Abstract
Early in programmed cell death (apoptosis), mitochondrial membrane permeability increases. This is at least in part due to opening of the permeability transition (PT) pore, a multiprotein complex built up at the contact site between the inner and the outer mitochondrial membranes. The PT pore has been previously implicated in clinically relevant massive cell death induced by toxins, anoxia, reactive oxygen species, and calcium overload. Here we show that PT pore complexes reconstituted in liposomes exhibit a functional behavior comparable with that of the natural PT pore present in intact mitochondria. The PT pore complex is regulated by thiol-reactive agents, calcium, cyclophilin D ligands (cyclosporin A and a nonimmunosuppressive cyclosporin A derivative), ligands of the adenine nucleotide translocator, apoptosis-related endoproteases (caspases), and Bcl-2–like proteins. Although calcium, prooxidants, and several recombinant caspases (caspases 1, 2, 3, 4, and 6) enhance the permeability of PT pore-containing liposomes, recombinant Bcl-2 or Bcl-XL augment the resistance of the reconstituted PT pore complex to pore opening. Mutated Bcl-2 proteins that have lost their cytoprotective potential also lose their PT modulatory capacity. In conclusion, the PT pore complex may constitute a crossroad of apoptosis regulation by caspases and members of the Bcl-2 family.
Two different major changes in mitochondrial membrane permeability have been observed during the effector phase of apoptosis. On the one hand, the electrochemical gradient built up on the mitochondrial inner membrane dissipates early during apoptosis (1–4). On the other hand, apoptogenic proteins that normally are sequestered in mitochondria are released via the outer mitochondrial membrane. Such proteins include cytochrome c (5–7) and apoptosis inducing factor (AIF)1 (8, 9). The protooncogene product Bcl-2 prevents the permeability increase in both mitochondrial membranes (4, 6–10). Based on the similarity of the effects of Bcl-2 and pharmacological inhibitors of the mitochondrial permeability transition (PT) pore, we have advanced the hypothesis that opening of the PT pore might be (co-)responsible for the apoptosis-associated changes in mitochondrial membrane function (2, 4, 8, 11). In isolated mitochondria, opening of the PT pore entails both the disruption of the inner mitochondrial transmembrane potential (Δψm) (12, 13) and the release of the apoptogenic proteins AIF (8, 9) and cytochrome c (14, 15), suggesting that the PT pore may have an important role in cell death control. Moreover, opening of the PT pore has been implicated in clinically relevant massive cell death of hepatocytes, neurons, and myocardiocytes induced by hepatotoxins, excitotoxins, calcium, reactive oxygen species, and anoxia (3, 4, 12, 13, 16–18 and references cited therein).
If the mitochondrion fulfilled a major role in apoptosis control, it should be capable of integrating very different proapoptotic signal transduction and damage pathways. In this context, it appears important that the PT pore is a dynamic multiprotein complex located at the contact site between the inner and the outer mitochondrial membranes, one of the critical sites of metabolic coordination between the cytosol, the mitochondrial intermembrane space, and the matrix. The PT pore participates in the regulation of matrix Ca2+, pH, Δψm, and volume and functions as a Ca2+-, voltage-, pH-, and redox-gated channel with several levels of conductance and little if any ion selectivity (12, 13, 19). Although the exact composition of the PT pore complex (PTPC) is unknown, it is thought to involve proteins from the cytosol (hexokinase), the outer membrane (voltage-dependent anion channel [VDAC]), the inner membrane (the adenine nucleotide translocator [ANT]), and the matrix (cyclophilin D) (12, 13, 20–23). As a consequence, the PT pore complex contains multiple targets for endogenous regulators. In intact cells and isolated mitochondria, PT pore opening is induced by several proapoptotic second messengers: Ca2+, prooxidants, nitric oxide, ceramide, and caspase 1 (1, 2, 8, 9, 12, 13, 19, 24–27). Moreover, it is regulated by the antiapoptotic oncoproteins Bcl-2 and Bcl-XL, which stabilize mitochondrial membranes (4, 8, 9, 28–31), and by the proapoptotic Bcl-2 analogue Bax, which disrupts the Δψm (32).
It has been unclear whether these effectors specifically act on PTPC, affect other mitochondrial structures not associated with PTPC (6, 7), or rather nonspecifically perturb membrane permeability, as this has been suggested for members of the Bcl-2 family (32–35). To distinguish these possibilities, we purified protein complexes containing PTPC, reconstituted them in liposomes, and created a reduced experimental system that shares properties of the PT pore studied in intact mitochondria or cells. Biochemical and functional data indicate that PTPC enriched from brain homogenates contain the proapoptotic Bcl-2 homologue Bax (but not Bcl-2 and Bcl-XL), in addition to proteins previously suggested to participate in the regulation of PT (ANT, VDAC, cyclophilin D, and hexokinase). The membrane permeability of PTPC liposomes was enhanced by several inducers of PT including Ca2+, prooxidants, and recombinant caspases. Recombinant Bcl-2 and Bcl-XL act as inhibitors of PT pore opening in this artificial system. Thus, PTPC constitutes the target of multiple apoptosis regulators, emphasizing its probable central role in cell death control.
Materials and Methods
Materials.
Recombinant human Bcl-XL (1–209), Bcl-2 (1– 218), mutant Bcl-2 (Gly145Ala), and Bcl-2Δα5/6 (Δ143–184), all lacking the hydrophobic transmembrane domain (Δ219–239 in the case of Bcl-2; Δ210–230 for Bcl-XL) and tagged NH2 terminally with His6, were produced and purified as described (34). Recombinant caspases were produced as active enzymes (36, 37). Caspase activity is defined as amount of enzyme required to cleave 1 μmol of the fluorogenic substrate Ac-DEVD.amc (acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin; caspases 3 and 6), Ac-YVAD.amc (acetyl-Tyr-Val-Ala-Asp-aminomethylcoumarin; caspase 1), or Ac-WEHD.amc (acetyl-Trp-Glu-His-Asp-aminomethylcoumarin; caspase 4) per hour. Caspase substrates and inhibitors (Ac-DEVD. cmk [acetyl-Asp-Glu-Val-Asp-chloromethylketone], Ac-YVAD. cmk [acetyl-Tyr-Val-Ala-Asp-chloromethylketone]) were purchased from Bachem (Basel, Switzerland). All remaining reagents were from Sigma Chemical Co. (St. Louis, MO), unless specified differently.
Reconstitution of PTPCs in Liposomes.
