Abstract
Human myeloma are incurable hematologic cancers of immunoglobulin-secreting plasma cells in bone marrow. Although malignant plasma cells can be almost eradicated from the patient's bone marrow by chemotherapy, drug-resistant myeloma precursor cells persist in an apparently cryptic compartment. Controversy exists as to whether myeloma precursor cells are hematopoietic stem cells, pre–B cells, germinal center (GC) B cells, circulating memory cells, or plasma blasts. This situation reflects what has been a general problem in cancer research for years: how to compare a tumor with its normal counterpart. Although several studies have demonstrated somatically mutated immunoglobulin variable region genes in multiple myeloma, it is unclear if myeloma cells are derived from GCs or post-GC memory B cells. Immunoglobulin (Ig)D-secreting myeloma have two unique immunoglobulin features, including a biased λ light chain expression and a Cμ–Cδ isotype switch. Using surface markers, we have previously isolated a population of surface IgM−IgD+CD38+ GC B cells that carry the most impressive somatic mutation in their IgV genes. Here we show that this population of GC B cells displays the two molecular features of IgD-secreting myeloma cells: a biased λ light chain expression and a Cμ–Cδ isotype switch. The demonstration of these peculiar GC B cells to differentiate into IgD-secreting plasma cells but not memory B cells both in vivo and in vitro suggests that IgD-secreting plasma and myeloma cells are derived from GCs.
Immunoglobulin D (IgD) is the major antigen receptor isotype coexpressed with IgM on the surface of mature naive B cells (1–9). Strikingly, while membrane IgD on human B cells is preferentially associated to κ light chain (1, 10), secreted IgD from myeloma cells is preferentially associated to λ light chain (11, 12). The ability of myeloma cells to secrete IgD appears to be the result of an unusual Cμ to Cδ switch mediated by DNA recombination between sequences within JH–Cμ intron and Cμ–Cδ intron (13–16).
One question has been which B cell differentiation window corresponds to the stage where IgD myeloma cells were originated. The answer for this will clarify the long standing controversial issues (17, 18) of whether the myeloma precursors are hematopoietic stem cells (19), pre–B cells (20), germinal center (GC)1 B cells (21), circulating memory cells (22, 23), or plasma blasts (24). Although several studies have demonstrated somatically mutated Ig variable region genes in multiple myeloma including IgD myeloma (23–33), it is unclear if myeloma cells are derived from GCs or post-GC memory B cells. Here, we report a population of IgM−IgD+ GC B cells that share three unique molecular features of IgD myeloma cells: (a) most impressive somatic hypermutation in IgVH genes, (b) Cμ–Cδ isotype switch, and (c) λ light chain expression. These GC B cells were shown to differentiate into plasma cells but not memory B cells, suggesting that IgD-secreting myeloma are derived from B cells at GC stage but not at memory stage.
Materials and Methods
Assay for Sμ–σ/δ Recombination.
Genomic DNAs were extracted from 3 × 107 EBV transformed cells or 105 fresh purified cells, according to the standard procedure. Genomic DNA was submitted to PCR amplification using the 5′ primer P3 (5′-CGGCAATGAGATGGCTTT-3′) and the 3′ primer P4 (5′-GGCAAACTGTCATGG GTT-3′), as shown in Fig. 1 A. All PCR reactions were performed with Taq polymerase (Perkin-Elmer Corp., Norwalk, CT) using the reaction buffer provided by the manufacturers and a DNA thermal cycler (Perkin-Elmer Corp.) with 35 cycles of 1 min denaturation at 94°C, 2 min primer annealing at 60°C, and 3 min extension at 72°C. Complete extension of the products was then performed by a final 10-min incubation at 72°C. For DNA sequencing, PCR products were cloned using the TA cloning kit (Invitrogen, Carlsbad, CA). Individual bacterial colonies were randomly picked and extracted plasmids were sequenced in an automated DNA sequencer (Applied Biosystems, Foster City, CA) on both strands.
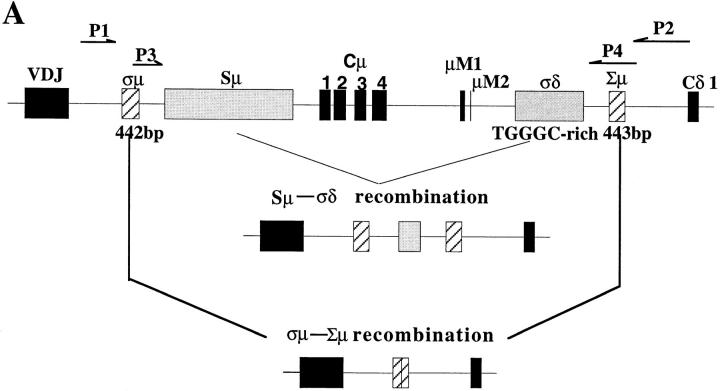
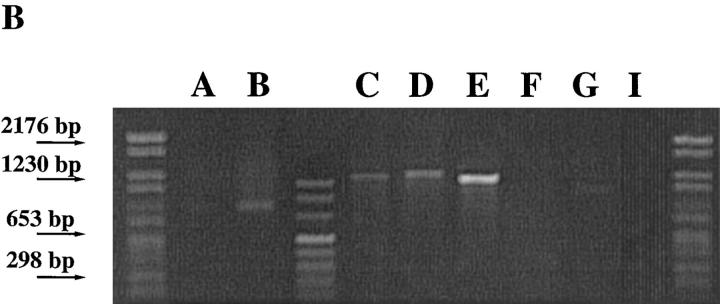
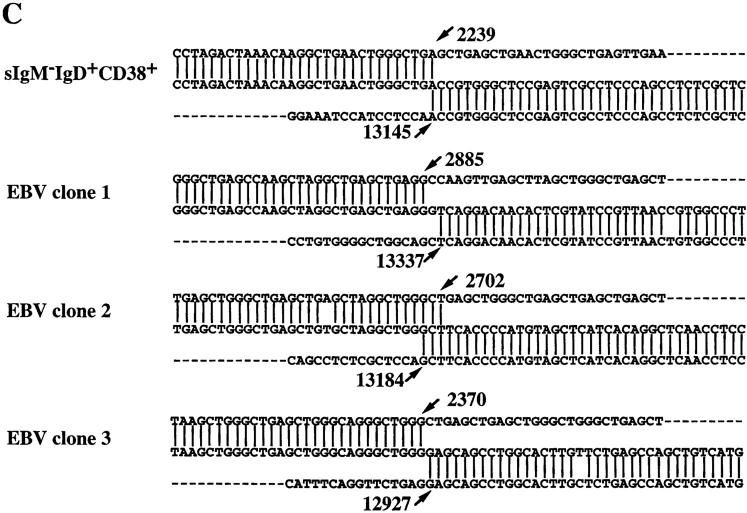
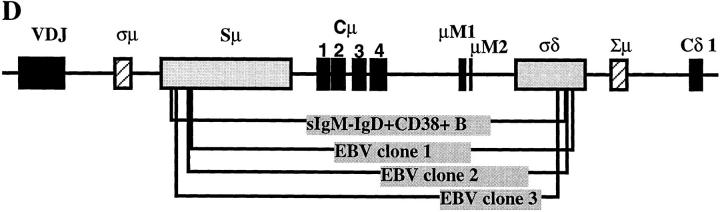
Figure 1.
