Abstract
Murine L929 fibrosarcoma cells treated with tumor necrosis factor (TNF) rapidly die in a necrotic way, due to excessive formation of reactive oxygen intermediates. We investigated the role of caspases in the necrotic cell death pathway. When the cytokine response modifier A (CrmA), a serpin-like caspase inhibitor of viral origin, was stably overexpressed in L929 cells, the latter became 1,000-fold more sensitive to TNF-mediated cell death. In addition, TNF sensitization was also observed when the cells were pretreated with Ac-YVAD-cmk or zDEVD-fmk, which inhibits caspase-1– and caspase-3–like proteases, respectively. zVAD-fmk and zD-fmk, two broad-spectrum inhibitors of caspases, also rendered the cells more sensitive, since the half-maximal dose for TNF-mediated necrosis decreased by a factor of 1,000. The presence of zVAD-fmk also resulted in a more rapid increase of TNF-mediated production of oxygen radicals. zVAD-fmk–dependent sensitization of TNF cytotoxicity could be completely inhibited by the oxygen radical scavenger butylated hydroxyanisole. These results indicate an involvement of caspases in protection against TNF-induced formation of oxygen radicals and necrosis.
Tumor necrosis factor (TNF) is an important mediator in many immunological and inflammatory responses, as well as in a number of pathological conditions. In vitro, TNF is able to induce cell death, activation of transcription factors, and proliferation (1–3). In murine L929 fibrosarcoma cells, TNF induces necrosis, a type of cell death characterized by swelling, finally leading to disruption of the plasma membrane (4). This cytotoxicity is due to an increase in oxygen radical accumulation; inhibition of this process by particular radical scavengers blocks TNF-induced cell death (5). On the other hand, L929 cells can be killed by apoptosis when human Fas antigen is expressed and triggered by agonistic antibodies (6, 7). Apoptosis is mainly characterized by membrane blebbing, DNA fragmentation, shrinking, and condensation of the cells and their organelles, and subsequent disintegration (8).
Recent research has documented that caspases play an important role in apoptosis (9–11). Inhibition of one or more caspases can block apoptotic cell death induced by several stimuli; current models implicate caspase-8, and possibly caspase-10b, as key mediators of TNF- and Fas-mediated apoptosis (12–15). In this study, we investigated the role of caspases in TNF-mediated necrosis. We found that treatment of L929 cells with caspase inhibitors sensitize rather than protect against this mode of cell death.
Materials and Methods
Cells.
L929 murine fibrosarcoma cells and HeLa H21 cervix carcinoma cells were cultured in DMEM supplemented with 5% newborn bovine serum and 5% FCS, penicillin (100 U/ml) streptomycin (0.1 mg/ml), and L-glutamine (0.03%). KYM rhabdomyosarcoma and PC60R55R75 murine T cell hybridoma cells were cultured in RPMI 1640, supplemented with 10% FCS, penicillin (100 U/ml), streptomycin (0.1 mg/ml), and L-glutamine (0.03%), and additionally 2-mercaptoethanol (5 × 10−5 M) and sodium pyruvate (1 mM) for PC60R55R75 cells.
Cytokines, Antibodies, and Reagents.
Recombinant murine TNF was produced in our laboratory and was purified to at least 99% homogeneity. The specific activity was 1.4 × 108 IU/mg, as determined in a standardized cytotoxicity assay on L929 cells. Actinomycin D, butylated hydroxyanisole (BHA),1 diethylmaleate (DEM), H2O2, and tert-butyl hydroperoxide (tBuOOH) were purchased from Sigma Chemical Co. (St. Louis, MO). Monochlorobimane was supplied by Molecular Probes (Eugene, OR). Dihydrorhodamine 123 (DHR123; Molecular Probes) was prepared as a 5 mM stock solution in DMSO and was used at 1 μM. Propidium iodide (PI; Becton Dickinson, San Jose, CA) was dissolved at 3 mM in PBS and was used at 30 μM.
The caspase peptide inhibitors zDEVD-fmk, zVAD-fmk, and zD-fmk were purchased from Enzyme Systems Products (Dublin, CA). Ac-YVAD-cmk and zAAD-cmk were supplied by Calbiochem–Novabiochem International (San Diego, CA). Anti–cytokine response modifier A (CrmA) antibodies were provided by D. Pickup (Durham, NC).
Plasmids.
Cowpox CrmA cDNA (a gift from D. Pickup, Durham, NC) was inserted as an EcoRI fragment into the EcoRI site of pCAGGS (16). pSV2neo, which contains the neomycin-resistant gene under control of the SV40 early promoter, was used as a selection marker (17).
Cytotoxicity Assays.
Cells were seeded on day −1 at 2 × 104 cells/well in a 96-well plate. The next day, inhibitors and TNF were added at the given concentrations. Typically, the cells were incubated with TNF or H2O2 for 18 h, and cell viability was assessed using staining with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide as previously described (18). The percentage of cell survival was calculated as follows: (A 595/655 treated cells − A 595/655 medium)/(A 595/655 untreated cells − A 595/655 medium) × 100.
Measurement of Oxygen Radical Formation and Cell Death by Flow Cytometry.
DHR123 was added at the same time as TNF to suspension cultures, obtained by seeding cells in uncoated 24-well tissue culture plates (Sarstedt, Newton, NC). Cell samples were taken at different time points and analyzed on a FACScalibur® flow cytometer equipped with a 488-nm argon ion laser. PI fluorescence was detected at 610 nm and served as a measure for cell death. Rhodamine 123 fluorescence, as a result of DHR123 oxidation, was analyzed on PI-negative cells and detected at 525 nm. Relative rhodamine 123 fluorescence is defined as the ratio between emitted fluorescence at a given time point and initial fluorescence for the same condition.
Quantitation of Free Thiol Groups in Cell Lysates.
Cells were seeded on day −1 at 2 × 104 cells/microwell. The next day, zVAD-fmk (25 μM) or DEM (3 mM) were added 2 h before or 3 h after the start of TNF treatment, respectively. After 0.5 h of incubation with DEM, the supernatants were replaced with 400 μM monochlorobimane (19) in PBS and incubated for 30 min. After washing the cells, fluorescence was measured in a spectrofluorometer (CytoFluor 2300; PerSeptive Biosystems, Cambridge, MA) at 480 nm, using an excitation wavelength of 360 nm.
