Abstract
The signals that direct differentiation of T cells to the CD4 or CD8 lineages in the thymus remain poorly understood. Although it has been relatively easy to direct differentiation of CD4 single positive (CD4+) cells using combinations of antibodies and pharmacological agents that mimic receptor engagements, equivalent stimuli do not induce efficient maturation of CD8+ cells. Here we report that, irrespective of the MHC-restriction specificity of the TCR, differentiation of mature CD8+ thymocytes can be induced by ligation of CD3 polypeptides on immature thymocytes with a F(ab′)2 reagent (CD3fos-F(ab′)2). The tyrosine phosphorylation patterns stimulated by CD3fos-F(ab′)2 have been shown to resemble those delivered to mature T cells by antagonist peptides, which are known to direct positive selection of CD8+ cells, and we can show that this reagent exhibits potent antagonistic-like activity for primary T cell responses. Our results suggest a distinction in the signals that specify lineage commitment in the thymus. We present a model of thymocyte differentiation that proposes that the relative balance of signals delivered by TCR engagement and by p56lck activation is responsible for directing commitment to the CD8 or CD4 lineages.
The question of whether distinct biochemical signals specify commitment to the CD4 or CD8 lineage in the thymus is currently unresolved. Receptor engagements that induce differentiation of CD4+ T cells appear to be relatively promiscuous, in that they can be mimicked by antibodies interacting with a variety of molecules on the immature thymocyte surface. Examples include hybrid antibodies that target thymocytes via their TCRs to thymic cortical epithelium (1), extensive engagement of TCR-β chains with antibody (2), and coligation of the TCR–CD3 complex on immature thymocytes with various surface molecules such as CD2 (1, 3), CD4, or CD8 (4), and even CD5, CD24, CD28, CD49d, and CD81 (3). Moreover, most of these receptor engagements are capable of inducing proliferation of mature T cells and thus resemble agonistic stimuli.
In contrast, positive selection of murine CD8+ T cells has been reproduced successfully in thymic organ cultures (TOCs)1 only by presentation of more specific stimuli, such as the positively selecting MHC class I molecule together with altered peptide ligands with antagonist (5) or partial agonist (6) activity. It should be noted that equivalent stimuli do not induce, but rather inhibit, differentiation of CD4+ cells (7). The selection of CD8+ T cells expressing MHC class I–restricted transgenic TCRs by low concentrations of the nominal peptide or a weak agonist has also been reported (6, 8, 9). However, T cells that had been selected on such ligands are aberrant in that they seem to have adjusted their stimulation threshold and are no longer responsive to the selecting peptide (9, 10). Thus, CD8+ cells are positively selected by stimuli that do not induce significant proliferation in mature T cells. Finally, evidence that different signaling pathways may be engaged during differentiation of the CD4 and CD8 lineages in the thymus has been suggested by studies that have shown that PMA stimulation of immature thymocytes in the presence of the Ca2+ ionophore ionomycin promotes full maturation of CD4 but not CD8 cells (11, 12).
To dissect the differential signaling requirements for positive selection of CD4 and CD8 lineages, our aim was to mimic the partial, antagonist-like signals perceived by a TCR upon engagement of some altered peptide ligand/ MHC complexes. CD3–TCR-specific F(ab′)2 reagents have been used as nonmitogenic alternatives to the intact parent antibodies for immunomodulation of autoimmune responses and graft rejection (13–15). These reagents, by virtue of their absent Fc portions, are capable of engaging the TCR–CD3 complex while failing to fully activate mature T cells to proliferate and secrete significant quantities of cytokines (13–15). Recently, the early signaling events triggered by such F(ab′)2 reagents were examined directly and shown to bear a striking resemblance to those induced by altered peptide ligands (16, 17). Furthermore, the inability of these reagents to induce full stimulation was shown to correlate with a lack of coreceptor-associated p56lck recruitment to the TCR–CD3 complex (16).
In the thymus it has been suggested that development of the CD4 lineage is favored by increasing the level of p56lck recruitment to the TCR complex (18). We have shown previously that coligation of CD3ε with either CD4 or CD8 with bispecific F(ab′)2s (BsAb) induces maturation of CD4+ cells in TOCs (4). The CD3/CD4 BsAb was more efficient than the CD3/CD8 BsAb at inducing CD4+ differentiation, consistent with the observation that CD4 associates more efficiently with lck (19), and can thus bring lck into proximity with the TCR more effectively, and that such recruitment favors CD4+ differentiation (18). A genetically engineered CD3ε-specific F(ab′)2 fragment derived from 2C11 V-regions (20) induced no CD4+ maturation in these experiments (4). Here we show that this CD3fos-F(ab′)2 reagent is able to mimic the signals required for CD8+ thymocyte differentiation, enabling us to investigate directly the role of partial signals in the differentiation of single positive thymocytes.
Materials and Methods
Mice.
The β2 microglobulin–deficient (β2m−) and I-Aβ– deficient (MHC class II−) mice have been previously described (references 21 and 22, respectively) and were intercrossed to obtain β2m−×class II− (MHC−) mice. TCR transgenic mice backcrossed onto a RAG-1–deficient (RAG-1−) background (23) were as follows: MHC class I–restricted F5 TCR (24), specific for influenza nucleoprotein (NP), restricted by H-2Db, and MHC class II–restricted A18 TCR (25), specific for C5 in the context of I-Ek. Male studs, homozygous for the TCR transgenes on the appropriate backgrounds (RAG-1−/β2m− for F5 and RAG-1− for A18), were bred with RAG-1−/β2m− or RAG-1− females, respectively, yielding neonates from which thymus lobes were obtained.
Antibodies.
Bi- and monospecific F(ab′)2 antibodies dimerized through Fos or Jun leucine zippers were prepared as previously described (20). V-regions with specificity for CD3ε, CD4, or CD8α were derived from 145.2C11, GK1.5, and YTS169. Monoclonal antibodies were purified and conjugated with FITC or biotin in our own laboratory, unless stated otherwise. The TCR-ζ–specific mAb, MCA146, was a gift from S. Ley (NIMR, London, UK), rabbit antiserum and mAb (3.3.1) to ZAP-70 was from J. Tite (Glaxo Wellcome, Stevenage, UK), and rabbit antiserum to p56lck was kindly provided by A. Magee (NIMR, London, UK).
Thymus Organ Culture.
Neonatal (day of birth) thymus lobes were cultured for 3–4 d as previously described (4) with the indicated antibodies or with medium only. Lobes were transferred to fresh filters in culture medium without antibody and cultured for a further 2–4 d to allow reexpression of downmodulated molecules. Single cell suspensions prepared from lobes after culture were stained with FITC-CD8α– (YTS169.4 or KT15; Fig. 5) or FITC-CD8β– (KT112-1), PE-CD4– (Boehringer Mannheim, Lewes, UK), and biotin–heat-stable antigen (HSA)– (YBM5.10), biotin-Vβ11– (KT11.5, for F5 TCR), biotin-Vβ8.3– (F23.1, for A18 TCR) or biotin-pan-TCR-β– (H57.597; see Fig. 2) specific antibodies, followed by Streptavidin-RED670 (GIBCO BRL, Paisley, UK) and 20,000 live events (gated on forward and side scatter profiles) were analyzed on a FACScan® (Becton Dickinson, Oxford, UK).
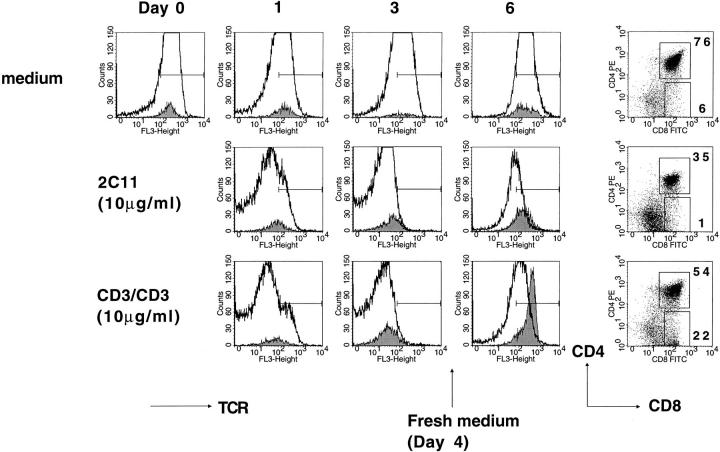
Figure 5.