Cμ–Cδ switch recombinations in IgM−IgD+CD38+ GC B cells. (A) Schematic representation of the Sμ–σ/δ and σ/μ–Σ/μ recombinations (14–16). (B) PCR amplification of Sμ–σ/δ recombination. Lane A, IgM+IgD+CD38+ GC founder cells; lane B, IgM−IgD+CD38+ GC B cells; lanes C, D, and E, EBV clones from IgM−IgD+CD38+ cells; lanes F, G, and I, EBV clones from IgM+IgD+CD38+ cells. (C) DNA sequences of Sμ–σ/δ junctional regions from IgM−IgD+CD38+ cells. Upper strings, germline sequences of the Sμ region; lower strings, germline sequences of the σ/μ–σ/δ intron. The central string represents the sequences of cloned PCR products. Homologous nucleotides are linked by vertical bars. Arrowed figures, base numbers, base No. 1 being the first 3′ of the VDJ region. (D) Schematic representation of the break points.
Measurements of Ig Secretion.
IgG, IgA, and IgM measurements were performed using ELISA as previously described (34). For IgD measurement, flat-bottomed 96-well plates were coated with 2 μg/ml of monoclonal anti-IgD antibodies (Nordic Immunological, Tiburg, The Netherlands) overnight at 4°C. After six washes, plates were first saturated for 3 h at 37°C with RPMI (GIBCO BRL, Gaithersburg, MD) containing 10% fetal calf serum (GIBCO BRL), and then incubated overnight at 4°C with appropriate dilutions of the assays and the standard purified myeloma IgD (The Binding Site, Birmingham, UK). Plates were washed six times and incubated with goat anti-IgD-biotin (Sigma Chemical Co., St. Louis, MO) at 2 μg/ml for 2 h at room temperature. After six washes, streptavidin–alkaline phosphatase (Sigma Chemical Co.) diluted 1/10,000 was added for 1 h at room temperature and enzymatic activity was revealed by p-nitrophenyl phosphate (Sigma Chemical Co.) and read at 490 nm on a Vmax spectrophotometer. The absence of cross-reactivities with human IgG, IgA, IgM, IgE, Igκ, and Igλ was checked, and values were reported to a standard curve using a purified myeloma IgD.
Analysis of Ig Light Chain Expression.
5 × 103 B cells were transformed by EBV during a 2-wk culture in a CD40 system with 10 μl of EBV containing B95-8 supernatant in a round-bottomed 96-well plate. Cloning was performed by culturing 1 cell/ well. The clones derived from surface (s)IgM−IgD+CD38+ GC B cells were selected by their sIgM−IgD+ phenotype and clones derived from sIgM+IgD+CD38+ B cells were selected according to their sIgM+ phenotype. VH gene expression by EBV clones was analyzed by VH–Cδ PCR amplification and sequence analyses using primers specific for each VH family and Cδ region. Among the 76 EBV clones, the V gene usage of 12 EBV clones derived from sIgM−IgD+CD38+ GC B cells were determined. One VH1, two VH5, fourVH3, and five VH4 were identified. Furthermore, light chain expression by EBV clones was analyzed by flow cytometry with anti-Igκ-FITC and anti-Igλ-FITC (Kallestad, Austin, TX).
Isolation of Tonsillar Plasma Cells.
In brief, tonsillar cells were centrifuged through 1.5% BSA at 10 g for 10 min. CD20− CD38++ plasma cells were then isolated by cell sorting. To isolate IgD+ and IgD− plasma cells, after centrifugation through 1.5% BSA, cells were first stained with anti-CD38-PE (Becton Dickinson, Mountain View, CA). They were permeabilized by an overnight incubation with 1% paraformaldehyde at 4°C. Intracellular IgD was stained with a mouse anti-IgD antibody-FITC (Dako Corp., Carpinteria, CA). CD382+ plasma cells were finally separated into intracellular IgD+ or IgD− plasma cells by cell sorting.
Sequence Analysis of the VH5 Transcripts.
This was done according to our established methods (35–37). In brief, messenger RNA was extracted from 2.5 × 104 plasma cells and cDNA was obtained by reverse transcription. Full-length VH5–δ transcripts were amplified with 5′LVH5 primer (5′-CCCGAATTCATGGGGTCAACCGCCATCCT-3′) with 3′ primer HCδ (5′-GGCGGCCGCTGGCCAGCGGAAGATCTCCTTCTT-3′), HCμ (5′-TGGGGCGGATGCACTCCC-3′), or HCγ (5′-CAGGGGAAGACCGATGG-3′) with Taq polymerase (Perkin-Elmer Corp.). PCR reaction was 35 cycles of 1 min denaturation at 94°C, 2 min of primer annealing at 60°C, and 30 min at 72°C. The frequency of Taq error in our lab is <2%. The PCR products were cloned, using the TA cloning kit (Invitrogen). Plasmids extracted from individual bacterial colonies were sequenced.
Results
Hypermutated sIgM−IgD+CD38+ GC B Cells Have Undergone Cμ–Cδ Switch by Recombination between Sμ and the Pentamer-rich σ/δ Region.
