Abstract
Paired immunoglobulin-like receptor B (PIR-B) (p91) molecule has been proposed to function as an inhibitory receptor in B cells and myeloid lineage cells. We demonstrate here that the cytoplasmic region of PIR-B is capable of inhibiting B cell activation. Mutational analysis of five cytoplasmic tyrosines indicate that tyrosine 771 in the motif VxYxxL plays the most crucial role in mediating the inhibitory signal. PIR-B–mediated inhibition was markedly reduced in the SH2-containing protein tyrosine phosphatases SHP-1 and SHP-2 double-deficient DT40 B cells, whereas this inhibition was unaffected in the inositol polyphosphate 5′-phosphatase SHIP-deficient cells. These data demonstrate that PIR-B can negatively regulate B cell receptor activation and that this PIR-B–mediated inhibition requires redundant functions of SHP-1 and SHP-2.
The ability of B cells to respond to antigen relies on signals transmitted through the B cell antigen receptor (BCR) complex. Activation of cytoplasmic protein tyrosine kinases is the earliest measurable biochemical response to BCR cross-linking. This initial event leads to the generation of secondary signals including Ras activation, phosphatidylinositol 3-kinase activation, turnover of phosphoinositides, and calcium mobilization. Both the strength and duration of the BCR-elicited signal are important in directing biological responses of B cells such as proliferation, differentiation, and apoptosis (for reviews see references 1–4). Thus, attenuation and termination of these activation signals are also critical components for B cell response.
B cell activation is inhibited by cross-linking FcγRIIB with the BCR (5, 6). The cytoplasmic domain of FcγRIIB contains an immunoreceptor tyrosine-based inhibitory motif (ITIM), which is necessary for the inhibitory function of the receptor (7, 8). Phosphorylation of the tyrosine in the ITIM by an activated protein tyrosine kinase(s) is critical to its inhibitory mechanism (7). Although the phosphorylated FcγRIIB ITIM associates with the SH2-containing protein tyrosine phosphatase SHP-1 and the SH2-containing inositol polyphosphate 5′-phosphatase SHIP (9, 10), functional evidence has shown that inhibition by FcγRIIB primarily involves SHIP (11–13). In B cells, in addition to FcγRIIB, a recently cloned p91 (PIR-B) is suggested to function as an inhibitory receptor. PIR-B, a member of the immunoglobulin superfamily, is a 91-kD transmembrane glycoprotein containing four potential ITIMs in its cytoplasmic region (14, 15).
A growing family of inhibitory receptors that can interrupt the activation process have generated interest in the mechanism of inhibition and raised questions about the similarity in this mechanism used by the different receptors. To test whether PIR-B can deliver inhibitory signals in B cells, and whether both PIR-B– and FcγRIIB-mediated inhibitory responses are dependent on the same signaling molecule SHIP, we have constructed chimeric FcγRIIB– PIR-B molecules with the cytoplasmic region of PIR-B and assessed their ability to inhibit BCR signaling. We report here that SHP-1 and SHP-2, but not SHIP, are required for PIR-B–mediated inhibitory signal.
Materials and Methods
Cells, Expression Construct, and Abs.
Various mutant DT40 cells, wild-type A20, and A20 IIA1.6 cells were maintained in RPMI 1640 supplemented with 10% FCS, penicillin, streptomycin, and glutamine. FcγRIIB–PIR-B chimera and its mutants were created by the PCR method. Resulting constructs were confirmed by DNA sequencing. The mutant and wild-type FcγRIIB–PIR-B cDNAs were subcloned into pApuro vector (16) and were electroporated into DT40 or A20 IIA1.6 cells as previously described (17). After selecting clones in the presence of puromycin (0.5 μg/ ml), cell surface expression levels of FcγRIIB–PIR-B were checked by flow cytometry analysis using anti–mouse FcγRIIB mAb, 2.4G2 (18). Anti–chicken IgM mAb M4, anti-SHIP Ab, intact rabbit anti–mouse IgM, F(ab′)2 rabbit anti–mouse IgM, and antiphosphotyrosine mAb 4G10 were as previously described (11). Anti–SHP-1 Ab and anti–PIR-B Ab were obtained by immunizing rabbits with bacterially expressed glutathione S-transferase fusion protein containing chicken SHP-1, and peptides in the mouse PIR-B cytoplasmic region, respectively. Anti–SHP-2 Ab, intact rabbit anti–mouse IgG, and F(ab′)2 rabbit anti–mouse IgG were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), Chemicon, Inc. (Temecula, CA), and Chemicon, Inc., respectively.