Fluorogenic Substrate Assay for Caspase Activity.
Cytosolic cell extracts were prepared by lysing the cells in a buffer containing 1% Nonidet P-40, 200 mM NaCl, 20 mM Tris/HCl, pH 7.4, 10 μg/ml leupeptin, aprotinin (0.27 trypsin inhibitory U/ml), and 100 μM PMSF. Caspase-1– or caspase-3–like activities were determined by incubation of cell lysate (containing 25 μg total protein) with 50 μM of the fluorogenic substrates Ac-YVAD-AMC or Ac-DEVD-AMC (Peptide Institute, Osaka, Japan), respectively, in 200 μl cell-free system buffer, comprising 10 mM Hepes, pH 7.4, 220 mM mannitol, 68 mM sucrose, 2 mM NaCl, 2.5 mM KH2PO4, 0.5 mM EGTA, 2 mM MgCl2, 5 mM pyruvate, 0.1 mM PMSF, and 1 mM dithiothreitol (12). The release of fluorescent 7-amino-4-methylcoumarin was measured for 1 h at 2 min intervals by spectrofluorometry; data are expressed as the increase in fluorescence as a function of time.
Measurement of Nuclear Factor (NF)-κB Activity.
L929 cells carried a reporter construct consisting of a luciferase gene under control of the minimal chicken conalbumin promoter preceded by three NF-κB sites (20). Cells were seeded on day −1 at 2 × 104/microwell. The next day, cells were pretreated with different caspase inhibitors for 2 h and stimulated with TNF. After 3 h of incubation, cells were lysed according to the luciferase assay protocol of Promega Biotec (Madison, WI); luciferin (Duchefa Biochemie, Haarlem, The Netherlands) was added and luciferase activity was measured on a Topcount Luminometer (Packard Instrument Co., Meriden, CT).
Results
Overexpression of CrmA Renders L929 Cells More Sensitive to TNF-mediated Necrosis.
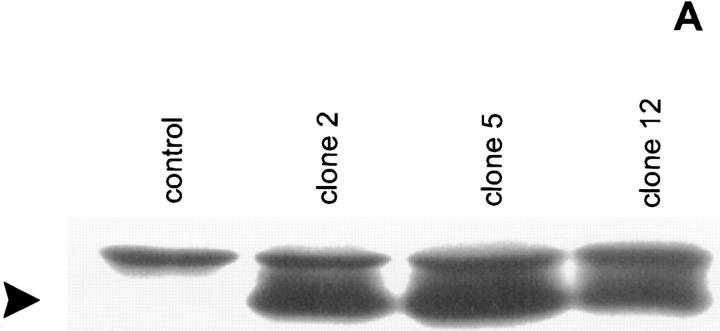
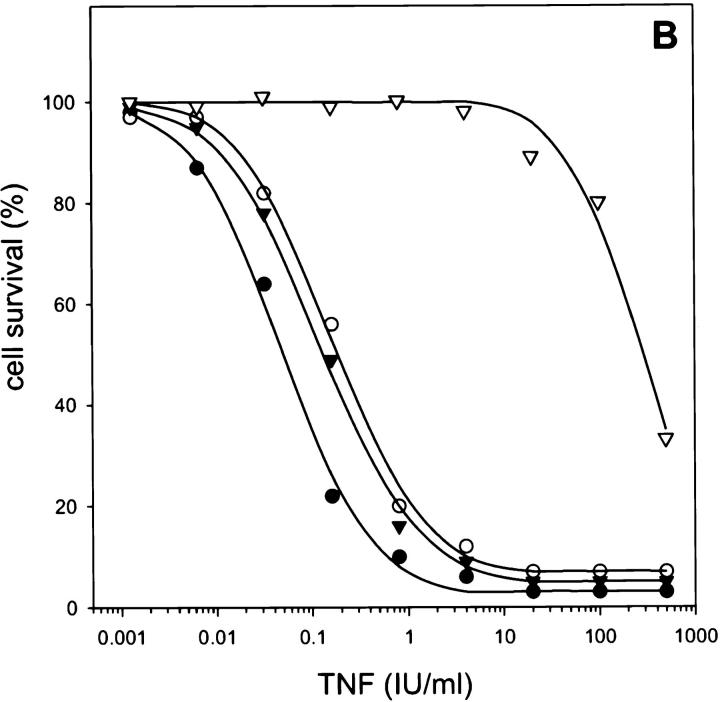
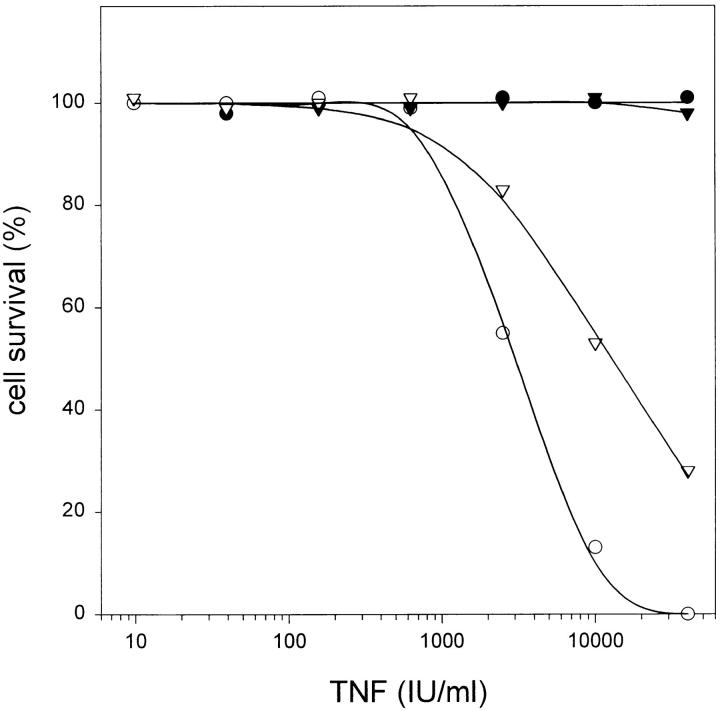
L929 cells were cotransfected with cDNA encoding CrmA from cowpox virus and a pSV2neo selection plasmid. Individual neomycin-resistant clones were screened for CrmA expression by Western analysis and tested for their sensitivity to TNF-mediated necrosis (Fig. 1). Cells expressing CrmA were up to 1,000 times more sensitive to TNF as compared to mock-transfected cells (LD50 of ∼0.05 IU/ml, as compared to ∼50 IU/ml for control clones). These results suggest a protective role for CrmA-sensitive caspases against TNF-induced production of oxygen radicals.
