Abstract
T cell line–tropic (T-tropic) HIV type 1 strains enter cells by interacting with the cell-surface molecules CD4 and CXCR4. We have generated transgenic mice predominantly expressing human CD4 and CXCR4 on their CD4-positive T lymphocytes (CD4+ T cells). Their primary thymocytes are susceptible to T-tropic but not to macrophage-tropic HIV-1 infection in vitro, albeit with a viral antigen production less efficient than human peripheral blood mononuclear cells. Interestingly, even without HIV infection, transgenic mice display a CD4+ T cell depletion profile of peripheral blood reminiscent of that seen in AIDS patients. We demonstrate that CD4+ T cell trafficking in transgenic mice is biased toward bone marrow essentially due to CXCR4 overexpression, resulting in the severe loss of CD4+ T cells from circulating blood. Our data suggest that CXCR4 plays an important role in lymphocyte trafficking through tissues, especially between peripheral blood and bone marrow, participating in the regulation of lymphocyte homeostasis in these compartments. Based on these findings, we propose a hypothetical model in which the dual function of CXCR4 in HIV-1 infection and in lymphocyte trafficking may cooperatively induce progressive HIV-1 infection and CD4+ T cell decline in patients.
Human immunodeficiency virus 1 (HIV-1) has been broadly categorized into three groups: macrophage-tropic (M-tropic)1 nonsyncytium-inducing, T cell line– tropic (T-tropic) syncytium-inducing, and dual tropic mixed phenotypes (1–4). Primary isolates from early asymptomatic HIV-1 infection are frequently M-tropic, suggesting that M-tropic HIV-1 strains play a critical role in establishing infection (1–6). During the clinical course, T-tropic strains emerge at a later stage associated with a decline in CD4 positive T lymphocytes (CD4+ T cells), suggesting that these strains may be related to AIDS pathogenesis (1–6).
To enter cells, M- and T-tropic strains use distinct G protein–coupled seven-transmembrane chemokine receptors in addition to their common receptor, human CD4 (4–6). M-tropic strains use receptors for CC-chemokines, including RANTES (regulated on activation, normal T cell expressed and secreted), macrophage inflammatory protein (MIP)-1α, and MIP-1β (7, 8), or other chemokine receptor–like orphan receptors (9, 10). T-tropic strains, in contrast, mainly use CXCR4 (11, 12), the receptor for CXC-chemokine stromal cell–derived factor 1 (SDF-1) (13, 14). These chemokines function as chemotactic factors for hematopoietic lineage cells, including granulocytes, monocytes, lymphocytes, and their progenitor cells (7, 8, 15). It has been suggested that these chemokines play roles in the migration of these cells to local inflammation sites and in their homing to distant organs during development. The function and regulation of expression of these chemokine receptors may underlie the mechanisms of HIV transmission, latency, and immunodeficiency.
Although these chemokine receptors have been shown to facilitate HIV entry into cell lines, it remains unknown whether they are able to render mouse primary lymphocytes susceptible to HIV infection. Transgenic mice whose CD4+ T cells have HIV infectability are expected to prove useful as a small-animal model for developing therapeutic reagents against HIV infection and for understanding the basis of AIDS pathogenesis. Here we report the generation of transgenic mice that predominantly express human CD4 and CXCR4 on their CD4+ T cells and CD4+ thymocytes. Thymocytes from transgenic mice were found to be susceptible to T- but not M-tropic HIV-1 infection in vitro. Interestingly, the tissue distribution of CD4+ T cells in these mice was deregulated with the severe loss of CD4+ T cells from peripheral blood coinciding with their accumulation in bone marrow, and this was shown to be essentially a result of CXCR4 overexpression. Our data suggest that CXCR4 plays an important role both in the regulation of lymphocyte trafficking and in T-tropic HIV-1 infection of primary lymphocytes and may provide insights into the pathogenesis of CD4+ T cell decline in AIDS patients.
Materials and Methods
Production of Transgenic Mice.
The human CXCR4 (hCXCR4) cDNA was obtained by PCR from the human PBL cDNA library (Human Peripheral Blood Leukocyte Quick-Clone™; Clontech, Palo Alto, CA) using the following primers: primer 1, 5′-TGAGTCGACTGAGTGCTCCAGTAGCCACC; primer 2, 5′-TAGGTCGACAGATCTTGTACAATATTGGTCAGTCTT. PCR was done using cloned PFU DNA polymerase (Stratagene Inc., La Jolla, CA) for 30 cycles each consisting of 1 min at 94°C, 1 min at 55°C, and 3 min 72°C. The cDNA product was digested with SalI restriction endonuclease and inserted into the SalI site of the mouse CD4 transgene designed for restricted expression of a transgene in CD4+ T cells (16). The 40-kb genomic fragment containing all exons as well as 5′ and 3′ region of the human CD4 (hCD4) gene was joined with the murine CD4 enhancer (17). These two transgenes were separated from the vectors on an agarose gel, purified, and coinjected into the nuclei of mouse oocytes of (B6D2)F1 mice. Transgenic founders were screened for the expression of hCD4 and hCXCR4 in their PBLs by FACS® (Becton Dickinson and Co., Mountain View, CA) analysis, as well as for the integration of transgenes (hCD4 and hCXCR4) in their genomic DNA by PCR analysis. The founders were mated with C57BL/6J mice to generate the progeny. Mice are maintained in the SPF facility (Department of Experimental Animal Research, RIKEN).
Cell Culture and Infection Assays.
Human PBMCs purified with the lymphosepar I buffer (IBL, Fujioka, Japan) and mouse thymocytes passed through a nylon mesh were washed with PBS, and then cultured at 5 × 105 (PBMCs) and 5 × 106 (thymocytes) cells/ml in RPMI complete medium containing 3 μg/ml PHA (Sigma Chemical Co., St. Louis, MO) and 4 ng/ml recombinant human IL-2 (PeproTech EC, London, UK) or murine IL-2 (R&D Systems, Inc., Minneapolis, MN). On day 2, cells were harvested, counted, and 106 (human PBMCs) or 5 × 106 (murine thymocytes) cells were inoculated with the medium (1 ml) containing HIV-1 viruses (5 ng of p24 antigen) at 37°C for 3 h in the presence of 5 μg/ml polybrene (Sigma Chemical Co.). After washing three times with PBS, cells were treated with trypsin (0.025%) EDTA (0.27 mM) (GIBCO BRL, Gaithersburg, MD) for 3 min at 37°C, and then washed with complete medium three times. Cells were cultured in the presence of human or murine IL-2 (4 ng/ml). IL-2 was added to the culture medium every 2 d. On indicated days after infection, the supernatant (200 μl) was removed for a p24 enzyme immunoassay (Abbott, Abbott Park, IL).