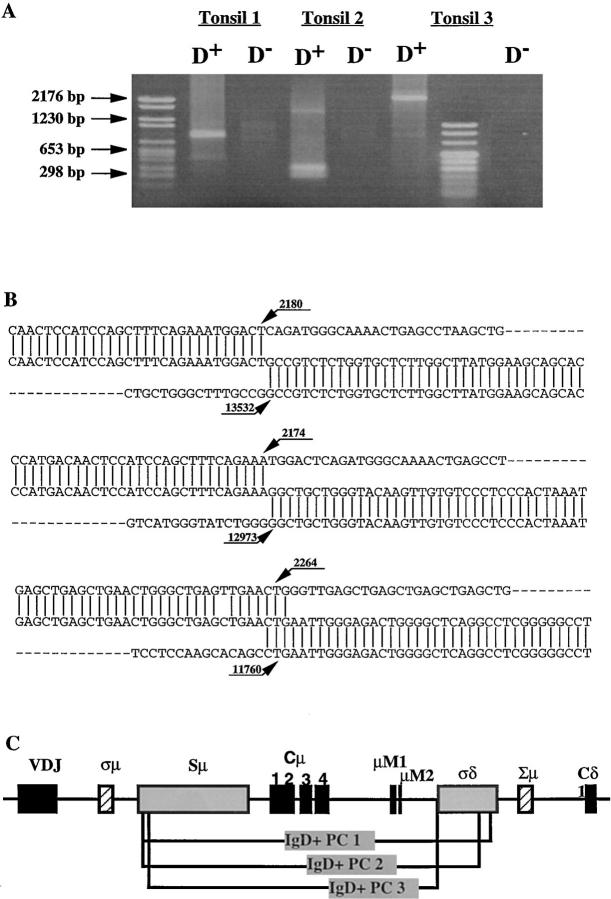
We have previously identified a population of sIgM−IgD+CD38+ GC B cells that contain extensively mutated Ig variable region genes (36). An intriguing link between these B cells and IgD-secreting myeloma cells is the rare single surface expression of IgD isotype of Igs. Such a phenotype can only be explained by either Cμ gene deletion as observed in IgD myeloma cells (14, 15) and in unfractionated cells from normal tonsils (16) of alternative splicing of μ–δ messenger RNAs, as observed in sIgM+IgD− B cells. To clarify this issue, PCR primers were designed for probing recombination events between the 442-bp σ/μ region and the 443-bp Σ/μ region or between Sμ and the pentamer-rich region σ/δ (Fig. 1 A). In three tonsillar samples, Sμ–σ/δ recombination but not σμ–Σμ recombination was detected in sIgM−IgD+CD38+ GC cells and their derived EBV transformed clones, but not in sIgM+IgD+CD38+ GC founder cells (37) and their EBV-derived clones (Fig. 1 B presents the result from one tonsil sample). To determine the Sμ–σ/δ break points, PCR-generated DNA products were cloned and sequenced. Fig. 1 C shows the sequences of four Sμ–σ/δ junctions obtained from freshly isolated sIgM−IgD+CD38+ GC B cells and their EBV clones. The four break points, which are presented in a schematic diagram in Fig. 1 D, demonstrate that the Cμ–Cδ switch had occurred in sIgM−IgD+CD38+ GC B cells.
Hypermutated sIgM−IgD+CD38+ GC B Cells Express λ Light Chains.
Since the second feature of IgD secreting myeloma was its preferential Igλ light chain expression (11, 12), we analyzed the light chain expression of a panel of EBV transformed clones derived from discrete B cell subsets of two tonsil samples (Table 1). Although 39 out of 83 EBV clones from sIgM+IgD+CD38+ GC founder cells and 17 out of 53 EBV clones from sIgD−CD38+ GC B cells express λ light chains, 75 out of 76 EBV clones from sIgM−IgD+CD38+ GC cells express λ light chains. VH sequence analysis showed that most clones were clonally independent (see Materials and Methods). These data demonstrate that sIgM−IgD+CD38+ GC B cells display the second feature of IgD myeloma cells: preferential expression of λ light chain.
Table 1.
Percentage and Number of Clones Expressing Igκ and Igλ Derived from Three CD38+ GC B Cell Subsets
| Clones derived from | Percentage of clones expressing Igλ | Number of clones | ||
|---|---|---|---|---|
| sIgM+IgD+CD38+ | 47 | 83 | ||
| sIgD−CD38+ | 32 | 53 | ||
| sIgM−IgD+CD38+ | 99 | 71 |
Hypermutated sIgM−IgD+CD38+ GC B Cells Display Poor Ability to Undergo Further Isotype Switch In Vitro.
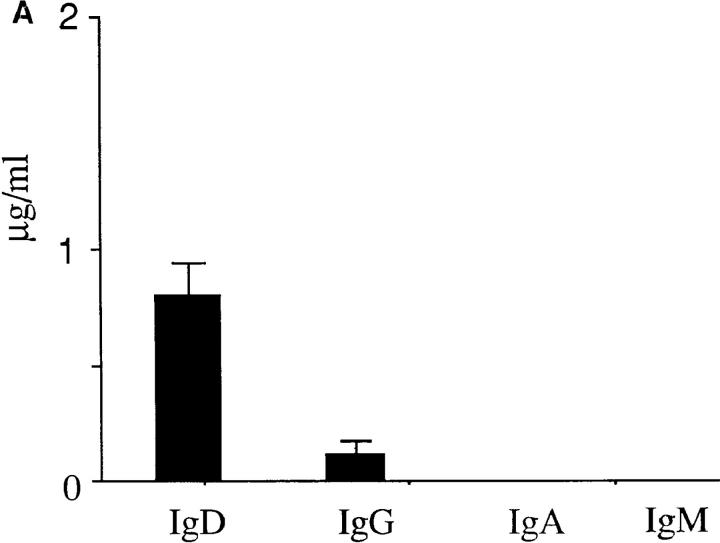
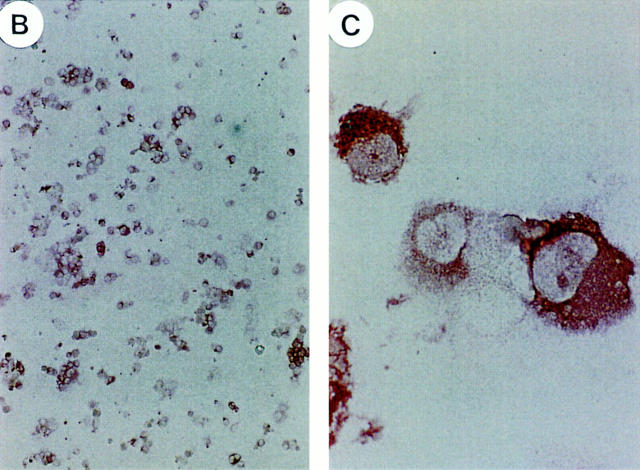
As sIgM− IgD+CD38+ GC B cells had lost a major part of the Sμ region after Cμ–Cδ switch (Fig. 1 D), it was anticipated that they would not undergo further isotype switch. Indeed, sIgM−IgD+CD38+ GC B cells differentiated mainly into IgD-secreting cells after 10 d of culture on CD40 transfected L cells with IL-2 and IL-10 (Fig. 2), a culture condition under which human naive B cells undergo isotype switch to IgG and differentiate into IgG-secreting cells (38, 39). Thus, sIgM−IgD+CD38+ GC B cells display two common features with IgD secreting myeloma cells, i.e., the Cμ–Cδ isotype switch and the preferential λ light chain expression, and they could differentiate into normal IgD-secreting cells in vitro.
Figure 2.