Generation of SHIP-, SHP-1–, SHP-2–, and SHP-1/SHP-2–deficient DT40 Cells.
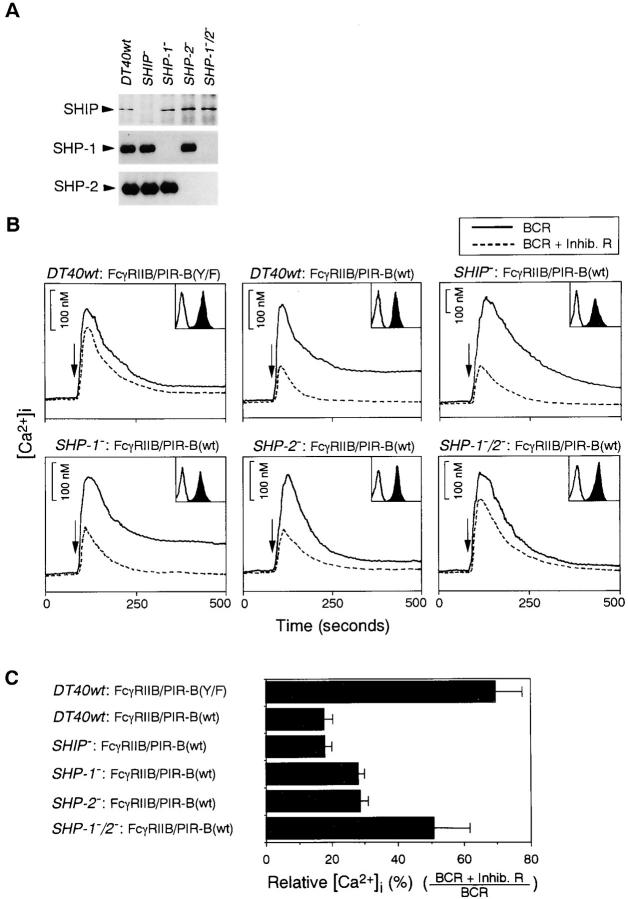
Chicken spleen cDNA library (Clontech, Palo Alto, CA) was screened by murine SHP-1 cDNA (provided by Dr. J.N. Ihle, St. Jude Children's Research Hospital, Memphis, TN) (19). Using the isolated chicken SHP-1 cDNA, the chicken genomic DNA library (Clontech) was screened to obtain genomic clones. After subcloning, the neo- or his-targeting constructs were made by replacing the genomic fragment containing exons corresponding to SHP-1 amino acid residues 420–520 with neo or his cassette. These constructs were sequentially transfected into wild-type DT40 cells by electroporation to obtain null mutants. Selection for drug-resistant clones was carried out by using G418 (2 mg/ml) and histidinol (1 mg/ml). Based on a previously published sequence of chicken SHP-2 (20), chicken SHP-2 cDNA and genomic clones were obtained by the PCR method. The targeting vectors, pSHP-2–bsr, pSHP-2–hisD, and pSHP-2–hygro were constructed by replacing the genomic fragment-containing exons that correspond to SHP-2 amino acid residues 472–533 with bsr, hisD, or hygro cassette. The targeting vector pSHP-2–bsr was linearized and introduced into wild-type DT40 cells. Selection was done in the presence of 50 μg/ml blasticidin S. Clones were screened by Southern blot analysis. pSHP-2–hisD was transfected into the bsr-targeted clone and selected with both blasticidin S (50 μg/ml) and histidinol (1 mg/ml). For the generation of SHP-1/ SHP-2 double-deficient DT40 cells, the targeting vectors pSHP-2–bsr and pSHP-2–hygro were sequentially transfected into SHP-1–deficient cells (11). Clones were selected in the presence of 50 μg/ml blasticidin S and 1.6 mg/ml hygromycin. The procedures to establish SHIP-deficient DT40 cells were described in reference 11. Evidence for null mutants of these knock-out DT40 cells were demonstrated by Western blotting analysis (see Fig. 5 A) as well as Northern blotting analysis (data not shown).
Figure 5.
Reduction of FcγRIIB–PIR-B–mediated inhibitory effect in SHP-1/SHP-2 double-deficient cells. (A) SHIP, SHP-1, or SHP-2 protein expression on various gene-targeted DT40 cells. Each protein was immunoprecipitated and was detected by Western blotting analysis using anti-SHIP, anti–SHP-1, or anti–SHP-2 Ab. (B) Calcium mobilization was measured after BCR cross-linking (solid line) and coligation (dashed line) in various DT40 mutants expressing FcγRIIB–PIR-B. Surface expression levels of FcγRIIB–PIR-B are indicated in inset boxes. (C) Comparison of inhibitory effect in various mutant DT40 cells. The results are expressed as the mean from three independent clones. Error bars represent SD from the mean.