Figure 1.
CrmA expression enhances TNF-induced necrosis. (A) Western blot analysis of transfected L929 clones. Lane 1, control L929 cells transfected with pSV2neo alone; lanes 2–4, different clones cotransfected with pCAGGS CrmA and pSV2neo. Arrowhead, CrmA expression. (B) Sensitizing effect on TNF-mediated necrosis in L929 cells. ▿, control; •, clone 2; ○, clone 5; and ▾, clone 12.
Blocking of Caspases by Oligopeptide Inhibitors Sensitizes L929 Cells to TNF-mediated Necrosis.
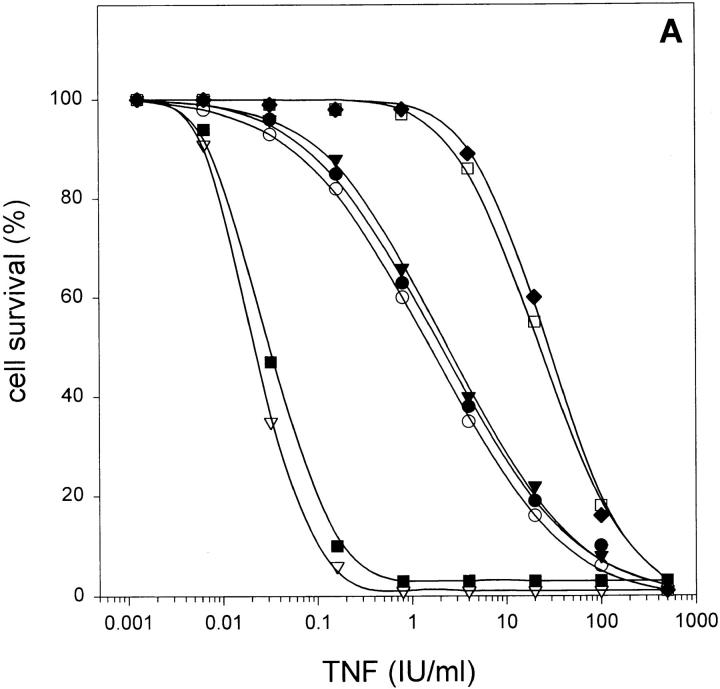
L929 cells were pretreated for 2 h with various caspase inhibitors, and their sensitivity to TNF was analyzed. When the cells were pretreated with Ac-YVAD-cmk or zDEVD-fmk (100 μM), which are tetrapeptide inhibitors of caspase-1 and caspase-3 subfamily members, respectively, they became significantly more sensitive to TNF-mediated cell death (with LD50 of 1 IU/ml as compared to 30 IU/ml for controls; Fig. 2 A). When Ac-YVAD-cmk and zDEVD-fmk were combined, no additional sensitization was observed, suggesting that they act on the same pathway. Two more broad-range caspase-blocking agents are zVAD-fmk and zD-fmk. When these inhibitors were added before TNF stimulation at a concentration of 25 μM, they drastically sensitized the cells to TNF (LD50 of 0.02 IU/ml). In contrast, zAAD-cmk, an inhibitor of granzyme B, did not alter TNF sensitivity, excluding nonspecific effects. Taken together, it is evident that members of the caspase family are responsible for protection against TNF-induced necrosis in L929 cells. Presumably additional caspases besides caspase-1 or caspase-3 are involved in this protective effect, as suggested by the weak sensitization by Ac-YVAD-cmk and zDEVD-fmk, compared to the strong effect of zVAD-fmk and zD-fmk.
Figure 2.
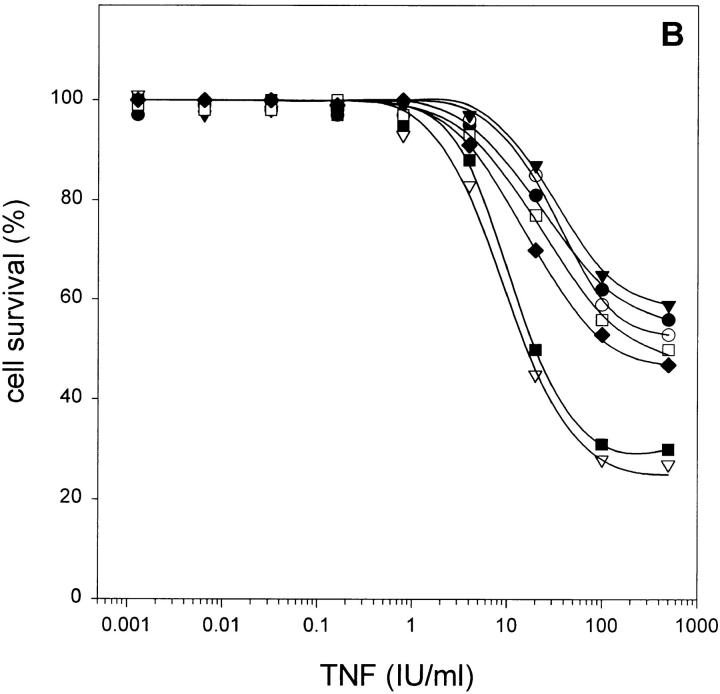
Sensitizing effect of peptide caspase inhibitors on TNF-induced necrosis in L929 cells, added 2 h before TNF treatment. (A) Without addition of BHA. •, Ac-YVAD-cmk (100 μM); ○, zDEVD-fmk (100 μM); ▾, Ac-YVAD-cmk + zDEVD-fmk (100 μM each); ▿, zVAD-fmk (25 μM); ▪, zD-fmk (25 μM); □, zAAD-cmk (100 μM); and ♦, control. (B) With BHA (100 μM) added at the same time as TNF (same symbols as in A).
