Abstract
CCR5, a chemokine receptor expressed on T cells and macrophages, is the principal coreceptor for M-tropic HIV-1 strains. Recently, we described an NH2-terminal modification of the CCR5 ligand regulated on activation, normal T cell expressed and secreted (RANTES), aminooxypentane-RANTES (AOP-RANTES), that showed potent inhibition of macrophage infection by HIV-1 under conditions where RANTES was barely effective. To investigate the mechanism of AOP-RANTES inhibition of HIV infectivity we examined the surface expression of CCR5 using a monoclonal anti-CCR5 antibody, MC-1. We demonstrate that AOP-RANTES rapidly caused >90% decrease in cell surface expression of CCR5 on lymphocytes, monocytes/ macrophages, and CCR5 transfected Chinese hamster ovary (CHO) cells. RANTES also caused a loss of cell surface CCR5, although its effect was less than with AOP-RANTES. Significantly, AOP-RANTES inhibited recycling of internalized CCR5 to the cell surface, whereas RANTES did not. When peripheral blood mononuclear cells are cultured for prolonged periods of time in the presence of RANTES, CCR5 expression is comparable to that seen on cells treated with control medium, whereas there is no CCR5 surface expression on cells cultured in the presence of AOP-RANTES. Immunofluorescence indicated that both AOP-RANTES and RANTES induced downmodulation of cell surface CCR5, and that the receptor was redistributed into endocytic organelles containing the transferrin receptor. When RANTES was removed, the internalized receptor was recycled to the cell surface; however, the receptor internalized in the presence of AOP-RANTES was retained in endosomes. Using human osteosarcoma (GHOST) 34/CCR5 cells, the potency of AOP-RANTES and RANTES to inhibit infection by the M-tropic HIV-1 strain, SF 162, correlated with the degree of downregulation of CCR5 induced by the two chemokines. These differences between AOP-RANTES and RANTES in their effect on receptor downregulation and recycling suggest a mechanism for the potent inhibition of HIV infection by AOP-RANTES. Moreover, these results support the notion that receptor internalization and inhibition of receptor recycling present new targets for therapeutic agents to prevent HIV infection.
Chemokine receptors, members of the heptahelical G protein–coupled receptor superfamily (GPCRs), act in concert with CD4 to enable the entry of HIV and simian immunodeficiency virus (SIV) into target cells. Several chemokine receptors have been identified in vitro as coreceptors for HIV. CCR5 is the major coreceptor for M-tropic HIV strains (1–3), whereas CXCR4 permits entry of T-tropic strains (4). Other chemokine receptors, such as CCR2b (5) and CCR3 (6), in addition to chemokine receptor-like orphan proteins such as STRL33 or Bonzo (7, 8), GRP-15 or BOB (8, 9), and GRP1 (9), when expressed on CD4-positive cell lines, can also function as coreceptors for M- and/or T-tropic HIV strains. A virally encoded seven transmembrane domain containing a chemokine receptor, CMV-US28 (10), may also act as a coreceptor in some cases.
The CCR5 ligands regulated on activation, normal T cell expressed and secreted (RANTES),1 macrophage inflammatory protein (MIP)-1α, and MIP-1β are able to block infection of M-tropic HIV strains (11). The inhibition of HIV entry and infection was only partial, and varied considerably between different M-tropic virus strains and target cells. Moreover, stimulatory effects of RANTES during infection of primary monocytes/macrophages have been reported (12). Several NH2-terminal modifications of RANTES have been described creating proteins with antagonist properties. Met-RANTES (13), RANTES(9–68; reference 14), and aminooxypentane (AOP)-RANTES (15) all antagonize cellular responses induced by chemokines. They also block infection by HIV-1 (15, 16), but the chemically modified AOP-RANTES is actually substantially more potent than RANTES itself.
Two theories have been proposed for the mechanism by which chemokines prevent chemokine receptor–dependent HIV entry. The chemokine could induce receptor downregulation from the cell surface, thereby removing the essential coreceptor. Alternatively, either an agonist or nonsignaling antagonist could sterically hinder the essential interaction between the HIV envelope glycoprotein-120 protein and the receptor. Inhibition of HIV infectivity by the three functional chemokine receptor antagonists initially suggested the second mechanism (15, 16). However, studies with CXCR4 and CCR5 have suggested that coreceptor internalization contributes to efficient chemokine inhibition of virus entry (17, 18). To investigate which mechanism AOP-RANTES efficiently inhibits HIV entry, we determined by FACS® (Becton Dickinson, Mountain View, CA) analysis the fate of CCR5 after binding of RANTES, Met-RANTES, or AOP-RANTES on Chinese hamster ovary (CHO) cells, stably transfected with CCR5, as well as on freshly isolated lymphocytes and monocytes. These three ligands induce varied degrees of downregulation of CCR5. However, we show here that the chemically conjugated AOP-RANTES is highly effective in preventing recycling of internalized CCR5 to the cell surface in both transfected CHO cells and primary PBMCs. Together, our results suggest that ligand-induced intracellular sequestration of HIV coreceptors can inhibit cellular infection by HIV.
Materials and Methods
Generation of the CHO/CCR5 Cell Line.
The cDNA sequence of CCR5 was amplified from genomic DNA of human PBMCs by PCR with Pfu-polymerase (Stratagene Corp., La Jolla, CA), ligated into the PCR-Script Amp Sk(+) script vector (Stratagene Corp.), and sequenced. After subcloning into the PEF-DHFR vector, DHFR-deficient CHO cells were transfected by electroporation and selected for stable expression in nucleoside free MEM medium with 10% dialyzed FCS as described (19). The CHO/CCR5 transfected cells were shown to be homogeneous by FACS® analysis.
PBMC Purification.
