Abstract
B cells from young lyn −/− mice are hyperresponsive to anti-IgM–induced proliferation, suggesting involvement of Lyn in negative regulation of B cell antigen receptor (BCR)-mediated signaling. Here we show that tyrosine phosphorylation of FcγRIIB and CD22 coreceptors, which are important for feedback suppression of BCR-induced signaling, was severely impaired in lyn −/− B cells upon their coligation with the BCR. Hypophosphorylation on tyrosine residues of these molecules resulted in failure of recruiting the tyrosine phosphatase SHP-1 and inositol phosphatase SHIP, SH2-containing potent inhibitors of BCR-induced B cell activation, to the coreceptors. Consequently, lyn −/− B cells exhibited defects in suppressing BCR-induced Ca2+ influx and proliferation. Thus, Lyn is critically important in tyrosine phosphorylation of the coreceptors, which is required for feedback suppression of B cell activation.
The Src family protein-tyrosine kinase, Lyn, is highly expressed in hematopoietic cells. Lyn physically associates with the BCR in B cells and with FcεRI in mast cells, and is rapidly activated upon cross-linking of the antigen receptors (1–3). Lyn interacts with and phosphorylates a number of substrates, such as the Syk kinase (4, 5), HS1 protein (6, 7), and Cbl protooncogene product (8). Taken together, Lyn is thought to play important roles in the antigen receptor–mediated positive signaling.
Recently, however, two groups reported that B cells from young lyn −/− mice were hyperresponsive to anti-IgM–induced proliferation due in part to the impairment of FcγRIIB-mediated feedback suppression of the B cell antigen receptor (BCR) signaling (9, 10). Therefore, it is suggested that Lyn may play some roles in the antigen receptor–mediated negative signaling, too. In this study, using splenic B cells or bone marrow–derived mast cells (BMMCs) from lyn −/− mice, we addressed molecular mechanisms by which the Lyn kinase would act as a key regulator of antigen receptor signaling.
Materials and Methods
Cells and Cell Culture.
All lyn− /− mice had been back-crossed at least six generations to C57BL/6J. Splenic B cells were isolated from 6–8-wk-old mice by T cell depletion with anti-Thy 1.2 mAb and with rabbit complement, followed by Percoll gradient purification (11). The resulting cells were >85% B220+ as determined by FACS® (Becton Dickinson, Mountain View, CA) analysis. BMMCs were obtained from bone marrow cells cultured with IL-3 for at least 4 wk as described (12).
Proliferative Responses.
For proliferation assay, B cells (105/ well) were cultured in 96-well flat-bottomed tissue culture plates either alone or in the presence of goat F(ab′)2 anti-IgM (Cappel, Durham, NC) or intact anti-IgM (Southern Biotechnology Assoc., Inc., Birmingham, AL). Cultured cells were pulse-labeled and assayed as described (13). All assays were performed in triplicate with <20% variation among assays.
Qualification of Total Ig by ELISA.
Amounts of each Ig isotype in sera and in culture supernatants were determined by ELISA with antibodies specific for each membrane-bound Ig (mIg) isotype (13).
Immunoprecipitation and Immunoblotting.
For the activation of B cells, splenic B cells were treated with goat F(ab′)2 anti-IgM (Cappel) or intact anti-IgM (Southern Biotechnology Assoc., Inc.) for 2 min at 37°C. For the activation of mast cells, BMMCs were sensitized for 1 h with antidinitrophenyl (anti-DNP) monoclonal IgE (10 μg/ml; Sigma Chemical Co., St. Louis, MO) followed by stimulation for 2 min at 37°C with 30 ng/ml DNP-conjugated human serum albumin (DNP-HSA; resulting in FcεRI cross-linking; Sigma Chemical Co.), or DNP-HSA/rabbit anti-HSA IgG immune complexes (resulting in FcγRII coligation to FcεRI). The stimulated splenic B cells (107) or BMMCs (107) were lysed in TNE (1% [vol/vol] Nonident P-40, 50 mM Tris-HCl [pH 8], 20 mM EDTA, and 0.2 mM sodium orthovanadate with aprotinin at 10 μg/ml) buffer and subjected to immunoprecipitation/immunoblotting using SDS-7.5% PAGE as described (14). Antibodies used in these experiments were 2.4G2 mAb (PharMingen, San Diego, CA), anti-FcγRIIB (α-mβ1, gift from J.V. Ravetch, The Rockefeller University, New York), anti-CD22 (gift from E.A. Clark, University of Washington, Seattle, WA), anti–SHP-1 (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-SHIP (gift from M.N. Lioubin, Fred Hutchinson Cancer Research Center, Seattle, WA). Biotinylated 4G10 antiphosphotyrosine antibody (Upstate Biotechnology Inc., Lake Placid, NY) was used to detect phosphorylated proteins in the immunoblotting experiments. The blots were treated with horseradish peroxidase (HRPO)-streptavidin, and then antibody-reacted bands were visualized by the use of enhanced chemiluminescence detection system (Amersham, Co., Arlington Heights, IL).