Differentiation of IgM−IgD+CD38+ B cells into IgD+ plasma cells in vitro. IgD, IgG, IgA, and IgM secretion (A) and IgD immunostaining (B and C) of IgM− IgD+CD38+ B cells cultured for 2 wk in the presence of IL-10, IL-2, and CD40 ligand transfected murine fibroblasts. Ig contents were measured by ELISA and results are given in μg/ml. Original magnifications of microphotographs are 100 (B) and 1,000 (C).
IgD-secreting Plasma Cells Represent a Major Population of Plasma Cells in Human Tonsils.
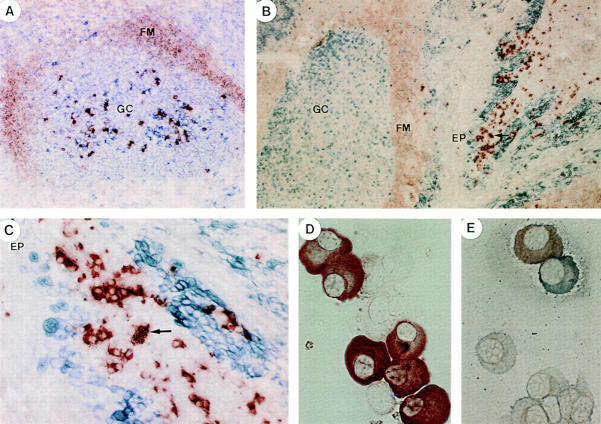
We have previously demonstrated that hypermutated sIgM−IgD+CD38+ GC B cells could not give rise to circulating memory cells in blood (36). However, IgD+ plasma cells were previously identified in human tonsils by immunohistology (40, 41), suggesting that sIgM−IgD+CD38+ GC B cells may differentiate into IgD-secreting plasma cells. An immunohistochemistry analysis performed on four randomly selected tonsillar samples with anti-IgD showed that IgD+ plasma cells represent an average of 16% (range 6–20%) of total CD382+ plasma cells. They were found either within GCs (Fig. 3 A) or within mucosal epithelium (Fig. 3, B and C), as reported earlier for IgA+ plasma cells (42). To further characterize IgD+ plasma cells, tonsillar plasma cells were isolated by cell sorting according to their CD382+CD20− phenotype as previously described. In agreement with the immunohistological analyses on tissue sections, plasma cells isolated from five tonsil samples contains 17% (3–48%) IgD+ and only 2–5% IgM+ cells (Fig. 3 D). Double anti-IgD and anti-IgM staining showed that plasma cells contain either IgM or IgD, but never both isotypes (Fig. 3 E). Furthermore, IgG, IgA, and IgD were the major Ig isotypes secreted by these plasma cells during overnight cultures (Table 2). The question is do IgD-secreting plasma cells in tonsils indeed represent the progeny of IgM−IgD+ GC B cells and the normal counterpart of IgD-secreting myeloma cells?
Figure 3.
Ig heavy chain expression by tonsillar plasma cells. Immunoenzymatic staining of tonsillar tissue sections (A, B, and C) or plasma cell cytospin preparations (D and E). (A) Double red anti-IgD and blue anti-CD38 staining showing purple IgD+ plasma cell within a GC and red IgD+ follicular mantle (FM) B cells. Original magnification: 400. (B and C) Double red anti-IgD and blue anti-Ki67 staining showing red IgD2+ plasma cells under the tonsillar epithelium (EP). Original magnifications are 100 (B) and 400 (C). (D) Single red anti-IgD staining showing IgD+ plasma cells among purified tonsillar plasma cells. Original magnification: 1,000. (E): Double blue anti-IgD and red anti-IgM staining showing exclusive expression by plasma cells of each isotype. Original magnification: 1,000.
Table 2.
Total Tonsillar PCs Secrete IgD
| Experiment No. 1 | Experiment No. 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| PCs | GCs | PCs | GCs | |||||
| IgM | <40 | <40 | <40 | <40 | ||||
| IgD | 11 ± 1 | <1 | 29 ± 1 | <1 | ||||
| IgG | 501 ± 64 | 25 ± 5 | 209 ± 15 | 43 ± 1 | ||||
| IgA | 205 ± 18 | 88 ± 1 | 116 ± 19 | 12 ± 1 | ||||
| IgE | <0.4 | <0.4 | <0.4 | <0.4 | ||||
CD20−CD382+ PCs and CD20+CD38+ GCs were isolated by FACS® sorting. 2 × 104 cells were cultured overnight in 200 μl Iscove medium. Ig contents were measured by ELISA assay. Data are given in ng/ml (mean ± SD).
IgD-secreting Plasma Cells Have Undergone Extensive Somatic Mutation and Display Striking Clonal Relatedness.
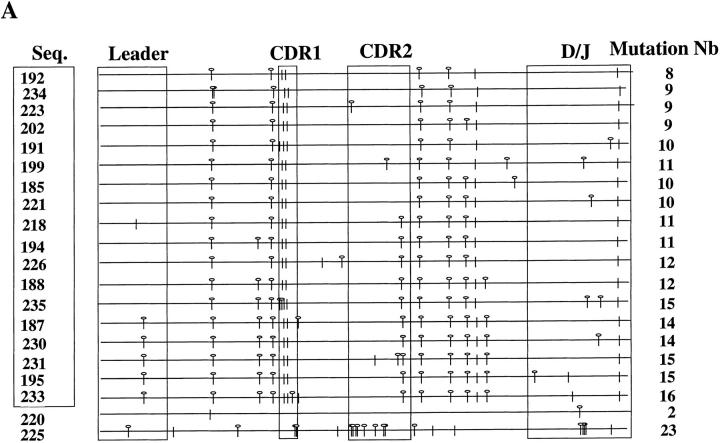
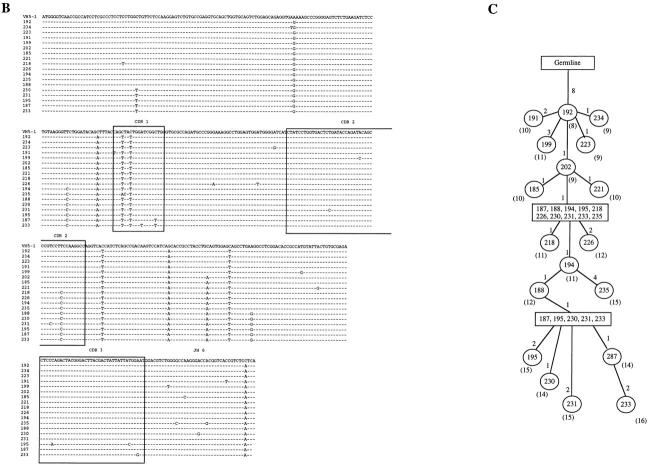
The first important feature of sIgM−IgD+CD38+ GC B cells is their extensively mutated IgV genes. Thus, VH5–δ, VH5–μ, and VH5-γ transcripts were amplified from 10,000 plasma cells of each of the three tonsil samples. The PCR products were then sequenced. Consistent with the surface or cytoplasmic Ig expression of different cell subsets, VH5–δ transcripts could only be amplified from IgD+CD38− naive B cells and CD382+CD20− plasma cells, but not from IgD− CD38+ GC B cells and IgD−CD38− memory B cells (data not shown). The 19 VH5–μ sequences had an average of four mutations per sequence and four sequences displayed clonal relatedness (Table 3). The 62 VH5–γ sequences had an average of 10 mutations/sequence and three sequences displayed clonal relatedness. These mutation frequencies are similar to that of the VH5–μ and VH5–γ transcripts of GC B cells and memory B cells previously described (35, 43), indicating the GC origin of these plasma cells. The 52 VH5–δ transcripts had accumulated an average of 21 mutations/sequence. 