PBMCs were prepared from fresh buffy coats of healthy blood donors by Ficoll density centrifugation. Buffy coats were diluted 1:2 in 0.9% NaCl, and 35 ml were layered onto 15 ml of Ficoll Paque and centrifuged for 25 min at 400 g. The white interphase was harvested and thrombocytes depleted by three subsequent washing and centrifugation steps at 100 g for 6 min in RPMI with 10% FCS. Freshly isolated monocytes expressed a very low level of CCR5, but expression was strongly induced after culture of PBMCs in RPMI with 10% FCS for 24 to 48 h at 37°C. The amount of FCS did not influence this induction. The expression of CCR5 on lymphocytes was not altered during culture.
Generation of the Monoclonal Anti-CCR5 Antibody.
To generate mAbs against human CCR5, five BALB/c mice were immunized intraperitoneally at 4-wk intervals, first with 107 PBMCs cultured for 10 d in IL-2 (100 U/ml) and six subsequent intraperitoneal injections of 107 CHO cells expressing high levels of CCR5. For this purpose, CCR5 transfected CHO cells were grown in the presence of 20 nM methotrexate to amplify expression of CCR5, and one clone expressing high levels of CCR5 was chosen. 4 d after the last intraperitoneal injection of CHO/ CCR5 cells, the spleens were removed and the cells fused with the P3X63-Ag8 cell line. Supernatants from ∼6,000 hybridomas were screened per fusion by flow cytometry on stable CHO/ CCR5 cells and an mAb against CCR5 (MC-1) was detected after the third fusion. The specificity of MC-1 (IgG1) was tested on CHO cells stably transfected with CCR1-4 and CXCR4. In all cases, no binding was detected. In addition, the antibody did not react against freshly isolated monocytes and cultured monocytes from a donor homozygous for the Δ32 deletion in the CCR5 gene.
Detection of Cell Surface CCR5.
5 × 105 CHO/CCR5 cells or PBMCs were incubated for 30 min at 37°C with various concentrations of chemokines diluted in RPMI with 10% FCS in a volume of 100 μl. Medium alone was used for controls. Cells were then cooled to 4°C and, after centrifugation, resuspended in 100 μl supernatant from the MC-1 hybridoma or medium as negative control. After 30 min at 4°C, cells were washed four times with PBS and incubated with an FITC-labeled rabbit anti– mouse F(ab)2 fragment (F313; Dako Corp., Carpinteria, CA) for 30 min at 4°C. After two washing steps, cells were analyzed on the FACScan®. Lymphocytes and monocytes were distinguished by their forward and side scatter properties. Relative fluorescence intensity was calculated as (mean channel fluorescence [chemokines] − mean channel fluorescence [negative control])/(mean channel fluorescence [medium] − mean channel fluorescence [negative control]).
CCR5 Recycling.
To study recycling of CCR5, PBMC or CHO/CCR5 cells were first incubated for 30 min at 37°C with 100 nM RANTES, 100 nM AOP-RANTES, or medium as control. Cells were washed four times in medium at room temperature and incubated further at 37°C. Aliquots were taken at the times indicated, stained, and analyzed as described above. When cycloheximide was used, cells were preincubated for 20 min with 100 μg/ml cycloheximide, which was also present during the subsequent procedures.
Calcium Mobilization.
The ability of the RANTES proteins to mobilize calcium was determined using 2 × 106 Fura-2 loaded CHO/CCR5 cells for each measurement as previously described (20).
Virus Infection Assays.
Human osteosarcoma (GHOST) 34/ CCR5 cells are derived from HOS/CD4 cells stably expressing CCR5 and were a gift from Dan Littman (Skirball Institute, New York). 2.5 × 104 cells in 48-well trays were exposed to 100 μl of chemokine at appropriate dilution for 30 min at 37°C. 100 μl of the nonsyncytium-inducing (NSI), CCR5-dependent HIV-1 strain, SF162, was added at 1,000 focus-forming units/ml and the cells were incubated for a further 3 h. The cells were then washed and incubated in medium containing the appropriate chemokine for 4 d before fixing, staining in situ for p24 production, and estimating foci of infection as previously described (21).
Immunofluorescence Microscopy.
CHO/CCR5 cells were cultured on glass coverslips in 4-well plates for 48 h. For experiments, the cells were washed in binding medium (BM; RPMI-1640 without bicarbonate, containing 0.2% BSA and 10 mM Hepes, pH 7.4) at room temperature. Subsequently, the cells were incubated in BM or BM containing either RANTES (250 nM), AOP-RANTES (125 nM), or Met-RANTES (250 nM) at 37°C. At the indicated times, the coverslips were placed on ice and washed with cold BM. For recycling, selected coverslips were incubated for a further hour in BM at 37°C. All cells were then fixed in PBS containing 3% paraformaldehyde for 10 min. The fixed cells were washed with PBS, quenched with 50 mM NH4Cl in PBS, and stained intact or after treatment with 0.05% Saponin for 15 min.
For intact cell staining, antibodies were diluted in PBS containing 0.2% gelatine. Cells were incubated with MC-1 (culture supernatant 1/10) for 1 h at room temperature and bound antibodies detected using a biotin-conjugated anti–mouse IgG antibody (1/100; Amersham Corp., Arlington Heights, IL) and streptavidin-FITC (1/100; Amersham Corp.). To stain permeabilized cells, the antibodies were diluted in PBS-0.2% gelatine containing 0.05% Saponin. The cells were labeled for 1 h with the anti-CCR5 mAb 45523.111 (10 μg/ml; R&D Systems Inc., Minneapolis, MN) and the anti-transferrin receptor mAb H68.4 (1/100; provided by Dr. Ian Trowbridge, Salk Institute, La Jolla, CA) or the mAb 3E9 against the lysosomal membrane glycoprotein Lgp-B (culture supernatant 1/2; provided by Dr. Bruce Granger, Montana State University, Bozeman, MO). The cells were washed in PBS containing 0.05% Saponin and then incubated with a mix of FITC-conjugated anti–mouse IgG2b (1/100; Nordic Immunological Laboratories, Maidenhead, UK) and biotin-conjugated anti–mouse IgG1 (1/100; Amersham Corp.) for 30 min at room temperature. Finally, the cells were stained with streptavidin–Texas red (1/100; Pierce, Chester, UK) for 30 min.