Sensitization of TNF-induced Necrosis by Peptide Caspase Inhibitors Is Abrogated by BHA.
Death of L929 cells after incubation with TNF follows excessive production of oxygen radicals in the mitochondria, and scavenging of these radicals by some antioxidants, such as BHA, protects the cells (5). When the effect of peptide caspase inhibitors on TNF-induced necrosis of L929 cells was analyzed in the presence of BHA, their sensitizing effect was completely abrogated in the case of zDEVD-fmk or Ac-YVAD-cmk, and to a great extent, when zVAD-fmk or zD-fmk were used (Fig. 2 B). This indicates that sensitization by caspase inhibitors enhances oxygen radical-dependent cytotoxicity.
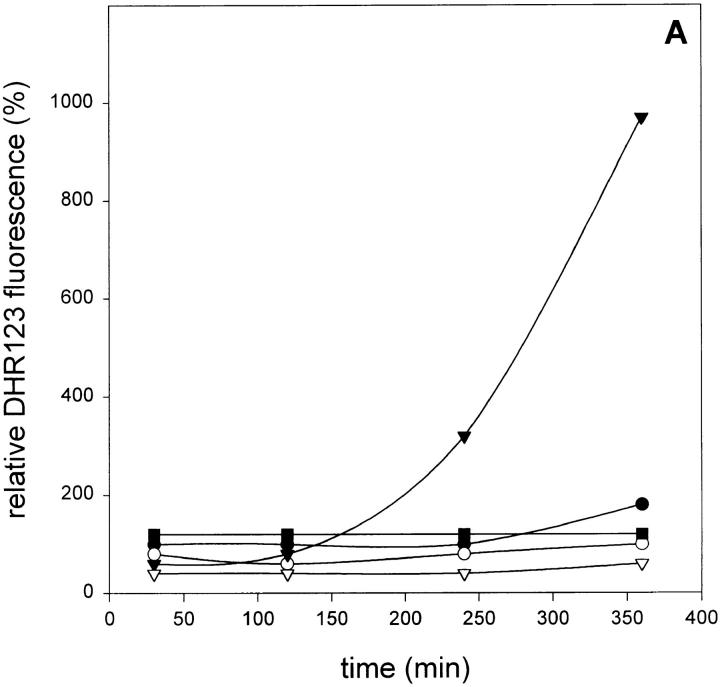
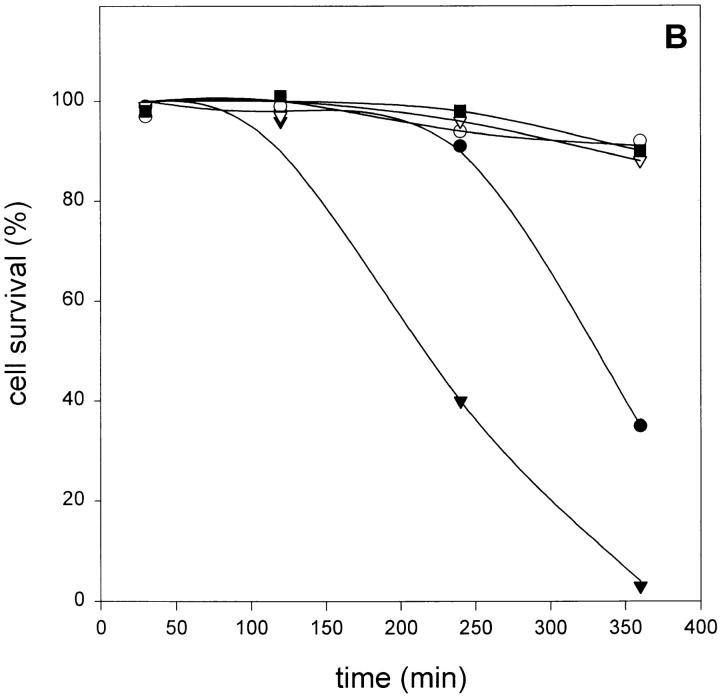
Enhanced Cytotoxicity in the Presence of zVAD-fmk Is Correlated with Increased Oxygen Radical Accumulation.
Oxygen radical accumulation was fluorimetrically measured using DHR123 oxidation as a specific marker. Since rhodamine 123 fluorescence was measured in cells with intact membranes (PI-negative), the influence of PI fluorescence could be ruled out. As shown in Fig. 3 A, incubation of L929 cells with TNF resulted in a small but significant increase of oxygen radicals, which could be blocked by BHA. However, when the cells were pretreated with zVAD-fmk, oxygen radical levels raised up to 10-fold after 6 h of treatment with TNF. Again, BHA (100 μM) could strongly inhibit this radical accumulation. zVAD-fmk alone had no effect on radical production after 6 h. Fig. 3 B shows cell killing of the same samples, as measured by PI uptake due to loss of cell membrane integrity, demonstrating the correlation between oxygen radical accumulation and cell death.
Figure 3.
Effect of zVAD-fmk on TNF-induced reactive oxygen formation and cell death. (A) Effect on TNF-induced oxygen radical production (relative DHR123 fluorescence as compared to untreated cells). •, TNF alone (500 IU/ml); ○, TNF + BHA (100 μM); ▾, TNF + zVAD-fmk (25 μM); ▿, TNF + zVAD-fmk + BHA; and ▪, zVAD-fmk alone. (B) Effect on TNF-induced cell killing determined on the basis of PI-negative cells (same experiment and symbols as in A).
Increased Oxygen Radical Accumulation After TNF + zVAD Treatment Is the Result of Higher Radical Production Rather than an Impaired Scavenging System.
