Abstract
To seek information on the role of Fas in negative selection, we examined subsets of thymocytes from normal neonatal mice versus Fas-deficient lpr/lpr mice injected with graded doses of antigen. In normal mice, injection of 1–100 μg of staphylococcal enterotoxin B (SEB) induced clonal elimination of SEB-reactive Vβ8+ cells at the level of the semi-mature population of HSAhi CD4+ 8− cells found in the thymic medulla; deletion of CD4+ 8+ cells was minimal. SEB injection also caused marked elimination of Vβ8+ HSAhi CD4+ 8− thymocytes in lpr/lpr mice. Paradoxically, however, elimination of these cells in lpr/lpr mice was induced by low-to-moderate doses of SEB (≤1 μg) but not by high doses (100 μg). Similar findings applied when T cell receptor transgenic mice were injected with specific peptide. These findings suggest that clonal elimination of semi-mature medullary T cells is Fas independent at low doses of antigen but Fas dependent at high doses. Previous reports documenting that negative selection is not obviously impaired in lpr/lpr mice could thus reflect that the antigens studied were expressed at only a low level.
Self-tolerance induction is largely a reflection of negative selection (clonal deletion) of immature T cells during maturation in the thymus (1–4). Despite the importance of central (thymic) tolerance, some self-antigens, e.g., tissue-specific antigens, are poorly represented in the thymus. Hence, unresponsiveness of T cells to these antigens is thought to involve peripheral mechanisms. Of the various mechanisms proposed to account for peripheral tolerance, considerable attention has been focused on the role of Fas (CD95) (5–8). This cell-surface molecule is upregulated after TCR stimulation and results in activation-induced cell death (AICD)1 through interaction with Fas ligand during the late stages of the primary response. In support of this view, the normal elimination of T cells after the primary response (9–11) is impaired in Fas-deficient lpr/lpr mice (11–16).
During later life lpr/lpr mice develop massive lymphadenopathy and auto-antibody production (17). This syndrome is considered to reflect a breakdown of peripheral tolerance as the result of defective AICD (8, 11–17). The possibility that lpr/lpr mice have a defect in central tolerance seems unlikely since most groups have failed to find evidence for impaired negative selection in the thymus of lpr/lpr mice (11, 12, 17–25). Nevertheless, a recent study found reduced apoptosis of cortical thymocytes in lpr/lpr mice after injection of specific peptides or anti-TCR mAb (26). However, this effect was only apparent within the first 24 h after injection.
Most studies on thymic tolerance have focused on negative selection occurring in the cortex. Recently, we obtained evidence that negative selection can operate at the level of the semi-mature subset of heat-stable antigen (HSA)hi CD4+ 8− cells found in the medulla (27). Thus, combined TCR/CD28 ligation in vitro induced rapid (<24 h) induction of apoptosis in HSAhi CD4+ 8− cells; by contrast, for fully mature HSAlo CD4+ 8− thymocytes TCR/CD28 ligation caused T cell activation rather than death. An unexpected finding in these experiments was that Fas played a decisive role in TCR/CD28-mediated apoptosis, but only when TCR ligation was induced with a high concentration of anti-TCR mAb. With a low-to-moderate concentration of this mAb, apoptosis induction was Fas-independent. Therefore, the implication is that Fas expression might play an important role in negative selection, but only for antigens expressed at a high level.
Since the above data were derived from a highly artificial in vitro model, the relevance of the data to normal negative selection in vivo is questionable. To seek direct evidence on the possible role of Fas in negative selection, we have now examined the effects of injecting normal versus lpr/lpr mice with various doses of Staphylococcus enterotoxin B (SEB), a soluble superantigen (SAg) recognized by Vβ8+ CD4+ T cells (28); previous studies have shown that injection of this antigen induces clonal elimination of T cells in the thymus (29). We also examined negative selection in D011 TCR transgenic mice (30) after injection of specific ovalbumin (ova) peptide. In each situation, injection of antigen caused elimination of HSAhi CD4+ 8− thymocytes. However, in lpr/lpr mice, negative selection failed to occur when the dose of antigen was raised to a high level.
Materials and Methods
Mice.
C3H/HeJ (C3H), MRL/Mpr-Faslpr (MRLlpr/lpr), C3H. MRL-Faslpr (C3Hlpr/lpr), C57BL/6J (B6), and B6-Faslpr (B6lpr/lpr) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in our animal facility. D011 TCR transgenic mice (30) were bred in our facility and backcrossed three times to MRLlpr/lpr; when used in experiments, neonatal mice were typed individually for Fas and TCR clonotype expression using specific mAbs.
Antibodies.
Antibodies specific for the following markers have been previously described (30, 31): CD4 (RL172, rat IgM), CD8 (3.163.8, rat IgM), CD25 (7D4, rat IgM), HSA (J11D, rat IgM), Thy 1.2 (J1j, rat IgM), D011 TCR (KJ1-26, mouse IgG), TCR Vβ8 (F23.1, mouse IgG), and class II (M5/114, rat IgG). Purified mAbs from ascites specific for TCR-β (H57-597, hamster IgG; reference 32) and CD28 (37.51, hamster IgG; reference 33) were used for stimulation of cells. FITC-conjugated anti-CD4 (H129.19, rat IgG) and PE-conjugated anti-CD8 (53-6.7, rat IgG) mAbs were purchased from GIBCO BRL (Gaithersburg, MD). The following mAbs were purchased from PharMingen (San Diego, CA): FITC-conjugated mAbs specific for HSA (M1/69, rat IgG); PE- or biotin-conjugated mAbs specific for TCR Vβ8.1,2 (MR5-2, rat IgG) and Vβ6 (RR4-7, rat IgG); and Cy-chrome–conjugated anti-CD4 (H129.19). FITC-conjugated anti-bromodeoxyuridine (BrdU) (B44, mouse IgG) mAb was purchased from Becton Dickinson (San Jose, CA). Cy5 conjugation to mAbs was performed using the Fluoro Link-Ab Cy5 Labeling Kit (Amersham Corp., Arlington Heights, IL).