Immunoprecipitation and Western Blotting Analysis.
IIA1.6 transformants and wild-type A20 cells (2 × 107) in 1 ml of RPMI 1640 medium were incubated for 3 min at 37°C with intact (50 μg) or F(ab′)2 fragment (25 μg) of rabbit anti-mouse IgG. Cells were solubilized in lysis buffer (1% NP-40, 150 mM NaCl, 20 mM Tris, pH 7.5, 1 mM EDTA, 10% glycerol) containing 50 mM NaF, 10 μM molybdate, 2 mM sodium vanadate supplemented with protease inhibitors as previously described (17). Precleared lysates were sequentially incubated with 2.4G2 and anti–rat IgG-agarose. Immunoprecipitates were separated by SDS-PAGE gel, transferred to nitrocellulose membrane, and detected by appropriate Abs and ECL system (Amersham Corp., Arlington Heights, IL).
Calcium measurements.
Cells (5 × 106) were suspended in PBS containing 20 mM Hepes (pH 7.2), 5 mM glucose, 0.025% BSA, and 1 mM CaCl2, and loaded with 3 μM Fura-2/AM at 37°C for 45 min. Cells were washed twice, and adjusted to 106 cells/ml. Continuous monitoring of fluorescence from the cell suspension was performed using Hitachi F-2000 fluorescence spectrophotometer (Hitachi Limited, Tokyo, Japan) at an excitation wavelength of 340 nm and an emission wavelength of 510 nm. Calibration and calculation of calcium levels were done as previously described (21). IIA1.6 cells expressing FcγRIIB–PIR-B cells were stimulated by adding intact or F(ab′)2 fragment of rabbit anti–mouse IgG, resulting in coligation of BCR to chimeric FcγRIIB–PIR-B receptor or cross-linking BCR alone. Stimulation of DT40 cells was carried out by adding intact or F(ab′)2 fragment of rabbit anti–mouse IgM followed by the anti–chicken IgM mAb, M4. Two independent IIA1.6 transformants expressing each FcγRIIB– PIR-B mutant were used for calcium measurement. In the case of DT40 cells expressing FcγRIIB–PIR-B, three different clones were used for this analysis. Calcium release from intracellular calcium store was measured in the buffer containing 1 mM EGTA.
Nuclear Factor in Activated T Cell Luciferase Assays.
24 h after transfection with 20 μg of nuclear factor in activated T cell (NF-AT) (Renilla luciferase control reporter vector) luciferase reporter gene and 2 μg of pRL-CMV (Promega, Madison, WI), 2 × 105 transfected cells were aliquoted into a 96-well plate and cultured in a final volume of 100 μl of RPMI 1640 medium. Cells were stimulated as previously described (11). After 5 h of stimulation, cells were lysed and luciferase activity was measured with the Dual-luciferase reporter assay system (Promega).
Flow Cytometric Analysis for Surface Expression of FcγRIIB–PIR-B.
Cells were washed, stained with FITC-conjugated 2.4G2 (PharMingen, San Diego, CA), and analyzed by FACScan® (Becton Dickinson, Mountain View, CA). The x and y axes for the histograms in inset boxes (Fig. 2 A, 4 A, and 5 B) indicate fluorescence intensity (4-decade-log scales) and relative cell number, respectively.
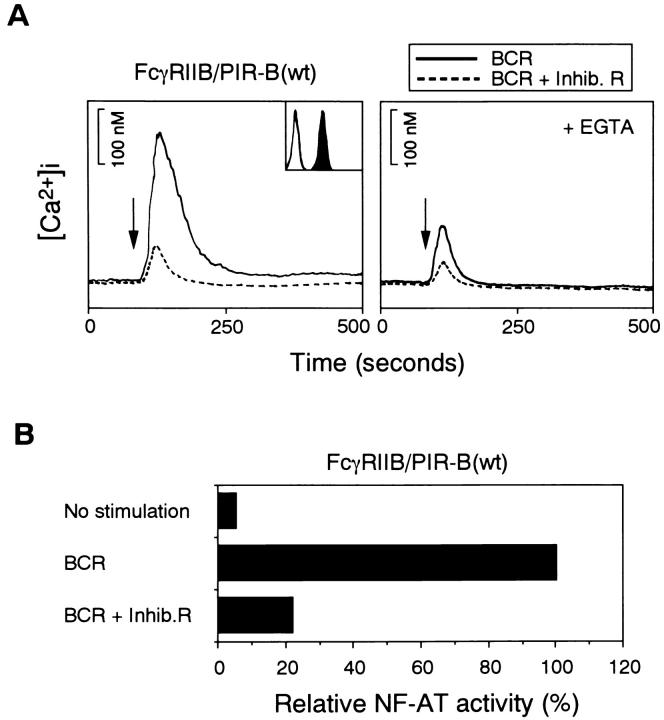
Figure 2.