Construction of SDF-1-Cγ1.
A cDNA clone encoding murine SDF-1α was cloned into pBluescript SK (−) (Stratagene Inc., La Jolla, CA) (18). The 3′ end of the coding sequences of mSDF-1α was mutated to delete the stop codon and to introduce an EcoRI site by PCR using the following primers: sense primer 5′-CCTCGAGGTCGACGGTATCGATA-3′ and antisense primer 5′-ATGGACCTCTTTCGAAATTTGTTCCCTTAAGCC-3′. After EcoRI digestion, the PCR product of expected size (310 bp) was subcloned into a pCγ1/Bluescript SK (−) vector containing human IgG1 constant region genomic sequences (provided by H. Yagita, Juntendo University School of Medicine, Tokyo, Japan) (19). Clones with mSDF-1α gene in right direction were chosen. The procedure resulted in an in-frame fusion gene between mSDF-1α and huCγ1(SDF-1–Cγ1). After confirming the sequences, a 1.7-kb XhoI–NotI fragment was subcloned into pBCMGSneo expression vector (20). A pSDF-1-Cγ1/BCMGSneo vector was transfected into Ag 8.653 B cell tumors using Lipofectin (GIBCO BRL) and stable transfectants were established after G418 selection.
Flow Cytometry Analysis.
FACS® analysis was performed on FACSort (Becton Dickinson and Co.). Peripheral blood from mice was incubated in red blood cell lysis buffer and washed with PBS, and 105 cells were stained with monoclonal antibodies on ice for 30 min in PBS containing FCS (5%) and the Fc block (PharMingen, San Diego, CA) for hCXCR4 staining, or normal mouse serum (10%) for hCD4 staining. For anti–hCXCR4 staining, anti–mouse IgG2a-FITC was used as the secondary antibody. After washing with PBS, cells were analyzed on a FACSort. For SDF-1–Cγ1 staining, cells were preincubated with 10% goat serum plus the Fc block. Cells were incubated with SDF-1–Cγ1 at 4°C for 40 min, washed once, and further reacted with FITC anti–huIgG goat (Fab′)2 antibody (Cappel, Aurora, Ohio). The monoclonal antibodies used in this study are as follows: rat anti– mouse CD4(L3T4)-PE (PharMingen), rat anti–mouse CD8 (Ly2)-TRICOLOR (Caltag Laboratories, Burlingame, CA), rat anti–mouse IgG2a-FITC (PharMingen), rat anti–mouse CD16/ CD32 (Fc block; PharMingen), mouse anti–hCXCR4 (12G5) (provided by J.A. Hoxie, University of Pennsylvania, Philadelphia, PA), mouse anti–hCD4-FITC (Dako SA, Glostrup, Denmark).
PCR Amplification of HIV Proviral DNA.
Thymocytes infected with HIV strains in vitro as described above were harvested on day 2 after infection. Genomic DNA was extracted from these cells (21). PCR was performed with primers (LTR5: 5′-GGCTAACTAGGGAACCCACTGCTT-3′, and LTR6: 5′-CTGCTAGAGATTTTCCACACTGAC-3′) for the early product of proviral DNA, or primers (LTR5 and 5NC2: 5′-CCGAGTCCTGCGTCGAGAGAGC-3′) for its late product (22), in combination with the IL-1 primers (5′-CTCTAGAGCACCATGCTACAGAC-3′, and 5′-TGGAATCCAGGGGAAACACTG-3′) of the murine IL-1 gene, which served as a control. Reactions were done in 50 μl of reaction mixture for 35 cycles. Southern hybridization was performed with the oligo nucleotide probes, LTR9 (5′-GCCTCAATAAAGCTTGCCTTG-3′) for HIV DNA and mIL-1 (5′-TGTAAGAATACCCAGACAGCTTTAAGGATGGGAGGG-3′) for the IL-1 gene, labeled with digoxigenin-ddUTP, and detection was performed using a DIG luminescent detection system (Boehringer Mannheim, Mannheim, Germany).
Chemotaxis Assay.
Chemotaxis assays for murine and human lymphocytes were performed as previously described (23). In brief, murine splenic mononuclear cells purified with lymphosepar II (IBL), or human PBMCs purified with lymphosepar I (IBL) were washed three times with PBS, resuspended in RPMI containing 0.25% BSA, and 106 cells in 100 μl were added to the top chambers of 50-μm–pore polycarbonate Transwell (Costar Corp., Cambridge, MA). For pretreatment of lymphocytes with pertussis toxin, cells were incubated with complete medium containing pertussis toxin (100 ng/ml) or medium alone for 2 h at 37°C under 5% CO2, and were then washed twice with PBS. Human SDF-1α (PeproTech, Inc., Rocky Hill, NJ) was added to the lower chambers containing 600 μl 0.25% BSA RPMI medium. In control experiments, other chemokines such as murine MIP-1α (Sigma Chemical Co.), murine monocyte chemoattractant protein (MCP)-1 (PharMingen), murine MCP-5 (PeproTech, Inc.), or human RANTES (85% amino acid sequence homology with murine RANTES; R&D Systems Inc.) were added to the lower chambers. After a 3-h incubation at 37°C with 5% CO2, the cells that migrated into the lower chambers were harvested and counted with a FACSort for 30 s at high speed, gating on the forward and side scatter of the lymphocytes. For murine spleen cells and human PBMCs, the input cells and the migrated cells were stained with antibodies for CD4 and CD8 and analyzed on FACSort to evaluate the migration activity of each T cell subset.
In Vivo Migration Assays.