43 out of 52 sequences displayed clonal relatedness. (Clonal relatedness means that more than two sequences within the same tonsil sample are derived from one cell by somatic mutation.) The VH5–δ sequences of plasma cells from one representative tonsil (Fig. 4 A) display three features previously observed in the VH5–δ sequences of sIgM−IgD+CD38+ GC B cells (36): (a) their mutation frequency being two- to threefold higher than that of μ and γ transcripts, (b) replacement mutations not being concentrated within complementarity determining regions (CDRs), and (c) a high frequency of clonal relatedness. The genealogical trees deduced from the clonally related sequences (Fig. 4, B and C) indicate that somatic mutations have been accumulated during the extensive clonal expansion of IgD plasma cell precursors, the sIgM−IgD+ CD38+ GC B cells. Since an average of 16% of tonsillar plasma cells secrete IgD and only ∼2–5% of human B cells use VH5 genes, each tonsil sample may contain only 30–80 IgD-VH5–expressing plasma cells. These cells may represent the descendents of a single GC and may explain the observed restricted V gene usage.
Table 3.
IgD Sequences from PC Show a High Rate of Somatic Mutations as Well as a Strong Clonal Relationship
| VDJ asso-ciated with | Mutations (number per VDJ segment) | Clonality (percent of related sequences) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cμ | 4 ± 2 | (1–9, n = 19) | 21 (4/19) | |||||
| Cγ | 10 ± 8 | (1–31, n = 62) | 5 (3/62) | |||||
| Cδ | 21 ± 12 | (1–65, n = 52) | 83 (43/52) | |||||
VH5–Cδ sequences were amplified from PC cDNA and compared to the VH5–Cμ and VH5–Cγ sequences. The number of mutations per VDJ segments is given as mean ± SD (range, n). The degree of clonality is represented by the percentage of related sequences analyzed. Cμ, Cγ, and Cδ derive from 1, 2, and 3 different tonsil samples, respectively.
Figure 4.
Analysis of Ig heavy chain variable region genes of IgD+ plasma cells. (A) Schematic representation of VH5–Cδ sequences from plasma cells of one representative tonsil. Leader, CDR1, CDR2, and D/J regions are boxed. Sequence names are listed on the left (Seq.) and boxed names represent clonally related sequences. Total mutation numbers are listed on the right. Germline sequences of D regions were not assigned. Mutations are represented as replacement (circle with stem) and silent (stem). (B) Nucleotide sequences from the largest clone. The upper string gives the nucleotides of the VH5–1 germline sequence, the D region from the less mutated sequence (192), and the JH6 germline sequence. CDR1, CDR2, and CDR3 are boxed. Dashes, matches to the first string. Mutated bases are indicated. (C) Genealogical tree from this clone. Each analyzed sequence is indicated by its circled name, whereas common predicted intermediates are boxed. The number of mutations between sequences are indicated on the line joining them, and total sequence mutations are within brackets.
IgD-secreting Plasma Cells Have Undergone Cμ–Cδ Switch.
To determine whether IgD-secreting plasma cells have undergone Cμ–Cδ switch, CD382+ total tonsillar plasma cells were separated into intracellular IgD+ and intracellular IgD− subsets by a two-color immunofluorescence cell sorter. Sμ–σ/δ junctions were amplified from DNA of 10,000 cells of each subset. Fig. 5 A shows that Sμ-σδ junction can be amplified from IgD+ plasma cells of three tonsil samples, but not from IgD− plasma cells. Fig. 5 B shows the sequences of three examples of Sμ–σ/δ junctions from IgD+ plasma cells. The corresponding break points are depicted in Fig. 5 C.
Figure 5.
Cμ–Cδ switch recombinations in IgD+ plasma cells of three tonsil samples. (A): PCR amplification of Sμ–σ/δ recombinations of genomic DNA from IgD+ (lanes D +) and IgD− (lanes D−) plasma cells. (B) DNA sequences of Sμ–σ/δ junctional regions from IgD+ plasma cells. Upper strings, germline sequences of the Sμ region; lower strings, germline sequences of the σ/μ–σ/δ intron. The central string represents the sequences of cloned PCR products. Homologous nucleotides are linked by vertical bars. Arrowed figures, the base numbers, base No. 1 being the first 3′ of the VDJ region. (C) Schematic representation of the break points.
IgD-secreting Plasma Cells Preferentially Express λ Light Chains.
To determine the light chain expression of normal IgD-secreting plasma cells, double staining with anti-IgD (blue) and anti-Igκ (red) as well as anti-IgD (blue) and anti-Igλ (red) were performed on serial sections of three tonsil samples. Although few IgD+ plasma cells expressed Igκ light chain (most cells are single stained blue; Fig. 6, A and B), >90% were shown to express Igλ light chain (double stained purple; Fig. 6, C and D).
Figure 6.
