Abstract
Alternative mRNA splicing is a rich source of transcript diversity in eukaryotic cells with broad roles in development and disease. Systems-wide experimental methods have started to define how global splicing regulation shapes complex biological properties and pathways. Here, we review these approaches, describe recent insights they have yielded, and discuss avenues of future investigation.
Keywords: mRNA splicing, systems biology, genome-wide, alternative splicing regulation, microarrays, RNA network
INTRODUCTION
Alternative splicing (AS) vastly augments the coding potential of complex genomes by allowing single genetic loci to encode multiple transcripts with different protein coding sequences and RNA regulatory elements. Roughly 75% of human genes are thought to encode two or more splice isoforms, with striking variation across tissue types and developmental stages (Johnson et al. 2003). Moreover, many human diseases arise from deficient splicing of crucial transcripts, or are marked by the appearance of aberrant splice isoforms in affected tissues (Wang and Cooper 2007).
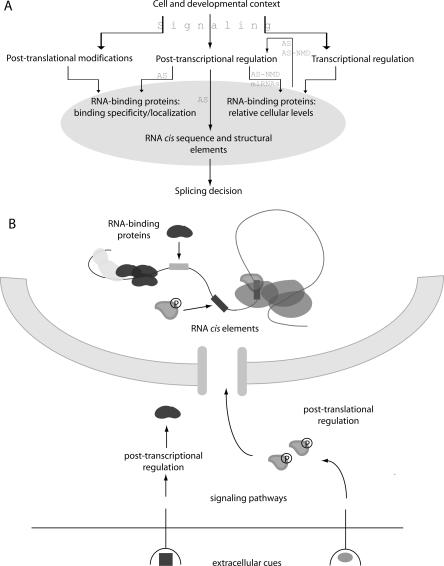
The mechanism and regulation of splicing have been informed mainly by genetic and biochemical studies in model organisms and human cells focusing on individual factors and events (Black 2003). Collectively these studies suggest that the use and/or suppression of splice sites are determined by combinatorial binding of RNA-binding proteins (RNABPs) to nearby regulatory elements. Deciphering this “splicing code” has proven a major experimental and bioinformatic challenge, which is further complicated by the many post-transcriptional and post-translational mechanisms that regulate RNABP expression and cellular localization (Fig. 1). These mechanisms are in turn responsive to signaling pathways and tissue-specific cues. With this hierarchical regulatory network in mind, efforts to characterize alternative splicing on a global scale have proceeded in several directions: (1) producing comprehensive catalogs of splice variants in different organisms and cell types, (2) defining cis regulatory elements that collectively comprise the splicing code, (3) identifying transcript networks that are regulated by individual trans-acting splicing factors, (4) elucidating the functional integration of splicing with transcription and mRNP surveillance and export, and (5) characterizing the response of splicing to signaling pathways, differentiation, and disease states. Many reviews have recounted various aspects of this rich source of biological diversity and regulation (Maniatis and Reed 2002; Matlin et al. 2005; Blencowe 2006; Keene 2007; Wang and Cooper 2007). Here, we focus on experimental methodologies that provide global views of splicing regulation and describe recent insights they have yielded.
FIGURE 1.
A hierarchy of splicing regulation. (A) Splicing patterns are a composite output of transcript-encoded cis elements and combinatorial binding of RNABPs to those elements. Shown are various upstream regulatory mechanisms that control the balance of nuclear RNABPs that regulate splicing decisions. These mechanisms are in turn responsive to signaling pathways that relay context-specific instructions. (B) The cellular organization of some regulatory mechanisms is shown. Signaling pathways affect levels, localization, and binding affinities of RNABPs via post-transcriptional mechanisms and post-translational modifications, including phosphorylation (P). Splicing patterns are determined by combinatorial binding of RNABPs to transcript-encoded cis elements (gray boxes).
EXPRESSION PROFILING
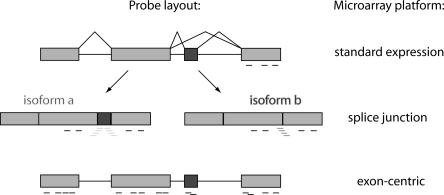
Microarrays permit the simultaneous measurement of all nucleic acids present in a biological sample, subject only to the technical constraint of how many sequences can be represented on a single chip (Stoughton 2005). Traditional expression profiling arrays are spotted with expressed sequence tag (EST)-derived cDNAs or 3′-clustered oligonucleotide sequences that probe total transcript abundance, but cannot distinguish between different splice isoforms (Fig. 2). However, several custom and commercial microarray platforms have been developed to measure variations in splicing. In general, microarrays with higher probe coverage have superior data resolution, but are more expensive and difficult to analyze due to sheer size of the data sets. Three microarray designs with various benefits and drawbacks for the large-scale analysis of splicing are described here: splice junction arrays, exon arrays, and tiled genomic arrays.