Donor lymphocytes were prepared from the spleen and the peritoneal lymph node of mice that were either doubly transgenic for hCD4 and hCXCR4 or singly transgenic for hCD4. The lymphocytes (5–9 × 106) were injected in the tail vein of normal mice (BDF1) (24). After 3 or 16 h, the recipient mice were killed, cells from their tissues were stained with anti–hCD4 FITC, anti–mCD4 PE, and anti–mCD8 TRICOLOR, and the cells were analyzed by three-color flow cytometry.
Results
Targeted Human CD4 and CXCR4 Expression in Mouse CD4+ T Cells.
Two transgenes containing either hCD4 genomic DNA or hCXCR4 cDNA were constructed under the control of CD4 gene cis elements to express them specifically in CD4+ T cells (16, 17). These constructs were comicroinjected into the nuclei of fertilized murine oocytes. Of transgenic offspring, mouse No. 12 displayed the highest level of both hCD4 and hCXCR4 expression in T lymphocytes. Both transgenes were cotransmitted to all transgene-positive progeny of this mouse, indicating that the two constructs were stably integrated into the same chromosome. Mouse No. 12's progeny were therefore analyzed in detail.
In FACS® profiles of their lymphocytes, human CD4 and hCXCR4 were coexpressed in the CD4 subset, but not in the CD8 subset, of peripheral T lymphocytes (Fig. 1, A and B). Both molecules were also detected specifically on CD4+CD8+ (double positive) and CD4+CD8− thymocytes of transgenic mice (Fig. 1, C and D, and data not shown). Human CD4, but not hCXCR4, was also detected in monocytes, B lymphocytes, and NK cells of transgenic mice, albeit at lower levels (data not shown). The hCD4 expression level in transgenic peripheral CD4+ T cells was higher than that seen in human PBLs. The hCXCR4 expression level (mean fluorescence intensity [MFI]: 12.44) was also higher than that seen in unstimulated human PBLs (MFI: 4.13), but lower than that in human PBLs stimulated by PHA and IL-2 (MFI: 20.19). Since SDF-1 is a natural ligand for CXCR4, SDF-1 binding assays were performed for transgenic and nontransgenic T lymphocytes using murine SDF-1α–huCγ1 fusion protein. Murine SDF-1α (mSDF-1α) is 98.6% identical to human SDF-1α (hSDF-1α) in its predicted amino acid sequences and is thought to interact with hCXCR4 (13, 25). Murine SDF-1α binding activity was significantly higher in transgenic CD4 single positive (SP) T lymphocytes and double positive thymocytes, but not in CD8 SP T lymphocytes, in line with the hCXCR4 expression pattern in transgenic mice (Fig. 1 E and data not shown). This result indicates that total receptors for murine SDF-1, including endogenous CXCR4 and hCXCR4, are overexpressed specifically in CD4+ T lymphocytes of transgenic mice. The level of SDF-1 receptor expression in transgenic CD4+ T cells (MFI; 76.82) was in the physiological range observed in control mouse CD4+ T cell blasts activated with PHA (5 μg/ml), Con A (2 μg/ml), and mIL-2 (5 ng/ml) for 1 d in vitro (MFI; 138.06). The levels of SDF-1 receptor in normal mouse thymocytes and peripheral T lymphocytes will be published elsewhere (Suzuki, G., Y. Nakata, Y. Dan, A. Uzawa, K. Nakagawa, T. Sato, K. Mita, and T. Shirasawa, manuscript in preparation).
Figure 1.
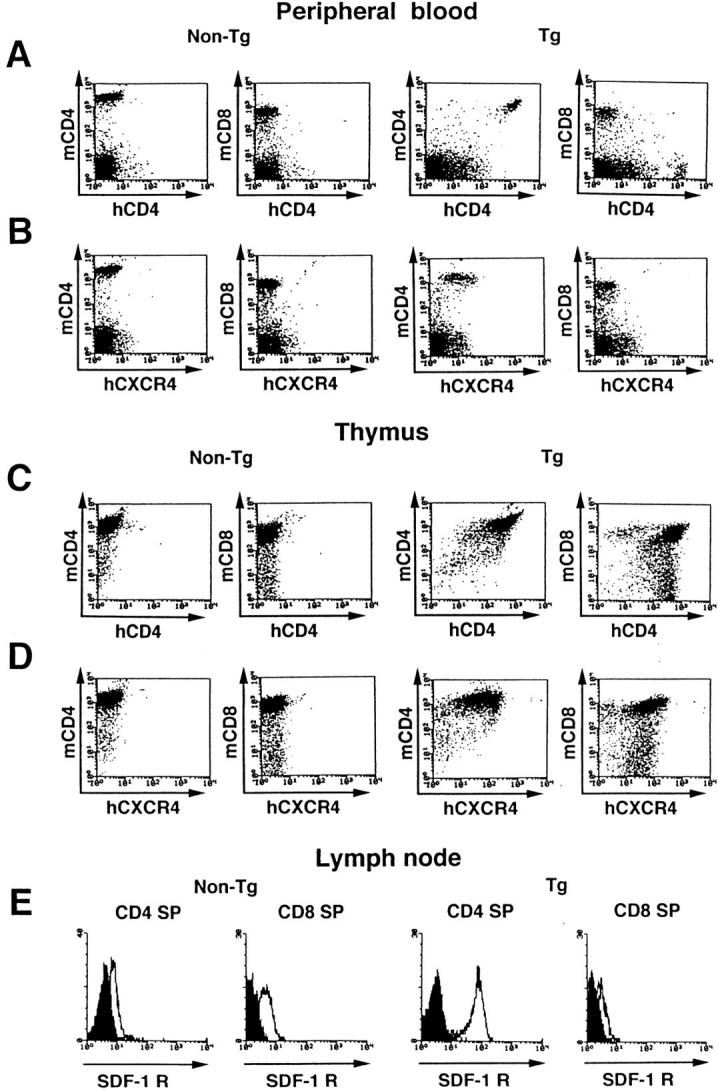
(A–D) hCD4 and hCXCR4 are expressed predominantly on CD4+ subsets of PBLs and thymocytes in transgenic mice. Three-color flow cytometric analysis of hCD4 and hCXCR4 expression on PBLs (A and B) and thymocytes (C and D) of a control nontransgenic mouse (Non-Tg) and of a transgenic litter mate (Tg). Cells were stained with FITC-conjugated monoclonal antibody against hCD4 (A and C) or hCXCR4 (B and D) in the presence of monoclonal antibodies against murine CD4 (PE) and CD8 (TRICOLOR). (E) Three-color analysis of receptors for murine SDF-1 (open histograms) on gated CD4+CD8− (CD4 SP) and CD4−CD8+ (CD8 SP) subsets of lymph node T cells from control and transgenic littermates. Background levels obtained with the FITC anti–huIgG alone are shown in solid histograms.