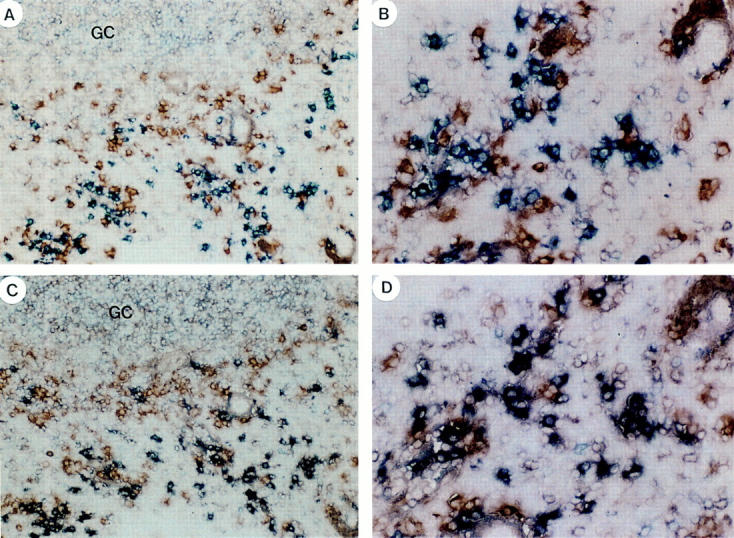
Ig light chain expression by tonsillar plasma cells. (A and B) Double blue anti-IgD and red anti-Igκ staining showing many single stained blue Igκ−IgD+ plasma cells and a few double stained purple Igκ+IgD+ plasma cells. (C and D) Double blue anti-IgD and red anti-Igλ staining on a serial section, showing many double stained Igλ+IgD+ plasma cells in purple and a few Igλ−IgD+ plasma cells single stained in blue. Original magnifications: 100 (A and C) and 400 (B and D).
IgD-secreting Myeloma Cells Have Undergone Somatic Mutation in Their Ig Variable Region Genes.
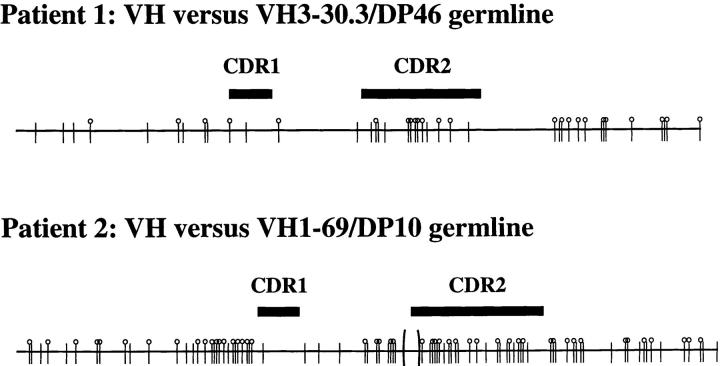
A previous study by Kiyoi et al. showed that four cases of IgD-secreting myeloma contained somatically mutated IgV genes (32). We analyzed the VH sequences of two well-characterized human IgD-secreting myeloma. VH sequence from patient 1 (15) displays 40 nucleotide differences from the three closest germline sequences (VH3-33/DP50, VH3-30.3/DP46, and VH3-30.5/DP49; Fig. 7). VH sequence from patient 2 (14) displays 85 nucleotide differences from the closest germline sequence (VH1-69/DP10; Fig 7). Exhaustive analyses and searches through different Ig databases from two different laboratories failed to identify other closest germline sequences. These data further suggest the GC origin of IgD-secreting myeloma cells.
Figure 7.
Analysis of Ig heavy chain variable region genes of IgD myeloma cells. Schematic representation of VH sequences from IgD myeloma cells of two samples. Patient 1 (15) shows 40 nucleotide substitutions, and patient 2 (14) shows 85 nucleotide differences plus one 9-bp deletion (between brackets). Mutations are represented as replacement (circle with stem) and silent (stem).
Discussion
IgD was first discovered by Rowe and Fahey as a unique myeloma protein (33). IgD-secreting myeloma cells were found to display two unusual features that could not fit into the current model of antigen-driven B cell development (44). First, while the membrane IgD on B cells shows a predominance of the Igκ type, more than two thirds of all known IgD myeloma proteins were shown to belong to the lambda type (11, 12). Second, IgD-secreting myeloma cells had undergone a unusual Cμ–Cδ switch. This raises the question of whether the features of IgD-secreting myeloma cells represent only a malignant event or reflect a normal B cell maturation pathway.
Here we demonstrate that hypermutated sIgM−IgD+ CD38+ GC B cells, which represent 2–5% of normal tonsillar B cells (36), may represent the precursors of normal and malignant IgD-secreting plasma cells. First, significant numbers of normal IgD-secreting plasma cells were identified in human tonsils (40, 41). In particular, both sIgM− IgD+CD38+ B cells and IgD+ plasma cells could be found within the same GCs. Second, CD40-activated sIgM− IgD+CD38+ GC B cells were shown to directly differentiate into IgD-secreting cells when cultured with IL-2 and IL-10. Third, both sIgM−IgD+CD38+ GC B cells and normal IgD-secreting plasma cells displayed a similar somatic hypermutation rate. Fourth, both sIgM−IgD+ CD38+ GC B cells and normal IgD-secreting plasma cells had been originated from a few cells that had undergone impressive clonal expansion and somatic mutation within GCs. Fifth, like IgD-secreting myeloma cells, both cell types preferentially expressed the Igλ light chain and had undergone Cμ–Cδ switch.
Previous studies have shown that IgVH and IgVL genes of IgG, IgA, and IgD myeloma contain extensive somatic mutation (23–33). These findings strongly suggest that IgD-secreting myeloma cells are not derived from the transformation of stem cells (19) or pre–B cells (20), but from GC B cells or post-GC memory B cells. Our previous study demonstrated sIgM−IgD+CD38+ GC B cells did not mature into blood memory B cells. This, together with the present finding that sIgM−IgD+CD38+ GC B cells differentiate into IgD-secreting plasma cells, suggests that IgD-secreting myeloma cells are derived from B cells at the GC B cell stage, but not at the post-GC memory B stage.
The identification of sIgM−IgD+CD38+ GC B cells and IgD+ plasma cells defines a novel GC B cell development pathway in human, characterized by (a) a nonclassical isotype switch from Cμ to Cδ, (b) a light chain shift from κ to λ, (c) the impressive oligoclonal expansion and somatic hypermutation, and (d) generation of IgD-secreting plasma cells. The molecular triggers and functional implications of the Cμ–Cδ switch, the κ–λ light chain shift, and the enormous clonal expansion and somatic mutation in sIgM−IgD+ CD38+ GC B cells are currently unknown. The κ-λ light chain shift may result from a secondary light chain rearrangement (receptor editing; references 45, 46) in GCs, as recently demonstrated in mouse GC B cells (47–50).
The identification of a significant number of IgD+ plasma cells in human tonsils also challenges the previous hypothesis that IgD functions simply as an antigen receptor, but not as a secreted antibody. This, together with recent identification of IgD+ memory B cells in human bone marrow (51) and virus-specific IgD-secreting plasma cells in the spleen of mice (52), strongly suggests that IgD plays an important role in certain types of humoral immune responses.
Acknowledgments
We thank J.-L. Preud'homme and E. Levievre (CNRS 1172, Poitiers, France) for helpful standardization of the IgD ELISA assay, M.-P. Lefrance (University Montpelier II, Montpelier, France) for help in mutation rate analyses of myeloma cells, E. Bates (Schering-Plough, Dardilly, France) for critical reading of the manuscript, and S. Bourdarel and M. Vatan (Schering-Plough, Dardilly, France) for editorial assistance. This paper is dedicated to Dr. J. Chiller, the late President of DNAX Research Institute, Palo Alto, CA.
