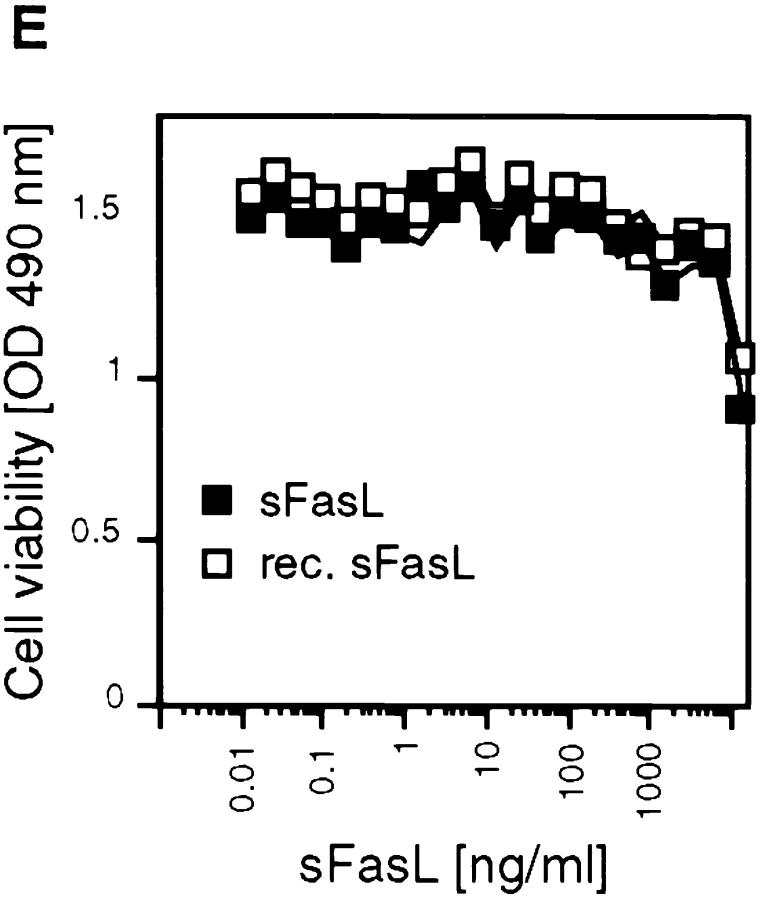
Recombinant and naturally processed sFasLs show marginal apoptotic activity on human cells. (A) Processing of membrane-bound FasL to sFasL. Cellular extracts of a 293 cell clone stably transfected with human FasL cDNA, were separated into Triton X-114–insoluble (Insoluble) and –soluble (Soluble) fractions. Cell supernatants (Medium) were separated into detergent and aqueous fractions by Triton X-114 phase separation. Samples were analyzed by Western blotting for the presence of FasL using an antibody specific for the extracellular segment of the ligand. The indicated FasL and sFasL bands were absent in mock transfected controls (D, and data not shown). The processed sFasL was purified by Fas/Fc affinity chromatography and visualized by Coomassie blue staining (CB). (B) 293 cells were transfected with mouse FasL cDNA. Total, unfractionated cellular extracts and cell supernatants were analyzed by Western blotting as described for A. (C) Site of proteinase-induced cleavage of FasL. Metalloproteinase(s) present in 293 cells cleave(s) membrane-bound FasL at the Ser126–Leu127 bond, liberating a soluble form of FasL. The cleavage site of the TNF-α precursor is shown for comparison. (D) The effect of cleavage site mutations on FasL processing. Wild-type (WT) FasL as well as FasL mutated at the P1 site (S126E), at the P1′ site (L127E), and at both the P1 and P1′ sites (S126E/L127E) were transiently transfected into 293T cells. Insoluble proteins (containing membrane-associated FasL) and medium (containing processed sFasL) were analyzed by Western blotting as described above. To achieve similar loading of soluble and cell-associated FasL, the supernatants corresponding to 10 times more cells were loaded on the gel. A nonspecific band at 60 kD indicates equal loading of the gel. In the control lane, cell extracts of nontransfected 293 cells were analyzed. (E) Activity of naturally processed (open squares) and recombinant (closed squares) sFasL on Jurkat T cells.