FcR Cross-linking and Degranulation Assay.
FcεRI-triggered mast cell activation was induced by 1 h of sensitization with 10 μg/ml biotinylated mouse IgE, followed by cross-linking with 10 μg/ml streptavidin (0% of FcγRII coligation). FcγRII was coligated to FcεRI by adding biotinylated anti-FcγRII monoclonal antibody (biotin-2.4G2) at the sensitization step (100% of FcγRII coligation). A mixture of biotinylated/nonbiotinylated 2.4G2 (1/9 for 10% or 1/99 for 1% coligation) was used to vary the extent of FcγRII coligation. The degree of degranulation was determined by measuring the release of β-hexosaminidase as described (12).
Measurement of Internal Ca2+ Concentration.
Splenic B cells and IgE-sensitized BMMCs from lyn +/+ and lyn −/− mice were incubated with 3 μM Fura-2/acetoxymethyl (AM; Dojindo, Osaka, Japan) in PBS containing 20 mM Hepes (pH 7.2), 5 mM glucose, 0.025% BSA, and 1 mM CaCl2 at 37°C for 45 min. After the reaction, the cells were resuspended in 500 μl of the same buffer at 2 × 106 cells/ml in a stirring cuvette. Then, by using CAF-110 fluorescence spectrophotometer (JASCO, Osaka, Japan), emission at 500 nm was monitored after excitation of the sample with two different wavelengths (340 and 380 nm).
Results and Discussion
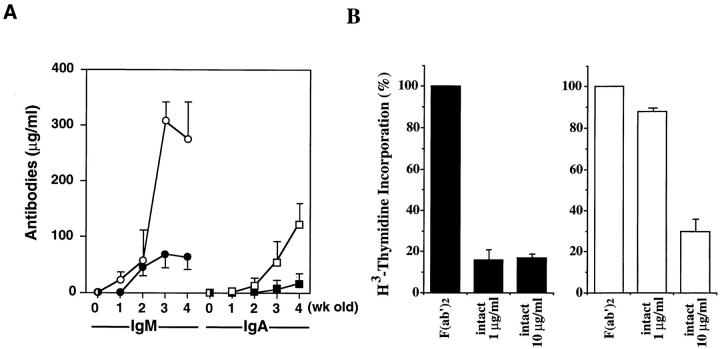
lyn −/− mice, generated by gene targeting, exhibit splenomegaly as they age. The enlarged spleen accumulates unusual lymphoblast-like cells and plasma cells (13, 15). lyn −/− mice with splenomegaly contain high concentrations of serum IgM and IgA, and often develop glomerulonephritis due to the production of autoreactive antibodies (13, 15). The lymphoblast-like cells are IgM+/−, IgD+/−, CD5−, Mac-1+, CD23−, B220+/−, and IL-5R+/−, and are therefore B-1b–like cells. These cells produce spontaneously a large amount of the antibodies. However, we found that serum levels of IgM and IgA were extremely high in lyn −/− mice compared to wild-type mice even at their early ages (<1 mo old) when the lymphoblast-like cells did not yet accumulate (Fig. 1 A). This suggests that Lyn is involved in negative regulation of antibody production by the normal B cells.
Figure 1.
(A) Serum levels of IgM and IgA in unimmunized infant mice. Wild-type mice (closed circles) and lyn −/− mice (open circles) were bled at 0–4 wk of age. Concentrations of IgM and IgA in their serum were determined by isotype-specific ELISA (13). Averages of the results from three experiments are shown. (B) Relative proliferative responses to BCR cross-linking. Splenic B cells from wild-type (left) or lyn −/− (right) mice were cultured with goat F(ab′)2 anti-IgM (5 μg/ml) or goat intact anti-IgM (1 or 10 μg/ml). After 42 h of incubation, cells were pulse labeled for 6 h with [3H]thymidine, and [3H]thymidine incorporation was determined (13). All assays were performed in triplicate. The proliferative responses were presented with [3H]thymidine incorporation expressed as a percentage of the response against F(ab′)2 anti-IgM stimulation.