2C11-derived CD3fos-F(ab′)2 (CD3/CD3) and intact 2C11 induce equivalent downmodulation of the TCR–CD3 complex on thymocytes in F5/RAG-1−β2m− neonatal lobes. Neonatal lobes were cultured as in Fig. 1 in the presence of 10 μg/ml 2C11 or CD3fos-F(ab′)2 (CD3/CD3) for 4 d, transferred to fresh medium on day 4, and cultured for a further 2 d. Individual lobes were harvested on days 1, 3, and 6 of culture and analyzed by flow cytometry. TCR expression for DP (thick line) and CD8+ (filled histograms) populations are shown for each time and treatment. CD4 and CD8 staining of thymocytes recovered on day 6 of culture is shown with the percentages of DP and CD8+ cells indicated.
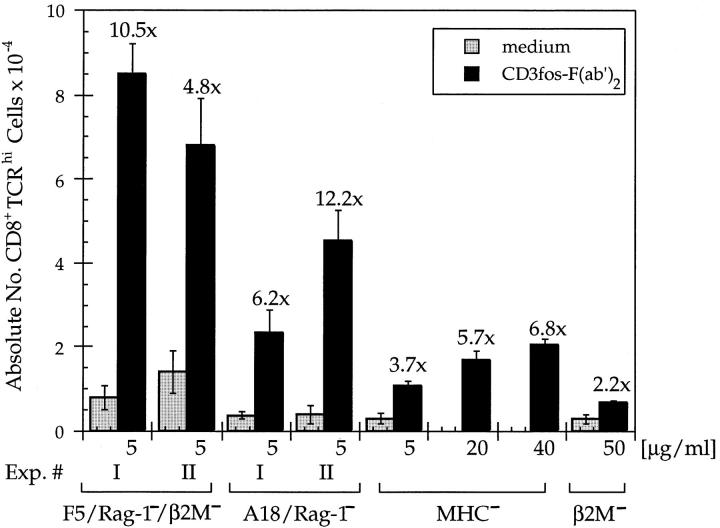
Figure 2.
CD3fos-F(ab′)2 induces a significant increase in the number of mature CD8+TCR-α/βhi T cells in neonatal thymus lobes. Lobes were cultured as in Fig. 1, with the indicated concentrations of CD3fos-F(ab′)2, whereupon thymocytes were stained for CD4, CD8, and TCR-β (KT11.5, F23.1, or H57.597) and analyzed by FACS®. Mature, TCRhi cells were gated and absolute numbers of CD8+TCRhi cells were calculated for each lobe. Data are presented as the means ± SE of CD8+TCRhi cells recovered from groups of 3-4 individual lobes. The extent of the increase in CD8+TCRhi cells in CD3fos-F(ab′)2–treated cultures compared with medium only is indicated above each experiment. Two independent experiments are shown for F5/RAG-1−/β2m− and A18/RAG-1− lobes and a titration of CD3fos-F(ab′)2 for MHC− lobes. The total numbers of thymocytes recovered from each group were, respectively: F5/RAG-1−/ β2m−: experiment 1, 53 ± 7 × 104 (medium) and 51 ± 6 × 104 (5 μg/ml); experiment 2, 14 ± 5 × 104 (medium) and 43 ± 10 × 104 (5 μg/ml); A18/RAG-1−: experiment 1, 10 ± 1 × 104 (medium) and 33 ± 4 × 104 (5 μg/ml); experiment 2, 20 ± 6 × 104 (medium) and 25 ± 3 × 104 (5 μg/ ml); MHC−: 108 ± 32 × 104 (medium), 91 ± 1 × 104 (5 μg/ml), 55 ± 5 × 104 (20 μg/ml) and 57±5 × 104 (40 μg/ml); and β2m− lobes: 42 ± 7 × 104 (medium) and 49 ± 1 × 104 (50 μg/ml).
CTL Assay.
Dendritic cells (DCs) were expanded by culture of B10 (H-2b) bone marrow in medium supplemented with GM-CSF for 7 d (26). Thymocytes from lobes cultured with either 10 μg/ml CD3fos-F(ab′)2 or medium were stimulated at 7 × 104/ well with 2.5 × 104 H-2b DCs/well and 1 μM NP68 peptide for 4 d at 37°C in medium plus 10% rIL-2 supernatant. On day 4, cells were harvested, resuspended to equivalent volumes, and titered with 103 51Cr-labeled NP68-loaded EL-4 targets/well for 4 h, after which 100 μl supernatant was removed for γ counting. Percentage of specific lysis was calculated as [(cpm experimental release − cpm spontaneous release)/(cpm maximum release − cpm spontaneous release)] × 100.
Proliferation and Antagonism Assays.
B10 thymocytes (106/well) were incubated for 72 h with 2C11, CD3/CD8, CD3/CD4, and CD3fos-F(ab′)2 in medium (triplicate cultures). In antagonism assays, splenocytes (2 × 105/well) from A18/RAG-1− or F5/ RAG-1− mice were cultured in the presence of C5 or NP68 peptides, respectively, at indicated concentrations (see legend to Fig. 6). CD3fos-F(ab′)2, a nonbinding control reagent, CD4/CD4, and an anti–Thy-1 antibody (YTS 154.7) were added at the concentrations indicated at the start of culture. Cultures were pulsed with 1 μCi [3H]thymidine per well for the last 18 h of culture, harvested, and counted in a beta counter with scintillation. The upregulation of CD69 during antagonism assays was measured over time by staining aliquots of the cultures with PE-CD4–, FITC-Vβ8.3– (A18), or PE-CD8α– (Sigma Chemical Co., Poole, UK) (F5) and biotin-CD69– (PharMingen, San Diego, CA) specific antibodies and CD69 expression on CD4+Vβ8.3+ (A18) or CD8+ (F5) cells analyzed by flow cytometry.
Figure 6.
CD3fos-F(ab′)2 antagonizes the proliferative response of mature CD4+ and CD8+ T cells to antigen. (A) Antagonist activity of CD3fos-F(ab′)2 is shown by its inhibitory effect at 1.25 μg/ml CD3fos-F(ab′)2 on the proliferation of spleen cells from A18/RAG− mice to various concentrations of C5 peptide and (B) at 12.5 μg/ml on the proliferation of F5/RAG-1− spleen cells to NP68 peptide. The same concentrations of a nonbinding F(ab′)2, CD4/CD4 (B only), and a binding, nonantagonistic antibody, anti–Thy-1 (YTS 154.7), are included as controls. (C) CD69 is upregulated on A18 and F5 spleen cells cultured with 200 nM C5 peptide for 22 h (A18) or 10 nM NP68 for 1.5 h (F5) (light fill), and to an equivalent extent in the presence of 62.5 ng/ml (for A18) or 625 ng/ml (for F5) CD3fos-F(ab′)2 plus peptide (solid line). No CD69 expression is observed in the absence of antigen (data not shown) or in cultures with CD3fos-F(ab′)2 alone (dark fill).
Phosphotyrosine Analyses.
Thymocytes from F5/RAG-1−/β2m− mice (95% double positive [DP], 5% double negative [DN] cells) were kept at 4°C on ice and stimulated for 5–20 min at 37°C with saturating amounts of antibody (100 μg/ml per 1–2 × 108 thymocytes). Cells were pelleted at 13,000 rpm for a few seconds and lysed for 1 h at 4°C in 1% Brij or Triton X-100 (for ZAP-70 immunoprecipitations) in 150 mM NaCl, 50 mM Tris (pH 7.5), 5 mM EDTA, 10 mM NaF, 10 mM disodiumpyrophosphate, 200 mM PMSF, 5 mM leupeptin, 1.5 mM pepstatin A, and 1 mM Na3VO4. Postnuclear supernatants were precipitated overnight at 4°C with 20 μl protein A–Sepharose (PAS), covalently coupled to anti–TCR-ζ mAb (MCA146); rabbit anti–ZAP-70 SH2 serum and PAS; or rabbit anti-lck peptide serum and PAS. Precipitates were washed 3× in lysis buffer, dissolved in reducing sample buffer, and resolved on a 7–15% gradient SDS-PAGE gel. Proteins were transferred electrophoretically onto a nylon membrane, blocked in 5% nonfat milk powder in PBS with 0.1% Tween 20, and blotted with antiphosphotyrosine (4G10, Upstate Biotechnology, Inc., Lake Placid, NY) or anti–ZAP-70 mAbs. Anti–mouse Ig-horseradish peroxidase conjugate antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL) developed with ECL (Boehringer Mannheim, Lewes, UK) were used for detection.
Results
CD3fos-F(ab′)2 Induces Differentiation of CD8+ Thymocytes.