FIGURE 2.
Microarray analysis of alternative splicing. Probe topographies for different array designs are schematized for a hypothetical transcript with one alternative (dark gray) and three constitutive (light gray) exons. Black probes are located wholly within exons, light gray and dark gray probes span exclusive exon junctions in isoforms a and b, respectively.
SPLICE JUNCTION MICROARRAYS
“Splice junction” arrays bear oligonucleotide probes spanning annotated exon–exon junctions exclusive to individual splice isoforms (Johnson et al. 2003). Junction probes are position-constrained and thus subject to noise arising from nonideal sequence content. Thus, most versions of these arrays include probes within constitutive exons so that transcripts can first be scored as present or absent from the sample, then assessed for AS based on junction probes. Splice junction arrays have proven valuable in functional classification of RNA processing mutants in yeast (Clark et al. 2002; Burckin et al. 2005) and definition of tissue-specific splicing patterns and regulatory motifs in mammalian cells (Johnson et al. 2003; Pan et al. 2004; Sugnet et al. 2006; Fagnani et al. 2007). The latter studies have produced the recurring observation that transcripts regulated at the level of alternative splicing comprise a distinct population from those regulated at the level of transcript abundance. This finding suggests that regulatory mechanisms governing transcript diversity and transcript abundance can evolve separately to influence distinct properties of a given cell type. Several studies have extended these observations to contexts of differentiation and disease. Junction profiling following T-cell activation identified many transcripts showing changes in total abundance, with functional enrichment for immune defense and cytoskeleton dynamics; however, transcripts undergoing changes in AS were enriched for cell-cycle functions (Ip et al. 2007). Similarly, many transcripts in prostate and breast cancer cells showed altered splice isoform levels relative to normal tissue without significant changes in total transcript abundance, notably the mRNA for cell adhesion molecule and metastatic effector CD44 (Li et al. 2006a,b). In addition, global patterns of splice isoform expression in various Hodgkin lymphoma tumors clustered according to how far the tumors had progressed (Relógio et al. 2005). These and related studies suggest that splice variant profiling may augment existing tools for tumor diagnosis.
Splice junction profiling upon removal of individual factors has revealed functionally coherent transcript networks controlled at the level of splicing. Individual knockdown of four broad-specificity RNABPs in Drosophila cells identified distinct sets of AS events regulated by each factor (Blanchette et al. 2005). In mouse, a subset of brain-specific exons regulated by the neuronal RNABP NOVA2 was discovered by comparing brain-derived transcript profiles of NOVA2-knockout to wild-type animals. Transcripts containing these exons encoded many synapse-specific factors, illuminating a clear biological function for this splicing network in synapse formation (Ule et al. 2005). Knockdown of splicing regulator PTBP1 and/or its neuronal paralog nPTB in mouse neuroblastoma cells identified exon sets that are differentially regulated by these proteins (Boutz et al. 2007). Since neuronal precursors express PTBP1, while differentiated neurons express nPTB, these data identified a “post-transcriptional switch” that reprograms AS during neuronal development.
Several studies have probed connections between AS and nonsense-mediated decay (AS–NMD) in light of estimates that some 35% of annotated alternative splicing events introduce premature termination codons (PTCs) (Green et al. 2003). Surprisingly, blocking NMD identified few changes in splice isoform levels in junction array profiles, suggesting that AS–NMD is confined to a small subset of AS events (Pan et al. 2006; Ni et al. 2007). However, transcripts encoding splicing regulators were highly enriched for AS–NMD regulation, with the cassette exons frequently overlapping “ultraconserved” DNA regions (Ni et al. 2007). These observations suggest a rigorously conserved post-transcriptional mechanism for controlling levels of key splicing regulators in mammals, with potential cross- and auto-regulatory organization.