PTPCs were purified and reconstituted in liposomes following published protocols (22), with several modifications (Fig. 1 A). In brief, four Wistar (Philadelphia, PA) rat brains (3–4-mo-old males, stored at −80°C) were homogenized in buffer 1 (1 mM α-monothioglycerol, 10 mM glucose, pH 8.0, 40 ml; sample 1 in Fig. 1) and centrifuged twice (15 min, 12,000 g, 4°C) to resuspend the pellet first in buffer 1 alone, and then in buffer 1 plus 0.5% Triton X-100 (Boehringer Mannheim, Indianapolis, IN) for 30 min at room temperature (RT) while stirring. Supernatants (40 min, 50,000 g, 4°C) of this mixture, the Triton-soluble protein fraction (sample 2 in Fig. 1), were mixed with 17 g DE52 resin previously equilibrated with buffer 2 (1.5 mM Na2HPO4, 1.5 mM K2HPO4, 100 mM glucose, 1 mM dithioerythitol, pH 8.0). These beads were packed into an FPLC column (XK16/20; Pharmacia Biotech, Uppsala, Sweden) and eluted with buffer 2 supplemented with 50 mM KCl (buffer 3) or 400 mM KCl (buffer 4). After equilibration with buffer 3 (0.8 ml/min, 6 ml), elution was performed on a linear gradient from 50 to 400 mM KCl (buffers 3 versus 4), followed by determination of hexokinase activity (sample 3 in Fig. 1). Lipid vesicles were prepared by mixing 300 mg phosphatidylcholine and 60 mg cholesterol in 3 ml chloroform, evaporation of the chloroform under nitrogen, and resuspension in 3 ml 125 mM sucrose + 10 mM Hepes (pH 7.4) + 0.3% n-octyl-β-d-pyranoside by vortexing (90 min, RT). These vesicles (6 ml) were incubated with 6 ml of PTPC-containing solution during 20 min at RT and dialyzed overnight (4°C) against 125 mM sucrose + 10 mM Hepes (pH 7.4). In several experiments, recombinant Bcl-2, Bcl-XL, or mutated Bcl-2 proteins were added during the dialysis step at a dose corresponding to 5% of the total PTPC proteins, as determined in each experiment. Liposomes recovered from dialysis were ultrasonicated (120 W, Ultrasonic Processor; Bioblock, Illkirch, France) during 7 s in 5 mM malate and 10 mM KCl, charged on a Sephadex G50 column (C16/20; Pharmacia Biotech), and eluted with 125 mM sucrose + 10 mM Hepes (pH 7.4, 0.8 ml/min) (Fig. 1 C). Proteins were extracted from the liposome preparation (1 ml) by mixing with 2 ml 880 ml KCl + 6 ml chloroform/methanol (2:1 vol/vol) and recovered from the interphase after standard methods (38), followed by resuspension in 0.1% SDS (sample 4 in Fig. 1). They were then precipitated with 80% (vol/vol) acetone for two-dimensional electrophoresis. A mean of 1.86 ± 0.24 μg protein/mg lipid (X ± SD, n = 5) was recovered from proteoliposomes. In several experiments, purified rat cytochrome c (25 μg/ml, corresponding to 500 ng cytochrome c/mg lipid) was added before the sonication step, followed by two washes on Sephadex G50 columns to remove excess cytochrome c from the supernatant.
Figure 1.
Enrichment of the PTPC. (A) Steps of the purification process. For details consult Materials and Methods. (B) Typical profile of an anion exchange chromatography performed on Triton-soluble proteins (A, 2). Hexokinase activity (solid line) elutes from the DE52 resin at a KCl concentration (linear gradient, dotted line) of 190 ± 10 mM. The most active fractions (bar) are recovered (A, 3) and reconstituted in liposomes as outlined in A. Cytochrome c elutes from the gradient with the major protein peak, at 70 ± 10 mM (arrow). (C) Typical profile of a molecular weight chromatography performed on liposomes reconstituted with the fractions recovered in B (A, 3). Note that hexokinase activity accumulates in a few fractions of the liposome–protein mixture. The fraction containing maximum hexokinase activity constitutes the PTPC liposomes (A, 4). (D) Immunochemical detection of proteins contained in PTPC. Proteins from successive steps of the purification procedure (A) were analyzed by Western blot for the presence of the indicated proteins. Proteins extracted from PTPC liposomes constitute the final step (A, 4) of the purification procedure. Results are representative for 22 (B and C), and two to three (D) independent determinations.
Determination of Calcein Efflux from PTPC Liposomes.
Liposomes were generated as described above with the sole difference that sonication was performed in 8 mM calcein (Molecular Probes Inc., Eugene, OR) + 10 mM cobalt chloride. 200 μl liposome suspension was incubated for 90 min with different concentration of atractyloside (Atr) and/or 50 μM bongkrekic acid (gift from Hans J. Duine, Delft University, Delft, The Netherlands). The supernatants of liposomes (4.5 × 106 g, 45 min, 4°C) were recovered, supplemented with EDTA (final concentration of 1 mM), and subjected to fluorometric analysis (excitation at 488 nm, emission at 520 nm) in a fluorescence spectrometer (F4500; Hitachi, Tokyo, Japan).
Western Blots and Two-dimensional Electrophoresis.
Total brain homogenates, Triton-soluble proteins, PTPC preparations from anion exchange columns, and proteins extracted from PTPC-reconstituted liposomes were separated by SDS-PAGE (10–15%, 30 μg protein/lane), followed by Western blot using monoclonal antibodies recognizing cytochrome c (PharMingen, San Diego, CA; reference 5), hsp60 (clone LK1; Sigma Chemical Co.), VDAC (gift from F. Thinnes, Molecular Pathology Institute for Experimental Medicine, Göttingen, Germany), or polyclonal rabbit antisera against the ANT (gift from T. Wallimann, Zürich, Switzerland; reference 22), and the NH2 terminus of cyclophilin D (gift from Paolo Bernardi, University of Padova, Padova, Italy; reference 20), F1 ATPase (provided by P.V. Vignais, Centre National de la Recherche Scientifique, Grenoble, France; reference 1), Bcl-2 (specific for residues 20–34; Calbiochem Corp., La Jolla, CA), Bcl-XL (Ab-1; Calbiochem Corp.), Bad, Bag-1, or Bax (Santa Cruz Biotechnology, Santa Cruz, CA). In one series of experiments, supernatants (3.5 × 105 g, 60 min, 4°C) of liposomes were supplemented with bovine serum albumin (6 μg/ml), precipitated with 80% acetone (80%, overnight, 4°C, centrifugation: 1.4 × 103 60 min at 4°C), and examined for the release of cytochrome c. Two-dimensional electrophoresis was performed after standard protocols using a Pharmacia (Piscataway, NJ) ampholyte (1%, pH 3–10; reference 39).
Cytofluorometric Analysis of PTPC Liposomes.