To investigate whether we could induce differentiation of CD8 thymocytes, we used a variety of mouse strains in which normal CD8 differentiation was impaired. Initially thymuses were obtained from mice transgenic for the MHC class I–restricted TCR, F5 (24), specific for influenza NP restricted by H-2Db and back-crossed to the RAG-1−/ β2m− background. The lack of MHC class I in these mice impairs maturation of significant numbers of CD8+ thymocytes, and those that fall within the CD8+ gate contain only a small fraction of mature HSAlo cells (Fig. 1 A, medium control). However, when newborn thymus lobes from F5/RAG-1−/β2m− mice were cultured in the presence of CD3fos-F(ab′)2, but not control CD8jun-(Fab′)2, reagents, differentiation of significant numbers of CD8+ cells occurred that had downregulated expression of HSA (Fig. 1 A). Furthermore, these cells also stained brightly for the CD8β chain (data not shown), indicating that they belong to the TCR-α/β and not the γ/δ lineage, since the majority of CD8+ T cells that belong to the γ/δ lineage preferentially express CD8α/α homodimers on their surface (27).
Figure 1.
CD3fos-F(ab′)2 (CD3/CD3) induces the differentiation of mature CD8+ cells and antagonizes the normal differentiation of CD4+ T cells in neonatal thymus lobes. Newborn thymus lobes from (A) MHC class I–restricted F5/RAG-1−/β2m− transgenic (24); (B) class II–restricted A18/RAG-1− transgenic (25); (C) β2m−; and (D) MHC− (class II−β2m−) mice were cultured with 5 μg/ml (A and B), 50 μg/ml (C), or 20 μg/ml (D) CD3fos-F(ab′)2 (CD3/CD3) or CD8jun-F(ab′)2 (CD8/CD8). Dot-plots show CD8 and CD4 expression of all thymocytes recovered from a single lobe. The percentage of total cells for individual subpopulations are indicated. HSA levels of CD4+ (thick line), DP (light fill) and CD8+ (dark fill) subpopulations are presented in histograms below each dot-plot. The data are representative of 13 (A), 8 (B), 6 (C), and 4(D) independent experiments.
The absolute number of TCRhi CD8+ cells generated from F5/RAG-1−/β2m−-derived thymus lobes was dependent on CD3fos-F(ab′)2 concentration, with 5–10 μg/ml inducing a 5–10-fold increase above background (Fig. 2). Therefore, the efficiency of positive selection induced by CD3fos-F(ab′)2 is directly comparable to that induced by altered peptide ligands for the OVA–tcr-1 transgenic mice on a β2m− background (3–9-fold increase) (5), a naturally occurring positively selecting peptide of the OT-1 TCR on a TAP-1− background (7-fold increase) (28) and more efficient than was reported for P14 TCR transgenic mice on a TAP-1− background (2–4-fold increase) (6) or for a mixture of thymic self-peptides in fetal TOCs from TAP-1− mice (3-fold increase) (29). Furthermore, the final percentages (20–30%) of CD8+ cells and corresponding numbers of mature, TCRhi CD8+ cells (∼7 × 104) recovered from F5/RAG-1−/β2m− thymuses after treatment with CD3 fos-F(ab′)2, are comparable to the level of positive selection observed under optimal selection conditions, i.e., in organ cultures of F5/RAG-1− lobes expressing the positively selecting ligand (H-2Db). Under identical culture conditions, ∼15% of thymocytes in F5/RAG-1− lobes are CD8+, which corresponds to ∼8 × 104 TCRhi CD8+ cells/lobe (not shown).
CD3ε Ligation Promotes CD8+ Differentiation in Thymuses Expressing MHC Class II–restricted and Polyclonal TCRs.
The effect of CD3fos-F(ab′)2 on CD8+ cell differentiation was not restricted to thymocytes expressing a transgenic MHC class I–restricted TCR, as CD8+ cells also differentiated to maturity in thymuses expressing a MHC class II– restricted TCR, A18 (25), on a RAG-1− background (Fig. 1 B) or polyclonal, endogenously rearranged TCRs in β2m− mice (Fig. 1 C). CD8+ cell maturation was found to be much less efficient in the latter, and even a 10-fold increase in the concentration of CD3fos-F(ab′)2 induced only a moderate 2–3-fold increase in mature TCR-α/βhi CD8+ cells (Fig. 2), considerably fewer than could be generated from mice with transgenic TCRs (5–10-fold increase, Fig. 2). These differences in efficiency of CD8+ generation may reflect the presence of the rearranged TCR in transgenic mice, but they also correlate with the extent of competition for differentiation to the CD4 lineage ongoing in these lobes. That is, although under normal culture conditions no CD4+ T cells differentiate in lobes from F5/RAG-1− mice, class II–restricted A18/RAG-1− lobes generate ∼30% CD4+ cells, whereas β2m− thymus lobes, which have endogenous TCR rearrangement, generate ∼70% CD4+ cells over an equivalent culture period. Commitment to the CD4 lineage in these β2m− lobes is so efficient that CD3fos-F(ab′)2 treatment may not be able to redirect these committed cells to the CD8 lineage.
We tested whether this was the case by examining whether we could improve the efficiency of CD8+ cell maturation by using thymus lobes from MHC− mice in which there is no competition for commitment to the CD4 lineage. As shown in Fig. 1 D, CD3fos-F(ab′)2 was able to promote more efficient maturation of thymocytes to the CD8 lineage in MHC− lobes than in β2m− lobes, such that the number of mature, TCR-α/βhi CD8+ cells generated approached that obtained with TCR transgenic lobes (Fig. 2). However the concentration of CD3fos-F(ab′)2,which promoted maximal CD8+ differentiation in MHC− mice, was ∼5-fold higher than was required for inducing differentiation of CD8+ cells in mice expressing a transgenic TCR (Fig. 2). It is likely that the requirement for higher concentrations of the F(ab′)2 reagent reflects differences in the level and timing of expression of the transgenic TCRs relative to mice expressing endogenous TCRs. For both β2m− and MHC− thymocytes, we could confirm that the CD8+ cells generated were of the α/β lineage as they showed upregulated TCR upon staining with a pan–TCR-β antibody, H57.597.
We have shown previously that CD3/CD4 or CD3/ CD8 BsAbs can promote differentiation of CD4+ cells in TOCs (4). CD4 maturation required a minimum of 16 h of incubation with BsAb followed by a further 24–48 h of incubation in medium alone before significant numbers of mature CD4+ cells were detected. In contrast, for maximal CD8+ thymocyte differentiation we routinely cultured the lobes for 3 d with CD3fos-F(ab′)2 followed by a further 3–4 d of culture in medium alone. Shorter incubation times with CD3fos-F(ab′)2 were not optimal for CD8+ differentiation. Although CD8+ cells could be detected in cultures at earlier times after the lobes were changed into medium without Ab, the cells that appeared were phenotypically immature, in that they expressed high levels of HSA and reduced levels of TCR (as we previously reported for MHC− mice [4]). We observed a gradual maturation of the population to TCRhi HSAlo phenotype in the 48–72 h after Ab removal. This difference in the timing required for maturation of CD4+ versus CD8+ cells is similar to that which has been reported in vivo (30).
CD3fos-F(ab′)2 Blocks Maturation of CD4+ Thymocytes.
It appeared that CD3fos-F(ab′)2 was less efficient at redirecting thymocytes to the CD8 lineage where there was overt competition for maturation to the CD4 lineage, particularly in β2m− lobes (Fig. 2). However, we also observed that there was a significant influence of CD3fos-F(ab′)2 treatment on the transition from DP to CD4+ cells in thymuses from A18/RAG-1− (Fig. 1 B) and β2m− (Fig. 1 C) mice. The percentage of CD4+ cells was reduced by four- to sixfold after culture with CD3fos-F(ab′)2 compared with medium, and, strikingly, the remaining CD4+ cells were largely immature, in that they failed to fully downregulate HSA (Fig. 1, B and C). This decrease in CD4 maturation generally corresponded to up to an increase of up to twofold in the number of DP thymocytes present in treated thymuses, suggesting that CD3fos-F(ab′)2 blocked normal differentiation of immature CD4-committed thymocytes. Thus, it appears that CD3fos-F(ab′)2 specifically inhibits the maturation of CD4+ cells in TOCs, similar to what has been reported for antagonistic MHC class II–restricted altered peptide ligands (7).
Unlike intact 2C11 Ab (31, 32), CD3fos-F(ab′)2 does not stimulate thymocyte transition from DN to DP subsets in organ cultures from RAG− mice (data not shown), nor induce proliferation of thymocytes (shown in Fig. 4), making it unlikely that CD3fos-F(ab′)2 actively promotes the generation of DP thymocytes. However, it is possible that CD3fos-F(ab′)2 to some extent promotes survival of DP thymocytes as there was a two- to threefold increase in total numbers of thymocytes recovered from treated lobes compared with medium controls in about half of the experiments (see legend to Fig. 2).
Figure 4.