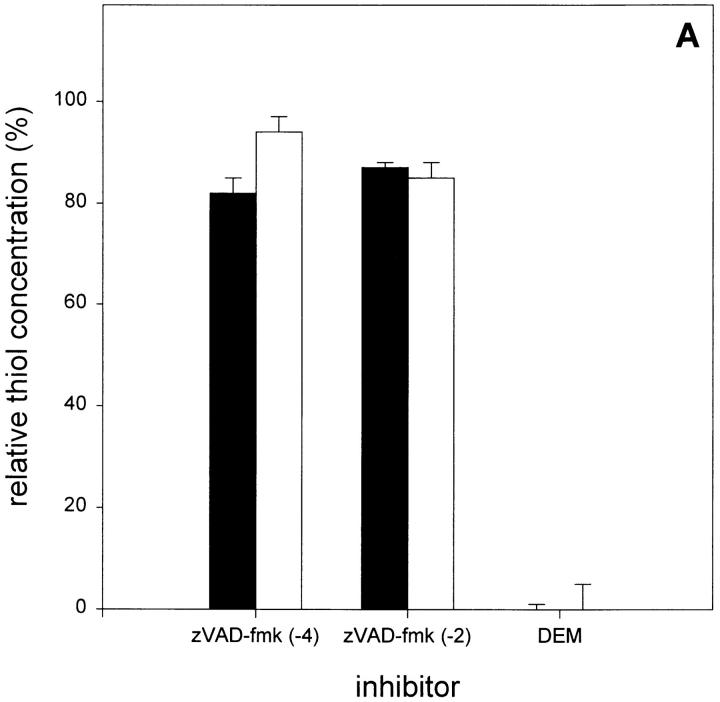
In the case of TNF-mediated radical production in L929 cells, it was previously shown that excess radicals are scavenged by the mitochondrial glutathione system (5). As the increased levels of oxygen radicals after TNF + zVAD-fmk treatment may result either from an enhanced production of radicals or an impaired mitochondrial glutathione system, we analyzed cellular thiol concentrations using monochlorobimane fluorescence after treatment with zVAD-fmk in the presence or absence of TNF. However, no significant decrease in fluorescence could be observed (Fig. 4 A). This suggests that the sensitizing effect of zVAD-fmk on TNF-mediated oxygen radical production is not the result of depleted thiol pools, such as mitochondrial glutathione.
Figure 4.
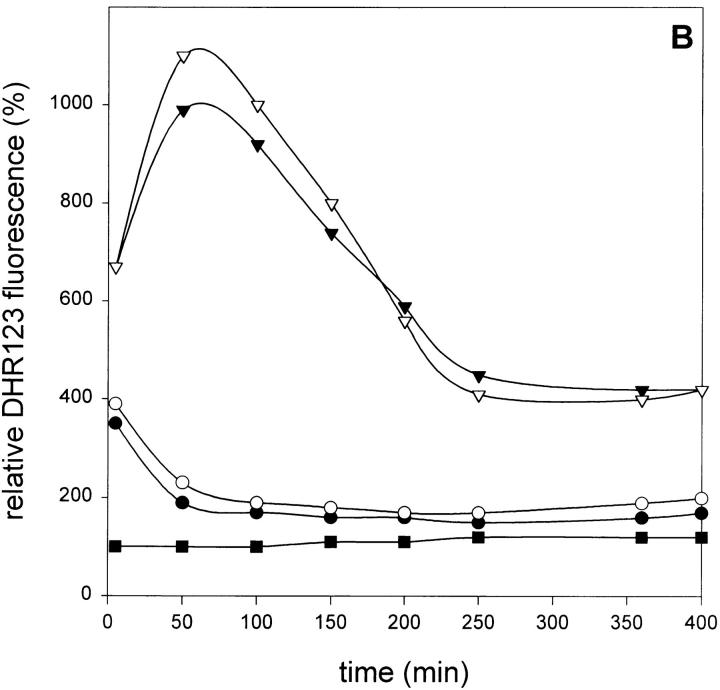
Effect of zVAD-fmk on radical scavenging in L929 cells. (A) zVAD-fmk does not alter free thiol concentrations. Cells were treated with zVAD-fmk (25 μM) for 4 h [zVAD-fmk (-4)] or 2 h [zVAD-fmk (-2)] before TNF addition, or with DEM 3 h after TNF addition. Open bars, without TNF; filled bars, 1,000 IU/ml TNF. (B) Effect of zVAD-fmk on H2O2- or tBuOOH-induced oxygen radical production (relative DHR123 fluorescence as compared to untreated cells). •, H2O2 (50 μM); ○, H2O2 + zVAD-fmk (25 μM); ▾, tBuOOH (100 μM); ▿, tBuOOH + zVAD-fmk; and ▪, zVAD-fmk alone.
Therefore, we tested whether zVAD-fmk had any effect on the accumulation of radicals induced by the addition of exogenous H2O2 or tBuOOH, which cause lipid peroxidation in the cells. As shown in Fig. 4 B, zVAD-fmk did not alter radical accumulation. Again, this indicates that the signaling pathway to radical formation, rather than the scavenging capacity of the cells, is affected by caspase inhibition.
TNF Treatment Does Not Result in Detectable Caspase Activity in L929 Cells.
To study whether caspase activity occurs after TNF treatment of L929 cells, lysates were prepared after several incubation periods. Caspase-3– and caspase-1–like activities were determined with the substrates Ac-DEVD-AMC and Ac-YVAD-AMC, respectively. As shown in Table 1, no significant 7-amino-4-methylcoumarin release was detected in L929 lysates. PC60R55R75 cells, which die in an apoptotic mode after TNF treatment (21), were used as a control. After 4 h, DEVD cleavage activity began to appear, peaking at ∼6 h. These results suggest that caspase activity is correlated with apoptotic and not with necrotic cell death. Furthermore, the sensitization by caspase inhibitors apparently is due to inhibition of low, constitutive levels of caspases.
Table 1.
Activation of Caspase-1– and Caspase-3–like Proteases in L929 and PC60R55R75 Cells
| TNF treatment (10,000 IU/ml) | L929 cells | PC60R55R75 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ac-YVAD-AMC | Ac-DEVD-AMC | Ac-YVAD-AMC | Ac-DEVD-AMC | |||||
| (h) | ||||||||
| 1 | 26 | 10 | 11 | 11 | ||||
| 2 | 22 | 14 | 15 | 15 | ||||
| 3 | 17 | 31 | 18 | 25 | ||||
| 4 | 20 | 25 | 22 | 57 | ||||
| 6 | 15 | 20 | 14 | 492 | ||||
| 11 | 15 | 15 | 8 | 431* | ||||
| 24 | 8 | 11 | 8 | 117* | ||||
Values given are maximal fluorescence releases (arbitrary U/min) measured during a 1-h incubation period with the substrates indicated.