10-μl aliquots (∼107) of liposomes were incubated during 15 min at RT in 125 mM sucrose + 10 mM Hepes (pH 7.4) supplemented with the indicated dose of PT inducers (Atr, CaCl2, diazenedicarboxylic acid bis 5N,N-dimethylamide [diamide], ter-butylhydroperoxide) and/or PT inhibitors (bongkrekic acid; cyclosporin A, N-methyl-Val-4-cyclosporin A [provided by Sandoz, Basel, Switzerland]; and monochlorobiman [Molecular Probes]). Alternatively, liposomes were incubated during 15 min at 37°C in the presence of the indicated dose of active or inhibitor-inactivated caspases. Diluted (1 ml) liposomes were incubated with 3,3′dihexylocarbocyanine iodide [DiOC6(3), 80 nM, 20–30 min at RT; Molecular Probes], followed by analysis of DiOC6(3) retention in a FACS®-Vantage cytofluorometer (Becton Dickinson, San José, CA). The forward scatter threshold was set at 30 (Amp 16) and the flow-rate at 1,500 events/s. The photomultiplyer of the side scatter and FL1 were set at 700 mV and 700–800 mV, respectively. The fluorescence was excited with an Argon laser (excitation wavelength 488 nm) and analyzed in FL-1 (wave length 530 ± 30 nm). The forward and side scatters were gated on the quantitatively most abundant population of liposomes while excluding background noise. Calibration with carboxylate microspheres (Fluoresbrite BB; Polyscience, Warrington, PA) of defined diameters was used to determine the diameter of liposomes that were gated on (gate: 150 to 300 nm; mean size of liposomes: 230 ± 60 nm; X ± SD for 5 × 104 events). Electron microscopy confirmed the presence of mostly unilamellar proteoliposomes of the expected size in the PTPC liposome preparation. Triplicates of 5 × 104 liposomes were analyzed for each data point. Results were expressed as percent of reduction of DiOC6(3) fluorescence (log scale, geometric mean), considering the reduction obtained with 0.25% SDS (15 min, RT) in PTPC liposomes as 100% value.
Evaluation of Caspase Effects on Isolated Mitochondria.
Purified mouse liver mitochondria were incubated in 10 mM Tris-MOPS + 100 mM NH4Cl + 10 μM EGTA (pH 7.2) during 30 min at RT in the presence of different caspases. The supernatant (1.5 × 105 g) of these mitochondria was stored at −80°C until testing for apoptogenic activity on isolated HeLa nuclei (90 min, 37°C, RT). DNA fragmentation was quantified by propidium iodine staining (10 μg/ml, ⩾5 min at RT) and cytofluorometric analysis in an EPICS Prolife II (Coulter, Hialeah, FL), as described (26). Results were expressed as the percentage of subdiploid nuclei, after subtraction of values obtained with buffer only (<20%). For control purposes, different caspase inhibitors (Ac-DEVD.cmk, Ac-YVAD.cmk, or Z-VAD.fmk (N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone); 100 μM final concentration) were added to the mitochondrial supernatant 15 min before determination of apoptogenic activity. Aliquots of caspase-treated mitochondria were resuspended in 400 mM mannitol, 50 mM Tris (HCl, pH 7.2), 5 mg/ml BSA, 10 mM KH2PO4, and 5 mM succinate, and then labeled with DiOC6(3) (100 nM, 15 min at RT) and subjected to cytofluorometric analysis using carbonylcyanide m-chlorophenylhydrazone (CCCP; 50 μM) or Atr (5 mM) as positive controls of maximum Δψm disruption.
Results
Reconstitution of the PTPC in Liposomes.
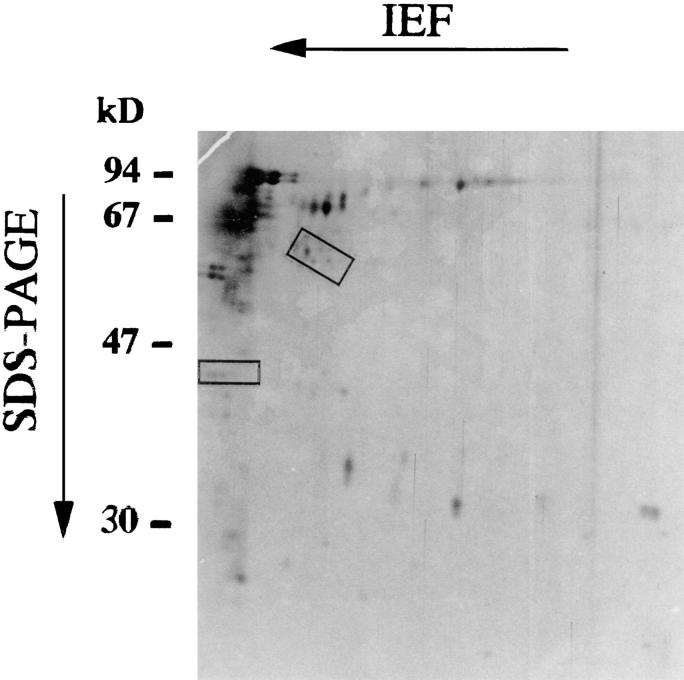
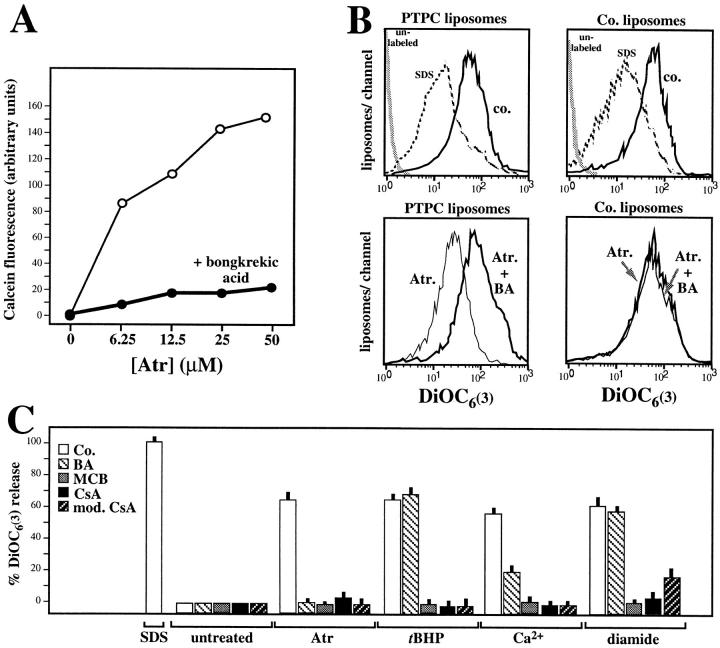
Hexokinase 1 is a cytosolic protein, part of which associates with the mitochondrial outer membrane where it binds to porin within the contact site (22, 40–42). Taking advantage of this fact, we traced the hexokinase activity copurifying with a protein complex that is water insoluble in brain homogenates, partitions into the triton-soluble fraction, elutes from an anion exchange FPLC column at a relatively high salinity, and incorporates into phosphatidylcholine/cholesterol liposomes (Fig. 1 A–C). When comparing the abundance of proteins extracted from liposomes incorporating hexokinase activity with that of the preceding purification steps, it appears that some proteins are selectively enriched (cyclophilin D, the ∼60-kD isoform of the ANT, Bax, Bag-1), whereas some are reduced (VDAC, F1 ATPase) or eliminated below the limit of detection (Bcl-XL, Bcl-2, Bad, cytochrome c, hsp60; Fig. 1 D). As shown by two-dimensional gel electrophoresis, PTPC liposomes contain a limited set of proteins whose complete identification is still in progress (Fig. 2). PTPC-containing liposomes can be treated with inducers of PT pore opening, which cause the release of encapsulated molecules such as malate (106 daltons) and ATP (509 daltons) (22, 43). Similarly, the fluorochrome calcein (622 daltons), a hydrophilic polyanionic fluorochrome previously used to measure PT pore opening in intact cells (44), can be encapsulated into PTPC liposomes and then released by incubation with the ANT ligand Atr, a potent inducer of PT pore opening (Fig. 3 A). This effect is prevented by another ANT ligand, bongkrekic acid, which inhibits PT pore opening (Fig. 3 A). We have developed another approach to quantify PT pore opening induced in PTPC liposomes. Liposomes were equilibrated with the amphophilic cationic fluorochrome DiOC6(3) (573 daltons). The retention of DiOC6(3) fluorescence was then monitored in a cytofluorometer (that is, in a flow in which liposomes are diluted and the external DiOC6(3) concentration approaches 0), whereas gating on a population of liposomes with defined forward and side scatter characteristics (estimated