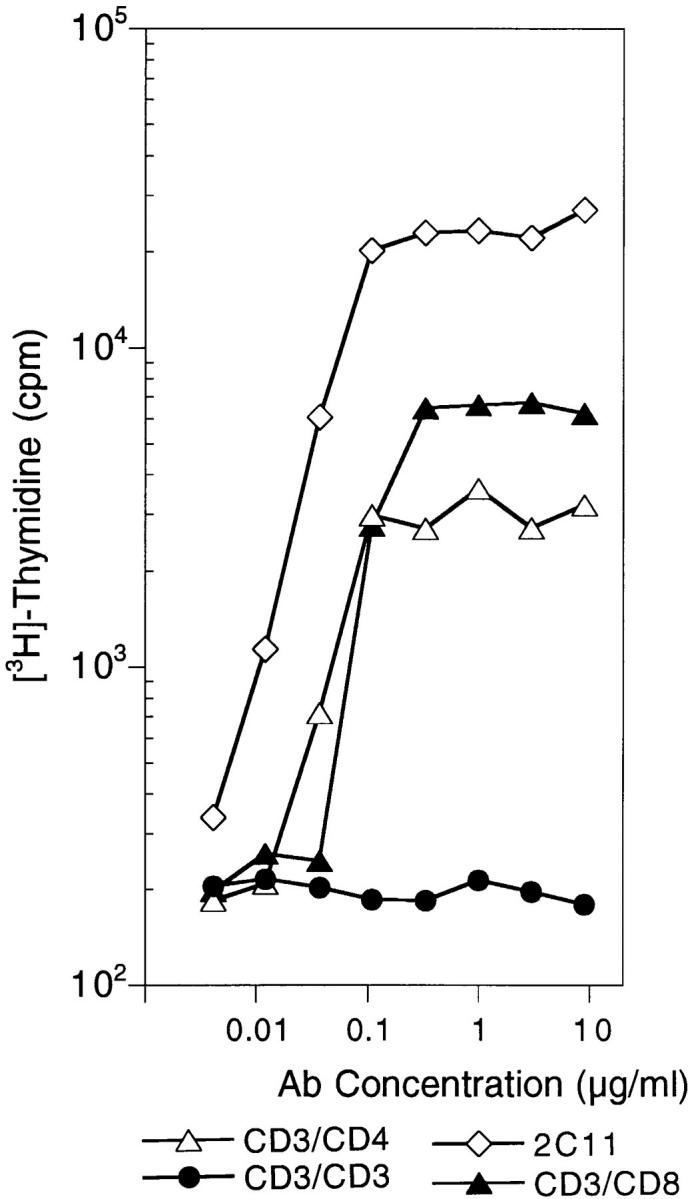
CD3fos-F(ab′)2 cannot induce proliferation in mature thymocytes. Shown is proliferation of B10 thymocytes to anti-CD3 mAb (2C11), CD3/ CD8 and CD3/CD4 BsAb, and CD3fos-F(ab′)2 (CD3/CD3), as indicated. B10 thymocytes (106/ well) were incubated for 72 h in 96-well plates with antibodies. Cultures were pulsed with 1 μCi [3H]thymidine/well for the last 18 h of culture, harvested, and counted in a beta counter with scintillation.
CD8+ Thymocytes Induced by CD3ε Ligation Can Differentiate into Effector CTLs.
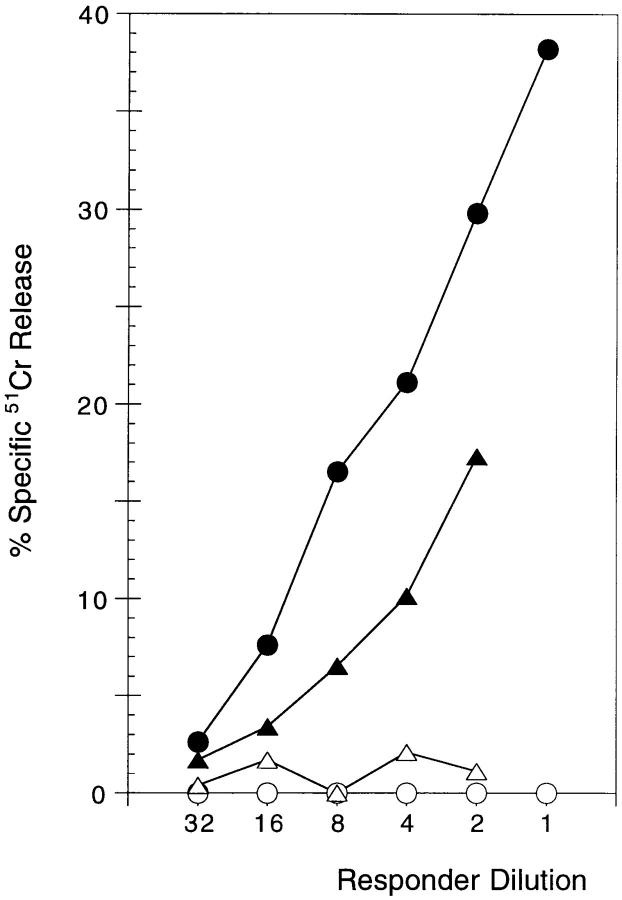
The CD8+ cells recovered from CD3fos-F(ab′)2–treated cultures looked phenotypically mature as indicated by downregulated HSA (Fig. 1 A) and high TCR levels (shown in Fig. 5). To test whether they were functionally mature, we took lobes from F5/RAG-1−/β2m− mice cultured with CD3fos-F(ab′)2 or medium as described above, and asked whether the mature CD8+ cells could differentiate to effector CTLs when stimulated with B10 (H-2b) DCs, loaded with the antigenic H-2Db-binding NP68 peptide. After 4 d of culture with stimulator cells in the presence of IL-2, efficient lysis of NP68-loaded H-2Db target cells, but not control targets, was detectable (Fig. 3). Control lobes from F5/RAG-1−/β2m− mice, cultured in medium alone, contained a few CD8+ T cells (as shown in Fig. 1 A), which were also capable of expanding upon stimulation with peptide-pulsed DCs in the presence of IL-2 and maturing into cytotoxic T cells.
Figure 3.
CD8+ T cells recovered from CD3fos-F(ab′)2– treated F5/RAG-1−β2m− thymuses are functionally mature CTL precursors. CTL activity shown is of cells recovered from CD3fos-F(ab′)2–treated F5/ RAG-1−β2m− thymuses (circles) or medium control thymuses (triangles). Thymocytes recovered from equivalent numbers of cultured lobes/group were stimulated with NP68 peptide-pulsed DCs for 4 d and tested for cytolytic activity on peptide-loaded (filled symbols) or control (open symbols) EL-4 target cells in a standard 4-h 51Cr–release assay. The effector ratio is presented as responder dilution as cells recovered from stimulation cultures were resuspended to the same volume.
CD3fos-F(ab′)2 Does Not Stimulate T Cell Proliferation but Causes TCR Downmodulation.
To investigate the signals induced by CD3fos-F(ab′)2 in thymocytes, we examined its ability to induce T cell proliferation. In contrast to native parental antibody, 145.2C11, which induces strong proliferation of mature thymocytes, CD3fos-F(ab′)2 did not stimulate any detectable proliferation over a wide concentration range (Fig. 4), as has been previously reported for peripheral T cells (16, 17). CD3/CD4 and CD3/CD8 F(ab′)2s were able to stimulate thymocyte proliferation also, although not as effectively as 2C11 Ab, indicating that coligating the coreceptor with CD3 can replace to some extent the contribution provided by the Fc portion of intact antibody.
As CD3fos-F(ab′)2 did not stimulate proliferation, we asked whether it caused downmodulation of the TCR–CD3 complex (33–35) when added to TOCs. Indeed, effective downregulation of TCR–CD3 complex on thymocytes was evident 18 h (day 1) after addition of the antibodies, and reached a maximum within 3 d (Fig. 5). TCR downmodulation was comparable for CD3fos-F(ab′)2 and the intact 2C11 antibody, suggesting that these reagents engaged equivalent numbers of CD3ε molecules (35). Removal of the antibodies on day 4 resulted in gradual reexpression of the TCR–CD3 complex on DP and CD8+ thymocytes, up to normal levels by day 6. Although extensive deletion of DP thymocytes was evident in 2C11-treated lobes, as previously described (31), none was observed after CD3fos-F(ab′)2 treatment, but rather differentiation of mature CD8+ cells with upregulated TCR (Fig. 5). It is also of interest to note that both CD3fos-F(ab′)2 and CD3/CD4 were effective at inducing TCR modulation on thymocytes in suspension culture (data not shown), and yet CD3fos-F(ab′)2 failed to induce any proliferation. Furthermore, this effect was not specific for thymocytes as CD3fos-F(ab′)2 caused equivalent TCR downmodulation on peripheral T cells (data not shown). These observations support data from Cai et al. (35), who showed that TCR modulation in response to TCR engagement does not necessarily correlate with the efficacy of T cell activation.