In vitro cross-linking of the BCR on resting B cells induces a proliferative response. The response is prominent only when F(ab′)2 anti-IgM is used as a stimulating antibody, whereas intact anti-IgM fails to fully activate B cells. This is due to the inhibitory signals induced by coligation of the BCR and FcγRIIB, a low-affinity immunoglobulin G receptor (16, 17). In contrast to wild-type B cells, lyn −/− B cells from young mice (<6 wk old) were highly responsive to the intact anti-IgM, exhibiting a proliferation level similar to that with F(ab′)2 anti-IgM. The data suggest that the lyn −/− B cells are defective in FcγRIIB-mediated suppression of BCR signaling (9, 10, and Fig. 1 B). Furthermore, the disorders of lyn −/− mice, such as the hyperresponse to proliferation after BCR ligation and high concentrations of serum Igs, are found in mice lacking FcγRIIB (18). Moreover, the phenotypes of mice deficient in SHP-1, an SH2-containing protein tyrosine phosphatase (19, 20), or CD22, a B-lineage specific surface molecule (21–24), overlap those of lyn −/− mice. Note that both SHP-1 and CD22 participate in the feedback suppression of BCR signaling, and that tyrosine phosphorylation of immunoreceptor tyrosine-based inhibitory motifs (ITIMs; reference 25) of CD22 and FcγRIIB is involved in the suppression (26–28). The expression levels of SHP-1, CD22, and FcγRII in lyn −/− mice did not differ from those in wild-type mice (data not shown). Therefore, the inhibitory signalings through SHP-1, CD22, and FcγRIIB are likely related to Lyn kinase activity.
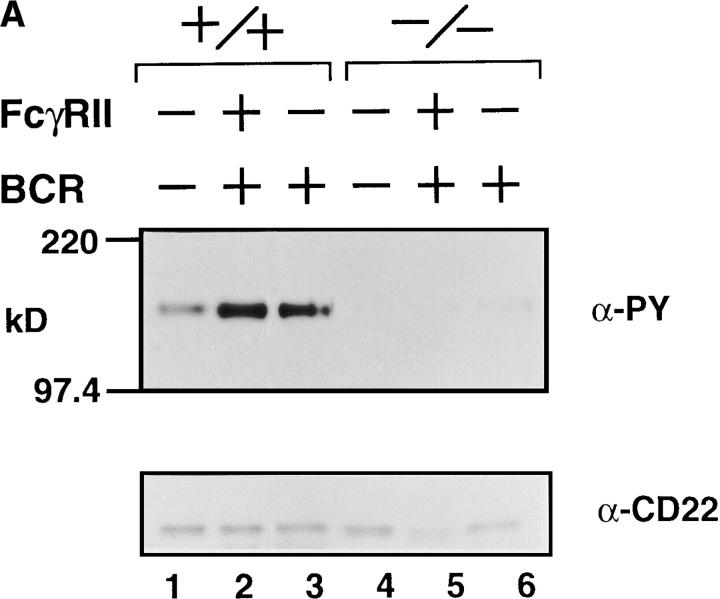
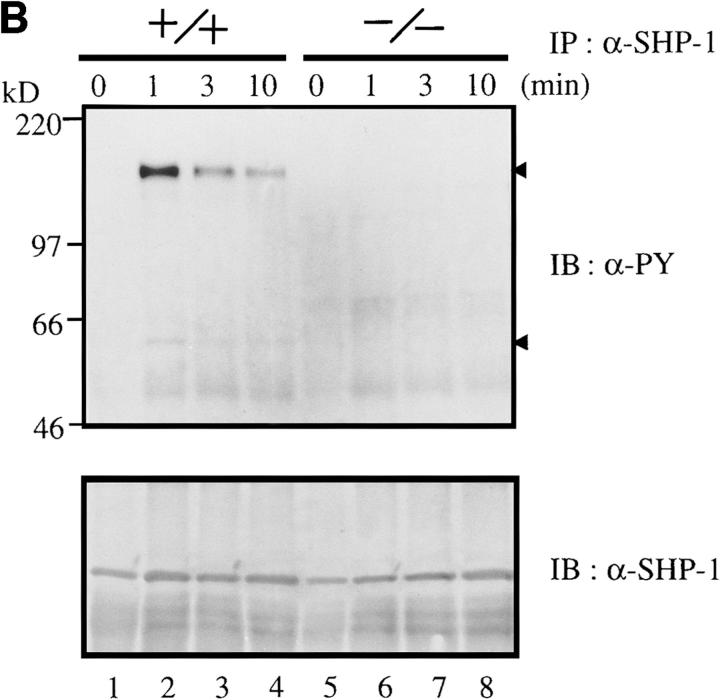
Upon stimulation of the BCR, CD22 and SHP-1 become rapidly tyrosine-phosphorylated (29) and associate with one another (30–32). To determine whether Lyn is involved in the tyrosine phosphorylation of CD22 and SHP-1, we immunoprecipitated these proteins from the lysates of BCR–cross-linked splenic B cells and probed the immunoprecipitates with antiphosphotyrosine antibody. As shown in Fig. 2 A, tyrosine phosphorylation of CD22 was extremely impaired in lyn −/− B cells (lanes 5 and 6), as compared with that in wild-type B cells (lanes 2 and 3). A level of tyrosine phosphorylation of SHP-1 was also low, and association of CD22 with SHP-1 was poor in the absence of Lyn (Fig. 2 B and data not shown).