diameter: 0.15–0.3 μm). When using this approach, we found an Atr-induced shift in DiOC6(3) retention, suggesting that most if not all proteoliposomes found in this population contain a PT pore. PT pore opening does not provoke a change in the average size (forward scatter) of liposomes, nor does it affect their ultrastructure. A good correlation was found between the release of small molecules (ATP, malate, calcein) measured in bulk experiments and the DiOC6(3) release measured by cytofluorometry (Fig. 3; references 22, 43; and unpublished data). This suggests that all these molecules can be released through the PT pore, in accord with its reported molecular cut off of 1,500 daltons (12). The baseline DiOC6(3) incorporation was the same in liposomes containing functional PTPC (mean fluorescence channel 593 ± 53, X ± SEM, n = 3) as that observed in control liposomes (mean channel 601 ± 43) (Fig. 3 B), indicating that the PT pore is constitutively closed. PTPC-containing liposomes release DiOC6(3) in response to several agents that induce PT in intact mitochondria (Atr, ter-butylhydroperoxide, Ca2+, diamide), with inhibitory effects of bongkrekic acid, monochlorobiman, cyclosporin A, and N-methyl-Val-4-cyclosporin A, a nonimmunosuppressive cyclophilin D ligand not acting on calcineurin (Fig. 3 C). These findings emphasize the functional similarity between the natural (mitochondrial) PTPC (8, 12) and the reconstituted (liposomal) PTPC (Fig. 3). Moreover, they confirm that the ANT (target of bongkrekic acid and atractyloside), cyclophilin D (target of cyclosporin A and N-methyl-Val-4-cyclosporin), and redox-sensitive SH groups (target of diamide and monochlorobiman), as well as Ca2+-sensitive sites, participate in the regulation of the PTPC (12, 13, 20–23).
Figure 2.
Two-dimensional gel electrophoresis of proteins extracted from PTPC liposomes (Fig. 1 A, 4). Silver-stained proteins whose abundance is consistently (three experiments) reduced upon digestion with caspase 1 (1.5 U/ml) are marked in rectangles. Results are representative for three independent experiments.
Figure 3.
Function of reconstituted PTPC pores. (A) Release of calcein from PTPC-containing liposomes incubated with two antagonistic ANT ligands. Calcein-loaded PTPC liposomes were incubated with the indicated dose of Atr and/or bongkrekic acid (50 μM), followed by fluorometric determination of the calcein release into the supernatant. (B) Cytofluorometric profile of liposomes labeled with the potential-sensitive fluorochrome DiOC6(3). Liposomes were reconstituted either in the presence of the hexokinase-containing fraction (PTPC liposomes) or in its absence (control liposomes), treated with SDS (0.25%), Atr (25 μM), and/or bongkrekic acid (BA; 50 μM), followed by DiOC6(3) staining and cytofluorometric analysis. (C) PTPC liposomes treated with PT inducers (Atr [25 μM], CaCl2[25 μM], diamide [500 μM] or ter-butylhydroperoxide [tBHP, 500 μM]) and/or PT inhibitors (bongkrekic acid [BA; 50 μM], cyclosporin A [CsA; 10 μM], N-methyl-4-Val-CsA [mod. CsA; 10 μM], or monochlorobiman [MCB; 50 μM]). Results are expressed as percentage (X ± SD of triplicates) of the DiOC6(3) release induced by 0.25% SDS. Results are representative for at least three independent determinations.
Effect of Recombinant Bcl-2 and Bcl-XL on PTPC.
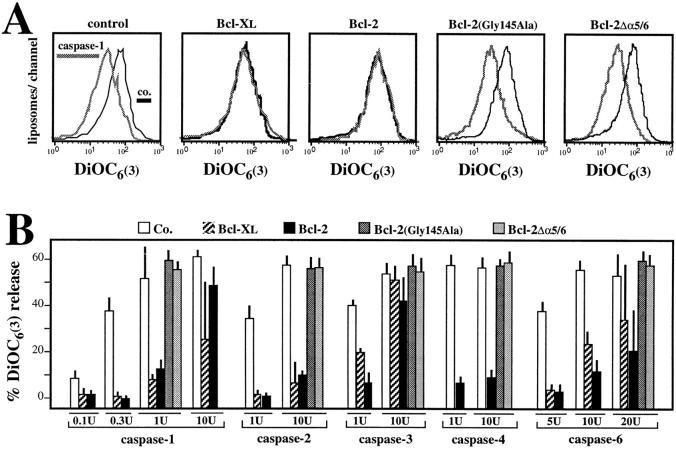
Under the conditions of fractionation described in Fig. 1, a proapoptotic member of the Bcl-2 family (Bax) selectively coenriches with components of PTPC, whereas several antiapoptotic members of the Bcl-2 family do not (Bcl-XL, Bcl-2; Fig. 1 D). We therefore investigated the effect of antiapoptotic members of the Bcl-2 family on PTPC. Recombinant Bcl-2 and Bcl-XL proteins, as well as mutant Bcl-2 proteins, were incorporated into liposomes together with PTPC via dialysis, a procedure that allows for the oriented, pH-independent incorporation of proteins into lipid membranes (45, 46). Irrespective of the presence of Bcl-2–like proteins, all liposome preparations consistently (n = 12) maintained a similar baseline DiOC6(3) fluorescence (mean fluorescence channel 620 ± 18 and 616 ± 19 in the presence or absence of Bcl-2, respectively, X ± SEM, 12 independent experiments), with comparable SDS-releasable DiOC6(3) release (Fig. 4 A) for as long as 8 h (not shown), suggesting that Bcl-2 does not augment the membrane permeability in this experimental system. Moreover, Bcl-2 does not perturb the ultrastructure of PTPC liposomes or their protein composition (not shown). The presence of Bcl-XL or Bcl-2 protected against the DiOC6(3) release induced by atractyloside, ter-butylhydroperoxide, as well as low doses of Ca2+, but not by diamide (Fig. 4 C). Similar results were obtained, when instead of DiOC6(3) retention, calcein efflux was studied (not shown). These effects correlate with the functional potency of Bcl-2, which protects cells against most PT inducers (8, 9, 47), but not against diamide (8, 9). A Bcl-2 deletion mutant lacking a putative channel-forming domain corresponding to the α5 and α6 helices, Bcl-2Δα5/6, which has lost its antiapoptotic function (34), failed to prevent the DiOC6(3) release. In addition, a Bcl-2 point mutant in the BH1 region, Bcl-2 (Gly145Ala), which does not interact with Bax, failed to protect against apoptosis (48) and had no inhibitory effect on PTPC liposomes (Fig. 4 C). Altogether, these findings suggest that Bcl-2 can regulate membrane permeability by acting on or in concert with PTPC.