Figure 2.
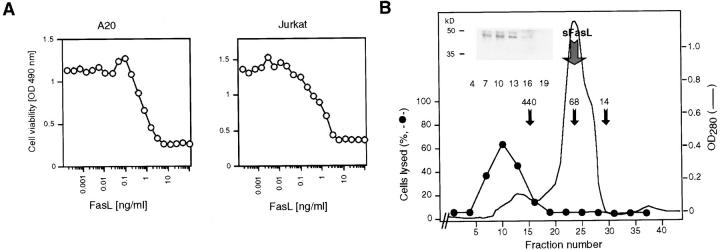
(A) Characterization of FasL in supernatants of Neuro-2a cells transfected with muFasL. Crude supernatants were assayed for FasL activity on mouse A20 B cells and human Jurkat T cells. (B) Gel filtration analysis of FasL. Supernatants were concentrated and loaded onto a column (Superdex 200; Pharmacia). Fractions were analyzed for FasL by Western blotting in the presence of SDS (inset), for cell death–inducing activity (closed circles), and for protein (lines). Molecular size markers and the position of sFasL elution are indicated.
Unexpectedly, the specific activity of sFasL after its purification and removal of residual membrane-bound FasL by Triton X-114 extraction was marginal. Human Jurkat T cells, which are highly sensitive to apoptosis induced by anti-Fas antibodies (34), remained viable at sFasL concentrations exceeding 1 μg/ml (Fig. 1 E). Recombinant sFasL corresponding in length to the naturally processed form was equally inactive (Fig. 1, C and E). This low activity was surprising in view of several previous reports showing that recombinant sFasL or supernatants of cells overexpressing FasL were very active. One well-described example is the highly apoptotic supernatant of Neuro-2a cells transfected with murine FasL (19), which induces apoptosis in human Jurkat or mouse A20 cells at concentrations as low as 1 ng/ml (Fig. 2 A). Western blot analysis (Fig. 2 B) revealed that the recombinant FasL detected in these supernatants was not the processed sFasL, but corresponded to the membrane-bound, unprocessed form (Fig. 2 B). Accordingly, the active fractions were eluted in the void volume (>500 kD) of a gel filtration column (Superdex 200; Pharmacia) and not at the expected molecular weight of trimeric sFasL (70 kD), indicating that the high apoptotic activity was associated with aggregated forms of FasL. It is likely that Neuro-2a cells express little or no processing proteases, and that membrane fragments or vesicles containing the membrane-bound FasLs are released into the supernatant. This conclusion was corroborated by the observation that after ultracentrifugation (100,000 g for 2 h), the activity was recovered in the pellet (data not shown). Moreover, most of the activity present in some batches of recombinant sFasL was attributable to small quantities of contaminating aggregates of sFasL (data not shown).
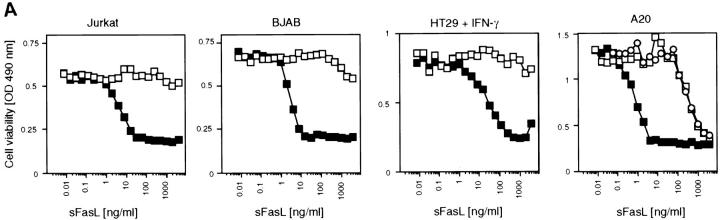
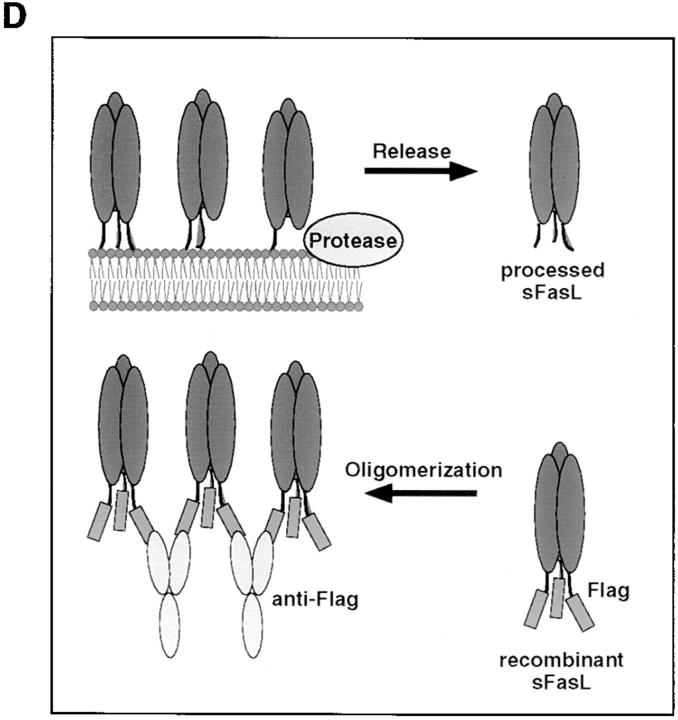