Figure 2.
(A) Anti-IgM–induced tyrosine phosphorylation of CD22. Splenic B cells were stimulated with F(ab′)2 anti-IgM (30 μg/ml) (lanes 3 and 6) or intact anti-IgM (50 μg/ml) (lanes 2 and 5). Anti-CD22 immunoprecipitates from the cell lysates were probed with α-PY (phosphotyrosine antibody; top) or anti-CD22 antibody (bottom) by immunoblotting. (B) Anti-IgM–induced tyrosine phosphorylation of SHP-1 and its associated molecules. Splenic B cells were stimulated with intact anti-IgM (50 μg/ml) for indicated times. Anti–SHP-1 immunoprecipitates from the cell lysates were probed with antiphosphotyrosine monoclonal antibody 4G10 (α-PY) by immunoblotting. About 150-kD proteins (upper arrowhead) coimmunoprecipitated with SHP-1 (lower arrowhead) corresponded to CD22.
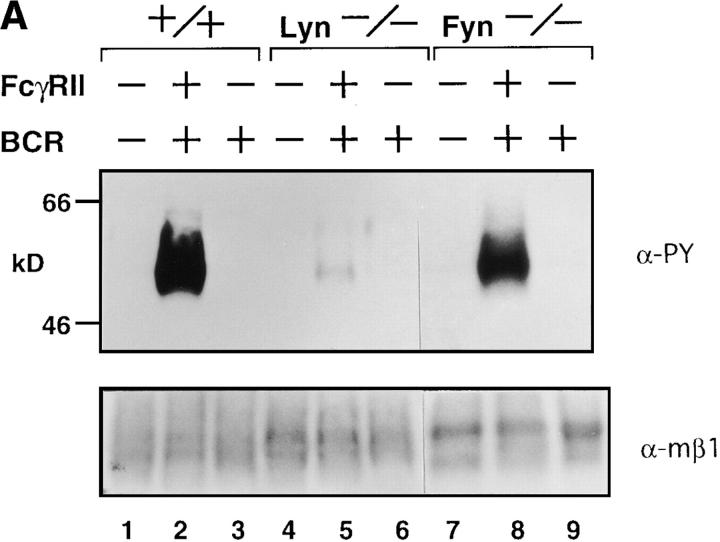
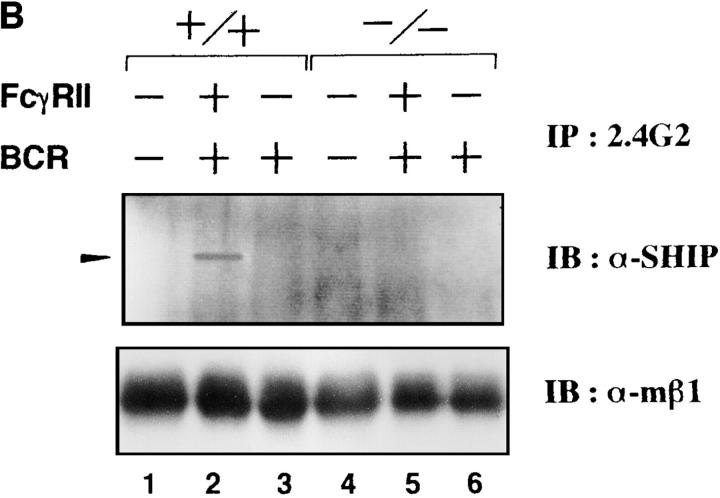
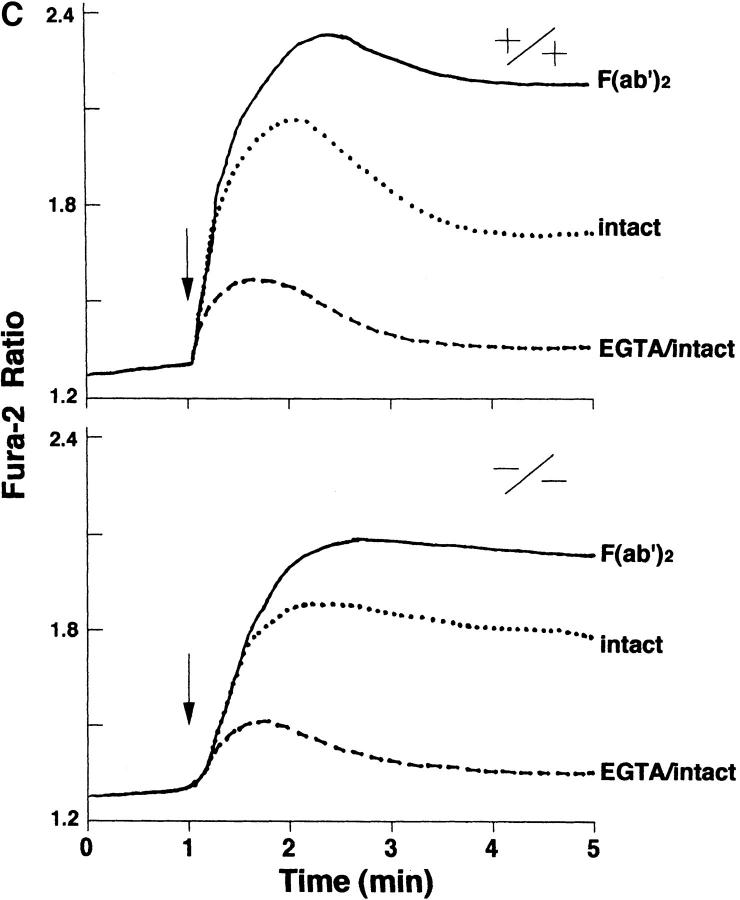
Next, we examined the level of tyrosine phosphorylation of anti-FcγRIIB immunoprecipitates prepared from the lysates of wild-type and lyn −/− splenic B cells on which FcγRIIB and BCR had been cross-linked. Immunoblot analysis of the precipitates with antiphosphotyrosine antibody (Fig. 3 A) showed a very low level of tyrosine phosphorylation of FcγRIIB in the absence of Lyn. The recruitment of SHIP, an inositol phosphatase important for the feedback suppression (26–28), from cytoplasm to membrane was also impaired in lyn −/− B cells (Fig. 3 B). Accordingly, the magnitude of BCR-induced calcium influx was less affected by coligated FcγRIIB in lyn −/− B cells than in wild-type B cells (Fig. 3 C). Moreover, as mentioned above, the proliferative response to