CD3fos-F(ab′)2 Is a Potent Antagonist of Primary T Cell Responses.
The biological effects of CD3fos-F(ab′)2 in TOCs are clearly reminiscent of the effects of some altered peptide ligands with antagonist/partial agonist activity, namely the following: the ability to promote the maturation of CD8+ thymocytes in vitro (5, 6, 9); to interfere with normal CD4+ thymocyte maturation, referred to as “competitive antagonism” (7); to downmodulate the TCR–CD3 complex (34); and the inability to induce proliferation of mature T cells (36–39). Therefore, we tested whether CD3fos-F(ab′)2, was able to antagonize an antigen-specific response at concentrations similar to those inducing CD8 differentiation. Fig. 6 shows that addition of CD3fos-F(ab′)2 inhibits proliferation of primary cultures of peripheral T cells from either class II–restricted, CD4+ A18 transgenic TCR-expressing cells (Fig. 6 A) or class I–restricted, CD8+ T cells from F5/ Rag-1− transgenic mice (Fig. 6 B), to a range of concentrations of the agonist peptide. For F5 spleen cells at suboptimal peptide concentration (1 nM), as little as 1.5 μg/ml CD3fos-F(ab′)2 gave >90% inhibition of the response (data not shown).
It has been reported previously that nonstimulatory anti-CD3 antibodies are able to induce functional anergy in T cell clones (17) by delivering a set of distinct intracellular signals (see below). Although unlikely, it was possible that CD3fos-F(ab′)2 was sterically interfering with TCR recognition of MHC/peptides. This did not appear to be the case for F5 TCR transgenic mice, as CD3fos-F(ab′)2 did not interfere with differentiation of CD8+ cells in organ culture (4). However, to exclude the possibility that steric hinderance was preventing TCR transgenic cells from interacting with MHC, we examined upregulation of CD69 in response to peptide when CD3fos-F(ab′)2 was present in the culture. As shown in Fig. 6 C, both CD4+ A18 T cells and CD8+ F5 T cells upregulated CD69 to a comparable extent in wells containing peptide or peptide plus CD3fos-F(ab′)2. Proliferation in parallel cultures was inhibited by ∼50% (A18) and 98% (F5) in the presence of CD3fos-F(ab′)2 for this particular experiment (data not shown). Therefore, CD3fos-F(ab′)2 does not appear to inhibit early TCR recognition events but may subsequently alter intracellular signals induced during antigen recognition by mature T cells (17).
Tyrosine Phosphorylation Signals Involved in CD8+ Lineage Commitment.
Early tyrosine phosphorylation events in mature T cells in response to CD3/CD4 and CD3fos-F(ab′)2 have been studied in detail (16, 17). Using identical reagents to those used in this study, it was shown that in T cell clones, CD3/CD4 BsAb induced tyrosine phosphorylation patterns characteristic of an agonist signal, whereas CD3fos-F(ab′)2 induced signals indistinguishable from those generated by partial agonist/antagonist ligands (16, 17).
We compared signals initiated by CD3/CD4 BsAb, which behaves as an agonist and induces CD4 maturation, with those transduced by the antagonist, CD3fos-F(ab′)2, in immature thymocytes from F5/RAG-1−/β2m− mice. As there are no mature CD4+ or CD8+ cells in ex vivo thymocytes from these mice, we were able to look at the effects of these reagents on unmanipulated immature cells, i.e., those which had not been subject to antibody-mediated selection. Perhaps not surprisingly, we observed some differences to what has been reported for T cell clones (16). Both CD3/CD4 and CD3fos-F(ab′)2 induced hyperphosphorylation of the constitutively phosphorylated TCR-ζ chain, p21 (40, 41), resulting in the appearance of the p23 isoform (Fig. 7 A). However, unlike CD3/CD4 BsAb, CD3fos-F(ab′)2 did not stimulate phosphorylation of p27, identified as CD3ε (16). This observation is of particular relevance given recently reported differences in the downstream effector molecules, which are able to interact with phosphorylated TCR-ζ versus CD3ε immunoreceptor tyrosine-based activation motif (42), which may have distinct consequences for engaging downstream signaling pathways (43).
Figure 7.


Distinct patterns of tyrosine phosphorylation are induced in immature F5/RAG-1−β2m− thymocytes by stimulation with CD3/CD4 BsAb and CD3fos-F(ab′)2. (A) Thymocytes were stimulated with F(ab′)2 reagents, as indicated, for 8 min at 37°C. Lysates were immunoprecipitated with anti–TCR-ζ mAb and the precipitates immunoblotted with antiphosphotyrosine mAb (4G10), to reveal differences in TCR-associated phosphotyrosine-containing proteins. (B) Total ZAP-70 proteins associated with TCR-ζ polypeptides were examined in unstimulated or F(ab′)2-stimulated samples (5 min at 37°C), as indicated. Lysates were immunoprecipitated with anti–TCR-ζ and immunoblotted with anti–ZAP-70 mAb. (C) Tyrosine phosphorylation of ZAP-70 proteins in control or F(ab′)2-stimulated thymocytes was visualized by immunoprecipitating cell lysates with polyclonal anti–ZAP-70 serum after stimulation of thymocytes for 5 and 20 min at 37°C, as indicated, and immunoblotting was with antiphosphotyrosine 4G10. Half of the 20-min precipitate was probed with anti–ZAP-70 mAb to confirm that equivalent amounts of protein were present. (D) p56lck immunoprecipitates demonstrate different association with TCR-associated phosphoproteins. Thymocytes were stimulated with F(ab′)2-reagents at 37°C for 5 min, as indicated, or left on ice throughout (4°C). Cell lysates were precipitated with p56lck-antiserum and immunoblotted with antiphosphotyrosine (4G10).
In all cases, similar amounts of ZAP-70 protein were found associated with phospho-ζ proteins in stimulated and unstimulated thymocytes (Fig. 7 B). However, phosphorylated ZAP-70 was present only in cells stimulated with CD3fos-F(ab′)2 and CD3/CD4 BsAb and not in control CD4jun-F(ab′)2–stimulated or unstimulated cells (Fig. 7 C). At 5 min of stimulation, tyrosine-phosphorylated ZAP-70 was apparent in the CD3fos-F(ab′)2–treated thymocytes, but at slightly lower levels than in the CD3/CD4 BsAb-treated group, and by 20 min levels of phospho–ZAP-70 had further diminished in the former, whereas they were maintained at relatively high levels in the latter (Fig. 7 C). These observations differ from studies in T cell clones, in which differences were noted in TCR-ζ phosphorylation and no ZAP-70 phosphorylation was detected after stimulation with altered peptide ligands (16, 38, 44, 45) or CD3fos-F(ab′)2 (16, 17), and may reflect the fact that TCR-ζ chains are constitutively phosphorylated in ex vivo thymocytes (40, 41). Given the essential role of ZAP-70 in the maturation of both CD4 and CD8 lineages in the mouse thymus (46), it is perhaps not surprising that both CD4 and CD8 differentiation signals should induce some level of ZAP-70 activation.
To examine whether differences observed in thymocytes after stimulation with CD3/CD4 or CD3fos-F(ab′)2, could be attributed to a lack of coreceptor-associated p56lck recruitment to the signaling complex by CD3fos-F(ab′)2, we examined p56lck immunoprecipitates from F5/RAG-1−/ β2m− thymocyte lysates for the presence of coprecipitating tyrosine phosphoproteins. Fig. 7 D clearly demonstrates the physical juxtaposition of p56lck with high levels of p21 as well as p23 phospho-ζ and p27 CD3ε in thymocytes after ligation with CD3/CD4, and to a lesser extent (p21 and p23 phospho-ζ) with CD3/CD8 BsAbs, in agreement with reported differences in the levels of p56lck associated with CD4 and CD8 (19). In contrast, only low levels of p21 phospho-ζ were detected in p56lck precipitates from CD3fos-F(ab′)2-stimulated lysates (Fig. 7 D). After treatment with all stimuli, a 70-kD phosphoprotein that was confirmed to be ZAP-70 by Western blotting with specific antisera (data not shown) was also found to be associated with p56lck precipitates. Note that low levels of phospho–ZAP-70 coprecipitated with p56lck in unmanipulated thymocytes kept on ice. ZAP-70 was rapidly dephosphorylated upon incubation at 37°C so that virtually no phospho–ZAP-70 could be detected in unstimulated thymocytes at 37°C. The low level of phospho–ZAP-70 was maintained in CD3fos-F(ab′)2–stimulated thymocytes and increased in CD3/CD4 and CD3/CD8–stimulated cells. Thus, although not completely devoid of p56lck upon CD3fos-F(ab′)2 stimulation, the extent to which phosphorylated CD3/TCR chains are associated with p56lck appears to be significantly less than after CD3/CD4 stimulation, and may be insufficient for sustaining high levels of phospho–ZAP-70 (47). As we have previously shown that CD3/CD4, and to a lesser extent CD3/CD8, BsAb promote differentiation of CD4+ thymocytes (4), and show here that CD3fos-F(ab′)2 efficiently promotes differentiation of CD8+ thymocytes, these data indicate that these differences in signaling underlie the differential commitment between the two lineages.