EXON ARRAYS
An alternative array strategy uses “exon-centric” probe (Fig. 2) sets consisting of multiple oligonucleotide probes within every annotated and predicted exon in the human, mouse, and rat genomes (GeneChip Exon Arrays, Affymetrix; Xing et al. 2006; Okoniewski et al. 2007b). In contrast to junction arrays, which probe only a predetermined set of alternative splicing events, “exon arrays” provide an unbiased profile of transcript architecture, permitting discovery of novel variations in splicing. Expression analysis of 16 human tissues with these arrays identified numerous tissue-specific exons and indicated that testis and brain show the highest level of alternative exon use among tissues examined (Clark et al. 2007). Time-course analysis of stimulus-induced exon use in a human cell culture model for neuron depolarization identified novel instances of alternative splicing in functionally relevant transcripts such as the voltage-gated potassium channel KCNH4 and proton pump subunit ATP6V o a4 (McKee et al. 2007). In agreement with previous genome-wide surveys, the authors identified distinct networks of regulation at the level of transcript abundance and alternative splicing showing dynamic changes over the course of measurements. Some functional groups, such as calcium ion binding, were regulated at both levels, while others showed preference for one form of regulation. Interestingly, transcripts encoding splicing regulators were significantly enriched for exon use regulation, but not transcript abundance, indicating possible auto-regulatory organization at the level of splicing. Profiling of human gliomas (French et al. 2007) and colon cancer cells (Gardina et al. 2006) identified exons that were differentially spliced in tumors relative to normal cells, suggesting these arrays will be useful in the de novo discovery of aberrant splicing events in diseased tissues. In general authors agreed that the Exon Array platform provided high quality transcript abundance data that benefited from high probe resolution (Kapur et al. 2007; Okoniewski et al. 2007a). Success in tracking individual exons was more variable, probably because individual exon probe sets may be constrained to nonideal sequences. RT-PCR validation rates for changes in individual exons ranged from 33% to 86% in the studies outlined here, and fluctuated somewhat based on filtering criteria. Thus, these arrays are capable of identifying novel regulated splicing, but experimental validation is necessary to infer true biological function.
TILED GENOMIC ARRAYS
Tiled oligonucleotide arrays spanning whole chromosomes or genomes provide comprehensive coverage and obviate the need for prior knowledge of exon coordinates. This probe topography is advantageous because it can detect patterns of alternative splicing, such as altered splice site use, intron retention, or use of nonannotated exons, which are difficult or impossible to detect on platforms limited to a defined set of annotated exons. The added expense and substantial computational challenges of analyzing tiled array data sets outweigh potential benefits in many experimental applications. However, some initial profiling efforts suggest tiled arrays can reveal transcript architecture that sequencing efforts and other microarray platforms have missed. Analysis of the Saccharomyces cerevisiae transcriptome on tiled arrays identified previously non-annotated introns and examples of regulated intron retention (Juneau et al. 2007; Zhang et al. 2007). A survey of human transcripts from 12 tissues on tiled ENCODE arrays spanning roughly 1% of the human genome found inclusion of non-annotated sequences for an astonishing 81.5% of the 399 protein-coding transcripts represented on the array, with 20% of novel variants having altered open reading frames (Denoeud et al. 2007). Use of distal 5′ transcription starts, extension of annotated exons, and appearance of novel exons in annotated introns contributed significantly to this tally, and a large degree of tissue-specific transcript architecture was observed. The biological significance of these observations is unclear, but these experiments highlight that our knowledge of the exon landscape of the human genome is far from complete. Since whole-genome tiling arrays for human and mouse are now available, comprehensive surveys of transcript architecture will address the ongoing need for a complete catalog of transcript diversity.
OTHER PROFILING TECHNIQUES
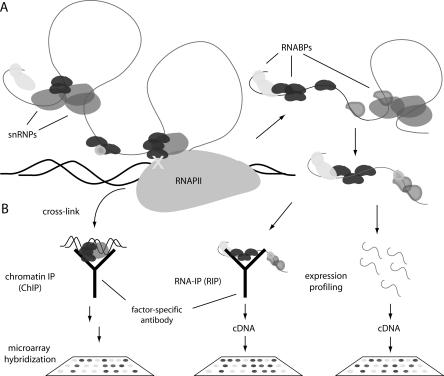
Coupling microarray analysis to molecular techniques such as chromatin and RNA immunoprecipitation (ChIP-chip and RIP-chip, respectively) can identify populations of genes and transcripts regulated by individual factors. ChIP-chip can provide a genome binding profile for processing factors that are recruited to mRNAs during transcription, thus allowing target identification (Fig. 3). In addition, this approach provides mechanistic insights by allowing comparative analysis of how and when different factors associate with genes. For instance, two recent genome localization studies in S. cerevisiae showed that factors required for early steps in spliceosome assembly are recruited co-transcriptionally, while late assembly steps are usually removed from transcription sites (Tardiff et al. 2006; Moore et al. 2006). This observation implies that most splicing catalytic events are post-transcriptional in yeast, in contrast to previous models. Moreover, early factors showed broad binding profiles that were heavily RNA-dependent, suggesting that sampling of nascent mRNAs by a subset of early splicing “commitment” factors forms the mechanistic basis of targeting the yeast spliceosome (Moore et al. 2006). Finally, a series of developmentally regulated splicing events were identified based on the fact that the spliceosome was specifically excluded from the corresponding genes. In human cells, ChIP-chip analysis of RNABPs demonstrated binding of splicing factor PTBP1 to promoter regions of genes, suggesting promoter loading and/or an uncharacterized role in transcription initiation (Swinburne et al. 2006). Further, PTBP1 showed distinct sites of accumulation along genes with a preference for 5′ and internal exons, supporting dynamic remodeling of mRNPs as they are coordinately synthesized and packaged.