Figure 4.
Effects of Bcl-2 on PTPC. Hexokinase-enriched fractions (Fig. 1 A, 3) were incorporated into liposomes by dialysis in the presence or absence of recombinant Bcl-2, Bcl-2 (Gly145Ala), Bcl-2Δα5/6, or Bcl-XL, followed by functional analysis. (A) Representative fluorescence profiles of control PTPC and Bcl-2 PTPC liposomes treated with buffer only (control), SDS, or Atr, followed by incubation with DiOC6(3). Note the absence of Atr effects in Bcl-2 PTPC liposomes. (B) Incorporation of native and mutant Bcl-2 proteins into liposomes. Proteins were extracted from PTPC liposomes prepared in the presence or absence of the indicated Bcl-2 mutant, followed by immunochemical quantitation of Bcl-2 with a monoclonal antibody that recognizes an epitope (residues 20-34) not affected by the mutations. (C) Functional impact of Bcl-2 and Bcl-XL. The different PTPC liposome preparations were treated with Atr (25 μM), CaCl2 (25 μM), diamide (500 μM), or ter-butylhydroperoxide (tBHP, 500 μM) to determine the DiOC6(3) release. Results are representative for three to five independent experiments. 100% DiOC6(3) release was defined as the SDS-induced reduction of DiOC6(3) fluorescence observed in PTPC liposomes generated in the absence of Bcl-2 or Bcl-XL.
Effect of Recombinant Caspases on PTPC.
Since caspases are involved at all stages of apoptosis (5, 26, 49–51), we tested whether caspases might act on PTPC. PTPC reconstituted in liposomes were exposed to recombinant caspases, followed by determination of the DiOC6(3) retention. Several caspases induced DiOC6(3) release in a dose-dependent fashion (Fig. 5, A and B). This effect was only obtained in PTPC-containing liposomes, but not in control liposomes (not shown). Tetrapeptide inhibitors of caspases (Ac-YVAD.cmk for caspases 1 and 4 and Ac-DEVD.cmk for caspases 2, 3, and 6) abolish caspase-induced DiOC6(3) release, suggesting that this effect involves proteolysis rather than nonenzymatic protein interactions (Fig. 5, A and B). Accordingly, two-dimensional gel electrophoresis of proteins extracted from PTPC liposomes suggest several unidentified proteins to be caspase 1 substrates (Fig. 2). The same caspases that release DiOC6(3) from PTPC liposomes also disrupt the Δψm in isolated liver mitochondria (Fig. 5 C) and release AIF, which causes isolated nuclei to undergo DNA fragmentation (Fig. 5 D). Bcl-2 and Bcl-XL incorporated into liposomes reduce the caspase-induced DiOC6(3) release, whereas inactive Bcl-2 mutants (Bcl-2Δα5/6 and Bcl-2(Gly145Ala) fail to stabilize PTPC (Fig. 6, A and B). This Bcl-2 effect can be at least partially overcome by high caspase concentrations. Thus, in addition to stabilizing PTPC liposomes exposed to Atr, ter-butylhydroperoxide and calcium (Fig. 4), Bcl-2, and Bcl-XL partially suppress caspase-induced DiOC6(3) release (Fig. 6).
Figure 5.
Effect of caspases on PTPC liposomes and isolated mitochondria. (A) Representative DiOC6(3) fluorescence histograms obtained after treatment of liposomes with various caspases (1.2 U/ml for caspase 1, 10 U/ml for caspase 6) in the presence or absence of the indicated caspase inhibitor (100 μM). (B) Dose dependency of effects obtained with different recombinant caspases on PTPC liposomes. (C) Effect of caspases on the Δψm. Mitochondria were treated during 30 min with 5 U caspase/200 μl, followed by determination of the Δψm using DiOC6(3). The protonophore m-chlorophenylhydrazone (50 μM) defined 100% Δψm disruption. (D) Release of AIF into the mitochondrial supernatant. Intact mitochondria were treated with the indicated caspase (5 U/200 μl), followed by centrifugation and removal of the supernatant that was tested for apoptogenic activity on isolated HeLa nuclei. The incubation was performed in the presence of tetrapeptide inhibitors (which inhibit caspases but not AIF) or in the presence of Z-VAD.fmk (which inhibits AIF) to exclude that nuclear DNA degradation is a direct caspase effect. Similar results were obtained with mouse and rat (not shown) hepatocyte mitochondria.
Figure 6.
Effect of Bcl-2 on the caspase induced DiOC6(3) release observed in PTPC liposomes. (A) Representative DiOC6(3) staining profiles. Liposomes were generated in the presence of recombinant Bcl-2, Bcl-XL, and the indicated Bcl-2 mutants, treated with 1 U caspase 1, and labeled with DiOC6(3) to determine the DiOC6(3) release. Results are representative for at least three independent determinations. (B) Dose response curves of caspase effects on liposomes containing Bcl-XL, Bcl-2, or Bcl-2 mutants.
Failure of PTPC to Release Cytochrome c.
Since induction of PTPC in intact mitochondria causes cytochrome c release (14, 15; and unpublished data), and since several groups have suggested that Bcl-2 primarily regulates the release of cytochrome c via the outer mitochondrial membrane rather than PT (6, 7, 31, 52), we investigated the putative relationship between PT pore opening and cytochrome c. Incorporation of purified cytochrome c into PTPC liposomes (which constitutively are devoid of cytochrome c, Fig. 1 D) does not alter their functional behavior. Thus, PTPC liposomes containing cytochrome c exhibit a normal baseline level of DiOC6(3) retention and release DiOC6(3) in response to Atr and caspases in a Bcl-2–inhibitable fashion (Fig. 7 A). Although such liposomes contain significant amounts of SDS-releasable cytochrome c, they fully retain cytochrome c when incubated with doses of Atr or caspase that cause DiOC6(3) release (Fig. 7 B). This indicates that PTPC are not directly responsible for the release of cytochrome c.
Figure 7.