HIV Infection In Vitro.
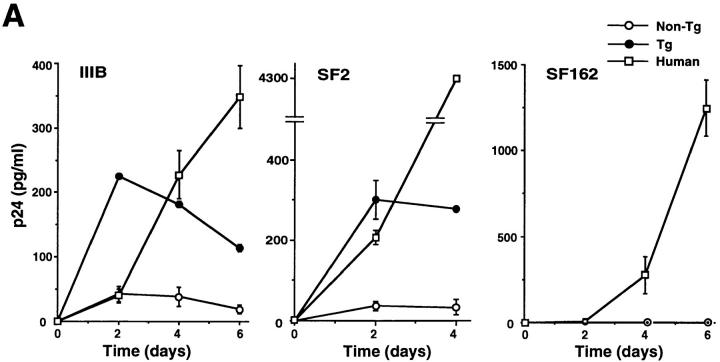
Although several chemokine receptors have been shown to facilitate HIV entry into cell lines, it remains to be determined whether they are able to render mouse primary immune cells susceptible to HIV infection. We therefore studied the susceptibility of transgenic lymphocytes to HIV-1 infection in vitro. Transgenic thymocytes express hCXCR4 at a higher level than peripheral lymphocytes (Fig. 1, B and D), so we used them for in vitro HIV infection. HIV gag p24 antigen production was clearly detected in the culture supernatant of transgenic thymocytes exposed to T-tropic strains IIIB and SF2, showing a time course more rapid than that of human PBMCs (Fig. 2 A). The p24 level produced by transgenic thymocytes was significantly lower, however, than that produced by human PBMCs after 4 d of infection. Barriers to efficient HIV replication may therefore still exist in transgenic mouse thymocytes (26), or, alternatively, this may reflect the poor viability of mouse thymocytes at this stage of culturing. In contrast, M-tropic HIV-1 strain SF162 neither allowed control nor transgenic thymocytes to produce the p24 antigen, in line with previous observations that M-tropic strains use different chemokine receptor classes to enter cells (5, 6, 9, 10). Genomic DNA from mouse thymocytes was subjected to PCR amplification using HIV primers. Both early- and late-stage products were detected specifically in DNA isolated from transgenic thymocytes exposed to the T-tropic HIV-1 strain (Fig. 2 B).
Figure 2.
T-tropic HIV infection in transgenic thymocytes. (A) PHA-stimulated thymocytes from 6-wk-old control nontransgenic mice, transgenic mice, or human PBMCs were exposed to T-tropic HIV strains IIIB and SF2, or an M-tropic SF162 for 3 h. After washing three times with PBS, cells were treated with trypsin-EDTA for 3 min, and then washed three times with complete medium. The p24 antigen was assayed in the medium immediately after cell washing (day 0) and in the culture supernatant harvested on the indicated days after infection. Background levels taken to be those on day 0 were subtracted from the amount of p24. Results shown are the mean ± SD of duplicated infections. (B) PCR amplification of HIV DNA in control or transgenic thymocytes infected with IIIB or SF162. Murine IL-1 gene primers were used for internal control. The product with LTR5/LTR6 represents the early HIV DNA product, whereas that with LTR5/5NC2 represents the late product (22). Anticipated DNA size is as follows: 139 bp (LTR5/LTR6), 202 bp (LTR5/5NC2), 308 bp (IL-1). Similar results were obtained in two independent experiments (A and B).
Compared with thymocytes, lower levels of p24 antigens were detected in the culture supernatants of spleen lymphocytes exposed to T-tropic HIV strains (data not shown). This may be due, at least in part, to their lower hCXCR4 expression compared with that in thymocytes.
Abnormal Tissue Distribution of CD4 SP T Lymphocytes.
Interestingly, transgenic mice had significantly lower CD4 SP (CD4+CD8−) T cell counts in peripheral blood (Fig. 3, A and B). CD8 SP (CD4−CD8+) T cell counts were also slightly reduced, but reduction varied with the individual mouse (data not shown). In contrast, the proportion and number of CD4 SP T cells in the thymus, and especially in bone marrow, were elevated (Fig. 3, A and B). Since bone marrow contains immature CD4 positive cells, three-color analysis was conducted using antibodies against murine CD3, CD4, and CD8. The number of mature CD3-positive CD4 SP T cells increased significantly in the bone marrow of transgenic mice (Fig. 3 B). Both the lymph nodes and spleen maintained relatively higher percentages of CD4 SP T cells than did peripheral blood (Fig. 3 B).
Figure 3.

Abnormal CD4+ T cell distribution in transgenic mice and transient normal distribution recovery after an injection of pertussis toxin. (A) Flow cytometric analysis of lymphocytes in tissues from 5-wk-old nontransgenic (left) and transgenic (right) litter mates. (B) Percentage of CD4 SP T cells in lymphoid cells from tissues of 5-wk-old litter mates (n = 9). CD3+ CD4 SP T cells are shown for bone marrow because it contained immature CD3− CD4+ cells. Bone marrow cells gated on lymphoid cells may include immature myeloid and erythroid lineage cells. Data is the averaged value obtained with three nontransgenic and six transgenic mice. The averaged number of lymphoid cells in each tissue is as follows: peripheral blood, 9.78 × 106/ml (Non-Tg) vs. 9.38 × 106/ml (Tg); thymus, 1.97 × 108 vs. 1.45 × 108; bone marrow, 2.52 × 107 vs. 1.95 × 107; spleen, 6. 07 × 107 vs. 3.71 × 107; lymph node, 7.27 × 107 vs. 6.15 × 107. PB, peripheral blood; LN, lymph node; BM, bone marrow. (C) Before and after intraperitoneal injection of pertussis toxin (500 ng) or control saline, murine peripheral blood was analyzed using a flow cytometer on indicated days. The percentage of CD4 SP T cells in blood lymphocytes is shown. Data represents mean values obtained with two mice for each experimental set. Similar results were obtained in two independent experiments. (D) 5 d after injection of pertussis toxin (500 ng) or control saline i.p., mice were analyzed for CD3+ CD4 SP T cell counts in blood and bone marrow (two femurs). Data is mean values ± SD obtained with two mice. PT, pertussis toxin. Similar results were obtained in three independent experiments.