FIGURE 3.
Genome-wide profiling methods. (A) mRNA splicing begins during transcription in the nucleus, and finishes prior to nuclear export. RNABPs dynamically associate with transcripts throughout nuclear and cytoplasmic processing and transport steps. (B) Three methods to probe splicing regulation are shown, including ChIP and RIP analysis of a RNABP (black) and genome-wide transcript profiling. ChIP protocols cross-link (X) co-transcriptional processing factors to DNA, which is then profiled on microarrays. RIP involves purification of native or cross-linked RNPs and profiling associated transcripts. These methods can identify coregulated splicing networks. Expression profiling with splicing-sensitive microarrays generates a genome-wide survey of alternative splicing by measuring total, steady-state RNA.
RIP-chip has been used to identify targets of RNABPs in several systems (Fig. 3; Keene et al. 2006). While technically challenging because of the fragile nature of RNP complexes, RIP-chip is advantageous because it can identify targets at all steps in mRNP biogenesis, whereas ChIP-chip only identifies co-transcriptional associations. This benefit was borne out in RIP-chip analysis of splicing factor U2AF65, which associated with spliced mRNAs both in the nucleus and cytoplasm of HeLa cells (Gama-Carvalho et al. 2006). This observation suggests functions for U2AF65 beyond its extensively characterized role in early steps of splicing, in agreement with previous implication of the Drosophila homolog of U2AF65 (dU2AF50) in the nuclear export of intronless mRNAs (Blanchette et al. 2004). Moreover, U2AF65 bound transcripts enriched for specific functions that were distinct from those bound by PTBP1.
Application of a related technique termed CLIP (cross-linking and immunoprecipitation) to the neuronal splicing factor Nova identified a network of targets encoding synaptic and neural inhibitory factors in mouse brain (Ule et al. 2003). CLIP is a modified RIP-chip protocol in which RNP complexes are cross-linked in vivo by UV exposure. This strategy allows stringent immunopurification ensuring that copurified RNAs likely represent directly bound targets. Since traditional RIP-chip requires gentle conditions to preserve native RNP complexes, it cannot unambiguously determine direct RNA–protein interactions. In addition, there is some evidence that RNP complexes can reorganize following cell lysis (Mili and Steitz 2004). These caveats for RIP-chip analysis bear consideration but have proven manageable with sufficient validation and quality control measures. A second difference between CLIP and RIP-chip is that targets were identified by sequencing rather than microarray analysis. This approach foreshadowed the more recent resurgence of high-throughput sequencing (HTS) technologies as an alternative to microarrays for massively parallel measurements of nucleic acids (Kim et al. 2007; Mardis 2007; Mikkelsen et al. 2007). HTS has the benefit of unbiased sequence determination and avoids problems with signal-to-noise and bad probes that are inescapable drawbacks of microarray hybridization. HTS platforms generate short sequence reads (∼30 bp). Since human exons average roughly 200 bp, high read numbers would be required to sufficiently profile exon boundaries and distinguish different splice isoforms levels. To date, the suitability and cost of HTS for AS analysis relative to microarrays have not been examined.
COMBINED COMPUTATIONAL AND EXPERIMENTAL APPROACHES
A number of recent studies have combined experimental and bioinformatic methods to yield incisive observations into global splicing regulation. Screening a random library of decanucleotides for splicing regulatory activity identified many novel exonic splicing silencing (ESS) motifs (Wang et al. 2004). Bioinformatic searches for these ESSs in endogenous human genes revealed positive enrichment in alternatively spliced exons and pseudoexons, and negative enrichment in constitutively spliced exons. Moreover, ESSs correlated to splice site “strength”: Constitutive exons with weak consensus splice sites contained fewer ESSs, which would presumably induce unwanted exon skipping. Subsequent work showed that the presence of ESSs inhibited the use of “decoy” 5′ and 3′ splice sites within or near regulated exons (Wang et al. 2006). In contrast, exonic splicing enhancers (ESEs) had either the opposite effect or no impact on decoy splice site use. In accordance with an endogenous role in regulating alternative splice site choice, ESSs were statistically enriched in alternatively spliced regions of regulated exons.