BCR cross-linking was not suppressed by coligated FcγRIIB in lyn −/− B cells (Fig. 1 B). Thus, in the case of B cells, Lyn is directly involved in the feedback suppression mechanisms as well as in the BCR positive signaling. The controversial observation that B cells from relatively older lyn −/− mice are hyporeactive to BCR stimulation (13, 15) may suggest that negative signaling pathways are differently tuned with age.
Figure 3.
(A) Anti-IgM–induced tyrosine phosphorylation of FcγRIIB. Splenic B cells from wild-type (lanes 1–3), lyn −/− (lanes 4–6), or fyn −/− (lanes 7–9) mice were stimulated with F(ab′)2 anti-IgM (30 μg/ml; lanes 3, 6, and 9) or intact anti-IgM (50 μg/ml; lanes 2, 5, and 8) for 2 min. Proteins were immunoprecipitated with 2.4G2 anti-FcγRIIB monoclonal antibody from the cell lysates, and the precipitates probed with α-PY (top) or anti-FcγRIIB antibody (α-mβ1; bottom) by immunoblotting. (B) Recruitment of SHIP to FcγRIIB. Splenic B cells from wild-type (lanes 1–3) or lyn −/− (lanes 4–6) mice were stimulated with F(ab′)2 anti-IgM (30 μg/ml; lanes 3 and 6) or intact anti-IgM (50 μg/ml; lanes 2 and 5) for 2 min. Anti-FcγRIIB (2.4G2) immunoprecipitates from the cell lysates were probed with anti-SHIP antibodies by immunoblotting. (C) Time course of Ca2+ flux in splenic B cells upon BCR coligation to FcγRIIB. Panels show real time Fura-2 ratios (340/380) for splenic B cells of wild-type mice (top) and lyn −/− mice (bottom). Splenic B cells were stimulated with F(ab′)2 anti-IgM (5 μg/ml; line), intact anti-IgM (10 μg/ ml; dotted line), or intact anti-IgM (10 μg/ml) in the presence of 1.5 mM EGTA (perforated line).