Cytochrome c retention in PTPC liposomes. Liposomes were generated in the absence or presence of recombinant Bcl-2, followed by generation of a KCl-dependent ion gradient and incorporation of cytochrome c during the sonication step. (A) Effect of SDS (0.25%), Atr (50 μM), or caspases 1 or 3 (1 U), as determined by flow cytometry after labeling with DiOC6(3). (B) Supernatants of the liposomes treated as in A were subjected to protein precipitation, followed by Western blot analysis of the release of cytochrome c. Note that the blot has been overexposed. The amount of cytochrome c released upon SDS treatment was estimated to be 1 μg, and the detection limit of the immunoblot is ∼10 ng/lane.
Discussion
Functional Equivalence of Natural and Reconstituted PTPC: A Target of Multiple Effectors Including Caspases.
PTPCs are formed at the mitochondrial inner/outer membrane contact site where they function as a Ca2+-, voltage-, pH-, and redox-gated channel with several levels of conductance (12, 13, 19). In this work, we report the functional analysis of PTPC enriched from brain homogenates and reconstituted in liposomes. Although the exact molecular composition of PTPC remains to be defined, the functional exploration of PTPC (Fig. 3) suggests that it does contain functionally interconnected sites of interaction with bongkrekic acid and Atr (two ligands of the ANT and perhaps other members of the mitochondrial carrier family), cytochrome c and N-methyl-4-Val cytochrome c (two ligands of the cyclophilin D), diamide, and monochlorobimane (which act on thiol residues), as well as Ca2+. Accordingly, we detected the ANT, cyclophilin D, and additional molecules previously suggested to associate with the ANT, namely porin and hexokinase, in the PTPC (Fig. 1). In addition to these molecules, PTPCs purify with Bax, Bag-1, F1-ATPase (Fig. 1 D), and several nonidentified proteins (Fig. 2) whose impact on PTPC remains unclear. However, the functional data indicate that PTPC liposomes regulate membrane permeability in a fashion that resembles the PT pore found in mitochondria. Thus, using a number of different inducers and inhibitors of PT, we found an approximate functional equivalence between the natural (mitochondrial) PTPC and the reconstituted (liposomal) PTPC (Fig. 3) in the regulation of membrane permeability. Both in mitochondria and in PTPC liposomes, a similar panel of agents acts to permeabilize membranes (Ca2+, Atr, prooxidants, and diamide) or to stabilize membrane function (cyclosporin A, monochlorobiman, and bongkrekic acid; references 8, 12, Fig. 3). Thus, the protocol for PTPC enrichment and incorporation into liposomes yields a reduced experimental system in which their function can be analyzed without interference by other mitochondrial structures.
The equivalence between the natural and the reconstituted PTPC also extends to the fact that caspases disrupt the membrane permeability in both PTPC liposomes (Fig. 5, A and B) and intact mitochondria (Fig. 5, C and D). This suggests, in line with previous observations (5, 26, 30, 49–51), that caspases act as facultative inducers of PT (e.g., caspase-1 activated after Fas/APO-1 cross-linking and perhaps caspase 3 in neuronal development) in specific signal transduction pathways. The molecular target(s) of caspases within the PTPC remain(s) to be defined. Of note, caspases are not only involved in the upstream premitochondrial phase, but also in the downstream postmitochondrial stage of apoptosis, when they are activated as a result of mitochondrial cytochrome c and AIF release (5–7, 26). Thus, mitochondria and caspases may engage in a positive amplification loop in which caspases cause mitochondrial membrane disruption, which in turn favors the release of caspase-activating factors.
Bcl-2–related Proteins Act on PTPC.
The data reported in this paper indicate that Bcl-2 and Bcl-XL regulate PT by directly acting on PTPC. It has been suggested that Bcl-2 and Bcl-XL would primarily act on the mitochondrial release of cytochrome c (6, 7, 52), which would be an event upstream of (6, 31) or independent from (7) PT pore opening. However, the PTPC reconstituted into liposomes do not contain cytochrome c (Fig. 1, B and D), yet are regulated by Bcl-2 and Bcl-XL, implying that Bcl-2/Bcl-XL affect certain mitochondrial functions in a cytochrome c–independent fashion. As shown in this work, the PTPC is not the structure responsible for cytochrome c release (Fig. 7), in line with previous estimations suggesting that the PTPC has a molecular cut off of ∼1,500 daltons (12, 13). Two speculative possibilities remain plausible. First, the primary regulatory target of Bcl2/BclXL in the mitochondrion could be the PTPC that, once opened, causes cytochrome c release in an indirect fashion, either by activating a yet unknown cytochrome c–specific transporter or by mechanically disrupting the integrity of the outer mitochondrial membrane, e.g., due to local distension of the mitochondrial matrix (12–14, 53). Second, Bcl2/BclXL might affect PTPC and cytochrome c independently from each other in a pleiotropic fashion. In favor of this latter hypothesis, BclXL has been reported to bind to cytochrome c (52), and Bcl-2 might interact with cytochrome c via the mammalian CED4 homologue (54).
Recombinant Bcl-2 and Bcl-XL incorporated into PTPC liposomes inhibit the induction of PT by a variety of inducers: the ANT ligand atractyloside, the prooxidant ter-butylhydroperoxide, Ca2+ (Fig. 4), and low doses of caspases (Fig. 6). In contrast, Bcl-2 and Bcl-XL fail to protect PTPC liposomes against diamide (Fig. 4 C), in line with the fact that Bcl-2 is an inefficient inhibitor of diamide-induced Δψm disruption, both in cells and in isolated mitochondria (8, 9). Moreover, Bcl-2 fails to prevent the effects of high doses of caspases (Fig. 6), in accord with our previous observation that Bcl-2 present in mitochondria from human CEM-C7 T lymphoma cells fails to counteract caspase 1–induced PT and apoptosis (26). The finding that these Bcl-2 and Bcl-XL effects can be overcome by high, but not by low, doses of caspses may resolve a controversy opposing models in which Bcl-2 homologues completely fail to prevent Fas/APO-1 (caspase 1–dependent) apoptosis (26, 55–58) or, on the contrary, efficiently counteract caspase 1–mediated (59) or Fas/APO-1–triggered apoptosis (30, 60). Moreover, the fact that Bcl-2 mitigates the PT induced by caspases that are broadly involved in apoptosis (e.g., caspases 3 and 6) suggests that it can interrupt a self-amplifying loop in which caspase effects on mitochondria favor the release of caspase activators. We have investigated whether Bcl-2 acts as an inhibitor of caspase-mediated digestion of PTPC proteins. Our preliminary findings indicate that Bcl-2 does not prevent the digestion of caspase 1 substrates (not shown), suggesting that it inhibits the functional consequence of caspase 1–mediated proteolysis rather than proteolysis itself. It has been shown recently that caspase 3 cleaves Bcl-2, thereby converting it from a death inhibitor to a death promoter (61). However, caspase 1 does not digest Bcl-2, at least in the conditions reported in Fig. 6 A, suggesting the functional relevance of additional caspase targets within the PTPC.