Footnotes
1 Abbreviations used in this paper: CDR, complementarity determining region; GC, germinal center; s, surface.
C. Arpin is the recipient of a grant from the Fondation Mérieux (Lyon, France).
Jacques Banchereau's present address is the Baylor Institute of Immunology Research, 3535 Worth St., Sammons Cancer Center, Suite 4800, Dallas, TX 75246.
References
- 1.Rowe DS, Hug K, Forni L, Pernis B. Immunoglobulin D as a lymphocyte receptor. J Exp Med. 1973;138:965–972. doi: 10.1084/jem.138.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pernis B, Brouet JC, Seligmann M. IgD and IgM on the membrane of lymphoid cells in macroglobulinemia. Evidence for identity of membrane IgD and IgM antibody activity in a case with anti-IgG receptors. Eur J Immunol. 1974;4:776–778. doi: 10.1002/eji.1830041114. [DOI] [PubMed] [Google Scholar]
- 3.Salsano F, Froland SS, Natvig JB, Michaelsen TE. Same idiotype of B-lymphocyte membrane IgD and IgM. Formal evidence for monoclonality of chronic lymphocytic leukemia cells. Scand J Immunol. 1974;3:841–846. doi: 10.1111/j.1365-3083.1974.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 4.Fu SM, Winchester RJ, Kunkel HG. Similar idiotypic specificity for the membrane IgD and IgM of human B lymphocytes. J Immunol. 1975;114:250–252. [PubMed] [Google Scholar]
- 5.Goding JW, Layton JE. Antigen-induced co-capping of IgM and IgD-like receptors on murine B cells. J Exp Med. 1976;144:857. doi: 10.1084/jem.144.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern C, McConnell I. Immunoglobulins M and D as antigen-binding receptors on the same cell, with shared specificity. Eur J Immunol. 1976;6:225–227. doi: 10.1002/eji.1830060316. [DOI] [PubMed] [Google Scholar]
- 7.Vitetta ES, Uhr JW. Cell surface immunoglobulin. XV. The presence of IgM and an IgD-like molecule on the same cell in murine lymphoid tissue. Eur J Immunol. 1976;6:140–143. doi: 10.1002/eji.1830060215. [DOI] [PubMed] [Google Scholar]
- 8.Parkhouse RM, Cooper MD. A model for the differentiation of B lymphocytes with implications for the biological role of IgD. Immunol Rev. 1977;37:105–126. doi: 10.1111/j.1600-065x.1977.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 9.Blattner FR, Tucker PW. The molecular biology of immunoglobulin D. Nature. 1984;307:417–422. doi: 10.1038/307417a0. [DOI] [PubMed] [Google Scholar]
- 10.Ligthart GJ, Schuit HR, Hijmans W. Subpopulations of mononuclear cells in aging: expansion of the null cell compartment and decrease in the number of T and B cells in human blood. Immunology. 1985;55:15–21. [PMC free article] [PubMed] [Google Scholar]
- 11.Fine JM, Rivat C, Lambin P, Ropartz C. Monoclonal IgD. A comparative study of 60 sera with IgD “M” component. Biomedicine. 1974;21:119–125. [PubMed] [Google Scholar]
- 12.Fibbe WE, Jansen J. Prognostic factors in IgD myeloma: a study of 21 cases. Scand J Haematol. 1984;33:471–475. doi: 10.1111/j.1600-0609.1984.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilliam AC, Shen A, Richards JE, Blattner FR, Mushinski JF, Tucker PW. Illegitimate recombination generates a class switch from C mu to C delta in an IgD-secreting plasmacytoma. Proc Natl Acad Sci USA. 1984;81:4164–4168. doi: 10.1073/pnas.81.13.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasui H, Akahori Y, Hirano M, Yamada K, Kurosawa Y. Class switch from μ to δ is mediated by homologous recombination between σμ and δ sequences in human immunoglobulin gene loci. Eur J Immunol. 1989;19:1399–1403. doi: 10.1002/eji.1830190808. [DOI] [PubMed] [Google Scholar]
- 15.White MB, Word CJ, Humphries CG, Blattner FR, Tucker PW. Immunoglobulin D switching can occur homologous recombination in human B cells. Mol Cell Biol. 1990;10:3690–3699. doi: 10.1128/mcb.10.7.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluin PM, Kayano H, Zani VJ, Kluin-Nelemans HC, Tucker PW, Satterwhite E, Dyer MJS. IgD class switching: identification of a novel recombination site in neoplastic and normal B cells. Eur J Immunol. 1995;25:3504–3508. doi: 10.1002/eji.1830251244. [DOI] [PubMed] [Google Scholar]
- 17.Bergsagel DE. Treatment of plasma cell myeloma. Annu Rev Med. 1979;30:431–443. doi: 10.1146/annurev.me.30.020179.002243. [DOI] [PubMed] [Google Scholar]
- 18.Greipp PR. Advances in the diagnosis and management of myeloma. Semin Hematol. 1992;29:24–45. [PubMed] [Google Scholar]
- 19.Epstein J, Xiao HQ, He XY. Markers of multiple hematopoietic-cell lineages in multiple myeloma. New Engl J Med. 1990;322:664–668. doi: 10.1056/NEJM199003083221005. [DOI] [PubMed] [Google Scholar]
- 20.Kubagawa H, Vogler LB, Capra JD, Conrad ME, Lawton AR, Cooper MD. Studies on the clonal origin of multiple myeloma. Use of individually specific (idiotype) antibodies to trace the oncogenic event to its earliest point of expression in B-cell differentiation. J Exp Med. 1979;150:792–807. doi: 10.1084/jem.150.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warburton P, Joshua DE, Gibson J, Brown RD. CD10-(CALLA)–positive lymphocytes in myeloma: evidence that they are a malignant precursor population and are of germinal centre origin. Leuk Lymphoma. 1989;1:11–18. doi: 10.3109/10428198909042453. [DOI] [PubMed] [Google Scholar]
- 22.Jensen GS, Mant MJ, Belch AJ, Berenson JR, Ruether BA, Pilarski LM. Selective expression of CD45 isoforms defines CALLA+ monoclonal B-lineage cells in peripheral blood from myeloma patients as late stage B cells. Blood. 1991;78:711–719. [PubMed] [Google Scholar]
- 23.Sahota SS, Leo R, Hamblin TJ, Stevenson FK. Ig VH gene mutational patterns indicate different tumor cell status in human myeloma and monoclonal gammopathy of undetermined significance. Blood. 1996;87:746–755. [PubMed] [Google Scholar]
- 24.MacLennan, I.C.M., and E.Y.T. Chan. 1991. The origin of bone marrow plasma cells. In Epidemiology and Biology of Multiple Myeloma. G.I. Obrams and M. Potter, editors. Springer-Verlag, Berlin. 129–135.