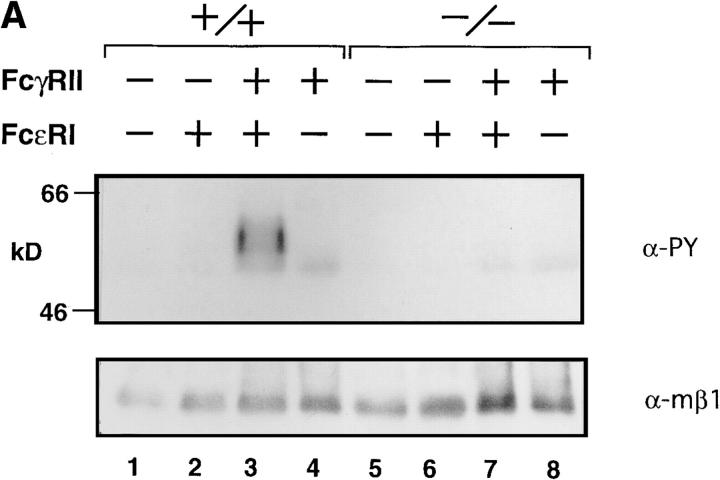
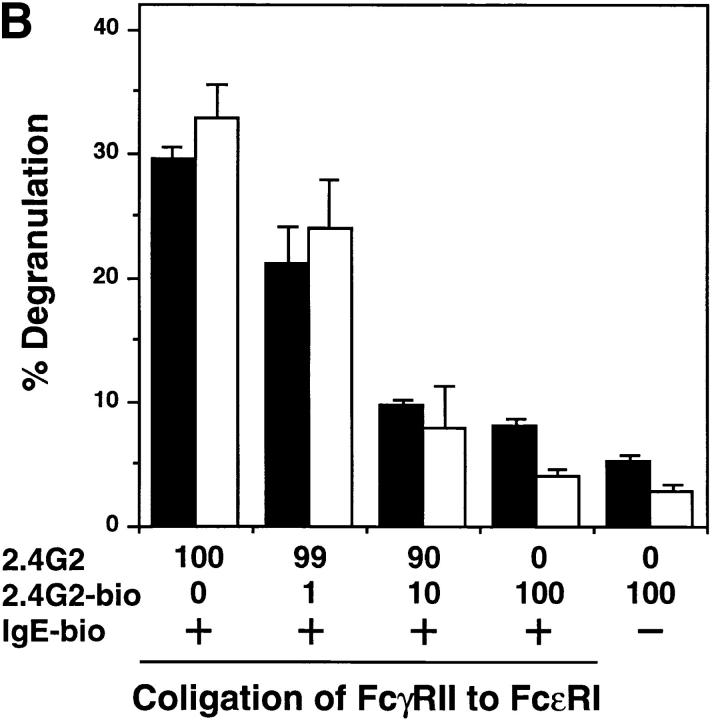
The similarities between the BCR- and FcεRI-mediated signaling prompted us to examine whether tyrosine phosphorylation of FcγRIIB coligated with FcεRI was impaired in lyn −/− mast cells. As shown in Fig. 4 A, tyrosine phosphorylation of coligated FcγRIIB was suppressed in the lyn −/− BMMCs. This likely results in the failure of recruitment of SHIP and/or SHP-1 to the ITIM of FcγRIIB, leading to the lack of suppression of FcεRI signaling by the inhibitory coreceptor. However, in both wild-type and lyn −/− BMMCs, the calcium response after IgE cross-linking was similarly suppressed by coligation of FcγRIIB with FcεRI–IgE complexes (data not shown). Furthermore, coligation of FcγRIIB with FcεRI–IgE complexes with increasing amounts of the cross-linking antibody resulted in increasing inhibition of degranulation, as measured by β-hexosaminidase release, in both wild-type and lyn −/− BMMCs (Fig. 4 B). Coligation of FcγRIIB with FcγRIII on both wild-type and lyn −/− BMMCs also inhibited receptor-triggered degranulation (the pair columns at the foremost right in Fig. 4 B). These results suggest that, unlike the case with the B cells, the negative signaling pathways appear to function similarly in wild-type and lyn −/− BMMCs.
Figure 4.