Crystallographic data (62) and studies of artificial membranes containing Bcl-XL or Bcl-2 (33, 34) suggest that Bcl-2–like proteins constitute ion channels. However, Bcl-XL and Bcl-2 incorporated into membranes containing PTPC, rather than increasing membrane permeability, stabilize PTPC liposomes and prevent PT pore opening. This apparent discrepancy may be explained by the composition of the artificial membranes, which only allow Bcl-2 to form channels when they contain, in addition to neutral lipids (as in this paper), an unusually high percentage (30– 40%) of acidic lipids (33, 34). At present, we cannot discriminate between the possibilities that the PT-inhibitory effect of Bcl-2 is due to interactions with and conformational effects on PTPC constituents, or rather due to the specific neutralization of Bax (35, 48), a proapoptotic molecule that is present in PTPC (Fig. 1 D) and favors PT (32). Irrespective of these possibilities, the Bcl-2 effect on PTPC correlates with its antiapoptotic potential in the sense that mutations or deletions abolishing the death antagonistic potential of Bcl-2 also abrogate its PT-inhibitory function.
In addition to its PT-inhibitory effect, which may account for at least part of its cytoprotective action, Bcl-2 has further pleiotropic effects (4, 10). Although some of these effects, including those concerning the capacity of Bcl-2 to affect redox regulation or intracellular Ca2+ partition, may be secondary to PT modulation; others are more difficult to accommodate in a model in which the major action of Bcl-2 would be PT regulation. This applies, in particular, to the participation of Bcl-2 participation in a multiprotein ensemble or “apoptosome” involving the mammalian CED-4 homologue(s), cytochrome c, and large prodomain caspases. As a possibility, Bcl-2 could exert a dual function in which it simultaneously or sequentially acts on PTPC and inactivates the apoptosome (10).
The Central Executioner of Apoptosis: Involvement of PTPC?
Changes in mitochondrial membrane function have been proposed to form part of the “central executioner” (63), colloquially also referred to as “great integrator” or “apostat” (2, 3, 6–8, 11). Activation of the central executioner during the effector stage would control the commitment to undergo cell death and unify the many private induction pathways of apoptosis into one common pathway. The findings reported herein indicate that PTPC can constitute a crossroad at which physiological modulators of PT (Ca2+, Mg2+, pH, ADP, ATP, NAD(P)H, glutathione, ceramide, lipid oxidation products, etc.; references 12, 13, 19; Fig. 3), caspases (Fig. 5), and Bcl-2 homologues (Fig. 4, 6) together influence the fate of the cell. Thus, PTPC may simultaneously collect information on the metabolic stage of the cell, signal transduction pathways, as well as on the composition of the Bcl-2 complex. Opening of the PT pore, which occurs almost universally during apoptosis, has lethal repercussions including the mitochondrial generation of reactive oxygen species, disruption of oxidative phosphorylation, and the mitochondrial release of apoptogenic proteins necessary for the activation of downstream caspases and endonuclease activation (1–10, 14, 15).
In conclusion, PTPC may be identical with or form part of the critical structure that integrates different apoptosis induction pathways, decides the fate of the cell, and coordinates the common death program. If this interpretation is correct, the future elucidation of the exact composition and fine tuning of PTPC should furnish invaluable clues to the understanding of the apoptotic process.
Acknowledgments
We thank Drs. A. Srinivasen and K. Tomaselli (Idun Pharmaceuticals, La Jolla, CA) for recombinant caspases 1, 3, and 6; N. Thornberry (Merck, Rahway, NJ) for caspases 1, 2, and 4; G. Salvesen (The Burnham Institute, La Jolla, CA) for caspases 3 and 6; Drs. S. Matsujama, C. Aimé-Sempé, and S. Takajama (The Burnham Institute) for Bcl-2 plasmid constructions. Electron microscopic analyses of PTPC liposomes were performed by Marie-Christine Prévost (Pasteur Institute, Paris, France).
This work has been supported by grants from the Agence Nationale pour la Recherche contre le SIDA, Association pour la Recherche contre le Cancer, Centre National de la Recherche Scientifique, Fondation pour la Recherche Médicale, Institut National de la Santé et de la Recherche Médicale, Ligue Nationale contre le Cancer (to G. Kroemer), University of California Breast Cancer Research Program (grant No. IRB-009B) and CaP-CURE Inc. (to J.C. Reed). I. Marzo and S.A. Susin receive fellowships from the Spanish Ministry of Science and from the European Commission, respectively.
Footnotes
Abbreviations used in this paper: Δψm, mitochondrial transmembrane potential; Ac-DEVD.cmk, acetyl-Asp-Glu-Val-Asp-chloromethylketone; A-YVAD.cmk, acetyl-Tyr-Val-Ala-Asp-chloromethylketone; AIF, apoptosis-inducing factor; ANT, adenine nucleotide translocator; Atr, atractyloside; diamide, diazenedicarboxylic acid bis 5N,N-dimethylamide; DiOC6(3), 3,3′dihexyloxacarbocyanine iodide; PT, permeability transition; PTPC, PT pore complex; RT, room temperature; VDAC, voltage-dependent anion channel; Z-VAD.fmk, N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone.