Since aggregated or membrane-bound FasL was considerably more active than trimeric sFasL, we reasoned that controlled cross-linking of sFasL, mimicking membrane-bound FasL (Fig. 3 D), should lead to an increase in activity. We took advantage of the presence of a Flag tag on the recombinant sFasL to induce sFasL aggregation. Fig. 3 A shows that the mere addition of cross-linking anti-FasL antibodies resulted in a >1,000-fold activity increase of recombinant sFasL on Jurkat cells. Other cell lines tested responded identically. Human cells that were insensitive to sFasL up to 1 μg/ml underwent apoptosis at concentrations as low as 1–10 ng/ml (BJAB B lymphoma cells, human colon carcinoma HT29) with aggregated sFasL. In agreement with the higher affinity of human sFasL to mouse Fas (26), we found that mouse cell lines were generally more sensitive to FasL-mediated apoptosis, as exemplified by the A20 B lymphoma cell. A20 cells underwent apoptosis in the absence of cross-linking antibodies at 100 ng/ml. This (and the presence of contaminating aggregates) may explain previous results showing that recombinant sFasL produced in yeast was active at 1–10 ng/ml concentrations on the murine W4 cell line (human cells were not tested; reference 17).
Figure 3.
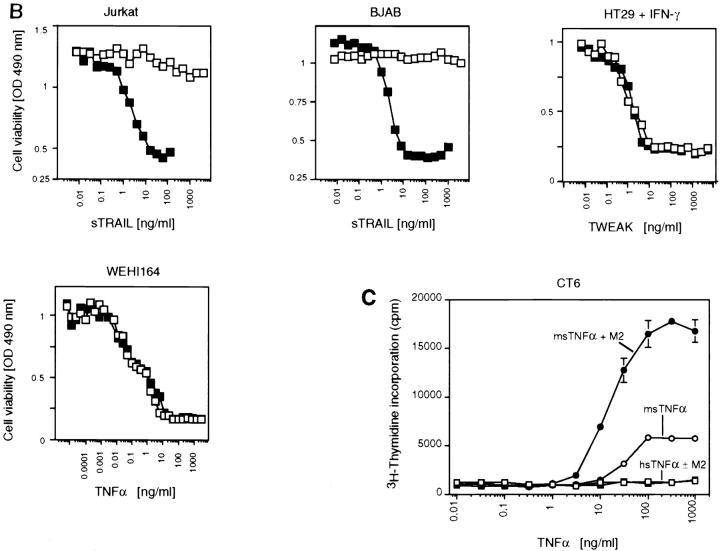
Increase of the specific apoptotic activity of sFasL and sTRAIL, but not sTWEAK, and differential modulation of sTNF-α activity upon cross-linking of the ligands. (A) Various cell lines (HT29, human colon carcinoma; Jurkat, human T cell leukemia; BJAB, human B cell lymphoma; A20, mouse B cell lymphoma) were assayed for their sensitivity to Flag tagged sFasL in the presence (closed squares) and absence (open squares) of aggregating anti-Flag antibodies. The effect of naturally processed sFasL on A20 cells is also shown (open circles). (B) The apoptosis-inducing effects of the ligands sTRAIL, sTNF-α, and sTWEAK were assayed in the presence (closed squares) and absence (open squares) of aggregating anti-Flag antibodies. Most cells are insensitive to the cytotoxic activity of these ligands, and the corresponding results were therefore not included. (C) The proliferative response of CT6 cells to murine (circles) and human (squares) sTNF-α was monitored in the presence (closed symbols) and absence (open symbols) of aggregating anti-Flag antibodies (M2) (the data shown are means ± SD, n = 3). (D) Model of FasL shedding by metalloproteases and oligomerization of Flag tagged sFasL by anti-Flag antibodies.