All coverslips were mounted in mowiol and examined using a microscope (Optiphot-2, Nikon, Melville, NY) equipped with a laser scanner (MRC 1024; BioRad Labs., Hercules, CA). The images were assembled in and printed directly from Adobe Photoshop.
Results
Downregulation of Surface CCR5.
CCR5 downregulation from the surface of CHO/CCR5 cells induced by RANTES, Met-RANTES, and AOP-RANTES as well as MIP-1α and MIP-1β is shown in Fig. 1. The expression of CCR5 on the surface of these cells was measured by FACS® analysis using the CCR5-specific mAb MC-1. Although the natural ligands RANTES, MIP-1α, and MIP-1β effectively caused disappearance of CCR5 from the cell surface, the downregulation was never >60% at a concentration of 100 nM. RANTES was more effective than MIP-1α and MIP-1β, showing 40% downregulation at 10 nM, whereas a significant downregulation induced by MIP-1α and MIP-1β was only observed at concentrations >30 nM. The two antagonist proteins demonstrated very different effects. Met-RANTES was very inefficient at receptor downregulation since at 100 nM only 20% of the receptors were removed from the surface, whereas AOP-RANTES at 10 nM caused 80% downregulation and <15% of CCR5 receptors were detectable at 100 nM. When the experiments were performed at 37°C in the presence of 1 M sucrose or at 4°C, conditions that in other systems inhibit receptor internalization (22), the disappearance of surface CCR5 was minimal, indicating that the induced decrease in cell surface CCR5 was due to receptor internalization and not to ligand-mediated inhibition of MC-1 binding (data not shown).
Figure 1.
Downregulation of CCR5 from the surface of stably transfected CHO cells. CHO-CCR5 cells were incubated for 30 min at 37°C with the concentrations indicated of the different chemokines: RANTES (•), MIP-1α (▴), MIP-1β (▾), AOP-RANTES (▪), and Met-RANTES (♦). Surface CCR5 was detected with a monoclonal CCR5 antibody (MC-1) and analyzed by flow cytometry. No downregulation was observed with monocyte chemotactic protein (MCP)-1, MCP-2, Gro-α, and SDF-1α at a concentration of 100 nM (data not shown).
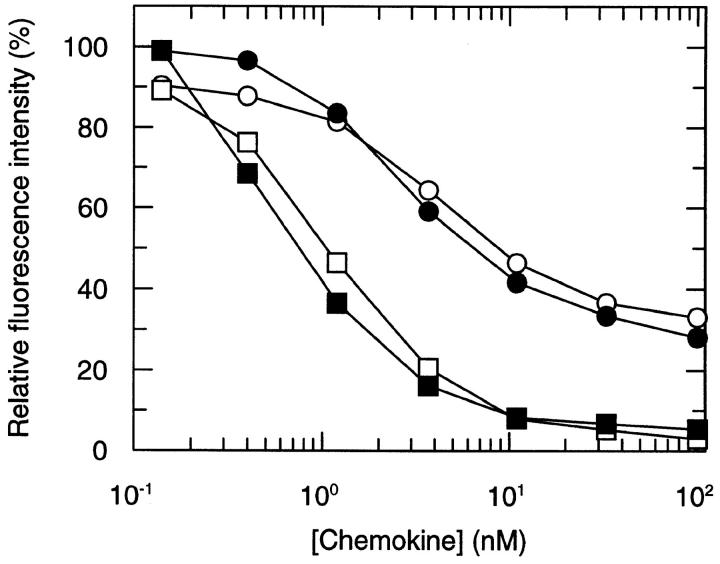
The potency of AOP-RANTES to downregulate CCR5 was even more striking when lymphocytes and cultured monocytes from several different donors were studied. Receptor loss was readily detectable after 5 min (data not shown) and reached a maximum after 20–30 min. Representative experiments are shown in Fig. 2. Using PBMCs from several different donors, AOP-RANTES caused >95% downregulation of CCR5 from lymphocytes and monocytes, whereas RANTES only achieved about 60– 70% reduction on these primary cells.
Figure 2.
Downregulation of CCR5 on lymphocytes (open symbols) and monocytes (solid symbols). PBMCs were incubated with various concentrations of RANTES (circles) and AOP-RANTES (squares) for 30 min at 37°C, labeled with a monoclonal CCR5 antibody (MC-1), and analyzed as described. It is noteworthy that PBMCs were cultured for 24 h at 37°C in RPMI with 10% FCS. This procedure strongly induces expression of CCR5 on monocytes, whereas expression on lymphocytes was unchanged (data not shown).