Decrease due to cell death.
TNF-mediated Apoptosis in HeLa H21 and KYM Cells Is Inhibited by Caspase Inhibitors.
To test whether only TNF-mediated necrosis was enhanced by inhibition of caspases, the effect of zVAD-fmk was also analyzed in HeLa H21 and KYM cells, which respond to TNF treatment by dying in an apoptotic way. When these cells were pretreated with zVAD-fmk for 2 h before TNF addition, complete protection against TNF was observed, even at 40,000 IU/ml TNF (Fig. 5). These results indicate that the antagonistic role of caspases is specific for TNF-induced reactive oxygen formation leading to necrosis.
Figure 5.
Inhibitory effect of zVAD-fmk on TNF-mediated apoptosis in HeLa H21 cells (• and ○; 1 μg/ml actinomycin D added) and KYM cells (▾ and ▿). Open symbols, TNF only; closed symbols, 25 μM zVAD-fmk added 2 h before TNF treatment.
Caspase Inhibitors Do Not Enhance NF-κB Activation.
Treatment of L929 cells with TNF also results in activation of the transcription factor NF-κB (22). Using a reporter construct consisting of two NF-κB sites and a minimal promoter linked to the luciferase gene, we checked whether NF-κB activation was affected by caspase inhibitors. Table 2 shows relative luciferase activities after 3 h, as compared to untreated cells. In contrast to the 1,000-fold sensitization of TNF-mediated necrosis, the presence of caspase inhibitors does not influence TNF-dependent activation of NF-κB. At 500 IU/ml TNF, luciferase activity in the presence of zVAD-fmk was even lower than in cells treated with TNF alone. However, as revealed by microscopic analysis, cells were already dying at that point. We conclude that the higher sensitivity to the cytotoxic activity of TNF on L929 cells in the presence of caspase inhibitors is not correlated with altered NF-κB activation.
Table 2.
NF-κB Activation Is Not Affected by Caspase Inhibitors
| TNF | Control | Ac-YVAD-cmk | zDEVD-fmk | zVAD-fmk | ||||
|---|---|---|---|---|---|---|---|---|
| IU/ml | ||||||||
| 0.032 | 1.2 | 1.1 | 1.1 | 1.2 | ||||
| 0.8 | 4.1 | 3.7 | 3.4 | 4.4 | ||||
| 20 | 9.3 | 9.5 | 8.8 | 8.3 | ||||
| 500 | 10.4 | 10.0 | 9.3 | 6.4* |
Values are relative luciferase activities as compared to unstimulated cells. Ac-YVAD-cmk and zDEVD-fmk were used at 100 μM; zVAD-fmk was used at 25 μM.
Decrease due to cell death.
Discussion
In this study, we investigated the role of caspases in TNF-mediated necrosis. First, we used the cowpox CrmA gene product as an inhibitor of a number of caspases. Surprisingly, expression of CrmA in L929 cells rendered them far more sensitive to TNF as compared to control cells not expressing CrmA. Furthermore, blocking of caspases by peptide inhibitors sensitized the cells to TNF-induced cytotoxicity. zDEVD-fmk and Ac-YVAD-cmk had moderate sensitizing activity, whereas zVAD-fmk and zD-fmk strongly potentiated TNF-mediated necrosis. In the latter case, the concentration of TNF required for half-maximal cytotoxicity decreased 1,000-fold. zDEVD-fmk and Ac-YVAD-cmk have a different specificity pattern, and when they were combined, they could not synergize with each other, suggesting the possibility that they inhibit consecutively acting caspases. TNF sensitization induced by zVAD-fmk was accompanied by an enhanced production of oxygen radicals, as measured by DHR123 oxidation. Scavenging of oxygen radicals by BHA completely abrogated the sensitizing effect of zVAD-fmk on TNF-induced necrosis. This indicates that enhanced oxygen radical production is the main cause of zVAD-fmk–mediated sensitization.
KYM and HeLa H21 cells respond to TNF treatment in an apoptotic way. When these cells were treated with TNF in the presence of zVAD-fmk, cell death was inhibited, revealing a fundamental difference between necrosis and apoptosis. In contrast to apoptosis, TNF-induced necrosis of L929 cells is not dependent on caspase activation; rather, the results shown here indicate a protective role for a low level of constitutively active caspase(s) in this mode of cell death. Alternatively, TNF may induce activation of a caspase that counteracts or deviates the pathway leading to mitochondrial oxygen radical production; this caspase activity would be at a level below the detection limit obtainable with fluorogenic substrates. TNF-induced cell death is primarily mediated by the p55 TNF receptor (21), which contains a death domain (DD) in its intracellular part. Upon ligand-induced clustering of receptor DDs, other DD-containing components of the signaling pathway are recruited, leading to cell death (3, 23–25). In the case of TNF-mediated necrosis in L929 cells, the DD of the p55 TNF receptor has been shown to be necessary and sufficient for fully active TNF signaling to necrosis (26). TRADD, which also has a DD, binds to the DD of clustered p55 TNF receptor, and is in turn necessary for recruiting the DD-containing FADD/MORT1 (25, 27, 28). The latter was first identified as a factor recruited by Fas, another DD-containing receptor, upon activation (14). In the case of the p55 TNF receptor, recruitment of FADD in the receptor complex has not yet been demonstrated at physiological receptor numbers. FADD/MORT1 possesses another domain that connects Fas and the TNF receptor complex to caspase-8 (12, 13) or the homologues caspase-10 and caspase-10b (15). Caspase-8 contains a COOH-terminal caspase-3–like domain that is proteolytically released into the cytosol after stimulation of Fas or the p55 TNF receptor (14). It is generally assumed that caspase-8 is the apex of a pathway leading to apoptosis in which the downstream executors are other caspases. The proteolytic activity of caspase-8 and caspase-10b is inhibited by zVAD-fmk, zDEVD-fmk, and CrmA, but not by Ac-YVAD-cmk (14, 29, 30). In this study, we show that neither zVAD-fmk, zDEVD-fmk, nor CrmA block TNF signaling to necrosis, but, on the contrary, considerably enhance cytotoxicity. Obviously, TNF-mediated necrosis in L929 cells is not dependent on caspase-8/caspase-10, but in fact is attenuated by one or more caspases.