We tested whether other members of the TNF ligand family known to induce apoptosis also required cross-linking for their activity (Fig. 3 B). Similar to FasL, TRAIL (35, 36) was active at low concentrations only in the presence of cross-linking antibodies (Fig. 3 B). No apoptosis was observed with sTRAIL at concentrations as high as 5 μg/ml. In contrast, cross-linking of sTWEAK (24) did not increase its activity (Fig. 3 B), suggesting that this ligand can exert its apoptotic activity in the soluble form. Interestingly, the activity of sTNF-α was differentially influenced by the presence of cross-linking antibodies depending on the receptor triggered. To distinguish between TNFR1- and TNFR2-mediated effects, we took advantage of the fact that human TNF-α only binds to murine TNFR1, but not to murine TNFR2 (37). When TNF-α was added to WEHI164 cells, which are known to undergo apoptosis upon stimulation of TNFR1, the activity was independent of the presence of cross-linking antibodies (Fig. 3 B). In contrast, the proliferative effect of TNF-α on mouse CT6 cells, which is mediated through TNFR2-dependent signaling pathways and is therefore only observed in response to murine TNF-α, was highly increased in the presence of cross-linking antibodies (Fig. 3 C). This is in line with results demonstrating that membrane-bound TNF-α is the prime activating ligand for TNFR2-mediated responses (38).
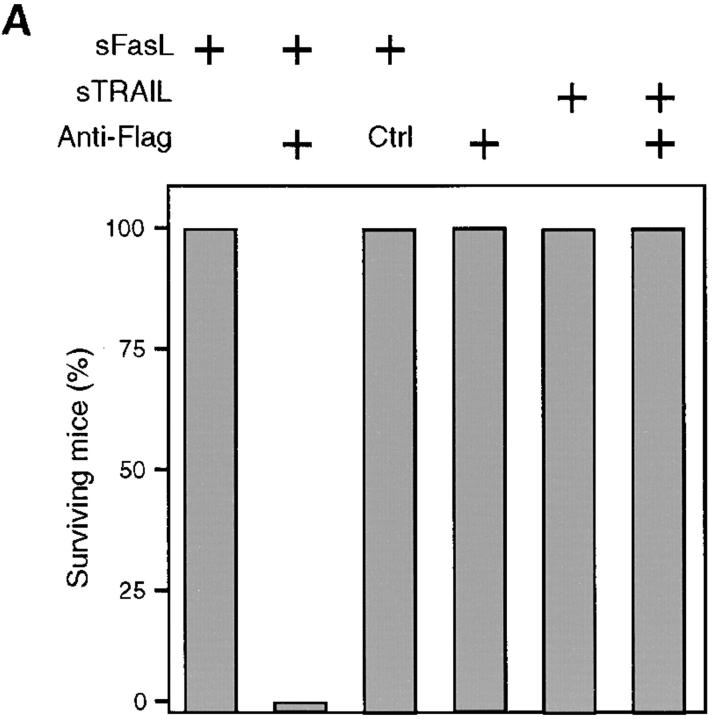
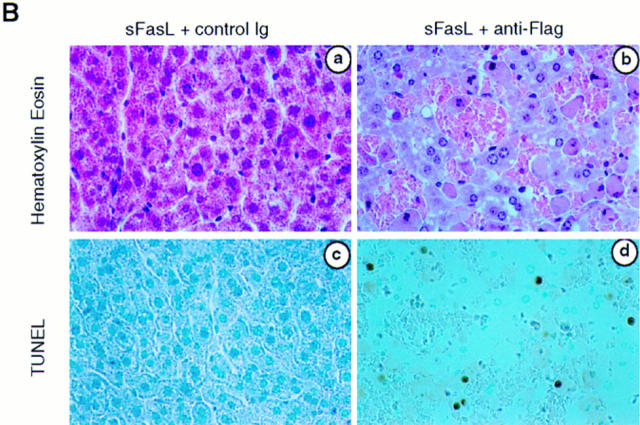
These results suggested that sFasL and sTRAIL should be either not or only poorly active in vivo. Indeed, mice injected with 12.5 μg of sFasL or sTRAIL survived and showed no signs of sickness (Fig. 4 A). In contrast, injection of sFasL followed by cross-linking anti-Flag antibodies was fatal and mice died within 3 h, similar to mice treated with agonistic anti-Fas antibodies (18) or with FasL contained in supernatants of transfected Neuro-2a cells (19). The cross-linked FasL induced lethal liver hemorrhages and hepatocyte apoptosis (Fig. 4 B), whereas in the absence of the anti-Flag antibody, hepatocytes had a normal morphology (Fig. 4 B). The administration of the anti-Flag antibody alone, or of sFasL with an isotype-matched irrelevant antibody, also had no effect. Interestingly, treatment of mice with cross-linked sTRAIL was not lethal, despite the broad expression of the two cytotoxic TRAIL receptors (39–42). Whether this is due to the expression of the inhibitory TRAILR3 (39, 43, 44), TRAILR4 (45), or other inhibitory molecules remains to be determined.