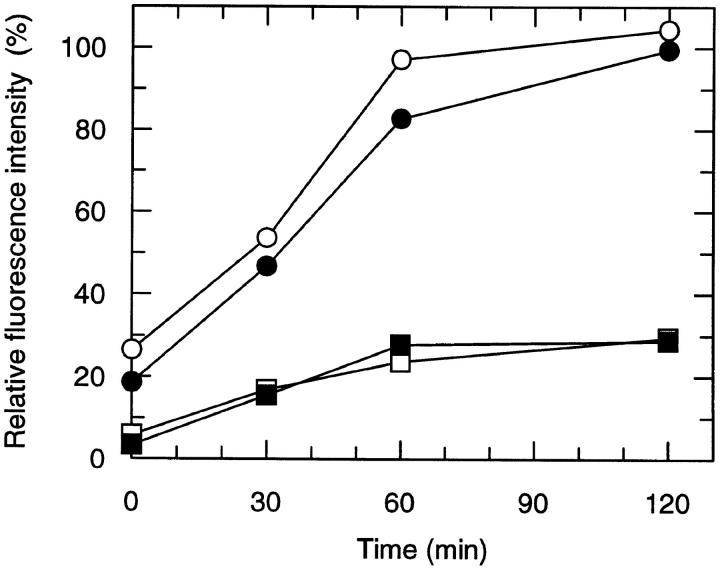
To examine the fate of internalized CCR5, RANTES– and AOP-RANTES–treated lymphocytes and monocytes were assessed for cell surface CCR5 expression after reculture after removal of the chemokine from the culture medium. As shown in Fig. 3, receptor cell surface expression returned to control levels after removal of RANTES. In contrast, surface reexpression of CCR5 was significantly lower after removal of AOP-RANTES. The experiments were performed with PBMCs from five donors. Exposure of PBMCs to AOP-RANTES from these donors resulted in 25–30% recycling compared to that of controls, whereas only 15% recycling was observed for the fourth, and 40% for the fifth. However, in the case of RANTES exposure, the receptor recycling consistently attained almost 100% of controls. To investigate whether the reappearance of CCR5 on the cell surface was due to de novo synthesis of the receptor, the experiments were repeated in the presence cycloheximide at a concentration of 100 μg/ml. The results were identical to those shown in Fig. 3. Recycled receptors were not refractory to internalization, as they could be downregulated by a second exposure to RANTES or AOP-RANTES (data not shown).
Figure 3.
Recycling of CCR5 on lymphocytes (open symbols) and monocytes (closed symbols) after downregulation with RANTES (circles) and AOP-RANTES (squares). PBMCs were first incubated for 30 min at 37°C with 100 nM RANTES or AOP-RANTES. After downregulation, the chemokines were removed by four washing steps with medium, and cells were further cultured in medium at 37°C for various periods of time and analyzed for CCR5 expression as described above.
The most impressive observation was the result of prolonged incubation of PBMC for up to 12 d in the presence of either RANTES or AOP-RANTES (Fig. 4). Cells cultured in the presence of 100 nM RANTES showed normal levels of surface expression CCR5. However, no CCR5-positive cells were observed when the cells were cultured with the same concentration of AOP-RANTES.
Figure 4.
Histogram analysis showing the surface expression of CCR5 on lymphocytes after 12 d of incubation with 100 nM RANTES and AOP-RANTES. CCR5 expression was measured as described in Fig. 2. Incubation with medium (solid line), 100 nM RANTES (dotted line), or 100 nM AOP-RANTES (dashed line). Shaded area, secondary antibody alone.
Morphological Examination of CCR5 Internalization.
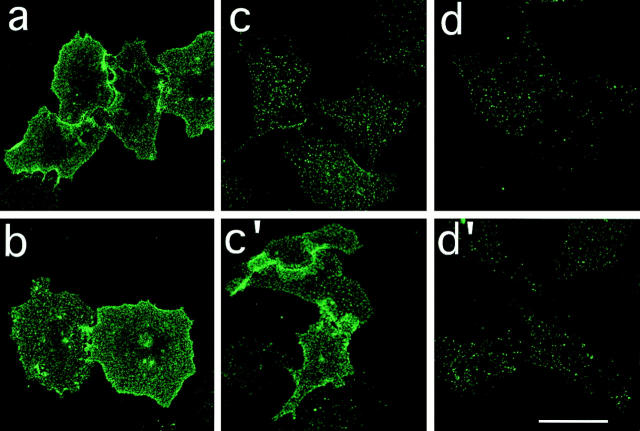
To further eliminate the possibility that the apparent disappearance of surface CCR5 was due to alteration of the MC-1 epitope induced by ligand binding, we examined CCR5 downmodulation morphologically. CHO/CCR5 cells, grown on glass coverslips, were treated with RANTES, AOP-RANTES, or Met-RANTES for 30 min at 37°C. Subsequently, the cells were fixed and cell surface CCR5 expression was determined using the anti-CCR5–specific mAb MC-1. Fig. 5 shows that cells incubated in medium alone (a) show prominent CCR5 cell surface expression. On cells treated with RANTES or AOP-RANTES, significantly less CCR5 staining was seen (c and d, respectively). By contrast, Met-RANTES induced little change in cell surface CCR5 expression compared to cells treated with medium alone (b).
Figure 5.
Downmodulation and recycling of CCR5. CHO/CCR5 cells were treated with medium (a) or medium containing either RANTES (250 nM; c and c′ ), AOP-RANTES (125 nM; d and d′ ), or Met-RANTES (250 nM; b) for 30 min at 37°C. The cells were then cooled on ice, washed extensively in cold medium, and either held on ice or reincubated at 37°C for a further 60 min in medium (c′ and d′ ). Subsequently, all cells were fixed and labeled with the anti-CCR5 mAb MC1. Bar, 40 mm.
Since removal of RANTES, but not AOP-RANTES, allowed reappearance of the receptor on the cell surface after downmodulation (Fig. 3), we examined RANTES– and AOP-RANTES–treated cells for surface CCR5 expression after reincubation in ligand-free medium for 1 h at 37°C. Fig. 5, c′ shows that after removal of RANTES, CCR5 reappeared at the cell surface. By contrast, in cells treated with AOP-RANTES, there was no observable recovery of cell surface receptor over the 1-h incubation period (d′ ). Together, these observations support the biochemical data indicating that RANTES and AOP-RANTES, but not Met-RANTES, can induce downmodulation of cell surface CCR5. Moreover, AOP-RANTES prevents the recycling of the receptor seen after removal of RANTES.