Inhibition of BCR-mediated cell activation by FcγRIIB– PIR-B. (A) Calcium mobilization stimulated by BCR cross-linking (solid line) and coligation of BCR to FcγRIIB–PIR-B (dashed line) in IIA1.6 cells expressing wild-type FcγRIIB–PIR-B. Calcium release from intracellular calcium store was measured in the presence of 1 mM EGTA, shown by right panel. Histogram in the inset box indicates the expression level of FcγRIIB–PIR-B by 2.4G2 staining. Unstained cells were used as a negative control (open histogram). Stimulation of FcγRIIB–PIR-B alone by 2.4G2 did not induce calcium mobilization. (B) NF-AT activation by BCR cross-linking and the coligation of BCR to FcγRIIB–PIR-B. The results are shown as relative NF-AT activity to BCR cross-linking alone.
Results and Discussion
The Cytoplasmic Domain of PIR-B Is Capable of Inhibiting BCR Activation.
To test whether the cytoplasmic domain of PIR-B may inhibit BCR activation, a chimeric molecule with the cytoplasmic domain of PIR-B and the extracellular domain of FcγRIIB was constructed (Fig. 1). This molecule was transfected into the FcγRIIB-negative mutant of the mouse A20 B cell lymphoma IIA1.6 (22) to obtain stable transformants. Expression level of this chimeric receptor was assessed by flow cytometry analysis using anti– mouse FcγRIIB mAb, 2.4G2 (Fig. 2 A). IIA1.6 cells expressing the FcγRIIB–PIR-B receptor was stimulated by BCR cross-linking alone (Fig. 2 A, solid line) or coligation of BCR and the FcγRIIB–PIR-B (Fig. 2 A, dashed line). Coligation of FcγRIIB–PIR-B to the BCR resulted in an inhibition of intracellular free calcium. Incubation with EGTA further decreased Ca2+ mobilization upon co–cross-linking of BCR with FcγRIIB–PIR-B, indicating that the FcγRIIB–PIR-B acts at least on calcium release from intracellular stores. Consistent with these results, transcriptional activation of the NF-AT luciferase reporter was inhibited by coligation of the BCR to this chimeric molecule (Fig. 2 B). Thus, the cytoplasmic domain of PIR-B can deliver a signal that inhibits BCR-mediated function in IIA1.6 B cells.
Figure 1.
Schematic diagram of FcγRIIB–PIR-B chimeric molecules. PIR-B cytoplasmic region contains four ITIM-like motifs (residues 688– 693, SLYASV; 717–722, ETYAQV; 769–774, VTYAQL; and 799–804, SVYATL). In addition to these tyrosines, the cytoplasmic domain of PIR-B contains one more tyrosine (residue 747). The open and shaded boxes represent portions of FcγRIIB and PIR-B, respectively.
SHP-1 and SHP-2 Associate with the Cytoplasmic Domain of PIR-B.