Discussion
The signals required for CD4 differentiation seem to be relatively promiscuous in that coengagement of the TCR with a large number of surface molecules can induce CD4+ differentiation in immature thymocytes even in the absence of MHC class II molecules (1, 3, 4). CD8 differentiation, on the other hand, seems to require specific signals and MHC class I expression (48). In this study we show that TCR engagement in the absence of coaggregation of other surface receptors (in particular those associated with p56lck) can exclusively induce differentiation of the CD8 lineage.
We show that the signal that induces CD8 differentiation, i.e., ligation with CD3fos-F(ab′)2, is unable to stimulate proliferation in mature T cells and can act as a functional antagonist for antigen-specific peripheral T cell responses. Moreover, this reagent induces a set of intracellular tyrosine phosphorylation patterns in thymocytes, namely, hyperphosphorylation of CD3ζ, no phosphorylation of CD3ε, and altered levels and kinetics of ZAP-70 phosphorylation, distinct from that induced by CD3/CD4 BsAb, which promotes CD4 differentiation. Furthermore, we can correlate these distinct signaling patterns to the association of lck with the TCR complex because, not surprisingly, CD3/CD4 coligation with BsAbs induces very stable TCR–lck association, whereas CD3 ligation alone does not. In mature T cells, recruitment of p56lck to the TCR complex seems to be an essential feature of TCR signaling leading to T cell activation, whereas TCR engagement in the absence of sufficient p56lck activation leads to partial, antagonist-like signals (16). Such partial signals can be generated in mature T cells either by altered peptide ligands or by CD3fos-F(ab′)2 reagents, identical to those used here (16, 17). Given the sparse knowledge on the integration of the various signaling pathways involved in thymocyte differentiation, at this stage we are unable to further correlate differences in p56lck recruitment to the activation of unique downstream signaling pathways. However, it is clear that these differences induce distinct biological effects both in immature thymocytes and mature T cells.
There have been a number of different models proposed over the years to explain how lineage choices are made in the thymus. Most recent models have explained these choices in terms of quantitative differences in signaling (18, 49). In essence they propose a linear gradation of signaling, starting with signals so low that thymocytes die of neglect, progressing through weak signals suitable for CD8 differentiation, to stronger signals that are suitable for CD4 differentiation, and finally, to yet stronger signals that induce apoptosis. Although a number of experimental observations fit well with such models, others do not. For example, in support of the quantitative models, it has been shown that constitutive expression of CD8 transgenes (50), and more particularly, hybrid CD8 transgenes with CD4 cytoplasmic domains (18), cause cells expressing class I–restricted transgenic TCRs to be selected into the CD4 lineage. These coreceptor transgenes would be predicted to increase the sensitivity of thymocytes to signals, with the observed consequence that they change their commitment from the CD8 to CD4 lineage. In contrast, other studies have shown that some altered peptide ligands, which induce efficient maturation of CD8+ TCR transgenic cells, do not alter the commitment of these cells to the CD4 lineage at higher peptide concentration, but instead induce deletion (5, 10, 51). A quantitative signaling model would predict that increasing the peptide concentration should provide a linear increase in signal strength which at some point should be suitable for CD4 commitment. The observation that the transgenic thymocytes are instead deleted, is difficult to reconcile with these current models.
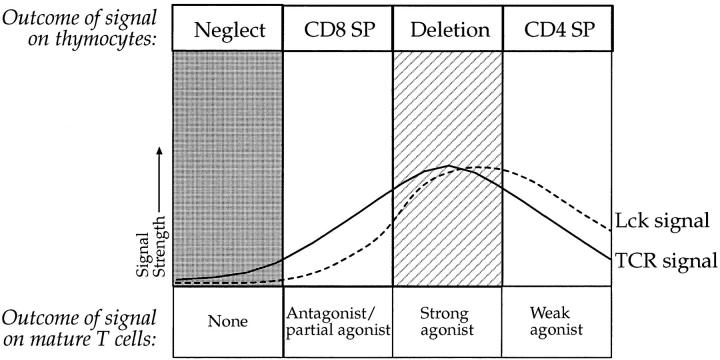
We suggest a novel model of thymocyte differentiation (illustrated in Fig. 8) incorporating the idea that antagonist/ partial agonist–like signals per se may be responsible for commitment to the CD8 lineage, as shown for some antagonist and partial agonist peptides (5, 39) and in the present study. The main difference from previous schemes is that we propose that T cells integrate two distinct signals, one provided by ligation of the TCR, and the second provided by the activation of lck. We suggest that it is the relative ratio of these two signals that leads to distinct differentiation decisions in the thymus. Thus, within a simple continuum, insufficient signals would lead to death by neglect, whereas CD8 differentiation would be signaled by TCR engagement combined with very little lck activation; if the combined TCR/lck signal is too strong, deletion results; but changing the ratio, to increase the lck signal relative to the TCR signal, promotes CD4 differentiation.
Figure 8.
A model of thymocyte differentiation in which the relative balance of signals delivered by TCR engagement and lck activation is responsible for directing commitment to the CD4 and CD8 lineages. Engagement of TCR with particular MHC/peptide ligands can give partial antagonist–like signals with relatively little lck activation that is appropriate for commitment to the CD8 lineage in the thymus. Increasing the concentration of such peptides may increase the TCR engagement and promote an lck signal, which results in deletion but not differentiation to the CD4 lineage. In contrast, MHC/peptide interactions that result in a relatively high lck to TCR signal ratio specify commitment to the CD4 lineage. Thus overexpression of coreceptors with class I TCR transgenes could disproportionately increase the lck signal relative to the TCR signal and result in commitment to the CD4 lineage. On the other hand, class II–restricted TCR transgenes on a CD4° background would not receive a CD4-associated lck signal upon MHC/peptide engagement, leading to a predominant TCR to lck signal, which would favor commitment to the CD8 lineage.
Therefore, as indicated in Fig. 8, CD8 differentiation is signaled by engagement of the TCR in the absence of overt lck signals, which is equivalent to the signals delivered in mature T cells by recognition of antagonist/partial agonist peptides. Although expression of CD8α/β heterodimers are obligatory for normal differentiation of this lineage (52–55), two recent papers (51, 56) together with our own data indicate that the participation of CD8 is not mandatory. We presume the primary role of CD8 in the thymus is to stabilize TCR–MHC interactions without contributing strong lck signals. This would fit both with the reported weak association of CD8α polypeptides with lck (19), and the observation that CD8α′/β heterodimers, which cannot associate with lck, are tolerated in mouse thymus (57). Interestingly, in CD4− thymocytes a number of class II–restricted transgenic TCRs have been shown to be selected efficiently into the CD8 lineage (49). According to our model, the absence of CD4 in these thymocytes would selectively reduce the lck signal, altering the balance in favor of a predominant TCR signal, thus changing the commitment of these cells to the CD8 lineage.
CD4 associates very efficiently with lck and engagement of TCR plus CD4 would favor an lck-biased signal, thus promoting CD4 lineage commitment. Given the prominent involvement of lck, we would predict that signals suitable for CD4 differentiation would tend to resemble weak agonist signals, capable of inducing full activation of mature T cells. Indeed CD4+ T cells selected on single MHC/ peptide ligands in the thymus have been shown to be able to proliferate in response to the selecting peptide (58). As lck is relatively promiscuous in its associations, engagement of a variety of molecules, e.g., CD4, CD8, or CD2, with the TCR could provide sufficient lck activity to favor CD4 differentiation (1, 3, 4). Furthermore, ligation of CD4 with anti-CD4 Abs, which has been shown to promote lck activity (59), enhances maturation of CD4+ thymocytes induced by anti-TCR or bispecific antibodies (4, 60). These results indicate that lck activation in the presence of weak TCR signals do indeed favor CD4 commitment.