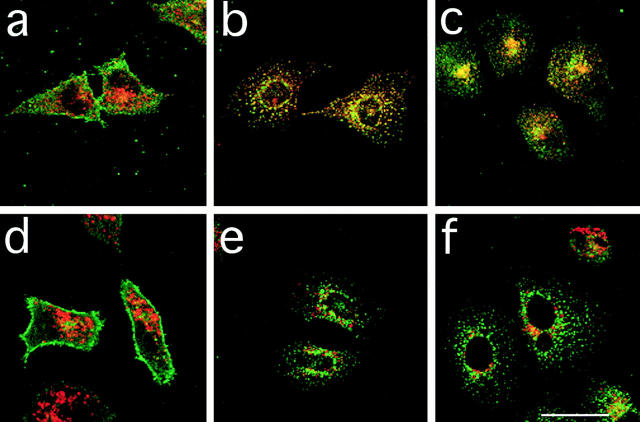
To determine whether the loss of cell surface CCR5 was due to internalization of the receptor, we examined the distribution of CCR5 on permeabilized cells after treatment with RANTES and AOP-RANTES. As illustrated above, cells incubated in medium alone showed prominent cell surface staining and little intracellular CCR5 staining (Fig. 6, a and d, green fluorescence). However, after treatment with RANTES or AOP-RANTES for 1 h, the cell surface staining was reduced considerably and prominent intracellular vesicular staining was seen (Fig. 6, b, c, e, and f ). In many cells, the vesicles were observed both in the periphery of the cell and in a cluster in the perinuclear region. To determine the nature of these internal vesicles, we costained cells with antibodies to either the transferrin receptor (TfR) that marks early endosomes (Fig. 6, a, b, and c, red) or a lysosomal membrane glycoprotein lgp-B that marks late endosomes and lysosomes (d, e, and f, red ). In cells treated with either RANTES or AOP-RANTES, some vesicles appeared that were either stained for CCR5 or TfR alone. However, we saw significant overlap of these two labels indicated in yellow (Fig. 6, b and c). In contrast, in cells costained for CCR5 and lgp-B, virtually all of the stained structures were either green or red, indicating little overlap in the distribution of the two proteins (Fig. 6, e and f ). Together, these data indicate (a) that the downmodulation of cell surface CCR5 by RANTES and AOP-RANTES is due to ligand-induced internalization of the receptor and (b) that the receptors internalized by both RANTES and AOP-RANTES appear to be largely associated with TfR containing early endosomes. No indication of the transfer of the receptors to lysosomes was seen, though we cannot exclude the possibility that some receptors were delivered to lysosomes and were rapidly degraded. However, within the time course of these experiments we saw no detectable loss of fluorescence signal. Moreover, in pulse chase experiments in which cells were treated with RANTES or AOP-RANTES for 1 h and then reincubated in ligand-free medium for a further hour, no colocalization of CCR5 with the lysosomal marker was observed (data not shown).
Figure 6.
Intracellular localization of downmodulated CCR5. CHO/CCR5 were treated with medium (a and d), medium with RANTES (250 nM; b and e), or AOP-RANTES (125 nM; c and f ) for 1 h at 37°C. After fixation and permeabilization, the cells were costained for CCR5 (green) and TfR (a, b, and c; red) or a lysosomal marker Lgp-B (d, e, and f; red). Scale bar, 40 mm.
Receptor Activation.
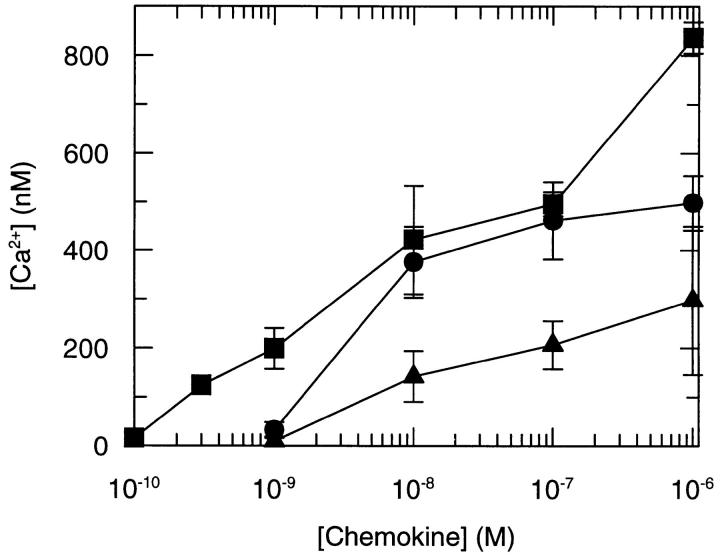
The ability of a ligand to downregulate its receptor depends on its ability to cause receptor activation. Modification of the NH2 terminus of RANTES has been reported by us and others to abrogate agonist activity, as judged by their capacity to elicit calcium mobilization and induce chemotaxis (13, 14). However, the ability of AOP-RANTES and RANTES(9–68) to induce receptor internalization indicates that these proteins are capable of receptor activation. Since the studies of agonist activity were performed on the promonocytic cell line, THP-1 (for Met-RANTES; reference 13) and freshly isolated monocytes (for AOP-RANTES; reference 15) where receptor expression may have been too low to allow signal detection, we decided to investigate the capacity of these ligands to mobilize calcium in cells expressing high levels of CCR5. As shown in Fig. 7, both modified proteins were capable of causing calcium mobilization, although neither achieved the potency and efficacy of RANTES itself. Met-RANTES was clearly the least effective in receptor activation, mirroring its inefficient downregulation of CCR5 and its inability to cause internalization of CCR1 in CHO cells (23).
Figure 7.
The induction of calcium mobilization induced by RANTES (•), AOP-RANTES (▪), and Met-RANTES (♦) in CHO/CCR5 cells. Calcium mobilization was determined as previously described (25) using 2 × 106 Fura-2–loaded cells for each measurement, with the concentration of chemokine as indicated.
Inhibition of Viral Infectivity.