(A) Tyrosine phosphorylation of FcγRIIB upon FcεRI and FcγRIIB coligation. BMMCs from wild-type (lanes 1–4), lyn −/− (lanes 5–8) mice were sensitized with anti-DNP monoclonal IgE (10 μg/ml) followed by stimulation for 2 min at 37°C with 30 ng/ml DNP-HSA (FcεRI cross-linking; lanes 2 and 6), or DNP-HSA/rabbit anti-HSA IgG immune complexes (FcγRII coligation to FcεRI; lanes 3 and 7). BMMCs were stimulated with DNP-HSA/rabbit anti-HSA IgG immune complexes without IgE sensitization (lanes 4 and 8). Anti-FcγRIIB (2.4G2) immunoprecipitates from the cell lysates were probed with α-PY (top) or anti-FcγRIIB antibody (α-mβ1) (bottom) by immunoblotting. (B) Inhibitory effect of FcγRIIB coligation to FcεRI in lyn −/− BMMCs. The mast cell was sensitized with biotinylated mouse IgE, followed by cross-linking with streptavidin (no FcγRII coligation). FcγRIIB was coligated to FcεRI by adding biotinylated anti-FcγRII monoclonal antibody (biotin-2.4G2) at the sensitization step (100% FcγRII coligation). A mixture of biotinylated/nonbiotinylated 2.4G2 (1/9 for 10% or 1/99 for 1% coligation) was used to vary the extent of FcγRII coligation. The degree of degranulation was determined by measuring the release of β-hexosaminidase as described (12). Closed columns, the results of wild-type mice; open columns, the results of lyn −/− mice. Standard errors of triplicate samples are indicated on each column.
The apparent contradiction between B cells and mast cells with regard to the FcγRIIB feedback function may be due to differences in their FcγRIIB-mediated signalings. Such differences could produce the cell-type specific balance between the positive and negative signalings. Present data together with previous observations (9, 10, 12, 13, 15) indicate that positive signaling is less affected than negative signaling in lyn −/− B cells, and vice versa in lyn −/− mast cells. The balance of the positive and negative signalings in lyn −/− cells could well be influenced by some other Src-like kinases and Syk kinase expressed in the cells. In B cells, the imbalance induced by lyn deficiency seems to severely affect the immune responses. As a consequence, lyn −/− mice, which have lost the control of positive and negative signalings, exhibit abnormal phenotypes such as hyperactivation of B cells (9, 10) and elevated serum levels of IgM and IgA (13, 15), resulting in development of autoimmune disease (13, 15).
Our data show that Lyn is critically involved in tyrosine phosphorylation of particular proteins such as CD22 and FcγRIIB that are involved in the feedback regulation in B cells and/or BMMCs (for review see references 33–35). Lyn is also important in tyrosine phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) family of proteins and the proteins involved in positive regulation of the BCR and FcεRI signaling events (4, 5, 12, 13, 15, 36). The other membrane molecules with the putative ITIMs have been described (37, 38). These include KIR on NK cells, glycoprotein 49B and MAFA-1 on mast cells, and CD23 and CD72 on B cells. To understand the molecular basis of the balance of the positive and negative signalings, it is important to address what kinases preferentially tyrosine phosphorylate and regulate these molecules. The candidates include the Src family kinases, JAK kinases, Tec family kinases, and Syk/ZAP-70 kinases. The balance, of course, could be affected by the absence of a critical kinase(s).
Acknowledgments
We thank J.V. Ravetch (Rockefeller University, New York), M. Ono (Rockefeller University, New York), E.A. Clark (University of Washington, Seattle, WA), and M.N. Lioubin (Fred Hutchinson Cancer Research Center, Seattle, WA) for antibodies, and A. Tanaka and Y. Yamanashi for critical reading of the manuscript.
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan, Human Frontier Science Program Organization, and Japan Society for the Promotion of Science.
References
- 1.Burkhardt AL, Brunswick M, Bolen JB, Mond JJ. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein kinases. Proc Natl Acad Sci USA. 1991;88:7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamanashi Y, Kakiuchi T, Mizuguchi J, Yamamoto T, Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991;251:192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- 3.Eisenman E, Bolen JB. Engagement of the high-affinity IgE receptor activates src-protein–related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 4.Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T, Yamamura H. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidorenko SP, Law C-L, Chandran KA, Clark EA. Human spleen tyrosine kinase p72Syk associates with the Src-family p53/56Lynand a 120-kDa phosphoprotein. Proc Natl Acad Sci USA. 1995;92:359–363. doi: 10.1073/pnas.92.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanashi Y, Okada M, Semba T, Yamori T, Umemori H, Tsunasawa S, Toyoshima K, Kitamura D, Watanabe T, Yamamoto T. Identification of HS1 protein as a major substrate of protein-tyrosine kinase(s) upon B-cell antigen receptor–mediated signaling. Proc Natl Acad Sci USA. 1993;90:3631–3635. doi: 10.1073/pnas.90.