Our model requires that different TCR-MHC/peptide ligand combinations can give different ratios of TCR versus lck signals, which is known to occur in mature T cells. The interaction of T cells with partial agonist/antagonist ligands has been shown to result in a distinct set of biochemical signals (61), which appear to differ from full activation signals due to insufficient recruitment of active lck to the TCR (49). Moreover, other TCR-MHC/peptide ligand interactions induce lck activity independently of the coreceptors. In these CD4- and CD8-independent peripheral T cell responses, full activation can be achieved when no coreceptors are present (62, 63). As lck has been shown to be required for T cell activation (64), it is generally supposed that these coreceptor-independent responses occur because particular TCR-MHC/peptide interactions are able either to recruit sufficient active lck to the TCR signaling complex, or that another src-family kinase, e.g., fyn, can cooperate to promote full activation. This is in contrast to many TCR-MHC/peptide combinations, which require coreceptors and TCRs to cooperatively bind MHC molecules in order to exceed the stimulation threshold. It is currently unknown whether interactions between specific TCR-MHC/peptide combinations produce these differential outcomes as a result of differences in ligand density, TCR affinity, the ability to induce specific conformational changes in the TCR upon ligand binding, inherent variability in the interaction between particular TCRs and intracellular signaling molecules, or combinations of these. For the purposes of the model, it is sufficient to be aware that examples exist in mature T cells of individual TCR-MHC/peptide engagements that can induce TCR signals with a range of lck activity from very little to high levels, and we propose the same holds true for thymocytes. We would account for the presence of MHC class II–restricted “CD4-manqué” cells in CD4− mice as examples of thymocytes expressing TCRs that behave in a coreceptor-independent fashion. That is, just as with some mature T cells, the engagement of their TCRs in the absence of coreceptor activates sufficient src-family kinases to direct maturation to the CD4 lineage.
Unlike quantitative signaling models, and as illustrated in Fig. 8, we can explain the failure of high concentrations of partial agonist peptides to drive class I–restricted transgenic TCRs into the CD4 lineage, as an inherent failure of these peptides to shift the TCR/lck signal balance to that required for CD4 commitment. Increasing the concentration of peptide simply increases the overall signal in these cells, shifting the curve to the right in Fig. 8, which drives the thymocytes to deletion. Similarly, some partial agonist peptides (56) may never be appropriate to induce CD8 differentiation, because when they engage the TCR too much lck is activated. An example may be the lymphocytic choriomeningitis virus variant peptide, A3V, which appears to be recognized by mature T cells in a CD8-independent manner (56) supporting the view that it induces relatively high lck activity, which would be inappropriate for CD8+ differentiation in our model.
A prediction of the scheme outlined in Fig. 8 is that antagonist peptides would not be appropriate for selection of CD4+ cells. Thus far, an MHC class II-binding antagonist peptide has been shown to inhibit, rather than promote, the maturation of specific TCR transgenic CD4+ T cells (7), similar to the results reported here after treatment of lobes with CD3fos-F(ab′)2. Furthermore, selection by a single peptide/MHC class II complex, where the peptide had been shown to possess antagonist properties, generated a repertoire of CD4+ cells that could not be antagonized by the same peptide in the periphery (65). It will be interesting to see whether this prediction will generally hold true.
In conclusion, our results suggest that engaging CD8α/β or CD4 coreceptors during thymocyte differentiation has distinct outcomes, resulting in differential recruitment of signaling mediators that direct commitment decisions. As the CD3fos-F(ab′)2 reagent mimics uniquely the receptor engagements appropriate for differentiation of CD8+ thymocytes, we are now in a position to be able to examine some of the downstream signaling cascades controlling lineage commitment.
Acknowledgments
We thank D. Kioussis, B. Stockinger, D. Mathis, C. Benoist, R. Jaenisch, S. Ley, A. Magee, and J. Tite for making mice and reagents available; J. Bluestone for the 145.2C11 and GK1.5 hybridomas; T. Norton for expert animal breeding and maintenance; and B. Stockinger, D. Kioussis, and P. Travers for critical comments on the manuscript.
This work was supported by the Medical Research Council and by a grant from the Leukemia Research Fund.
Footnotes
1 Abbreviations used in this paper: BsAb, bispecific F(ab′)2; DC, dendritic cell; DN, double negative; DP, double positive; HSA, heat-stable antigen; NP, nucleoprotein; TOC, thymic organ culture.
References
- 1.Müller K-P, Kyewski BA. T cell receptor (TcR) targeting in mice lacking CD4 or major histocompatibility complex (MHC) class II: rescue of CD4 T cell lineage without co-engagement of TcR/CD4 by MHC class II. Eur J Immunol. 1995;25:896–902. doi: 10.1002/eji.1830250406. [DOI] [PubMed] [Google Scholar]
- 2.Takahama Y, Suzuki H, Katz KS, Grusby MJ, Singer A. Positive selection of CD4+T cells by TcR ligation without aggregation even in the absence of MHC. Nature. 1994;371:67–70. doi: 10.1038/371067a0. [DOI] [PubMed] [Google Scholar]
- 3.Cibotti R, Punt JA, Dash KS, Sharrow SO, Singer A. Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity. 1997;6:245–255. doi: 10.1016/s1074-7613(00)80327-1. [DOI] [PubMed] [Google Scholar]
- 4.Bommhardt U, Cole MS, Yun J, Tso, Zamoyska R. Signals through CD8 or CD4 can induce commitment to the CD4 lineage in the thymus. Eur J Immunol. 1997;27:1152–1163. doi: 10.1002/eji.1830270516. [DOI] [PubMed] [Google Scholar]
- 5.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 6.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher H-P, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 7.Spain LM, Jorgensen JL, Davis MM, Berg LJ. A peptide antigen antagonist prevents the differentiation of T cell receptor transgenic thymocytes. J Immunol. 1994;152:1709–1717. [PubMed] [Google Scholar]
- 8.Sebzda E, Wallace VA, Mayer J, Yeung RSM, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 9.Sebzda E, Kundig TM, Thomson CT, Aoki K, Mak S-Y, Mayer JP, Zamborelli T, Nathenson SG, Ohashi PM. Mature T cell reactivity altered by peptide agonist that induces positive selection. J Exp Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+T cells. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 11.Ohoka Y, Kuwata T, Tozawa Y, Zhao Y, Mukai M, Motegi Y, Suzuki R, Yokoyama M, Iwata M. In vitro differentiation and commitment of CD4+CD8+thymocytes to the CD4 lineage without TcR engagement. Int Immunol. 1996;8:297–306. doi: 10.1093/intimm/8.3.297. [DOI] [PubMed] [Google Scholar]
- 12.Takahama Y, Nakauchi H. Phorbol ester and calcium ionophore can replace TCR signals that induce positive selection of CD4 T cells. J Immunol. 1996;157:1508–1513. [PubMed] [Google Scholar]
- 13.Henrickson M, Reid J, Bellet JS, Sawchuk SS, Hirsch R. Comparison of in vivo efficacy and mechanism of action of antimurine monoclonal antibodies directed against TCR αβ (H57-597) and CD3 (145-2C11) Transplantation (Baltimore) 1995;60:828–835. [PubMed] [Google Scholar]
- 14.Sawchuk SS, Gates R, Hirsch R. Contrasting in vivo effects on T helper cell functions induced by mitogenic (intact) versus nonmitogenic (F(ab′)2) anti-CD3 monoclonal antibody. Transplantation (Baltimore) 1995;60:1331–1337. [PubMed] [Google Scholar]
- 15.Chatenoud L, Primo J, Bach J-F. CD3 antibody-induced dominant self-tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 16.Madrenas J, Chau LA, Smith J, Bluestone JA, Germain RN. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide–MHC molecule ligands. J Exp Med. 1997;185:219–229. doi: 10.1084/jem.185.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JA, Yun J, Tso, Clark MR, Cole MS, Bluestone JA. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J Exp Med. 1997;185:1413–1422. doi: 10.1084/jem.185.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itano A, Salmon P, Kioussis D, Tolaini M, Corbella P, Robey E. The cytoplasmic domain of CD4 promotes the development of CD4 lineage T cells. J Exp Med. 1996;183:731–741. doi: 10.1084/jem.183.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veillette A, Zuniga-Pflucker JC, Bolen JB, Kruisbeek AM. Engagement of CD4 and CD8 expressed on immature thymocytes induces activation of intracellular tyrosine phosphorylation pathways. J Exp Med. 1989;170:1671–1680. doi: 10.1084/jem.170.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostelny SA, Cole MS, Yun J, Tso Formation of a bispecific antibody by the use of leucine zippers. J Immunol. 1992;148:1547–1553. [PubMed] [Google Scholar]