Other integrated approaches have focused on deciphering networks regulated by specific splicing factors. Ule and colleagues developed a comprehensive “RNA map” of NOVA binding sites proximal to regulated exons in mouse brain (Ule et al. 2006). This map not only correlated well with previous genome-scale assessments of NOVA-dependent splicing (Ule et al. 2003, 2005), but allowed highly accurate prediction of NOVA-dependent regulation for many previously unidentified targets. Several novel binding sites for Drosophila splicing regulator SXL were identified by a genome-wide computational search, and sex-specific, regulated splicing was experimentally verified in some instances (Robida et al. 2007). Since many splicing regulators bind highly degenerate RNA sequences or sequences that appear very frequently in the transcriptome (Singh and Valcárcel 2005), de novo searches in this mold have met with variable success. However, in vitro selection techniques such as SELEX have produced refined characterizations of RNA regulatory motifs for several splicing regulators, which may aid related efforts (Fairbrother et al. 2002; Hui et al. 2005; Paradis et al. 2007). Bioinformatic strategies identified significant overlap between “ultraconserved” DNA elements and alternative exons containing PTCs within genes for splicing regulators (Lareau et al. 2007). These findings revealed the highly conserved employment of AS–NMD as a means to regulate cellular levels of splicing regulators, as independently deduced from microarray analysis (Ni et al. 2007). Broadly, these studies demonstrate that skilled application of experimental techniques and computational analysis can reveal features of the “splicing code”. As more genome-wide data sets become available, bioinformatics analysis will improve predictive power in the analysis of splicing regulation.
SUMMARY AND FUTURE DIRECTIONS
Systems-wide approaches have yielded novel insights into mRNA splicing as a means of biological regulation, engendering several salient themes. First, the regulation of transcript abundance and transcript diversity are distinct processes that can influence different biological properties in the same cell. Accordingly these interconnected modes of regulation encompass distinct, though often overlapping, transcript populations. Second, patterns of alternative splicing comprise a reproducible signature for specific cell types, consistent with a crucial role in splicing regulation in determining cell identity. These patterns are dynamic and show extensive reprogramming during differentiation and disease, and in response to intra- and extra-cellular signals. Finally, functionally related transcripts can be coregulated in splicing networks to shape specific biological functions.
Though recent studies have defined fundamental themes in splicing regulation, many questions, especially relating to the roles of splicing in disease and development, remain unanswered. Global insights into mRNA splicing will continue to benefit from the publication and rigorous mining of genome-wide expression and ChIP/RIP binding profiles, which can form an empirical basis for testable, predictive models of splicing regulation. Large-scale proteomic profiling upon knockdown of PTB paralogs recently identified novel targets of those splicing regulators (Spellman et al. 2007). The success of this approach suggests that proteomic analysis, though assessing a downstream readout of alternative splicing, is a promising means to identify regulated splicing. Finally, the increasing availability of high-throughput RNAi and small molecule-based screening platforms opens the door to systematic screening for splicing regulators in mammalian cells. The current view, supported by the work reviewed here, is that cell- and context-specific splicing patterns are a composite output of the RNA-encoded “splicing code”, the differential binding specificities of RNABPs, and the myriad regulatory mechanisms that control RNABP expression and localization (Matlin et al. 2005). Continued advances along the diverse and complementary experimental avenues described here hold promise in further illuminating each of these crucial areas.
ACKNOWLEDGMENTS
We thank Jessica Hurt, Natalie Gilks-Farny, and Ian Swinburne for critiques of the manuscript. M.J.M. is supported by a National Science Foundation Graduate Fellowship and grants from the National Institutes of Health to P.A.S.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.868008.