- 25.Bakkus MH, Heirman C, Van Riet I, Van Camp B, Thielemans K. Evidence that multiple myeloma Ig heavy chain VDJ genes contain somatic mutations but show no intraclonal variation. Blood. 1992;80:2326–2335. [PubMed] [Google Scholar]
- 26.Baker BW, Deane M, Gilleece MH, Johnston D, Scarffe JH, Norton JD. Distinctive features of immunoglobulin heavy chain variable region gene rearrangement in multiple myeloma. Leuk Lymphoma. 1994;14:291–301. doi: 10.3109/10428199409049681. [DOI] [PubMed] [Google Scholar]
- 27.Wagner SD, Martinelli V, Luzzatto L. Similar patterns of V kappa gene usage but different degrees of somatic mutation in hairy cell leukemia, prolymphocytic leukemia, Waldenstrom's macroglobulinemia, and myeloma. Blood. 1994;83:3647–3653. [PubMed] [Google Scholar]
- 28.Kosmas C, Stamatopoulos K, Loukopoulos D. Antigen selection of multiple myeloma clonogenic B cells as evidenced by V(H) and V(L) gene mutations. Blood. 1997;90:1334–1335. [PubMed] [Google Scholar]
- 29.Biggs DD, Kraj P, Goldman J, Jefferies L, Carchidi C, Anderson K, Silberstein LE. Immunoglobulin gene sequence analysis to further assess B-cell origin of multiple myeloma. Clin Diagn Lab Immunol. 1995;2:44–52. doi: 10.1128/cdli.2.1.44-52.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosmas C, Viniou NA, Stamatopoulos K, Courtenay-Luck NS, Papadaki T, Kollia P, Paterakis G, Anagnostou D, Yataganas X, Loukopoulos D. Analysis of the kappa light chain variable region in multiple myeloma. Br J Haematol. 1996;94:306–317. doi: 10.1046/j.1365-2141.1996.d01-1815.x. [DOI] [PubMed] [Google Scholar]
- 31.Rettig MB, Vescio RA, Cao J, Wu CH, Lee JC, Han E, DerDanielian M, Newman R, Hong C, Lichtenstein AK, Berenson JR. VH gene usage in multiple myeloma: complete absence of the VH4.21 (VH4-34) gene. Blood. 1996;87:2846–2852. [PubMed] [Google Scholar]
- 32.Kiyoi H, Naito K, Ohno R, Naoe T. Comparable gene structure of the immunoglobulin heavy chain variable region between multiple myeloma and normal bone marrow lymphocytes. Leukemia (Baltimore) 1996;10:1804–1812. [PubMed] [Google Scholar]
- 33.Sahota SS, Leo R, Hamblin TJ, Stevenson FK. Myeloma VL and VH gene sequences reveal a complementary imprint of antigen selection in tumor cells. Blood. 1997;89:219–226. [PubMed] [Google Scholar]
- 34.Flückiger AC, Garrone P, Durand I, Galizzi JP, Banchereau J. Interleukin 10 (IL-10) upregulates functional high affinity IL-2 receptors on normal and leukemic B lymphocytes. J Exp Med. 1993;178:1473–1481. doi: 10.1084/jem.178.5.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascual V, Liu YJ, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu YJ, de Bouteiller O, Arpin C, Brière F, Galibert L, Ho S, Martinez-Valdez H, Banchereau J, Lebecque S. Normal human IgD+IgM− germinal center B cells can express up to 80 mutations in the variable region of their IgD−transcripts. Immunity. 1996;4:603–613. doi: 10.1016/s1074-7613(00)80486-0. [DOI] [PubMed] [Google Scholar]
- 37.Lebecque S, de Bouteiller O, Arpin C, Banchereau J, Liu YJ. Germinal center founder cells display propensity for apoptosis before onset of somatic mutation. J Exp Med. 1997;185:563–571. doi: 10.1084/jem.185.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rousset F, Garcia E, Defrance T, Péronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malisan F, Brière F, Bridon J-M, Harindranath N, Mills FC, Max EE, Banchereau J, Martinez-Valdez H. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J Exp Med. 1996;183:937–947. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrarini M, Corte G, Viale G, Durante ML, Bargellesi A. Membrane Ig on human lymphocytes: rate of turnover of IgD and IgM on the surface of human tonsil cells. Eur J Immunol. 1976;6:372–378. doi: 10.1002/eji.1830060513. [DOI] [PubMed] [Google Scholar]
- 41.Brandtzaeg P, Halstensen TS. Immunology and immunopathology of tonsils. Adv Oto-Rhino-Laryngol. 1992;47:64–75. doi: 10.1159/000421721. [DOI] [PubMed] [Google Scholar]
- 42.Merville P, Déchanet J, Desmoulière A, Durand I, de Bouteiller O, Garrone P, Banchereau J, Liu YJ. Bcl-2+ tonsillar plasma cells are rescued from apoptosis by bone marrow fibroblasts. J Exp Med. 1996;183:227–236. doi: 10.1084/jem.183.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein U, Küppers R, Rajewsky K. Variable region gene analysis of B cell subsets derived from a 4-year-old child: somatically mutated memory B cells accumulate in the peripheral blood already at young age. J Exp Med. 1994;180:1383–1393. doi: 10.1084/jem.180.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowe DS, Fahey JL. A new class of human immunoglobulins. I. A unique myeloma protein. J Exp Med. 1965;121:171–184. doi: 10.1084/jem.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han S, Zheng B, Schatz DG, Spanopoulou E, Kelsoe G. Neoteny in lymphocytes: Rag-1 and Rag-2 expression in germinal center B cells. Science. 1996;274:2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 48.Hikida, M., M. Mori, T. Takai, K.I. Tomochika, K. Hamatani, and H. Ohmori. 1996. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 2092–2094. [DOI] [PubMed]
- 49.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 50.Papavasiliou F, Casellas R, Suh H, Qin XF, Besmer E, Pelanda R, Nemazee D, Rajewsky K, Nussenzweig MC. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997;5336:298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- 51.Paramithiotis E, Cooper MD. Memory B lymphocytes migrate to bone marrow in humans. Proc Natl Acad Sci USA. 1997;94:208–212. doi: 10.1073/pnas.94.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moskophidis D, Moskophidis M, Löhler J. Virus-specific IgD in acute viral infection of mice. J Immunol. 1997;158:1254–1261. [PubMed] [Google Scholar]