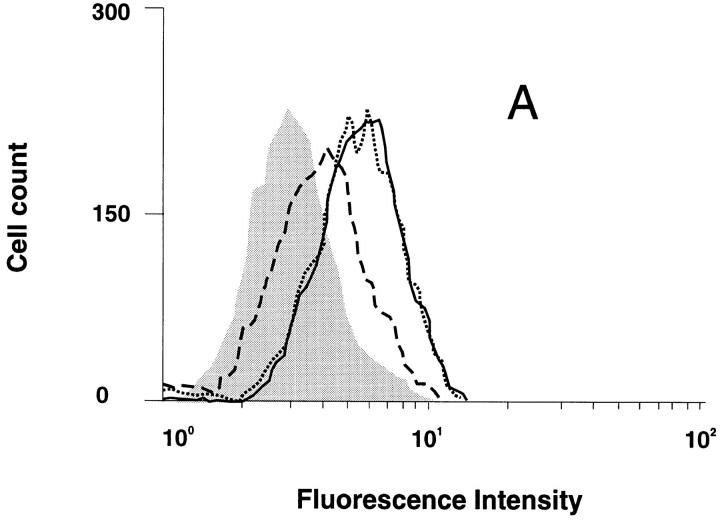
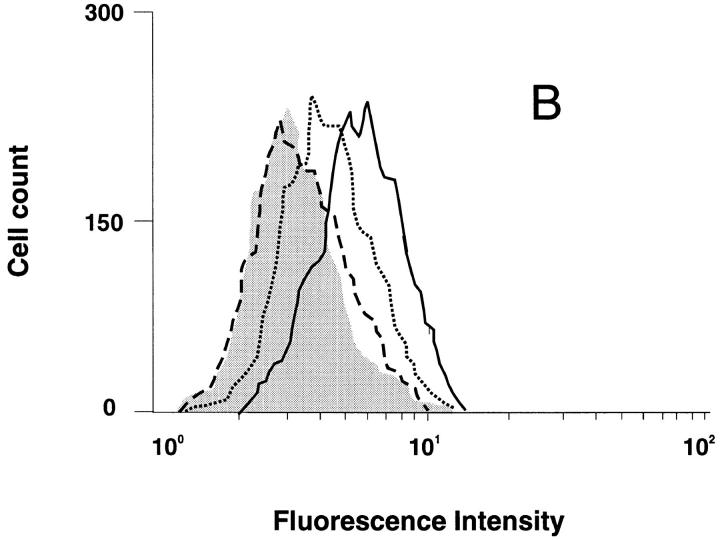
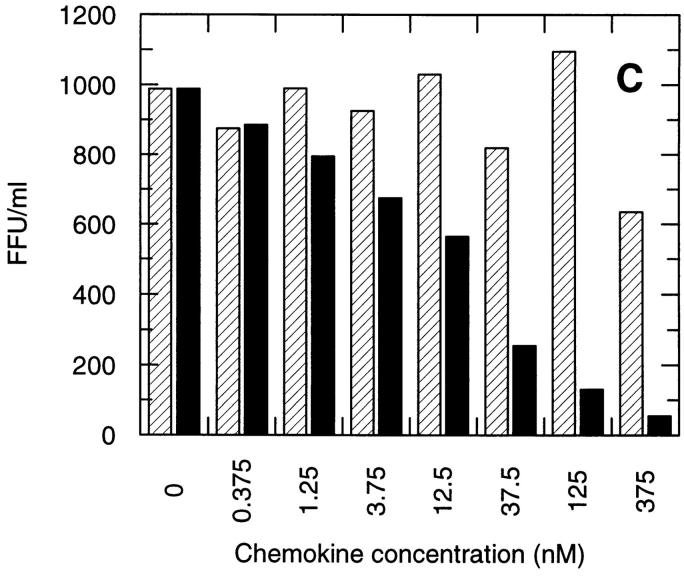
CCR5 downregulation was analyzed on GHOST 34/CCR5 cells in parallel with infection by the M-tropic HIV-1 strain, SF 162. Upon incubation with 100 nM RANTES, no internalization of CCR5 was observed in these cells after 30 min of incubation, but after 3.5 h, 50% internalization could be detected (Fig. 8 A). In contrast, ∼50% of CCR5 receptors were internalized after 30 min of incubation with AOP-RANTES and no receptors were detectable after 3.5 h (Fig. 8 B). The inhibition of viral infection by the M-tropic strain SF 162 (Fig. 8 C ) correlated very well with the loss of CCR5 surface receptors induced by AOP-RANTES, whereas RANTES showed almost no inhibition of infection. AOP-RANTES caused almost 90% inhibition at 125 nM, which corresponds well to the degree of removal of cell surface CCR5 seen on monocytes and lymphocytes shown in Fig. 2. Although 100 nM RANTES induces 70% CCR5 downregulation over 30 min, the recycling experiments shown in Fig. 3 may explain why inhibition of infection by RANTES only starts to be effective at higher concentrations.
Figure 8.
Downregulation of CCR5 from the surface of GHOST 34/ CCR5 cells and the inhibition of their infection by the M-tropic HIV-1 strain SF 162 by RANTES and AOP-RANTES. (A) Downregulation of CCR5 induced by 100 nM RANTES. The medium control is shown by the solid line and surface expression of CCR5 after 30 min by the dotted line and after 3.5 h by the dashed line. (B) Downregulation of CCR5 induced by 100 nM AOP-RANTES. The medium control is shown by the solid line and the surface expression of CCR5 after 30 min by the dotted line and after 3.5 h by the dashed line. (C) The inhibition of infection of GHOST 34/CCR5 cells by SF 162 by increasing concentrations of RANTES (hatched bars) and AOP-RANTES (solid bars).