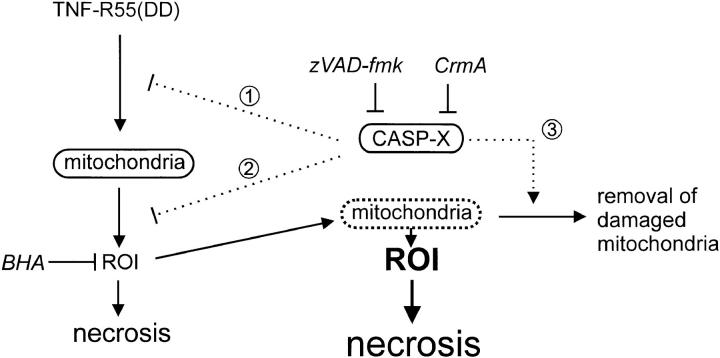
Our results suggest a new role for caspases as negative regulators of TNF-induced oxygen radical production and consequent necrosis. As shown previously, TNF-induced radical formation in L929 cells depends on an intact electron transport system in the mitochondria, and probably involves O2 −·, H 2O2, and/or lipid hydroperoxides (5). Although evidence for the existence of mitochondrial caspases has recently been reported (31, 32), a role for caspases in the electron transport system has not yet been demonstrated. However, since CrmA is probably located in the cytosol, it is unlikely that mitochondrial caspases are involved. Rather, it seems that one or more caspases interfere with the signal from the triggered receptor to the mitochondria. Alternatively, the production of oxygen radicals may be counteracted by caspases at the level of the mitochondria themselves (Fig. 6).
Figure 6.
Possible mechanisms of action in caspase inhibitor-mediated sensitization of TNF-induced necrosis in L929 cells. A putative caspase (CASP-X ), inhibited by CrmA or zVAD-fmk, acts as a negative regulator of premitochondrial signaling (1) or mitochondrial production of reactive oxygen intermediates (ROI; 2). Alternatively, damaging of mitochondria by ROI could impair normal functioning, resulting in an even higher radical production; normally, the cell possesses a mechanism to remove these damaged mitochondria by a process involving one or more caspases (3). Interference with this clean-up process enhances necrosis.
A third hypothetical model is the following. Degradation of mitochondrial proteins has been documented both in physiological and pathological conditions (33). This is especially the case when membrane proteins are damaged by oxygen radicals. In mitochondria of rat liver cells, increasing the radical production results in enhanced protease activity (34). In addition, oxidative damage to intracellular proteins increases their susceptibility to proteolysis (35). Although it is known that in some of these turnover processes, mitochondrial and/or cytosolic ATP-dependent protease complexes play an important role, there is also evidence for involvement of ATP-independent proteases in mitochondrial catabolism. Possibly, caspases could be key elements in such an intracellular mitochondrial quality control system. As cells increase their production of oxygen radicals in the mitochondria after p55 TNF receptor stimulation, oxidative damage of lipids and proteins accumulates; this results in occasional failure of the electron transport system, which leads to an amplified radical production. It is conceivable that such defective mitochondria are recognized and eliminated by a specific cellular mechanism, and this is where caspases could play a role. Elimination of such deficient but oxygen radical–producing mitochondria should then be beneficial for the cell to survive the deadly TNF signal. By inhibiting cytosolic caspase activity, this “rescue mechanism” would be impaired, and hence the cells would accumulate excessive reactive oxygen-producing mitochondria and would be far more sensitive to TNF-induced necrosis. Whatever the exact mechanism is, a low activity of caspases is implied, stressing the importance of a stringent control mechanism of caspase activity in healthy cells. Fig. 6 illustrates alternative mechanisms for possible interference by caspases in TNF-induced mitochondrial production of reactive oxygen intermediates.
The results reported here prompt us to add a cautionary note. Indeed, caspases have already been shown to be essential mediators in illness-related cell death, such as neuronal damage following hypoxic-ischemic insult (36) or fulminant liver destruction after anti-Fas injection (37), and evidence exists for the implication of caspases in amyotrophic lateral sclerosis (38) and Alzheimer's disease (39). In the first two indications, inhibition of caspases by tripeptide derivatives protects treated mice against injury and death. However, considering the 1,000-fold sensitization of TNF-induced necrotic cell death by inhibitors of caspases, one should be cautious in cases where reactive oxygen-mediated necrosis may be involved, such as neutrophil-induced endothelial cell necrosis in the systemic inflammatory response syndrome (40); liver necrosis after reperfusion, alcoholic liver disease, or hemochromatosis (iron overload) and Wilson's disease (copper overload) (41); and myocardial ischemia and reperfusion injury (42). It is not excluded that in these indications, administration of caspase inhibitors may rather have an adverse effect. Therefore, the mechanism leading to cell death should be taken into account when the use of caspase inhibitors would be considered as disease treatment.
Acknowledgments
The authors thank W. Burm, A. Meeus, and M. Van den Hemel for technical assistance. They are indebted to Dr. D. Pickup for donating CrmA cDNA and antiserum.
Abbreviations used in this paper
- BHA
butylated hydroxyanisole
- CrmA
cytokine response modifier A
- DD
death domain
- DEM
diethylmaleate
- DHR123
dihydrorhodamine 123
- NF
nuclear factor
- PI
propidium iodide
- tBuOOH
tert-butyl hydroperoxide
Footnotes
Research was supported by the Interuniversitaire Attractiepolen and the Fonds voor Geneeskundig Wetenschappelijk Onderzoek, as well as by a European Community Biomed Program grant BMH4-CT96-0300. R. Beyaert is a postdoctoral researcher, G. Denecker a research assistant, and P. Vandenabeele a postdoctoral researcher with the Fonds voor Wetenschappelijk Onderzoek–Vlaanderen. G. Van Loo is a fellow with the Vlaams Instituut voor de Bevordering van het Wetenschappelijk-technologisch Onderzoek in de Industrie.