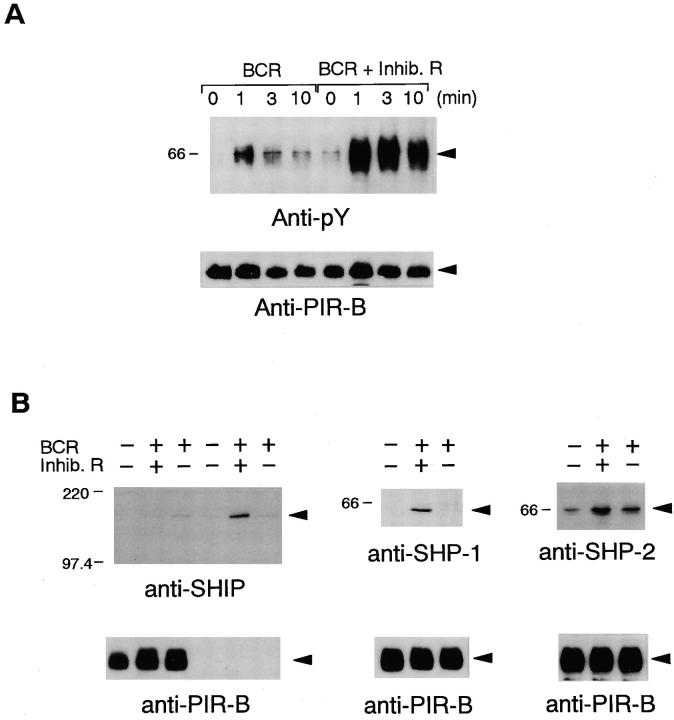
The presence of ITIM-related sequences in the PIR-B cytoplasmic domain (Fig. 1) suggests that the PIR-B may become tyrosine phosphorylated, resulting in its association with SH2-containing signaling molecules such as SHP-1, SHP-2, and/or SHIP. Using IIA1.6 cells transfected with FcγRIIB–PIR-B, the status of tyrosine phosphorylation of FcγRIIB–PIR-B was determined by immunoprecipitation followed by Western blotting with antiphosphotyrosine mAb 4G10. As shown in Fig. 3 A, the chimeric receptor was slightly tyrosine phosphorylated upon BCR cross-linking alone and this phosphorylation was markedly augmented by coligation of BCR and FcγRIIB–PIR-B. The FcγRIIB–PIR-B phosphorylation was due to tyrosine residues located in the cytoplasmic domain of PIR-B, since FcγRIIB–PIR-B(Y/F) (Fig. 1) did not undergo tyrosine phosphorylation upon coligation of BCR and FcγRIIB– PIR-B(Y/F) (data not shown). After co–cross-linking of BCR with FcγRIIB–PIR-B, the chimeric molecule was immunoprecipitated followed by Western blotting with anti–SHP-1 or anti–SHP-2 Ab (Fig. 3 B, middle and right), demonstrating that SHP-1 and SHP-2 are recruited to FcγRIIB–PIR-B. In contrast, recruitment of SHIP to FcγRIIB–PIR-B could not be detected (Fig. 3 B, left, lanes 1–3). As a positive control, recruitment of SHIP to phosphorylated FcγRIIB molecule was clearly observed under the same experimental conditions (Fig. 3 B, left, lanes 4–6).
Figure 3.
Recruitment of SHP-1 and SHP-2 to FcγRIIB–PIR-B after coligation of BCR and the chimeric receptor. (A) Tyrosine phosphorylation of FcγRIIB–PIR-B by BCR cross-linking and coligation of BCR to FcγRIIB–PIR-B. IIA1.6 cells expressing wild-type FcγRIIB–PIR-B were incubated with F(ab′)2 rabbit anti–mouse IgG, or with intact rabbit anti–mouse IgG. FcγRIIB–PIR-B was then immunoprecipitated with 2.4G2, separated by SDS-PAGE gel, transferred to membrane, and immunoblotted with antiphosphotyrosine mAb 4G10 (top). The same membrane was reprobed with anti–PIR-B Ab (bottom). (B) Immunoblotting with anti–SHP-1 and anti–SHP-2 Abs after BCR cross-linking and the coligation of BCR to FcγRIIB–PIR-B. IIA1.6 cells expressing wild-type FcγRIIB–PIR-B were stimulated with either F(ab′)2 rabbit anti–mouse IgG or intact rabbit anti–mouse IgG and were lysed. FcγRIIB–PIR-B was then immunoprecipitated with 2.4G2, resolved by SDS-PAGE gel, transferred to membrane, and probed with anti–SHP-1 or anti–SHP-2 Ab. In the case of immunoblotting with anti-SHIP, in addition to IIA1.6 cells expressing wild-type FcγRIIB–PIR-B (lanes 1–3), wild-type A20 cells expressing FcγRIIB were stimulated similarly (lanes 4–6). FcγRIIB– PIR-B or FcγRIIB was immunoprecipitated with 2.4G2 followed by Western blotting with anti-SHIP Ab. Membranes were reprobed with anti–PIR-B Ab (bottom).
Tyrosine 771 in the Cytoplasmic Domain of PIR-B Is Essential for Inhibition.