These findings suggest that CD4 SP T cell reduction in peripheral blood was due to neither reduced production in the thymus nor elimination from peripheral tissues, but was due, rather, to abnormal distribution in tissue compartments. Other double transgenic mouse lines expressing hCD4 and undetectable levels of hCXCR4 in peripheral CD4 SP T cells showed normal CD4 SP T cell counts in peripheral blood (data not shown). Human CD4 expression alone at high levels in CD4 SP T cells in hCD4 single transgenic mice did not reduce the number of these cells in peripheral blood (data not shown; see references 17 and 27). Moreover, the administration of pertussis toxin, a specific G protein blocker, to transgenic mice predominantly increased the CD4 subset in blood, and, reciprocally, decreased levels observed in bone marrow (Fig. 3, C and D). These results suggest that signaling through hCXCR4, rather than through hCD4, is critical in developing this phenotype.
Augmented Chemotactic Response to SDF-1.
It was recently found that SDF-1, the natural ligand for CXCR4, functions as a potent chemoattractant for murine and human T lymphocytes in vitro, and for murine lymphocytes in vivo (23, 28). In vitro chemotaxis assay demonstrated that transgenic CD4 SP T cells, but not CD8 SP T cells, were more hyperresponsive to SDF-1 than were nontransgenic cells, indicating that hCXCR4 expression in CD4 SP T cells increases their responsiveness to SDF-1 (Fig. 4 A). They were even more sensitive to SDF-1 than human CD4 SP T cells, which may be explained by the hCXCR4 expression level in transgenic CD4 SP T cells, which is higher than that of unstimulated human PBLs. Cell migration was eliminated by pretreatment with pertussis toxin, indicating that G-protein participates in signaling through CXCR4, similar to other seven-transmembrane receptors (Fig. 4 B). The response of CD4 SP T cells to several other lymphocyte chemoattractants was, however, similar between transgenic and nontransgenic mice (Table 1).
Figure 4.
Hyperresponse of transgenic CD4+ T cells to SDF-1 in a chemotaxis assay. (A) Spleen mononuclear cells from nontransgenic and transgenic mice or from human PBLs were analyzed for migration toward the SDF-1 gradient. The percentage of migrated CD4 SP or CD8 SP T cells to input cells of each subset was calculated (Materials and Methods). (B) SDF-1-induced chemotaxis of murine CD4 SP T cells was eliminated with pertussis toxin. After 2 h pretreatment with medium or pertussis toxin (100 ng/ml), chemotaxis of lymphocyte subsets in response to SDF-1 (40 ng/ml) was analyzed. Results (A and B) are the mean ± SD of duplicated experiments. Similar results were obtained in two independent experiments (A and B).
Table 1.
Chemotaxis of Murine Spleen CD4 T Lymphocytes to Different Chemokines
| Chemokines (ng/ml) | Migrated CD4 T cells (percentage of input CD4 T cells) | |||
|---|---|---|---|---|
| Non-Tg | Tg | |||
| SDF-1α (100) | 16.6 ± 2.9 | 63.5 ± 2.1 | ||
| mMIP-1α (10) | 3.2 ± 0.1 | 3.1 ± 0.3 | ||
| mMCP-1 (30) | 4.2 ± 0.3 | 3.8 ± 0.1 | ||
| mMCP-5 (50) | 4.1 ± 0.2 | 3.1 ± 0.6 | ||
| hRANTES (100) | 4.2 ± 0.4 | 3.3 ± 0.5 | ||
| Medium alone | 2.5 ± 0.3 | 2.4 ± 0.0 | ||
Spleen mononuclear cells from nontransgenic and transgenic mice were tested for in vitro migration toward active chemokine concentrations. Input and migrated cells were counted and stained with antibodies for CD4 and CD8 to evaluate CD4 SP T cell migration activity. Results are the mean ± SD of duplicate experiments.
Based on these in vivo and in vitro findings, it is tempting to speculate that large numbers of transgenic CD4 SP T cells migrate and become trapped in mouse tissues, such as bone marrow (29), whose stromal cells produce high levels of SDF-1, resulting in the loss of circulating CD4 SP T cells from the blood. It is important to note that the predicted amino acid sequences of both SDF-1 and CXCR4 are highly conserved between the human and mouse, and that murine SDF-1 induces signals through human CXCR4 (13, 25, 30, and see Discussion).
Biased Migration of Transgenic CD4+ T Cells.
We analyzed the in vivo migration of transgenic CD4+ T cells in cell transfer experiments. Peripheral lymphocytes of transgenic mice were injected intravenously into normal mice. Those of another transgenic mouse line, which expressed hCD4 but not hCXCR4 on its CD4 subset of T cells, were also injected into mice as controls. 3 or 16 h after injection, human CD4-positive murine CD4+CD8− T lymphocytes in tissues of recipient mice were analyzed and counted using three-color flow cytometry (Fig. 5, A and B). The number of circulating double transgenic CD4 SP T cells in peripheral blood was significantly lower than that of single transgenic CD4 SP T cells. In contrast, double transgenic CD4 SP T cell migration to bone marrow was higher than that of single transgenic control CD4 SP T cells. This discrepancy appeared increasingly pronounced when tissues were analyzed 16 h after cell transfer. Both transgenic CD4 SP T cells migrated to the spleen in similar numbers (data not shown). These results are compatible with the tissue distribution of CD4 SP T cells seen in double transgenic mice, and support the notion that the expression of hCXCR4 altered CD4 SP T cell homeostasis in blood by enhancing their migration to specific tissues, such as bone marrow.
Figure 5.