Figure 4.
sFasL is not active in vivo. (A) BALB/c mice (female, 8 wk old) were injected intravenously with sFasL or sTRAIL (12.5 μg). Within 10 min, anti-Flag antibodies (50 μg) or control IgG1 (Ctrl) antibodies (50 μg) were injected using the same route of administration. Mice receiving sFasL and anti-Flag antibodies (four mice) died within 3 h (mean survival: 2.25 ± 0.5 h). Injection of sFasL alone (four mice), sFasL followed by control IgG1 (two mice), anti-Flag alone (two mice), sTRAIL alone (four mice), or sTRAIL plus anti-Flag antibody (four mice) did not lead to death or to any sign of sickness of the animals. (B) Hepatic injury of mice treated with sFasL in the presence of anti-Flag antibodies or of a murine control antibody (IgG1). 24 h later (sFasL + control antibodies) or immediately after death (sFasL + anti-Flag antibodies), livers were fixed with 4% formaldehyde and embedded in paraffin. Sections were either stained with hematoxylin and eosin, or used for in situ DNA end labeling using the terminal uridyl transferase nicked ends labeling (TUNEL) method. Normal liver tissue is seen in a, whereas hepatocytes with pyknotic and fragmented nuclei are surrounded by red blood cells in b. In situ DNA end labeling confirms apoptotic cell death in d.
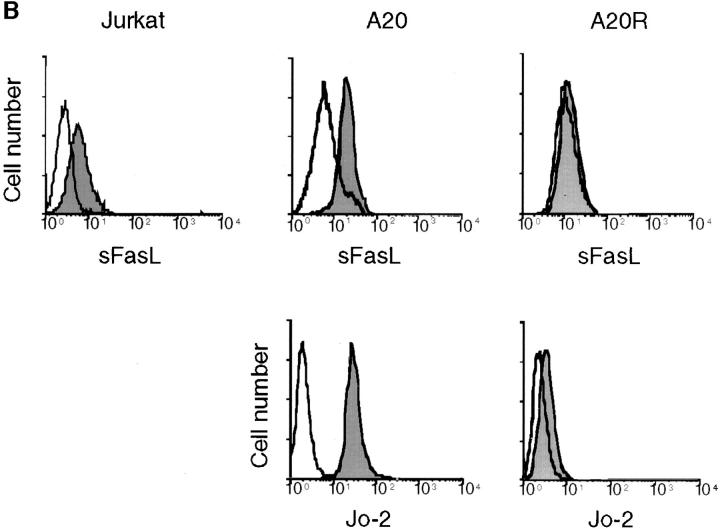
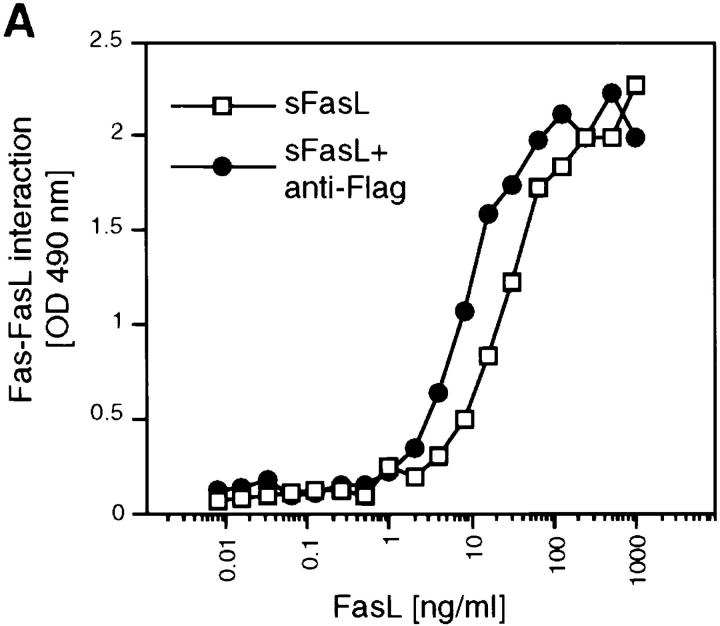
There are two possibilities to explain the absence of apoptotic activity of sFasL. Either trimeric sFasL displays a low affinity for its receptor Fas, which is increased upon aggregation and multimeric binding as seen for many protein– protein interactions involving multivalent partners (46), or trimerization of Fas is not sufficient to transmit FasL-mediated death signal (in contrast to TWEAK receptor and TNFR1). We measured sFasL binding to Fas fused to the human constant region of human IgG (Fas/Fc) in the presence or in the absence of aggregating antibody (Fig. 5 A), and to cells (Fig. 5 B). Binding of trimeric sFasL was observed in both cases, and aggregation of sFasL did not significantly increase binding efficiency to Fas/Fc.