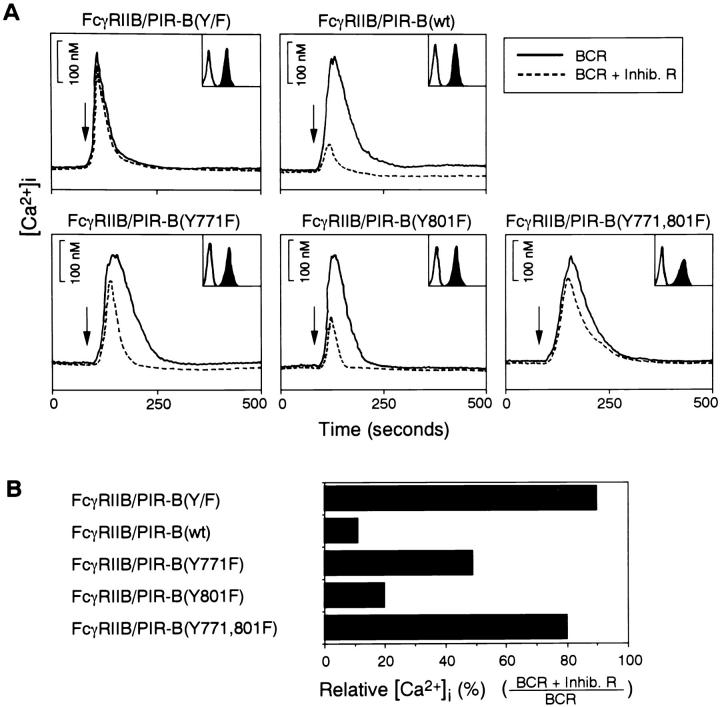
The cytoplasmic tail of PIR-B contains four copies (residues 688–693, 717–722, 769–774, and 799–804) of potential ITIMs (14, 15, 23). The inhibition assay described above allowed us to assess the structural importance of each phosphotyrosine for the inhibitory signal. Various tyrosine mutants were made (Fig. 1) and transfected into IIA1.6 cells to obtain stable transformants. IIA1.6 cells expressing comparable levels of chimeric receptors (Fig. 4 A) were stimulated by BCR alone or coligation of BCR and the chimeric mutants. FcγRIIB–PIR-B(Y/F) almost reverted the inhibitory effect of PIR-B. This result demonstrates that phosphorylation of cytoplasmic tyrosines of PIR-B plays an important role in the inhibitory signal and that the residual inhibition may be independent of tyrosine phosphorylation on PIR-B (Fig. 4 B). Similar to FcγRIIB–PIR-B(Y/F), inhibition of the BCR-induced calcium mobilization was almost abrogated by coligating of BCR and FcγRIIB–PIR-B(Y771, 801F). Analysis of the single tyrosine mutants, FcγRIIB–PIR-B(Y771F) and FcγRIIB–PIR-B(Y801F), indicates that these tyrosines have different degrees of importance in the inhibitory signal mediated by FcγRIIB–PIR-B. FcγRIIB–PIR-B(Y801F) inhibited calcium mobilization to a lesser degree than did wild-type chimeric receptor, whereas the FcγRIIB–PIR-B(Y771F) mutant inhibited it to a much lesser degree than wild-type FcγRIIB–PIR-B (Fig. 4, A and B). Thus, these data indicate that both tyrosines contribute to the inhibitory effect and that tyrosine 771 plays a more critical role in inhibiting the BCR-induced calcium mobilization.
Figure 4.
Requirement of cytoplasmic tyrosine residues in inhibitory response. (A) Calcium mobilization was monitored after BCR cross-linking (solid line) and coligation (dashed line) in IIA1.6 cells expressing various FcγRIIB–PIR-B mutants. Surface expression levels of FcγRIIB–PIR-B mutants are indicated in inset boxes. (B) Comparison of inhibitory effect by various FcγRIIB–PIR-B mutants. The percentage of [Ca2+]i was given by the total calcium mobilization elicited by coligation of BCR to FcγRIIB–PIR-B over that by BCR stimulation alone. The results are expressed as the mean from two independent clones.
SHP-1 and SHP-2 Are Redundantly Required for PIR-B–mediated Inhibition.