In vivo migration of transgenic CD4+ T cells. 9 × 106 (A) or 5 × 106 (B) lymphocytes from two transgenic mice lines expressing either hCD4 alone (hCXCR4−) or both hCD4 and hCXCR4 (hCXCR4+) were injected intravenously into normal BDF1 mice. The proportions of hCD4+, mCD4+, and mCD8− in each lymphocyte preparation were similar (16–23%). Values are mean numbers (±SD) of recovered hCD4+, mCD4+, and mCD8− donor T cells in peripheral blood (per 1 ml) or bone marrow (per two femurs) of recipient mice 3 (A) or 16 (B) h after injection. Two (A) and five (B) mice were used for each cell transfer. *P < 0.05, **P < 0.01, and ***P < 0.0001 determined by Student's t test. Similar results were obtained in three independent experiments.
Discussion
In this study, we analyzed transgenic mice coexpressing human CD4 and CXCR4 predominantly in their CD4+ T cells. The expression of these molecules facilitated the infection of mouse primary thymocytes with T-tropic strains of HIV-1 in vitro. Transgenic mice also displayed severe CD4+ T cell depletion in peripheral blood, with a concomitant increase of these cells in bone marrow. We demonstrated that trafficking of transgenic CD4+ T cells was biased toward bone marrow, inducing the CD4+ T cell loss from the blood pool. This phenotype has not been observed in transgenic mouse lines expressing hCD4 alone and was normalized transiently upon administration of a specific G-protein inhibitor, pertussis toxin. These findings and in vivo cell migration analysis indicated that signaling through hCXCR4 was a primary factor in developing this phenotype.
T-tropic HIV-1 Susceptibility of Transgenic Mouse Thymocytes.
The simultaneous expression of hCD4 and hCXCR4 in mouse CD4 T lymphocytes removed the restriction of HIV-1 infection in these cells to some extent. The level of p24 antigens, however, was significantly lower than that produced by human PBMCs after 4–6 d of infection (Fig. 2 A). This suggests that additional cellular factors are required to efficiently replicate viruses in mouse lymphocytes and presumably for the generalized HIV-1 infection in mice. It has been reported, for example, that cellular elements required for Tat and Rev functions during postentry HIV life cycle processes are lacking in mouse cell lines (26). Unlike T-tropic strains, M-tropic strain SF162 was shown to be noninfectious for transgenic thymocytes, which agrees with previous observations that M-tropic strains use different classes of chemokine receptors to enter cells (5, 6, 9, 10).
Human CD4 expression alone in mice does not confer sensitivity to T-tropic HIV-1 infection (31). The T-tropic HIV-1 sensitivity of hCD4+ single transgenic thymocytes was similar to that of nontransgenic thymocytes in our experiments (data not shown). Human CXCR4 expression, therefore, appears to be required for susceptibility, and structural differences in CXCR4 between the human and mouse may critically affect virus entry in primary T lymphocytes. However, rodent CXCR4 expression in nonpermissive hCD4+ cell lines has been shown to render these cells susceptible to T-tropic HIV-1 (32–34). Since the quantity of SDF-1 receptors was significantly higher in CD4+ T cells of double transgenic mice than in controls (Fig. 1 E), it is alternatively possible that the CXCR4 quantity expressed on the cell surface, irrespective of the species, may determine HIV-1 susceptibility and that endogenous CXCR4 levels expressed in lymphocytes from hCD4 single transgenic mice are not high enough for susceptibility.
Role of CXCR4 in Lymphocyte Trafficking and Homeostasis.
Lymphocyte migration through tissues is thought to involve various surface molecules such as adhesion molecules and chemokine receptors at multiple steps, and its physiological regulation appears complex (35, 36). CXCR4 molecules are expressed on hematopoietic cells such as lymphocytes and monocytes (28). Although mRNA of SDF-1, a natural ligand for CXCR4, can be detected in lymphoid and nonlymphoid tissues, its expression in bone marrow stromal cells is especially high (29). Since SDF-1 can function as a potent chemoattractant for lymphocytes (23) and hematopoietic progenitor cells (37), it has been proposed that it plays a role in lymphocyte trafficking through tissues for immune surveillance (23, 28) and in the homing of hematopoietic progenitor cells to bone marrow microenvironments (37). In SDF-1 null mice, hematolymphopoiesis was abolished in bone marrow but not in fetal liver, consistent with the proposed role for the SDF-1/CXCR4 pathway in hematopoietic cell development (38). Since these mice die perinatally, however, the in vivo role of SDF-1 for lymphocytes in adult mice has not been clarified.
The primary structures of murine and human SDF-1 are identical except for a single amino acid change of Ile to Val at position 18 (13), and mSDF-1 induces signals through hCXCR4 and vice versa (13, 25). CXCR4 is also highly conserved between the mouse and human, especially in its transmembrane and cytoplasmic amino acid sequences (96% identical) (30). Transgenic CD4+ T cells that express hCXCR4 showed, in fact, significantly higher mSDF-1 binding activity than those of controls (Fig. 1 E), indicating that transgenic mice overexpress receptors for endogenous SDF-1, specifically in their CD4+ T cells. This is compatible with the functional analysis showing that CD4, but not CD8, SP T cells of transgenic mice had a heightened chemotactic response to SDF-1 (Fig. 4). These mice thus provide an SDF-1 receptor-overexpression model for clarifying the in vivo role of the SDF-1/CXCR4 pathway in lymphocyte lineage cells. SDF-1 binding activity of transgenic CD4+ T cells was in a physiological range similar to the upregulated level in nontransgenic T cells upon in vitro activation.