REFERENCES
- Black, D.L. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- Blanchette, M., Labourier, E., Green, R.E., Brenner, S.E., Rio, D.C. Genome-wide analysis reveals an unexpected function for the Drosophila splicing factor U2AF50 in the nuclear export of intronless mRNAs. Mol. Cell. 2004;14:775–786. doi: 10.1016/j.molcel.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Blanchette, M., Green, R.E., Brenner, S.E., Rio, D.C. Global analysis of positive and negative pre-mRNA splicing regulators in Drosophila . Genes & Dev. 2005;19:1306–1314. doi: 10.1101/gad.1314205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe, B.J. Alternative splicing: New insights from global analyses. Cell. 2006;126:37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Boutz, P.L., Stoilov, P., Li, Q., Lin, C.H., Chawla, G., Ostrow, K., Shiue, L., Ares M., Jr, Black, D.L. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes & Dev. 2007;21:1636–1652. doi: 10.1101/gad.1558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckin, T., Nagel, R., Mandel-Gutfreund, Y., Shiue, L., Clark, T.A., Chong, J.-L., Chang, T.-H., Squazzo, S., Hartzog, G., Ares M., Jr Exploring functional relationships between components of the gene expression machinery. Nat. Struct. Mol. Biol. 2005;12:175–182. doi: 10.1038/nsmb891. [DOI] [PubMed] [Google Scholar]
- Clark, T.A., Sugnet, C.W., Ares M., Jr Genomewide analysis of mRNA processing in yeast using splicing-specific microarrays. Science. 2002;296:907–910. doi: 10.1126/science.1069415. [DOI] [PubMed] [Google Scholar]
- Clark, T.A., Schweitzer, A.C., Chen, T.X., Staples, M.K., Lu, G., Wang, H., Williams, A., Blume, J.E. Discovery of tissue-specific exons using comprehensive human exon microarrays. Genome Biol. 2007;8:R64. doi: 10.1186/gb-2007-8-4-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoeud, F., Kapranov, P., Ucla, C., Frankish, A., Castelo, R., Drenkow, J., Lagarde, J., Alioto, T., Manzano, C., Chrast, J., et al. Prominent use of distal 5′ transcription start sites and discovery of a large number of additional exons in ENCODE regions. Genome Res. 2007;17:746–759. doi: 10.1101/gr.5660607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnani, M., Barash, Y., Ip, J.Y., Misquitta, C., Pan, Q., Saltzman, A.L., Shai, O., Lee, L., Rozenhek, A., Mohammad, N., et al. Functional coordination of alternative splicing in the mammalian central nervous system. Genome Biol. 2007;8:R108. doi: 10.1186/gb-2007-8-6-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother, W.G., Yeh, R.-F., Sharp, P.A., Burge, C.B. Predictive identification of exonic splicing enhancers in human genes. Science. 2002;297:1007–1013. doi: 10.1126/science.1073774. [DOI] [PubMed] [Google Scholar]
- French, P.J., Peeters, J., Horsman, S., Dujim, E., Siccama, I., van den Bent, M.J., Luider, T.M., Kros, J.M., van der Spek, P., Sillevis Smitt, P.A. Identification of differentially regulated splice variants and novel exons in glial brain tumors using exon expression arrays. Cancer Res. 2007;67:5635–5642. doi: 10.1158/0008-5472.CAN-06-2869. [DOI] [PubMed] [Google Scholar]
- Gama-Carvalho, M., Barbosa-Morais, N.L., Brodsky, A.S., Silver, P.A., Carmo-Fonseca, M. Genome-wide identification of functionally distinct subsets of cellular mRNAs associated with two nucleocytoplasmic-shuttling mammalian splicing factors. Genome Biol. 2006;7:R113. doi: 10.1186/gb-2006-7-11-r113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardina, P.J., Clark, T.A., Shimada, B., Staples, M.K., Yang, Q., Veitch, J., Schweitzer, A., Awad, T., Sugnet, C., Dee, S., et al. Alternative splicing and differential gene expression in colon cancer detected by whole genome exon array. BMC Genomics. 2006;7:325. doi: 10.1186/1471-2164-7-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, R.E., Lewis, B.P., Hillman, R.T., Blanchette, M., Lareau, L.F., Garnett, A.T., Rio, D.C., Brenner, S.E. Widespread predicted nonsense-mediated mRNA decay of alternatively-spliced transcripts of human normal and disease genes. Bioinformatics. 2003;19(Suppl. 1):i118–i121. doi: 10.1093/bioinformatics/btg1015. [DOI] [PubMed] [Google Scholar]