To test directly the role of SHIP, SHP-1, and SHP-2 in the inhibitory signal delivered by PIR-B cytoplasmic tail, B cell lines deficient in SHIP, SHP-1, or SHP-2 individually and in a combination of SHP-1 and SHP-2 were established using a chicken DT40 B cell line as a consequence of homologous recombination (Fig. 5 A). A detailed analysis of BCR signal transduction in these mutants will be presented elsewhere. FcγRIIB– PIR-B chimeric molecule was transfected into DT40 wild-type and mutant cells, and compared for its ability to mediate the inhibitory signaling response. In contrast to IIA1.6 B cells, a more residual inhibition by FcγRIIB–PIR-B(Y/F) was observed in DT40 cells (Fig. 5, B and C). Various genetic background DT40 cells expressing similar levels of FcγRIIB– PIR-B were stimulated with BCR cross-linking alone (Fig. 5, solid line) or coligation of BCR and the FcγRIIB–PIR-B (dashed line). As shown in Fig. 5 B, FcγRIIB–PIR-B–mediated inhibition of calcium mobilization was unperturbed in SHIP-deficient DT40 cells, but was substantially reduced in SHP-1/SHP-2 double-deficient cells. The reduction of the chimeric receptor-mediated inhibition was also observed by loss of either SHP-1 or SHP-2 alone. However, this reduction was less than that in double-deficient cells. These results demonstrate that redundant functions of SHP-1 and SHP-2 are required for the FcγRIIB–PIR-B–mediated inhibitory signal.
Signals from the BCR and coreceptors such as CD19, CD22, and FcγRIIB (24, 25) are integrated inside the cell, allowing the B cell to mount a response appropriate to the source of the antigen and the lymphocyte environment. In this study, we provide evidence that the cytoplasmic region of PIR-B is capable of inhibiting B cell activation by a phosphotyrosine-dependent manner in IIA1.6 and DT40 B cells. Coligation of BCR and FcγRIIB–PIR-B induced tyrosine phosphorylation of the cytoplasmic domain of PIR-B and this phosphorylation was abrogated by the FcγRIIB– PIR-B(Y/F) mutant. Moreover, this mutant markedly reduced the inhibitory effects on BCR signaling, suggesting that the SH2-containing proteins are involved in PIR-B–mediated inhibitory signals.
The finding that the residual inhibition by FcγRIIB– PIR-B still occurs in SHP-1 and SHP-2 double-deficient DT40 cells, implicates that, in addition to SHP-1 and SHP-2, another SH2-containing protein(s) may participate in this inhibitory signal to some extent. Nevertheless, the substantial reduction of the inhibition by loss of SHP-1 and SHP-2 clearly indicates that the redundant functions of these SH2-containing phosphatases are required for PIR-B–mediated inhibition. Supporting this conclusion, both SHP-1 and SHP-2 were recruited to the phosphorylated FcγRIIB–PIR-B. In contrast to requirement of SHIP for an inhibitory response by FcγRIIB, our biochemical and functional data demonstrate that SHIP is dispensable for the PIR-B–mediated inhibitory response.
Mutational analysis of five cytoplasmic tyrosines indicates that tyrosine 771 in the motif VTYAQL plays the most crucial role in mediating the inhibitory signal and that tyrosine 801 in the motif SVYATL also contributes. The more functional importance of tyrosine 771 may reflect the fact that the motif surrounding tyrosine 771 matches better with the consensus I/VxYxxL sequence than that of tyrosine 801 (26). Binding of SH2 domains of SHP-1 and SHP-2 to tyrosine phosphorylated PIR-B would serve to relocate SHP-1 and SHP-2 to membrane (11), where they may gain access to potential substrates. Simultaneously, these phosphatases may be converted to an open conformation by binding to phosphorylated PIR-B, thereby leading to an increase of their enzymatic activity (27).
The ligand of PIR-B is still unknown. Significant homology of PIR-B to recently isolated LIR-1 (28), together with the evidence that LIR-1 is able to bind to the MHC class I molecule, suggests that PIR-B may be a receptor for MHC class I or class I–related molecules. Thus, similar to inhibition of NK cytotoxicity by interaction between class I molecules on target cells and KIRs on NK cells (29–32), cell–cell interactions may bring PIR-B into the close proximity of antigen-bound BCR, resulting in attenuation of BCR signaling. Alternatively, interaction of PIR-B with a putative ligand may remove it from the vicinity of BCR in order to release the B cells from the negative regulatory effects. Identification of the ligand for PIR-B as well as substrates for SHP-1 and SHP-2 will further clarify biological roles of PIR-B in the immune response and its mechanism of inhibition.
Acknowledgments
This work was supported by grants to T. Kurosaki from the Ministry of Education, Science, Sports, and Culture of Japan, the Science Research Promotion Fund of the Japan Private School Promotion Foundation, and the Sumitomo Foundation, and a grant to T. Takai from CREST.
Footnotes
We would like to acknowledge Dr. J.N. Ihle for providing us with the mouse SHP-1 cDNA.