I. Marzo and C. Brenner contributed equally to this work.
References
- 1.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssière J-L, Petit PX, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 4.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. . Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 7.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 8.Zamzami N, Susin SA, Marchetti P, Hirsch T, Gómez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1342. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 11.Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Haeffner A, Hirsch F, Geuskens M, Kroemer G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoratti M, Szabò I. The mitochondrial permeability transition. Biochim Biophys Acta Rev Biomembranes. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 13.Bernardi P, Petronilli V. The permeability transition pore as a mitochondrial calcium release channel; a critical appraisal. J Bioenerg Biomembr. 1996;28:129–136. doi: 10.1007/BF02110643. [DOI] [PubMed] [Google Scholar]
- 14.Kantrow SP, Piantadosi CA. Release of cytochrome cfrom liver mitochondria during permeability transition. Biochem Biophys Res Commun. 1997;232:669–671. doi: 10.1006/bbrc.1997.6353. [DOI] [PubMed] [Google Scholar]
- 15.Ellerby HM, Martin SJ, Ellerby LM, Naiem SS, Rabizadeh S, Salvese GS, Casiano CA, Cashman NR, Green DR, Bredesen DE. Establishment of a cell-free system of neuronal apoptosis: comparison of premitochondrial, mitochondrial, and postmitochondrial phases. J Neurosci. 1997;17:6165–6178. [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths EJ, Halestrup AP. Protection by cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 17.Trost LC, Lemasters JJ. The mitochondrial permeability transition: a new pathophysioligal mechanism for Reye's syndrome and toxic liver injury. J Pharmacol Exp Ther. 1996;278:1000–1005. [PubMed] [Google Scholar]
- 18.Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichas F, Jouavill LS, Mazat J-P. Mitochondria are excitable organelles capable of generating and conveying electric and calcium currents. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 20.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with mitochondrial inner membrane and regulation of the permeability transition pore, a cyclosporin A–sensitive channel. J Biol Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 21.Brustovetsky N, Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ Biochemistry. 1996;35:8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]
- 22.Beutner G, Rück A, Riede B, Welte W, Brdiczka D. Complexes between kinases, mitochondrial porin, and adenylate translocator in rat brain resemble the permeability transition pore. FEBS Lett. 1996;396:189–195. doi: 10.1016/0014-5793(96)01092-7. [DOI] [PubMed] [Google Scholar]
- 23.Halestrup AP, Woodfield K-Y, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocator. J Biol Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 24.White RJ, Reynolds IJ. Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastorino JG, Simbula G, Yamamoto K, Glascott PAJ, Rothman RJ, Farber JL. The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem. 1996;271:29792–29799. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]
- 26.Susin SA, Zamzami N, Castedo M, Daugas E, Wang H-G, Geley S, Fassy F, Reed J, Kroemer G. The central executioner of apoptosis. Multiple links between protease activation and mitochondria in Fas/Apo-1/CD95– and ceramide–induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hortelano S, Dallaporta B, Zamzami N, Hirsch T, Susin SA, Marzo I, Bosca L, Kroemer G. Nitric oxide induces apoptosis via triggering mitochondrial permeability transition. FEBS Lett. 1997;410:373–377. doi: 10.1016/s0014-5793(97)00623-6. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y. Bcl-2 blocks loss of mitochondrial membrane potential while ICE inhibitors act at a different step during inhibition of death induced by respiratory chain inhibitors. Oncogene. 1996;13:21–29. [PubMed] [Google Scholar]
- 29.Decaudin D, Geley S, Hirsch T, Castedo M, Marchetti P, Macho A, Kofler R, Kroemer G. Bcl-2 and Bcl-XL antagonize the mitochondrial dysfunction preceding nuclear apoptosis induced by chemotherapeutic agents. Cancer Res. 1997;57:62–67. [PubMed] [Google Scholar]
- 30.Boise LH, Thompson CB. Bcl-XL can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Proc Natl Acad Sci USA. 1997;94:3759–3764. doi: 10.1073/pnas.94.8.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CN, Wang XD, Huang Y, Ibrado AM, Liu L, Fang GF, Bhalla K. Overexpression of Bcl-x(L), inhibits Ara-C–induced mitochondrial loss of cytochrome cand other perturbations that activate the molecular cascade of apoptosis. Cancer Res. 1997;57:3115–3120. [PubMed] [Google Scholar]
- 32.Xiang J, Chao DT, Korsmeyer SJ. Bax-induced cell death may not require interleukin 1beta-converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minn AJ, Vélez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB. Bcl-XL forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 34.Schendel S, Xie Z, Montal MO, Matsuyama S, Montal M, Reed JC. Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi M, Bernard A, Mermod J-J, Mazzei G, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–376. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 36.Mittl PRE, Dimarco S, Krebs JF, Bai X, Karanewsky DS, Priestle JP, Tomaselli KJ, Grutter MG. Structure of recombinant human CPP32 in complex with the tetrapeptide Acetyl-Asp-Val-Ala-Asp fluoromethyl ketone. J Biol Chem. 1997;272:6539–6547. doi: 10.1074/jbc.272.10.6539. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes-Alnemri T, Armstrong RC, Krebs J, Srinivasula SM, Wang L, Bullrich F, Fritz LC, Trapani JA, Tomaselli KJ, Litwack G, Alnemri ES. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folch J, Lees M, Stanley GMS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:447–506. [PubMed] [Google Scholar]
- 39.Colas des Francs-Small C, Ambard-Bretteville F, Darpas A, Sallantin M, Huet J-C, Pernollet J-C, Rémy R. Variation of the polypeptide composition of mitochondria isolated from different potato tissues. Plant Physiol (Lond) 1992;98:273–278. doi: 10.1104/pp.98.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knull HR, Taylor WF, Wells WW. Effects of energy metabolism on in vivo distribution of hexokinase in brain. J Biol Chem. 1973;248:5414–5417. [PubMed] [Google Scholar]
- 41.Arora KK, Parry DM, Pedersen PL. Hexokinase receptors: preferential enzyme binding in normal cells to nonmitochondrial sites and in transformed cells to mitochondrial sites. J Bioenerg Biomembr. 1992;24:47–53. doi: 10.1007/BF00769530. [DOI] [PubMed] [Google Scholar]
- 42.Gelb B, Adams V, Jones S, Griffin L, MacGregor G, McCabe E. Targeting of hexokinase 1 to liver and hepatoma mitochondria. Proc Natl Acad Sci USA. 1992;89:202–206. doi: 10.1073/pnas.89.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997;414:253–257. doi: 10.1016/s0014-5793(97)01045-4. [DOI] [PubMed] [Google Scholar]
- 44.Nieminen AL, Byrne AM, Herman B, Lemasters JI. Mitochondrial permeability transition induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am J Physiol. 1997;41:C1286–C1294. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- 45.Rigaud JL, Paternostre MT, Bluzat A. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 2. Incorporation of the light-driven proton pump bacteriorhodopsin. Biochemistry. 1988;27:2677–2688. doi: 10.1021/bi00408a007. [DOI] [PubMed] [Google Scholar]
- 46.New, R.R.C. 1990. Peparation of liposomes. In Liposomes: a Practical Approach. R.R.C. New, editor. Oxford University Press, Oxford. 33–104.
- 47.Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci USA. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ying XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 49.Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 50.Kuida K, Zheng TS, Na S, Kyan C-Y, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 51.Fraser A, Evan G. A license to kill. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 52.Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yan Z-M, Saxena S, Weichselbaum R, et al. Role for Bcl-XL as an inhibitor of cytosolic cytochrome c accumulation in DNA damage-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.vander Heiden MG, Chandal NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-XL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 54.Zhou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegansCed-4, participates in cytochrome c–dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 55.Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO (Eur Mol Biol Organ) J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiu VK, Walsh CM, Chau-Ching L, Reed JC, Clark WR. Bcl-2 blocks degranulation but not Fas-based cell-mediated cytotoxicity. J Immunol. 1995;154:2023–2029. [PubMed] [Google Scholar]
- 57.Memon SA, Moreno MB, Petrak D, Zacharchuk CM. Bcl-2 blocks glucocorticoid- but not Fas- or activation-induced apoptosis in a T cell hybridoma. J Immunol. 1995;155:4644–4652. [PubMed] [Google Scholar]
- 58.Huang DCS, Cory S, Strasser A. Bcl-2, Bcl-XL, and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene. 1997;14:405–414. doi: 10.1038/sj.onc.1200848. [DOI] [PubMed] [Google Scholar]
- 59.Miura M, Zhu H, Rotello R, Hartwieg EA, Yan Y. Induction of apoptosis in fibroblasts by IL-1β–converting enzyme, a mammalian homolog of the C. eleganscell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 60.Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, et al. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 61.Cheng EHY, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 62.Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong S-L, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 63.Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? . Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]