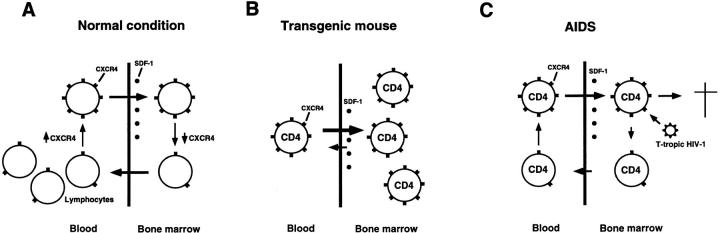
Our findings on transgenic mice are summarized as follows: (a) CD4 SP T cell depletion in peripheral blood accompanying their accumulation in bone marrow (Fig. 3, A and B); (b) transient normalization of this abnormal CD4 SP T cell distribution upon administration of a G-protein blocker, pertussis toxin (Fig. 3, C and D); and (c) preferential migration of hCD4+hCXCR4+ double transgenic CD4 SP T cells toward bone marrow and their rapid disappearance from peripheral blood, compared with hCD4+ single transgenic CD4 SP T cells, in cell transfer experiments (Fig. 5). These findings suggest that the CXCR4 pathway participates in lymphocyte trafficking through tissues and, specifically, may play a key role in lymphocyte circulation between peripheral blood and bone marrow whose stromal cells produce high levels of SDF-1 (29). It has been shown that CXCR4 expression in T lymphocytes is regulated by activation (28). The regulation of CXCR4 expression may therefore modulate lymphocyte trafficking between the blood pool and bone marrow reservoir (39, 40), influencing lymphocyte homeostasis in these compartments under physiological and pathological conditions such as viral infection and autoimmune diseases (Fig. 6 A). In our transgenic mice, such homeostasis appears to be specifically disrupted in CD4 SP T cells that constitutively express hCXCR4 (Fig. 6 B).
Figure 6.
Models for the CXCR4 role in lymphocyte trafficking and in AIDS pathogenesis. (A) CXCR4high lymphocytes such as activated lymphocytes (28) preferentially migrate to bone marrow or other tissues/microenvironments whose stromal cells produce high levels of SDF-1 (29). CXCR4 expression regulation may thus involve lymphocyte trafficking between peripheral blood and bone marrow, influencing lymphocyte homeostasis in these compartments. (B) In transgenic mice, hCXCR4 is constitutively expressed on CD4+ T cells, and SDF-1 receptors are overexpressed in these cells (see text). CD4+ T cells are therefore trapped in bone marrow, resulting in their loss from peripheral blood. (C) Model for the CD4+ T cell decline in AIDS patients. When active HIV-1 replication loci are present in bone marrow (42, 43), T-tropic HIV-1 infection accelerates because of preferential recruitment of CXCR4 high lymphocytes to bone marrow. Migrated CXCR4high CD4+ T cells are destroyed after HIV-1 infection and do not return to the blood pool. Note that the trafficking balance of CD4+ T cells through bone marrow mimics that in transgenic mice in B.
Other molecule(s) involved in cell migration rather than CXCR4 may play a role in developing the transgenic mouse phenotype. Forced CXCR4 expression, for example, would modulate other pertussis-toxin-sensitive chemokine receptors that actually induce the phenotype. The in vitro chemotaxis of transgenic CD4 SP T cells to several lymphocyte chemoattractants other than SDF-1, however, did not significantly differ from that of nontransgenic CD4 SP T cells (Table 1), favoring the possibility that signaling through hCXCR4 upon binding endogenous SDF-1 changed the CD4 SP T cell distribution in transgenic mice.
Hypothetical AIDS Pathogenesis Model.
The resemblance of blood lymphocyte profiles of transgenic mice and AIDS patients prompts us to surmise a link between CXCR4 function and AIDS pathogenesis. CXCR4high lymphocytes (e.g., activated lymphocytes; reference 28), are expected to migrate preferentially to bone marrow, as suggested by our transgenic mice, and presumably to other tissues or microenvironments that produce critical SDF-1 levels. The CXCR4 expression level appears correlated with T-tropic HIV-1 infectability (41). When bone marrow contains active HIV replication loci (42, 43), such a milieu may provide selective pressure to accumulate viruses that use CXCR4 for cell entry, and would accelerate T-tropic HIV-1 infection by recruiting susceptible CXCR4high CD4+ T cells. This would lead to a severe CD4+ T cell trafficking imbalance in the bone marrow compartment, where infected cells would be destroyed and unable to return to the blood pool, and the situation would eventually resemble that resulting from “trapping” in the bone marrow of transgenic mice (Fig. 6 C). Such an absorption of CD4+ T cells from circulating blood may also finally drain peripheral lymphoid organs.
Clinical observations that HIV-1 infection and activity in bone marrow correlate with CD4 cell depletion in progressive stages (43–45) appear to support this hypothesis. According to this view, the recovery of CD4+ T cell homeostasis in the peripheral blood of patients after potent anti–HIV therapy (46) could be due, at least in part, to an improved trafficking imbalance. Observations that SDF-1 inhibits T-tropic HIV-1 replication in vitro (13, 14, 41) may contradict this model. It is conceivable, however, that SDF-1 gradients in bone marrow microenvironments generate local regions displaying levels that enable T-tropic HIV-1 to replicate. It has been shown that the concentration of SDF-1 required to inhibit HIV-1 infection appears to be significantly higher than that required for receptor signaling or binding (47). Many CXCR4 using primary HIV-1 isolates were also shown to be resistant to SDF-1-mediated suppression of PBMC infection in contrast to laboratory-adapted, T cell line-tropic strains (48). The analysis of in situ SDF-1 expression, CXCR4 expression in lymphocyte subsets, and the status of HIV replication in tissues, including bone marrow, during HIV-1 infection may therefore help further our understanding of AIDS pathogenesis.
Acknowledgments
We thank S. Ishii for support and comments on the manuscript, H. Nakauchi for support, D.R. Littman, J.A. Hoxie, and H. Yagita for transgenic vectors, an 12G5 antibody, and pCγ1/Bluescript SK (−) vector, respectively, and T. Yamaguchi and T. Obina for technical assistance.
This work was supported by Japan Science and Technology Corporation (S. Sawada).