References
- 1.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 2.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 3.Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 4.Pleiman CM, D'Ambrosio D, Cambier JC. The B-cell antigen receptor complex: structure and signal transduction. Immunol Today. 1994;15:393–399. doi: 10.1016/0167-5699(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 5.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 6.Ravetch JV. Fc receptors. Curr Opin Immunol. 1997;9:121–125. doi: 10.1016/s0952-7915(97)80168-9. [DOI] [PubMed] [Google Scholar]
- 7.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of FcγRIIB modulates B-cell receptor signalling. Nature. 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 8.Amigorena S, Bonnerot C, Drake JR, Choquet D, Hunziker W, Guillet J-G, Webster P, Sautes C, Mellman I, Fridman WH. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 9.D'Ambrosio D, Hippen KL, Minskoff SA, Mellman I, Pani G, Siminovitch KA, Cambier JC. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by FcγRIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 10.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 11.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 12.Gupta N, Scharenberg AM, Burshtyn DN, Wagtmann N, Lioubin MN, Rohrschneider LR, Kinet J-P, Long EO. Negative signaling pathways of the killer cell inhibitory receptor and FcγRIIb1 require distinct phosphatases. J Exp Med. 1997;186:473–478. doi: 10.1084/jem.186.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadler MJS, Chen B, Anderson JS, Wortis HH, Neel BG. Protein-tyrosine phosphatase SHP-1 is dispensable for FcγRIIB-mediated inhibition of B cell antigen receptor activation. J Biol Chem. 1997;272:20038–20043. doi: 10.1074/jbc.272.32.20038. [DOI] [PubMed] [Google Scholar]
- 14.Hayami K, Fukuta D, Nishikawa Y, Yamashita Y, Inui M, Ohyama Y, Hikida M, Ohmori H, Takai T. Molecular cloning of a novel murine cell– surface glycoprotein homologous to killer cell inhibitory receptors. J Biol Chem. 1997;272:7320–7327. doi: 10.1074/jbc.272.11.7320. [DOI] [PubMed] [Google Scholar]
- 15.Kubagawa H, Burrows PD, Cooper MD. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc Natl Acad Sci USA. 1997;94:5261–5266. doi: 10.1073/pnas.94.10.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor–coupled Ca2+mobilization through distinct pathways. EMBO (Eur Mol Biol Organ) J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2, and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B cell antigen receptor. EMBO (Eur Mol Biol Organ) J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi T, Cleveland JL, Ihle JN. Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol Cell Biol. 1992;12:836–846. doi: 10.1128/mcb.12.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park CY, LaMontagne KR, Tonks NK, Hayman MJ. Cloning and expression of the chicken protein tyrosine phosphatase SH-PTP2. Gene. 1996;177:93–97. doi: 10.1016/0378-1119(96)00278-8. [DOI] [PubMed] [Google Scholar]
- 21.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 22.Jones B, Tite JP, Janeway CA., Jr Different phenotypic variants of the mouse B cell tumor A20/2J are selected by antigen- and mitogen-triggered cytotoxicity of L3T4-positive, I-A–restricted T cell clones. J Immunol. 1986;136:348–356. [PubMed] [Google Scholar]
- 23.Cambier JC. Inhibitory receptors abound? . Proc Natl Acad Sci USA. 1997;94:5993–5995. doi: 10.1073/pnas.94.12.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Rourke L, Tooze R, Fearon DT. Co-receptors of B lymphocytes. Curr Opin Immunol. 1997;9:324–329. doi: 10.1016/s0952-7915(97)80077-5. [DOI] [PubMed] [Google Scholar]
- 25.Unkeless JC, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 26.Vivier E, Daëron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 27.Pluskey S, Wandless TJ, Walsh CT, Shoelson SE. Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains. J Biol Chem. 1995;270:2897–2900. doi: 10.1074/jbc.270.7.2897. [DOI] [PubMed] [Google Scholar]
- 28.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, Hsu M-L. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 29.Leibson PJ. MHC-recognizing receptors: they're not just for T cell anymore. Immunity. 1995;3:5–8. doi: 10.1016/1074-7613(95)90153-1. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama WM. Hybrid resistance and the Ly-49 family of natural killer cell receptors. J Exp Med. 1995;182:273–277. doi: 10.1084/jem.182.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanier LL. Natural killer cells: from no receptors to too many. Immunity. 1997;6:371–378. doi: 10.1016/s1074-7613(00)80280-0. [DOI] [PubMed] [Google Scholar]
- 32.Long EO, Wagtmann N. Natural killer cell receptors. Curr Opin Immunol. 1997;9:344–350. doi: 10.1016/s0952-7915(97)80080-5. [DOI] [PubMed] [Google Scholar]