- Hui, J., Hung, L.H., Heiner, M., Schreiner, S., Neumuller, N., Reither, G., Haas, S.A., Bindereif, A. Intronic CA-repeat and CA-rich elements: A new class of regulators in mammalian alternative splicing. EMBO J. 2005;24:1988–1998. doi: 10.1038/sj.emboj.7600677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip, J.Y., Tong, A., Pan, Q., Topp, J.D., Blencowe, B.J., Lynch, K.W. Global analysis of alternative splicing during T-cell activation. RNA. 2007;13:563–572. doi: 10.1261/rna.457207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J.M., Castle, J., Garrent-Engle, P., Kan, Z., Loerch, P.M., Armour, C.D., Santos, R., Schadt, E.E., Stoughton, R., Shoemaker, D.D. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–2144. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- Juneau, K., Palm, C., Miranda, M., Davis, R.W. High-density yeast-tiling array reveals previously undiscovered introns and extensive regulation of meiotic splicing. Proc. Natl. Acad. Sci. 2007;104:1522–1527. doi: 10.1073/pnas.0610354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur, K., Xing, Y., Ouyang, Z., Wong, W.H. Exon arrays provide accurate assessments of gene expression. Genome Biol. 2007;8:R82. doi: 10.1186/gb-2007-8-5-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene, J.D. RNA regulons: Coordination of post-transcriptional events. Nat. Rev. Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- Keene, J.D., Komisarow, J.M., Friedersdorf, M.B. RIP-Chip: The isolation and identification of mRNAs, microRNAs, and protein components of ribonucleoprotein complexes from cell extracts. Nat. Protoc. 2006;1:302–307. doi: 10.1038/nprot.2006.47. [DOI] [PubMed] [Google Scholar]
- Kim, J.B., Porrca, G.J., Song, L., Greenway, S.C., Gorham, J.M., Church, G.M., Seidman, C.E., Seidman, J.G. Polony multiplex analysis of gene expression (PMAGE) in mouse hypertrophic cardiomyopathy. Science. 2007;316:1481–1484. doi: 10.1126/science.1137325. [DOI] [PubMed] [Google Scholar]
- Lareau, L.F., Inada, M., Green, R.E., Wengrod, J.C., Brenner, S.E. Unproductive splicing of SR genes associated with highly conserved and ultraconserved DNA elements. Nature. 2007;446:926–929. doi: 10.1038/nature05676. [DOI] [PubMed] [Google Scholar]
- Li, C., Kato, M., Shiue, L., Shively, J.E., Ares M., Jr, Lin, R.-J. Cell type and culture-dependent alternative splicing in human breast cancer cells revealed by splicing-sensitive microarrays. Cancer Res. 2006a;66:1990–1999. doi: 10.1158/0008-5472.CAN-05-2593. [DOI] [PubMed] [Google Scholar]
- Li, H.R., Wang-Rodriguez, J., Nair, T.M., Yeakley, J.M., Kwon, Y.S., Bibikova, M., Zheng, C., Zhou, L., Zhang, K., Downs, T., et al. Two-dimensional transcriptome profiling: Identification of mRNA isoform signatures in prostate cancer from archived paraffin-embedded cancer specimens. Cancer Res. 2006b;66:4079–4088. doi: 10.1158/0008-5472.CAN-05-4264. [DOI] [PubMed] [Google Scholar]
- Maniatis, T., Reed, R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- Mardis, E.M. ChIP-seq: Welcome to the new frontier. Nat. Methods. 2007;4:613–614. doi: 10.1038/nmeth0807-613. [DOI] [PubMed] [Google Scholar]
- Matlin, A.J., Clark, F., Smith, C.W. Understanding alternative splicing: Towards a cellular code. Nat. Rev. Mol. Cell Biol. 2005;6:386–398. doi: 10.1038/nrm1645. [DOI] [PubMed] [Google Scholar]
- McKee, A.E., Neretti, N., Carvalho, L.E., Meyer, C.A., Fox, E.A., Brodsky, A.S., Silver, P.A. Exon expression profiling reveals stimulus-mediated exon use in neural cells. Genome Biol. 2007;8:R159. doi: 10.1186/gb-2007-8-8-r159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen, T.S., Ku, M., Jaffe, D.B., Issac, B., Lieberman, E., Giannoukos, G., Alvarez, P., Brockman, W., Kim, T.K., Koche, R.P., et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cell. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mili, S., Steitz, J.A. Evidence for reassociation of RNA-binding proteins after cell lysis: Implications for the interpretation of immunoprecipitation analyses. RNA. 2004;10:1692–1694. doi: 10.1261/rna.7151404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M.J., Schwartzfarb, E.M., Silver, P.A., Yu, M.C. Differential recruitment of the splicing machinery during transcription predicts genome-wide patterns of mRNA splicing. Mol. Cell. 2006;24:903–915. doi: 10.1016/j.molcel.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Ni, J.Z., Grate, L., Donohue, J.P., Preston, C., Nobida, N., O'Brien, G., Shiue, L., Clark, T.A., Blume, J.E., Ares M., Jr Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and nonsense-mediated decay. Genes & Dev. 2007;21:708–718. doi: 10.1101/gad.1525507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoniewski, M.J., Hey, Y., Pepper, S.D., Miller, C.J. High correspondence between Affymetrix exon and standard expression arrays. Biotechniques. 2007a;42:181–185. doi: 10.2144/000112315. [DOI] [PubMed] [Google Scholar]