Abbreviations used in this paper
- CD4 (and CD8) SP
CD4 (and CD8) single positive
- h
human
- m
murine
- M-tropic
macrophage-tropic
- MIP
macrophage inflammatory protein
- RANTES
regulated on activation, normal T cell expressed and secreted
- SDF-1
stromal cell–derived factor 1
- T-tropic
T cell line–tropic
References
- 1.Cheng-Mayer C, Seto D, Tateno M, Levy JA. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 2.Tersmette M, de Goede REY, Al BJM, Winkel IN, Gruters RA, Cuypers HT, Huisman HG, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncitium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenyo EM, Morfeldt-Manson L, Chiodi F, Lind B, von Gegerfelt A, Albert J, Olausson E, Asjo B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988;62:4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1977;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Souza MP, Harden VA. Chemokines and HIV-1 second receptors. Nat Med. 1996;2:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 6.Unutmaz D, Littman DR. Expression pattern of HIV coreceptors on T cells: implications for viral transmission and lymphocyte homing. Proc Natl Acad Sci USA. 1997;94:1615–1618. doi: 10.1073/pnas.94.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schall TJ. Biology of the RANTES/SIS cytokine family. Cytokine. 1991;3:165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- 8.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 9.Lias F, Alkhatib G, Peden KWC, Sharma G, Berger EA, Farber JM. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng H, Unutmaz D, Kewal-Ramani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G-protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 12.Berson JF, Long D, Doranz BJ, Rucker J, Jirik FR, Doms RW. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type I strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 14.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler DF, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 15.Mackay CR. Chemokine receptors and T cell chemotaxis. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 17.Killeen N, Sawada S, Littman DR. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO (Eur Mol Biol Organ) J. 1993;12:1547–1553. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomura M, Matsuda Y, Itoh H, Hori T, Suzuki G. Genetic mapping of the mouse stromal cell-derived facto gene (Sdf 1) to mouse and rat chromosomes. Cytogenet Cell Genet. 1996;73:286–289. doi: 10.1159/000134357. [DOI] [PubMed] [Google Scholar]
- 19.Yamada A, Murakami M, Ijima K, Yagita H, Okumira K, Komatsu S, Uede T. Long-term acceptance of major histocompatibility complex–mismatched cardiac allograft induced by a low dose of CTLA4IgM plus FK506. Microbiol Immunol. 1996;40:513–518. doi: 10.1111/j.1348-0421.1996.tb01102.x. [DOI] [PubMed] [Google Scholar]
- 20.Karasuyama H, Kudo A, Melchers F. The proteins encoded by the VpreB and λ5 pre-B cell-specific genes can associate with each other and with μ heavy chain. J Exp Med. 1990;172:969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 23.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamann, A., and P. Jonas. 1997. Lymphocyte migration in vivo: the mouse model. In Immunology Methods Manual. I. Lefkovits, editor. Academic Press Inc., San Diego, CA. 1333–1341.
- 25.Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O, Matsushima K, Yoshida N, Springer TA, Kishimoto T. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell–derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci USA. 1997;93:14726–14729. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winslow BJ, Trono D. The blocks to human immunodeficiency virus type I tat and revfunctions in mouse cell lines are independent. J Virol. 1993;67:2349–2354. doi: 10.1128/jvi.67.4.2349-2354.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barzaga-Gilbert E, Grass D, Lawrance SK, Peterson PA, Lacy E, Engelhard VH. Species specificity and augmentation of responses to class II major histocompatibility complex molecules in human CD4 transgenic mice. J Exp Med. 1992;175:1707–1715. doi: 10.1084/jem.175.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 30.Heesen M, Berman MA, Benson JD, Gerard C, Dorf ME. Cloning of the mouse fusin gene, homologue to a human HIV-1 co-factor. J Immunol. 1996;157:5455–5460. [PubMed] [Google Scholar]
- 31.Lores P, Boucher V, Mackay C, Pla M, von Boehmer H, Jami J, Barre-Sinoussi F, Weill J-C. Expression of human CD4 in transgenic mice does not confer sensitivity to human immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1992;8:2063–2070. doi: 10.1089/aid.1992.8.2063. [DOI] [PubMed] [Google Scholar]
- 32.Tachibana K, Nakajima T, Sato A, Igarashi K, Shida H, Iizasa H, Yoshida N, Yoshie O, Kishimoto T, Nagasawa T. CXCR4/fusin is not a species-specific barrier in murine cells for HIV-1 entry. J Exp Med. 1997;185:1865–1870. doi: 10.1084/jem.185.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pleskoff O, Sol N, Labrosse B, Alizon M. Human immunodeficiency virus strains differ in their ability to infect CD4+cells expressing the rat homolog of CXCR-4 (fusin) J Virol. 1997;71:3259–3262. doi: 10.1128/jvi.71.4.3259-3262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bieniasz PD, Fridell RA, Anthony K, Cullen BR. Murine CXCR-4 is a functional coreceptor for T-cell-tropic and dual-tropic strains of human immunodeficiency virus type-1. J Virol. 1997;71:7097–7100. doi: 10.1128/jvi.71.9.7097-7100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 36.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 37.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 39.Benner, R. 1981. The bone marrow: a major site of antibody production. In The Immune System 1. Past and Future. C.M. Steinberg and I. Lefkovits, editors. Karger, Basel. 362–371.
- 40.Osmond DG. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 41.Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J-L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α–dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiley EL, Nightingale SD. Opportunistic events and p17 expression in the bone marrow of human immunodeficiency virus-infected patients. J Infect Dis. 1994;169:617–620. doi: 10.1093/infdis/169.3.617. [DOI] [PubMed] [Google Scholar]
- 43.Weiser B, Burger H, Campbell P, Donelan S, Mladenovic J. HIV type 1 RNA expression in bone marrows of patients with a spectrum of disease. AIDS Res Hum Retroviruses. 1996;12:1551–1558. doi: 10.1089/aid.1996.12.1551. [DOI] [PubMed] [Google Scholar]
- 44.Davis BR, Schwartz DH, Marx JC, Johnson CE, Berry JM, Lyding J, Merigan TC, Zander A. Absent or rare human immunodeficiency virus infection of bone marrow stem/progenitor cells in vivo. J Virol. 1991;65:1985–1990. doi: 10.1128/jvi.65.4.1985-1990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanley SK, Kessler SW, Justement JS, Schnittman SM, Greenhouse JJ, Brown CC, Musongela L, Musey K, Kapita B, Fauci AS. CD34+bone marrow cells are infected with HIV in a subset of seropositive individuals. J Immunol. 1992;149:689–697. [PubMed] [Google Scholar]
- 46.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 47.Crump MP, Gong J-H, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier J-L, Baggiolini M, Sykes BD, Clark-Lewis I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO (Eur Mol Biol Organ) J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng HK, Malnati MS, Plebani A, Siccardi AG, Littman DR, Fenyo EM, Lusso P. In vivoevolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]