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanashi Y, Fukuda T, Nishizumi H, Inazu T, Higashi K, Kitamura D, Ishida T, Yamamura H, Watanabe T, Yamamoto T. Role of tyrosine phosphorylation of HS1 in B cell antigen receptor–mediated apoptosis. J Exp Med. 1997;185:1387–1392. doi: 10.1084/jem.185.7.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tezuka T, Umemori H, Fusaki N, Yagi T, Takata M, Kurosaki T, Yamamoto T. Physical and functional association of the cbl protooncogene product with an Src-family protein tyrosine kinase, p53/56l yn, in the B cell antigen receptor–mediated signaling. J Exp Med. 1996;183:675–680. doi: 10.1084/jem.183.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Koizumi T, Watanabe T. Altered antigen receptor signaling and impaired Fas-mediated apoptosis of B cells in Lyn-deficient mice. J Exp Med. 1996;184:831–838. doi: 10.1084/jem.184.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signaling initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 11.Nathanson SD, Zamfirescu PL, Drew SI, Wilbur S. Two-step separation of human peripheral blood monocytes on discontinuous density gradients of colloidal silica-polyvinylpyrrolidinone. J Immunol Methods. 1977;18:225–234. doi: 10.1016/0022-1759(77)90176-4. [DOI] [PubMed] [Google Scholar]
- 12.Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow–derived mast cells. J Immunol. 1997;158:2350–2355. [PubMed] [Google Scholar]
- 13.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 14.Yamanashi Y, Fukui Y, Wongsasant B, Kinoshita Y, Ichimori Y, Toyoshima K, Yamamoto T. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor–mediated signaling. Proc Natl Acad Sci USA. 1992;89:1118–1122. doi: 10.1073/pnas.89.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 16.Doody GM, Dempsey PW, Fearon DT. Activation of B lymphocytes: integrating signals from CD19, CD22 and FcγRIIb1. Curr Opin Immunol. 1996;8:378–382. doi: 10.1016/s0952-7915(96)80128-2. [DOI] [PubMed] [Google Scholar]
- 17.Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 18.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 19.D'Ambrosio D, Hippen KL, Minskoff SA, Mellman I, Pani G, Siminovitch KA, Cambier JC. Recruitment and activation of PTP1C in the negative regulation of antigen receptor signaling by FcγRIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 20.Pani G, Kozlowski M, Cambier JC, Mills GB, Siminovitch KA. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor–associated protein involved in the regulation of B cell signaling. J Exp Med. 1995;181:2077–2084. doi: 10.1084/jem.181.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 22.Otipoby KL, Anderson KB, Draves KE, Klaus SJ, Garr AG, Kerner JD, Perlmutter RM, Law C-L, Clark EA. CD22 regulates thymus independent responses and the lifespan of B cells. Nature. 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 23.Sato S, Miller AS, Inaoki M, Bock CB, Jansen PJ, Tang ML, Tedder TF. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 24.Nitschke L, Caresetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signaling. Curr Biol. 1997;7:133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 25.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13–amino-acid motif in the cytoplasmic domain of FcγRIIB modulates B-cell receptor signaling. Nature. 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 26.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 27.Scharenberg AM, Kinet JP. The emerging field of receptor-mediated inhibitory signaling: SHP or SHIP? . Cell. 1996;87:961–964. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]
- 28.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 29.Schulte RJ, Campbell MA, Fischer WH, Sefton BM. Tyrosine phosphorylation of CD22 during B cell activation. Science. 1992;258:1001–1004. doi: 10.1126/science.1279802. [DOI] [PubMed] [Google Scholar]
- 30.Campbell MA, Klinman NR. Phosphotyrosine-dependent association between CD22 and protein tyrosine phosphatase 1C. Eur J Immunol. 1995;25:1573–1579. doi: 10.1002/eji.1830250616. [DOI] [PubMed] [Google Scholar]
- 31.Doody GM, Justement LB, Delibrias CC, Matthews RJ, Kin J, Thomas ML, Fearon DT. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 32.Law CL, Sidorenko SP, Chandran KA, Zhao Z, Shen SH, Fischer EH, Clark EA. CD22 associates with protein tyrosine phosphatase 1C, Syk, and phospholipase C-γ1 upon B cell activation. J Exp Med. 1996;183:547–560. doi: 10.1084/jem.183.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cyster JG, Goodnow CC. Tuning antigen receptor signaling by CD22: integrating cues from antigens and the microenvironment. Immunity. 1997;6:509–517. doi: 10.1016/s1074-7613(00)80339-8. [DOI] [PubMed] [Google Scholar]
- 34.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 35.Tedder TF, Tuscano J, Sato S, Kehrl JH. CD22, a B lymphocyte–specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz R, Baumann G, Gram H. Catalytic specificity of phosphotyrosine kinases Blk, Lyn, c-Src and Syk as assessed by phage display. J Mol Biol. 1996;260:664–677. doi: 10.1006/jmbi.1996.0429. [DOI] [PubMed] [Google Scholar]
- 37.Cambier JC. Inhibitory receptors abound? . Proc Natl Acad Sci USA. 1997;94:5993–5995. doi: 10.1073/pnas.94.12.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unkeless JC, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]