References
- 1.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 2.Fiers, W. 1995. Biologic therapy with TNF: preclinical studies. In Biologic Therapy of Cancer. 2nd ed. V.T. DeVita, Jr., S. Hellman, and S.A. Rosenberg, editors. J.B. Lippincott, Philadelphia. 295–327.
- 3.Wallach D, Boldin M, Varfolomeev E, Beyaert R, Vandenabeele P, Fiers W. Cell death induction by receptors of the TNF family: towards a molecular understanding. FEBS Lett. 1997;410:96–106. doi: 10.1016/s0014-5793(97)00553-x. [DOI] [PubMed] [Google Scholar]
- 4.Grooten J, Goossens V, Vanhaesebroeck B, Fiers W. Cell membrane permeabilization and cellular collapse, followed by loss of dehydrogenase activity: early events in tumour necrosis factor–induced cytotoxicity. Cytokine. 1993;5:546–555. doi: 10.1016/s1043-4666(05)80003-1. [DOI] [PubMed] [Google Scholar]
- 5.Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor–induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 7.Vercammen D, Vandenabeele P, Beyaert R, Declercq W, Fiers W. Tumour necrosis factor–induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine. 1997;9:801–808. doi: 10.1006/cyto.1997.0252. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G, Petit P, Zamzami N, Vayssière J-L, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- 9.Henkart PA. ICE family proteases: mediators of all apoptotic cell death? . Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 10.Martins LM, Earnshaw WC. Apoptosis: alive and kicking in 1997. Trends Cell Biol. 1997;7:111–114. doi: 10.1016/S0962-8924(96)10053-2. [DOI] [PubMed] [Google Scholar]
- 11.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 12.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1– and TNF receptor–induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 13.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD homologous ICE/ CED-3–like protease, is recruited to the CD95 (Fas/Apo-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 14.Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO (Eur Mol Biol Organ) J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincenz C, Dixit VM. Fas-associated death domain protein interleukin-1β–converting enzyme 2 (FLICE2), an ICE/Ced-3 homologue, is proximally involved in CD95- and p55-mediated death signaling. J Biol Chem. 1997;272:6578–6583. doi: 10.1074/jbc.272.10.6578. [DOI] [PubMed] [Google Scholar]
- 16.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 17.Southern PJ, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1:327–341. [PubMed] [Google Scholar]
- 18.Tada H, Shiho O, Kuroshima K, Koyama M, Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986;93:157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- 19.Shrieve DC, Bump EA, Rice GC. Heterogeneity of cellular glutathione among cells derived from a murine fibrosarcoma or a human renal cell carcinoma detected by flow cytometric analysis. J Biol Chem. 1988;263:14107–14114. [PubMed] [Google Scholar]
- 20.Kimura A, Israël A, Le Bail O, Kourilsky P. Detailed analysis of the mouse H-2Kbpromoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986;44:261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- 21.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Lin J-X, Vilček J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a κB-like sequence. Mol Cell Biol. 1990;10:3818–3823. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartaglia LA, Ayres TM, Wong GHW, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 24.Song HY, Dunbar JD, Donner DB. Aggregation of the intracellular domain of the type 1 tumor necrosis factor receptor defined by the two-hybrid system. J Biol Chem. 1994;269:22492–22495. [PubMed] [Google Scholar]
- 25.Boldin MP, Mett IL, Varfolomeev EE, Chumakov I, Shemer-Avni Y, Camonis JH, Wallach D. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J Biol Chem. 1995;270:387–391. doi: 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- 26.Vandevoorde V, Haegeman G, Fiers W. Induced expression of trimerized intracellular domains of the human tumor necrosis factor (TNF) p55 receptor elicits TNF effects. J Cell Biol. 1997;137:1627–1638. doi: 10.1083/jcb.137.7.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 28.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1–associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 31.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssière J-L, Petit PX, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rep M, Grivell LA. The role of protein degradation in mitochondrial function and biogenesis. Curr Genet. 1996;30:367–380. doi: 10.1007/s002940050145. [DOI] [PubMed] [Google Scholar]
- 34.Dean RT, Pollak JK. Endogenous free radical generation may influence proteolysis in mitochondria. Biochem Biophys Res Commun. 1985;126:1082–1089. doi: 10.1016/0006-291x(85)90296-7. [DOI] [PubMed] [Google Scholar]
- 35.Davies KJA, Lin SW, Pacifici RE. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J Biol Chem. 1987;262:9914–9920. [PubMed] [Google Scholar]
- 36.Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz MA. Inhibition of interleukin 1β converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez I, Matsuura K, Ody C, Nagata S, Vassalli P. Systemic injection of a tripeptide inhibits the intracellular activation of CPP32-like proteases in vivoand fully protects mice against Fas-mediated fulminant liver destruction and death. J Exp Med. 1996;184:2067–2072. doi: 10.1084/jem.184.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedlander RM, Brown RH, Gagliardini V, Wang J, Yuan J. Inhibition of ICE slows ALS in mice. Nature. 1997;388:31. doi: 10.1038/40299. [DOI] [PubMed] [Google Scholar]
- 39.Kim T-W, Pettingell WH, Jung Y-K, Kovacs DM, Tanzi RE. Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science. 1997;277:373–376. doi: 10.1126/science.277.5324.373. [DOI] [PubMed] [Google Scholar]
- 40.Wang JH, Redmond HP, Watson RWG, Duggan S, McCarthy J, Barry M, Bouchier-Hayes D. Mechanisms involved in the induction of human endothelial cell necrosis. Cell Immunol. 1996;168:91–99. doi: 10.1006/cimm.1996.0053. [DOI] [PubMed] [Google Scholar]
- 41.Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252–275. doi: 10.1016/0016-5085(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 42.Maxwell SRJ, Lip GYH. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol. 1997;58:95–117. doi: 10.1016/s0167-5273(96)02854-9. [DOI] [PubMed] [Google Scholar]