- 21.Zijlstra M, Bix M, Simister N, Loring J, Raulet D, Jaenisch R. β2-microglobulin deficient mice lack CD4−8+cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 22.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 23.Spanopoulou E, Roman C, Corcoran L, Schlissel M, Silver D, Nemazee D, Nussenweig M, Shinton S, Hardy R, Baltimore D. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1 deficient mice. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 24.Mamalaki C, Elliot J, Norton T, Yannoutsos N, Townsend AR, Chandler P, Simpson E, Kioussis D. Positive and negative selection in transgenic mice expressing a T-cell receptor specific for influenza nucleoprotein and endogenous superantigen. Dev Immunol. 1993;3:159–174. doi: 10.1155/1993/98015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II–restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony–stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bluestone JA, Cron RQ, Barrett TA, Houlden B, Sperling AI, Dent A, Hedrick S, Rellahan B, Matis LA. Repertoire development and ligand specificity of murine TCRγδ cells. Immunol Rev. 1991;120:5–33. doi: 10.1111/j.1600-065x.1991.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 28.Hogquist KA, Tomlinson AJ, Kieper WC, McGargill MA, Hart MC, Naylor S, Jameson SC. Identification of a naturally occurring ligand for thymic positive selection. Immunity. 1997;6:389–399. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 29.Ashton-Rickardt PG, Van Kaer L, Schumacher TNM, Ploegh HL, Tonegawa S. Peptide contributes to the specificity of positive selection of CD8+T cells in the thymus. Cell. 1993;73:1041–1049. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- 30.Lucas B, Vasseur F, Penit C. Normal sequence of phenotypic transitions in one cohort of 5-bromo-2′-deoxyurine-pulse-labelled thymocytes. J Immunol. 1993;151:4574–4582. [PubMed] [Google Scholar]
- 31.Levelt CN, Ehrfield A, Eichmann K. Regulation of thymocyte development through CD3. I. Timepoint of ligation of CD3ε determines clonal deletion or induction of developmental program. J Exp Med. 1993;177:707–716. doi: 10.1084/jem.177.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinkai Y, Alt F. CD3ε-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2−/−mice in the absence of TCRβ chain expression. Int Immunol. 1994;6:995–1001. doi: 10.1093/intimm/6.7.995. [DOI] [PubMed] [Google Scholar]
- 33.Luton F, Buferne M, Davoust J, Schmitt-Verhulst A-M, Boyer C. Evidence for protein tyrosine kinase involvement in ligand-induced TCR/CD3 internalization and surface redistribution. J Immunol. 1994;153:63–72. [PubMed] [Google Scholar]
- 34.Valitutti S, Müller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 35.Cai Z, Kishimoto H, Brunmark A, Jackson MR, Peterson PA, Sprent J. Requirements for peptide-induced T cell receptor downregulation on naive CD8+T cells. J Exp Med. 1997;185:641–651. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 37.De Magistris MT, Alexander J, Coggeshal M, Altman A, Gaeta FCA, Grey HM, Sette A. Antigen-analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 38.Sloan-Lancaster J, Shaw AS, Rothbard J, Allen PM. Partial T cell signalling: altered phospho-ζ and lack of ZAP70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 39.Jameson SC, Bevan MJ. T cell receptor antagonists and partial agonists. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama T, Singer A, Hsi ED, Samelson LE. Intrathymic signalling in immature CD4+CD8+thymocytes results in tyrosine phosphorylation of the T-cell receptor zeta chain. Nature. 1989;341:651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- 41.van Oers NSC, Tao W, Watts JD, Johnson P, Aebersold R, Teh H-S. Constitutive tyrosine phosphorylation of the T cell receptor (TCR) ζ subunit: regulation of TCR-associated protein kinase activity by TCR ζ. Mol Cell Biol. 1993;13:5771–5780. doi: 10.1128/mcb.13.9.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osman N, Turner H, Lucas S, Reif K, Cantrell DA. The protein interactions of the immunoglobulin receptor family tyrosine-based activation motifs present in the T cell receptor ζ subunits and the CD3 γ, δ and ε chains. Eur J Immunol. 1996;26:1063–1068. doi: 10.1002/eji.1830260516. [DOI] [PubMed] [Google Scholar]
- 43.Jensen WA, Pleiman CM, Beaufils P, Wegener A-MK, Malissen B, Cambier JC. Qualitatively distinct signaling through T cell antigen receptor subunits. Eur J Immunol. 1997;27:707–716. doi: 10.1002/eji.1830270320. [DOI] [PubMed] [Google Scholar]
- 44.Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. ζ-phosphorylation without ZAP-70 activation induced by TcR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 45.Reis e Sousa C, Levine EH, Germain RN. Partial signaling by CD8+T cells in response to antagonist ligands. J Exp Med. 1996;184:149–157. doi: 10.1084/jem.184.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negishi I, Motoyama N, Nakayama K, Senju S, Hatakeyama S, Zhang Q, Chan AC, Loh DY. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 47.van Oers NSC, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J Exp Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki H, Punt JA, Granger LG, Singer A. Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+T cell lineages: a new perspective on thymic commitment and selection. Immunity. 1995;2:413–425. doi: 10.1016/1074-7613(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 49.Matechak EO, Killeen N, Hedrick SM, Fowlkes BJ. MHC class II–specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity. 1996;4:337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- 50.Corbella P, Moskophidis D, Spanopoulou E, Mamalaki C, Tolaini M, Itano A, Lans D, Baltimore D, Robey E, Kioussis D. Functional commitment to helper T cell lineage precedes positive selection and is independent of T cell receptor MHC specificity. Immunity. 1994;1:269–276. doi: 10.1016/1074-7613(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 51.Goldrath AW, Hogquist KA, Bevan MJ. CD8 lineage commitment in the absence of CD8. Immunity. 1997;6:633–642. doi: 10.1016/s1074-7613(00)80351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fung-Leung W-P, Schillham M, Rahemtulla A, Kündig T, Vollenweider M, Potter J, van Ewijk W, Mak T. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 53.Fung-Leung W-P, Kündig T, Ngo K, Panakos J, De Souza-Hitzler J, Wang E, Ohashi P, Mak T, Lau C. Reduced thymic maturation but normal effector function of CD8+T cells in CD8β gene-targeted mice. J Exp Med. 1994;180:959–967. doi: 10.1084/jem.180.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crooks M, Littman D. Disruption of T lymphocyte positive and negative selection in mice lacking the CD8β chain. Immunity. 1994;1:277–286. doi: 10.1016/1074-7613(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 55.Nakayama K, Nakayama K, Negishi I, Kuida K, Louie MC, Kanagawa O, Nakauchi H, Loh DY. Requirement for CD8 β chain in positive selection of CD8-lineage T cells. Science. 1994;263:1131–1133. doi: 10.1126/science.8108731. [DOI] [PubMed] [Google Scholar]
- 56.Sebzda E, Choi M, Fung-Leung WP, Mak TW, Ohashi PS. Peptide-induced positive selection of TCR transgenic thymocytes in a co-receptor-independent manner. Immunity. 1997;6:643–653. doi: 10.1016/s1074-7613(00)80352-0. [DOI] [PubMed] [Google Scholar]
- 57.Zamoyska R, Derham P, Gorman SD, von Hoegen P, Bolen JB, Veillette A, Parnes JR. Inability of CD8α′ polypeptide to associate with p56lckcorrelates with impaired function in vitro and lack of expression in vivo. Nature. 1989;342:278–281. doi: 10.1038/342278a0. [DOI] [PubMed] [Google Scholar]
- 58.Nakano N, Rooke R, Benoist C, Mathis D. Positive selection of T cells induced by viral delivery of neopeptides to the thymus. Science. 1997;275:678–683. doi: 10.1126/science.275.5300.678. [DOI] [PubMed] [Google Scholar]
- 59.Veillette A, Bookman M, Horak E, Samelson L, Bolen J. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck . Nature. 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- 60.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 61.Sloan-Lancaster J, Allen PM. Altered peptide ligand–induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 62.Miceli M, Parnes J. Role of CD4 and CD8 in T cell activation and differentiation. Adv Immunol. 1993;53:59–122. doi: 10.1016/s0065-2776(08)60498-8. [DOI] [PubMed] [Google Scholar]
- 63.Kwan Lim, G., T. Ong, F. Aosai, H. Stauss, and R. Zamoyska. Is CD8 dependence a true reflection of TCR affinity for antigen? . Int Immunol. 1993;5:1219–1228. doi: 10.1093/intimm/5.10.1219. [DOI] [PubMed] [Google Scholar]
- 64.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 65.Ignatowicz L, Kappler J, Parker D, Marrack P. The responses of mature T cells are not necessarily antagonized by their positively selecting peptide. J Immunol. 1996;157:1827–1831. [PubMed] [Google Scholar]