Figure 5.
Trimeric sFasL binds to Fas receptor. (A) Interaction of Flag– sFasL and Fas/Fc. Wells of microtiter plates were coated with fusion proteins of Fas/Fc and incubated with either Flag-sFasL or anti-Flag–induced cross-linked Flag–sFasL. After several washings, binding of the ligands was determined by the addition of anti-Flag antibodies. (B) Binding of sFasL to Fas on Jurkat T cells, A20 B cell lymphomas, and an A20 cell that has lost Fas surface expression (A20R) was analyzed by flow cytometry using tagged FasL or anti-muFas Jo-2 antibodies. The open curves were obtained when the ligand or Jo-2 antibody was omitted.
Most of the members of the TNF ligand family are initially membrane bound, but can undergo processing and shedding by metalloendoproteinases. The efficacy of the ligand release varies. Membrane-bound TNF-α is immediately processed by proteolytic cleavage and the soluble form released into the circulation can exert its various activities locally and systemically. On the other end of the spectrum stands CD40L, whose physiological activities, such as B cell help, completely depend on the membrane form, and for which processing has not been reported (47). Local cell to cell contact appears to be critical for T cell– specific immunosurveillance and, indeed, FasL-mediated killing of virus-infected or tumor target cells is a highly specific process (4) that assures that neighboring cells or tissues are not affected. This specificity can only be guaranteed if the cytotoxic ligand remains associated with the lymphocytes. However, FasL is rapidly released from cells and detectable levels of sFasL have been found in sera from patients with large granular lymphocytic leukemias, NK lymphomas (17), or melanoma (11). Considering the hepatic toxicity of FasL, circulating sFasL, if active, would be pathological. Neither patients with tumors nor individuals with high numbers of activated T cells (for example, after a viral infection) do suffer from hepatitis, implying that sFasL in vivo is at best poorly active, in agreement with our results. We cannot exclude, however, that high local concentrations of sFasL, in conjunction with other cytotoxic agents, may lead to cell death.