Discussion
It is now clearly established that chemokine receptors are required for primate lentiviruses to fuse with and infect target cells either in combination with CD4 (24, 25) or independently (26, 27). Furthermore, it is clear that under certain circumstances agonistic and antagonistic ligands for these receptors can inhibit virus entry (11, 15, 16, 28). However, the precise mechanism through which these agents inhibit infection remains to be established. Two models have been proposed (29). The first proposes that the binding of the ligand to the requisite chemokine receptor sterically blocks the interaction of the viral envelope, or envelope–CD4 complex. Initial experiments performed with antagonists that were described as nonsignaling (15, 16) suggested that this might be the prime mode of action. The second model proposes that chemokine receptor ligands induce internalization of the coreceptor molecule from the cell surface, thereby removing a key element of the fusion complex. Two recent studies examining the ability of stromal cell–derived factor (SDF)-1 to block CXCR4-dependent virus entry have concluded that receptor internalization may, in fact, contribute to efficient inhibition of virus infection, although steric effects are not excluded by these studies (17, 18). Furthermore, Amara et al. (17) showed that the antagonist RANTES(9–68), although having been previously reported not to cause receptor activation as estimated by its inability to mobilize calcium and induce chemotaxis (14), was in fact able to induce downregulation of cell surface CCR5.
We have shown that chemical modification of the NH2 terminus of RANTES to form AOP-RANTES, while producing a potent antagonist of RANTES– and MIP-1β– induced monocyte chemotaxis (15), is very potent in inducing downregulation of surface CCR5. Furthermore, the downregulation of CCR5 achieved by AOP-RANTES is significantly higher than with the agonist, both in CHO cells and in freshly isolated primary cells such as lymphocytes and monocytes. Met-RANTES, on the other hand, was only able to weakly downregulate surface CCR5. In general, internalization of the G protein–coupled receptor superfamily is believed to require binding of an agonist to the receptor in order to activate the events that initiate receptor endocytosis (for reviews see references 30 and 31). In brief, this pathway is initiated by phosphorylation of the intracellular COOH-terminal domain by G protein receptor kinases and subsequent interaction with the arrestin proteins causing sequestration of the phosphorylated receptors into clathrin-coated pits. Thus, receptor internalization is an agonist-driven event, and the NH2-terminally modified RANTES ligands that are able to drive receptor downregulation must be able to activate the receptor. We have now shown that on CHO cells that express high numbers of recombinant receptors, G protein coupling, as indicated by calcium mobilization, is in fact measurable. However, it should be noted that the relative efficacies of AOP-RANTES and RANTES in eliciting downregulation of CCR5 do not correlate with their capacity to mobilize calcium. In both calcium mobilization and downregulation of CCR5, Met-RANTES is the least potent, and we previously reported that it was not able to cause internalization of CCR1 in CHO/CCR1 cells (23).
The enhanced potency of AOP-RANTES in receptor downregulation may be explained by its greater affinity for CCR5. It has been shown to have significantly different binding properties to CCR5 expressed in human embryonic kidney (HEK) 293 cells compared to both RANTES and Met-RANTES (15). RANTES and Met-RANTES show biphasic binding to the receptor, and are not able to completely compete for the radiolabeled ligand at a concentration of 100 nM. AOP-RANTES achieves complete competition at 10 nM, and shows a single phase competition curve. These binding characteristics are identical when the receptor is expressed in CHO cells (T. Schwartz, unpublished results). This difference in affinity could therefore explain, in part, the significantly increased potency of AOP-RANTES to induce CCR5 downregulation though the means by which this increase in binding affinity affects the endocytic trafficking of the CCR5 molecule has still to be established.
To our knowledge, this is the first report of inhibition of recycling of a receptor that normally will reappear on the cell surface after removal of the ligand. Although endocytosis of the chemokine receptors has not been studied in great detail to date, there is evidence that these receptors can either recycle or enter a degradation pathway via the lysosomal compartment. CXCR4 has been shown to recycle to the surface after both phorbol ester and SDF-1 induced endocytosis (17, 18), as we have also shown here for CCR5. On the other hand, CXCR2 has been reported to undergo degradation after internalization (32). It is believed that recycling of the receptors from endocytic organelles requires dissociation of the ligand from the receptor and subsequent dephosphorylation. The reason why AOP-RANTES prevents this process from occurring is not clear, and effects other than the greater affinity for the receptor will be subject for further investigation.
Receptor internalization is not directly responsible for the entry of the HIV virus into the cell (33), since mutant CCR5 molecules that prevent receptor endocytosis can still function as a coreceptor for viral entry (17, 18, 34). However, our results suggest that molecules that induce internalization and subsequently interfere with recycling of chemokine receptors contribute significantly to the inhibition of HIV-1 infectivity. An approach based on gene targeting in which the CCR5 ligands, RANTES, and MIP-1α are modified such that a KDEL sequence added to their COOH terminus prevents a newly synthesized receptor from trafficking from the endoplasmic reticulum to the cell surface renders these cells resistant to HIV infection (35).
Support for the notion that anti-HIV therapeutics aimed at removing cell surface CCR5 will be well tolerated and without significant side effects is suggested by the apparent healthy state of individuals homozygous for the Δ32 CCR5 allelle who appear highly resistant to HIV infection (36). Although adverse effects of this Δ32 CCR5 allele in the rate of mortality after infection have been suggested (37), a therapy based on prevention of CCR5 recycling could be envisaged in conjunction with the other therapies currently in use. To this end we are continuing to elucidate the biochemical and cellular mechanisms through which AOP-RANTES prevents CCR5 recycling.
Acknowledgments
We thank S. Chilla (University of Munich, Munich, Germany) for excellent technical assistance, B. Dufour (University of Geneva, Geneva, Switzerland) and R.E. Offord (University of Geneva, Geneva, Switzerland) for the synthesis of AOP-RANTES, Drs. H. Wagner and K.M. Heeg for the gift of recombinant IL-2, and Drs. R. Solari and M. Kosco-Vilbois for critical reading of the manuscript.
Footnotes
P.R. Clapham and G. Simmons were supported by the Medical Research Council (UK) and B. Luckow and P.J. Nelson were supported by grants from the Deutsche Forschungsgemeinschaft (Lu 612/1-1 and SFB 464).