- Okoniewski, M.J., Yates, T., Dibben, S., Miller, C.J. An annotation infrastructure for the analysis and interpretation of Affymetrix exon array data. Genome Biol. 2007b;8:R79. doi: 10.1186/gb-2007-8-5-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Q., Shai, O., Misquitta, C., Zhang, W., Saltzman, A.L., Mohammad, N., Babak, T., Siu, H., Hughes, T.R., Morris, Q.D., et al. Revealing global regulatory features of mammalian alternative splicing using a quantitative microarray platform. Mol. Cell. 2004;16:929–941. doi: 10.1016/j.molcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Pan, Q., Saltzman, A.L., Kim, Y.K., Misquitta, C., Shai, O., Maquat, L.E., Frey, B.J., Blencowe, B.J. Quantitative microarray profiling provides evidence against widespread coupling of alternative splicing with nonsense-mediated mRNA decay to control gene expression. Genes & Dev. 2006;20:153–158. doi: 10.1101/gad.1382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis, C., Cloutier, P., Shkreta, L., Toutant, J., Klarskov, K., Chabot, B. hnRNP I/PTB can antagonize the splicing repressor activity of SRp30c. RNA. 2007;13:1287–1300. doi: 10.1261/rna.403607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relógio, A., Ben-Dov, C., Baum, M., Ruggiu, M., Gemund, C., Benes, V., Darnell, R.B., Valcárcel, J. Alternative splicing microarrays reveal functional expression of neuron-specific regulators in Hodgkin lymphoma cells. J. Biol. Chem. 2005;280:4779–4784. doi: 10.1074/jbc.M411976200. [DOI] [PubMed] [Google Scholar]
- Robida, M.D., Rahn, A., Singh, R. Genome-wide identification of alternatively spliced mRNA targets of specific RNA-binding proteins. PLoS One. 2007;2:e250. doi: 10.1371/journal.pone.0000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R., Valcárcel, J. Building specificity with nonspecific RNA-binding proteins. Nat. Struct. Mol. Biol. 2005;12:645–653. doi: 10.1038/nsmb961. [DOI] [PubMed] [Google Scholar]
- Spellman, R., Llorian, M., Smith, C.W.J. Crossregulation and functional redundancy between the splicing regulator PTB and its paralogs nPTB and ROD1. Mol. Cell. 2007;27:420–434. doi: 10.1016/j.molcel.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoughton, R.B. Applications of DNA microarrays to biology. Annu. Rev. Biochem. 2005;74:53–82. doi: 10.1146/annurev.biochem.74.082803.133212. [DOI] [PubMed] [Google Scholar]
- Sugnet, C.W., Srinivasan, K., Clark, T.A., O'Brien, G., Cline, M.S., Wang, H., Williams, A., Kulp, D., Blume, J.E., Haussler, D., et al. Unusual intron conversation near tissue-regulated exons found by splicing microarrays. PLoS Comput. Biol. 2006;2:e4. doi: 10.1371/journal.pcbi.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburne, I.A., Meyer, C.A., Liu, X.S., Silver, P.A., Brodsky, A.S. Genomic localization of RNA binding proteins reveales links between pre-mRNA processing and transcription. Genome Res. 2006;16:912–921. doi: 10.1101/gr.5211806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff, D.F., Lacadie, S.A., Rosbash, M. A genome-wide analysis indicates that yeast pre-mRNA splicing is predominantly posttranscriptional. Mol. Cell. 2006;24:917–929. doi: 10.1016/j.molcel.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ule, J., Jensen, K.B., Ruggiu, M., Mele, A., Ule, A., Darnell, R.B. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302:1212–1215. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- Ule, J., Ule, A., Spencer, J., Williams, A., Hu, J.S., Cline, M., Wang, H., Clark, T., Fraser, C., Ruggiu, M., et al. Nova regulates brain specific splicing to shape the synapse. Nat. Genet. 2005;37:844–852. doi: 10.1038/ng1610. [DOI] [PubMed] [Google Scholar]
- Ule, J., Stefani, G., Mele, A., Ruggiu, M., Wang, X., Taneri, B., Gaasterland, T., Blencowe, B.J., Darnell, R.B. An RNA map predicting Nova-dependent splicing regulation. Nature. 2006;444:580–586. doi: 10.1038/nature05304. [DOI] [PubMed] [Google Scholar]
- Wang, G.-S., Cooper, T.A. Splicing in disease: Disruption of the splicing code and the decoding machinery. Nat. Rev. Genet. 2007;8:749–761. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Rolish, M.E., Yeo, G., Tung, V., Mawson, M., Burge, C.B. Systematic identification and analysis of exonic splicing silencers. Cell. 2004;119:831–845. doi: 10.1016/j.cell.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Xiao, X., Van Nostrand, E., Burge, C.B. General and specific functions of exonic splicing silencers in splicing control. Mol. Cell. 2006;23:61–70. doi: 10.1016/j.molcel.2006.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, Y., Kapur, K., Wong, W.H. Probe selection and expression index computation of Affymetrix Exon Arrays. PLoS ONE. 2006;1:e88. doi: 10.1371/journal.pone.0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Hesselberth, J., Fields, S. Genome-wide identification of spliced introns using a tiling microarray. Genome Res. 2007;17:503–509. doi: 10.1101/gr.6049107. [DOI] [PMC free article] [PubMed] [Google Scholar]