Our experiments also suggest that the mere trimerization of Fas by FasL may not suffice to transmit death signals. It is thought that the trimeric FasL induces Fas trimer formation, followed by the binding of Fas-associated death domain–containing protein (FADD) to the death domain of Fas (48–50). FADD then recruits the caspase FADD homologous IL-1β converting enzyme (ICE)-like protease (FLICE)/MACH/caspase 8 (51, 52), providing the connection of death receptors to caspases (53). The exact stoichiometry of adapters mediating caspase activation has, however, never been determined, and it is possible that multiple Fas trimers are required to allow the assembly of multiple FADD adapters bringing several FLICE proteins together and allowing reciprocal cleavage and subsequent activation of the caspase. The requirement of the formation of large aggregates of Fas for signal transduction is also suggested by the efficacy of agonistic antibodies of the IgM and IgG3 type, which are known to be multimeric or to form aggregates (54).
It could be predicted from the results presented here that circulating cells expressing membrane-bound FasL are more potent inducers of systemic tissue damage, and that the conversion of the ligand into the soluble form serves to downregulate the potentially harmful apoptotic activity. If true, the proposed use of metalloproteinase inhibitors to treat systemic tissue damage caused by circulating sFasL (17), which has proved efficient in the case of TNF-α (1, 2), could therefore result in an exacerbation rather than an amelioration of the disease in the case of FasL.
Acknowledgments
We thank S. Hertig (University of Lausanne, Epalinges, Switzerland), T. Bornand (University of Lausanne, Epalinges, Switzerland), S. Muljana (University Hospital, Zürich, Switzerland), and S. Aslan (University of Lausanne, Epalinges, Switzerland) for technical and secretarial assistance, S. Foley (Biogen, Cambridge, MA) and J. Browning (Biogen, Cambridge, MA) for protein sequence analysis, and S. Belli (University of Lausanne, Epalinges, Switzerland) for reading the manuscript.
This work was supported by grants from the Swiss National Science Foundation (to J. Tschopp and A. Fontana; NFP38), the Swiss Federal Office of Public Health (to P. Schneider and J. Tschopp), and from the Human Frontier Science (to M. Hahne).
Footnotes
1 Abbreviations used in this paper: aa, amino acids; DTAF, dichlorotriazinglaminofluorescein; FADD, Fas-associated death domain–containing protein; Fas/Fc, Fas with human immunoglobulin Fc; h, human; L, ligand; mu, murine; s, soluble; TRAIL, TNF-related apoptosis-inducing ligand; TWEAK, TNF-related ligand that weakly induces apoptosis.
References
- 1.Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS, Torrance DS, Otten-Evans C, Greenstreet T, Weerawarna K, et al. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- 2.McGeehan GM, Becherer JD, Bast RC, Jr, Boyer CM, Champion B, Connolly KM, Conway JG, Furdon P, Karp S, Kidao S, et al. Regulation of tumour necrosis factor–α processing by a metalloproteinase inhibitor. Nature. 1994;370:558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- 3.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell–mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 4.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 5.Braun MY, Lowin B, French L, Acha-Orbea H, Tschopp J. Cytotoxic T cells deficient in both functional Fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J Exp Med. 1996;183:657–661. doi: 10.1084/jem.183.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell JH, Rush B, Weaver C, Wang R. Mature T cells of the autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA. 1993;90:4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 8.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 9.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand–induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 10.French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 12.Strand S, Hofmann WJ, Hug H, Müller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR. Lymphocyte apoptosis induced by CD95 (Apo-1/Fas) ligand-expressing tumor cells: a mechanism of immune evasion? . Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO (Eur Mol Biol Organ) J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariani SM, Matiba B, Baumler C, Krammer PH. Regulation of cell surface APO-1/Fas (CD95) ligand expression by metalloproteases. Eur J Immunol. 1995;25:2303–2307. doi: 10.1002/eji.1830250828. [DOI] [PubMed] [Google Scholar]