The present address of F. Borlat and T.N.C. Wells is Sereno Pharmaceutical Research Institute, 14 Chemin des Aulx, 1228 Plan-les-Ouates, Geneva, Switzerland.
Abbreviations used in this paper: AOP, aminooxypentane; BM, binding medium; CHO, Chinese hamster ovary; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T cell expressed and secreted; TfR, transferrin receptor.
References
- 1.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major coreceptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 2.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein–coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 5.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 6.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Alkhatib G, Liao F, Berger EA, Farber JM, Peden KC. A new SIV coreceptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 8.Deng HK, Unutmaz D, Kewalramani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 9.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, et al. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 12.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 13.Proudfoot AEI, Power CA, Hoogewerf AJ, Montjovent MO, Borlat F, Offord RE, Wells TNC. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem. 1996;271:2599–2603. doi: 10.1074/jbc.271.5.2599. [DOI] [PubMed] [Google Scholar]
- 14.Gong JH, Uguccioni M, Dewald B, Baggiolini M, Clark-Lewis I. RANTES and MCP-3 antagonists bind multiple chemokine receptors. J Biol Chem. 1996;271:10521–10527. doi: 10.1074/jbc.271.18.10521. [DOI] [PubMed] [Google Scholar]
- 15.Simmons G, Clapham PR, Picard L, Offord RE, Rosenkilde MM, Schwartz TW, Buser R, Wells TNC, Proudfoot AEI. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 16.Arenzana-Seisdedos F, Virelizier JL, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 17.Amara A, Legall S, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier JL, Arenzanaseisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1-α–dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Signoret N, Oldridge J, Pelchenmatthews A, Klasse PJ, Tran T, Brass LF, Rosenkilde MM, Schwartz TW, Holmes W, Dallas W, et al. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack M, Riethmuller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci USA. 1995;92:7021–7025. doi: 10.1073/pnas.92.15.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lusti-Narasimhan M, Power CA, Allet B, Alouani S, Bacon KB, Mermod JJ, Proudfoot AEI, Wells TN. Mutation of Leu25 and Val27 introduces CC chemokine activity into interleukin-8. J Biol Chem. 1995;270:2716–2721. doi: 10.1074/jbc.270.6.2716. [DOI] [PubMed] [Google Scholar]
- 21.Clapham PR, McKnight A, Weiss RA. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J Virol. 1992;66:3531–3537. doi: 10.1128/jvi.66.6.3531-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roettger BF, Rentsch RU, Pinon D, Holicky E, Hadac E, Larkin JM, Miller LJ. Dual pathways of internalization of the cholecystokinin receptor. J Cell Biol. 1995;128:1029–1041. doi: 10.1083/jcb.128.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solari R, Offord RE, Remy S, Aubry JP, Wells TNC, Whitehorn E, Oung T, Proudfoot AEI. Receptor-mediated endocytosis of CC-chemokines. J Biol Chem. 1997;272:9617–9620. doi: 10.1074/jbc.272.15.9617. [DOI] [PubMed] [Google Scholar]
- 24.Moore JP. Coreceptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 25.Berger EA. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11:S3–S16. [PubMed] [Google Scholar]
- 26.Endres MJ, Clapham PR, Marsh M, Ahuja M, Turner JD, McKnight A, Thomas JF, Stoebenau-Haggarty B, Choe S, Vance PJ, et al. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 27.Martin KA, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard NP. CD4-independent binding of SIV gp120 to rhesus CCR5. Science. 1997;278:1470–1473. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- 28.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/FUSIN and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 29.Wells TNC, Proudfoot AEI, Power CA, Marsh M. Chemokine receptors–the new frontier for aids research. Chem Biol. 1996;3:603–609. doi: 10.1016/s1074-5521(96)90126-x. [DOI] [PubMed] [Google Scholar]
- 30.Bohm SK, Grady EF, Bunnett NW. Regulatory mechanisms that modulate signaling by G-protein–coupled receptors. Biochem J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koenig JA, Edwardson JM. Endocytosis and recycling of G protein–coupled receptors. Trends Pharmacol Sci. 1997;18:276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- 32.Mueller SG, Schaw WP, Richmond A. Activation of protein kinase C enhances the phosphorylation of the type B interleukin-8 receptor and stimulates its degradation in non-hematopoietic cells. J Biol Chem. 1995;270:10439–10448. doi: 10.1074/jbc.270.18.10439. [DOI] [PubMed] [Google Scholar]
- 33.Pelchen-Matthews A, Clapham P, Marsh M. The role of CD4 endocytosis in HIV infection. J Virol. 1995;69:8164–8168. doi: 10.1128/jvi.69.12.8164-8168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aramori I, Zhang J, Ferguson SSG, Bieniasz PD, Cullen BR, Caron MG. Molecular mechanism of desensitization of the chemokine receptor CCR-5–receptor signaling and internalization are dissociable from its role as an HIV-1 coreceptor. EMBO (Eur Mol Biol Organ) J. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang AG, Bai XF, Huang XF, Yao CP, Chen SY. Phenotypic knockout of HIV type 1 chemokine coreceptor CCR-5 by intrakines as potential therapeutic approach for HIV-1 infection. Proc Natl Acad Sci USA. 1997;94:11567–11572. doi: 10.1073/pnas.94.21.11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paxton WA, Martin SR, Tse D, O'Brien TR, Skurnick J, VanDevanter NL, Padian N, Braun JF, Kotler DP, Wolinsky SM, Koup RA. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 37.Garred P, Eugen-Olsen J, Iversen AK, Benfield TL, Svejgaard A, Hofmann B. Dual effect of CCR5 delta 32 gene deletion in HIV-1–infected patients. Copenhagen AIDS study group. Lancet. 1997;349:1884. doi: 10.1016/s0140-6736(05)63874-3. [DOI] [PubMed] [Google Scholar]