- 15.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariani SM, Matiba B, Sparna T, Krammer PH. Expression of biologically active mouse and human cd95/apo-1/fas ligand in the baculovirus system. J Immunol Methods. 1996;193:63–70. doi: 10.1016/0022-1759(96)00051-8. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, et al. Fas ligand in human serum. Nat Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 18.Ogasawara J, Watanabe FR, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 19.Rensing-Ehl A, Frei K, Flury R, Matiba B, Mariani SM, Weller M, Aebischer P, Krammer PH, Fontana A. Local Fas/APO-1 (CD95) ligand-mediated tumor cell killing in vivo. Eur J Immunol. 1995;25:2253–2258. doi: 10.1002/eji.1830250821. [DOI] [PubMed] [Google Scholar]
- 20.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 21.Galle PR, Hofmann WJ, Walzak H, Schaller H, Otto G, Stremmel W, Krammer HP, Runkel L. Involvement of the CD95 (Apo-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ameisen JC, Estaquier J, Idziorek T, DeBels F. The relevance of apoptosis to AIDS pathogenesis. Trends Cell Biol. 1995;5:27–32. doi: 10.1016/s0962-8924(00)88933-3. [DOI] [PubMed] [Google Scholar]
- 23.Hahne M, Peitsch MC, Irmler M, Schroter M, Lowin B, Rousseau M, Bron C, Renno T, French L, Tschopp J. Characterization of the non-functional Fas ligand of gld mice. Int Immunol. 1995;7:1381–1386. doi: 10.1093/intimm/7.9.1381. [DOI] [PubMed] [Google Scholar]
- 24.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 25.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 26.Schneider P, Bodmer JL, Holler N, Mattmann C, Scuderi P, Terskikh A, Peitsch MC, Tschopp J. Characterization of Fas (Apo-1, CD95)-Fas ligand interaction. J Biol Chem. 1997;272:18827–18833. doi: 10.1074/jbc.272.30.18827. [DOI] [PubMed] [Google Scholar]
- 27.Ranges GE, Bombara MP, Aiyer RA, Rice GG, Palladino MA., Jr Tumor necrosis factor-alpha as a proliferative signal for an IL-2–dependent T cell line: strict species specificity of action. J Immunol. 1989;142:1203–1208. [PubMed] [Google Scholar]
- 28.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 29.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 30.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, et al. Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 31.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor–α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 32.Rosendahl MS, Ko SC, Long DL, Brewer MT, Rosenzweig B, Hedl E, Anderson L, Pyle SM, Moreland J, Meyers MA, et al. Identification and characterization of a pro-tumor necrosis factor-α–processing enzyme from the ADAM family of zinc metalloproteases. J Biol Chem. 1997;272:24588–24593. doi: 10.1074/jbc.272.39.24588. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 34.Dhein J, Walczak H, Bäumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 35.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 36.Marsters SA, Pitti RM, Donahue CJ, Ruppert S, Bauer KD, Ashkenazi A. Activation of apoptosis by Apo-2 ligand is independent of FADD but blocked by CrmA. Curr Biol. 1996;6:750–752. doi: 10.1016/s0960-9822(09)00456-4. [DOI] [PubMed] [Google Scholar]
- 37.Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EY, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 39.Pan G, Ni J, Wei Y-F, Yu G-L, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain–containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 40.Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329–334. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- 41.Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003–1006. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- 42.Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO (Eur Mol Biol Organ) J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 44.Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-κB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 46.Wright JK, Tschopp J, Jaton JC, Engel J. Dimeric, trimeric and tetrameric complexes of immunoglobulin G fix complement. Biochem J. 1980;187:775–780. doi: 10.1042/bj1870775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jumper MD, Nishioka Y, Davis LS, Lipsky PE, Meek K. Regulation of human B cell function by recombinant CD40 ligand and other TNF-related ligands. J Immunol. 1995;155:2369–2378. [PubMed] [Google Scholar]
- 48.Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 49.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent Apo-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO (Eur Mol Biol Organ) J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 51.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/ FADD-interacting protease, in Fas/APO-1– and TNF receptor–induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 52.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/ CED-3–like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 53.Nicholson DW, Thornberry NA. Caspases: killer proteases. TIBS (Trends Biochem Sci) 1997;8:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 54.Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]