Ova Peptide.
Ova 323–339 peptides (ISQAVHAAHAEINEAGR) were synthesized on a synthesizer (model 431A; Applied Biosystems, Foster City, CA) by standard solid phase peptide synthesis method (tBoc chemistry) and purified with C18 reverse-phase HPLC. The concentration of peptides was determined by quantitative amino acid analysis.
Cell Purification.
Purification of HSAhi or HSAlo CD4+ 8− thymocytes was performed as previously described (27). In brief, for purification of HSAhi CD4+ 8− cells, thymocytes were treated with mAbs specific for CD8 (3.168.8) and CD25 (7D4) plus guinea pig complement (C) for 45 min at 37°C, positively panned with anti-CD4 (RL172) mAb, then positively panned with anti-HSA (J11d) mAb; panning was performed at 4°C. HSAlo CD4+ 8− cells were purified by treating thymocytes with mAbs specific for HSA, CD8, and CD25 plus guinea pig C at 37°C followed by positive panning with anti-CD4 mAb. CD8− thymocytes were purified by negative panning with anti-CD8 mAb. Control experiments established that the procedures used for purifying thymocyte subsets did not alter the sensitivity of the cells to TCR-mediated apoptosis (27). Splenic APCs were purified by treating spleen cells with mAbs specific for Thy 1.2 (J1j), CD4 (RL172), CD8 (3.168.8) and HSA (J11D) plus guinea pig C for 45 min at 37°C.
In Vivo Treatment for the Deletion of Immature Thymocytes.
Newborn (1–6-d-old) mice and adult (7–8-wk-old) mice were injected intraperitoneally with SEB (Sigma Chemical Co., St. Louis, MO), anti-TCR mAb (H57-597), or ova 323–339 peptide at the dose specified. At 20 (day 1), 44 (day 2), or 68 (day 3) h after injection, the mice were killed and cell surface markers of thymocytes were analyzed.
In Vivo BrdU Labeling.
Mice (4–6 d) were intraperitoneally injected with 0.3 mg of BrdU (Sigma Chemical Co.) in PBS twice, at 20 and 12 h before the mice were killed (34, 35).
Culture Conditions.
As described previously, purified cells (2–3 × 105) were cultured in 0.2 ml of RPMI medium supplemented with 5 × 10−5 M 2-mercaptoethanol, l-glutamine, and 10% FCS in 96-well tissue culture plates coated with anti-TCR (H57-597) ± anti-CD28 (37.51) mAbs or medium alone for 20 h (27). For in vitro negative selection of Vβ8+ HSAhi CD4+ 8− cells, purified cells (2 × 105) were cultured with splenic APCs (5 × 104) with or without SEB (10 μg/ml) in 96-well tissue culture plates for 20 h. After harvesting, cells were stained and analyzed on a FACSsort® (Becton Dickinson).
Flow Cytometry Analysis.
For the in vivo studies, thymocytes were incubated with FITC-conjugated anti-HSA mAb, PE-conjugated anti-CD8 (53-6.7), Cy5-conjugated anti-CD4 (GK1.5), and biotinylated anti-Vβ8 (F23.1), anti-Vβ6 (RR4-7), or KJ1-26 mAbs, followed by TRI-COLOR conjugated streptavidin (Caltag Lab., Burlingame, CA). For TUNEL (TdT-mediated dUTP-biotin nick end labeling) staining of apoptotic cells, purified cells were stained with PE-conjugated anti-Vβ8.1,2 (MR5-2) or anti-Vβ6 mAbs, Cy-Chrome–conjugated anti-CD4 (H129.19), and Cy5 conjugated-anti-HSA (J11D) mAbs, and then TUNEL stained after cell fixation. TUNEL staining has been previously described (36). For BrdU-labeled cells, purified CD8− thymocytes were stained with a mixture of biotinylated anti-Vβ8 or anti-Vβ6 mAbs, Cy-chrome–conjugated anti-CD4 and Cy5-conjugated anti-HSA mAbs, followed by PE-streptavidin (GIBCO BRL). After staining for surface markers, cells were fixed with ethanol and paraformaldehyde, treated with DNase, and then incubated with FITC-conjugated anti-BrdU mAb as previously described (35).
Results
Effects of SEB Injection in Adult versus Neonatal C3H Mice.
To examine the effects of SEB on negative selection, SEB was injected intraperitoneally into normal C3H versus Fas-deficient MRLlpr/lpr mice. In these immunogenic I-E+ strains, SEB is recognized by Vβ8+ T cells, especially by CD4+ cells (29). In a previous study, injection of SEB was reported to cause a twofold reduction in the proportion of Vβ8+ cells in unseparated thymocytes 1 d later both in normal and lpr/lpr mice (16); whether this finding was Vβ specific was unclear. The Vβ-specific effects of SEB injection on subsets of thymocytes in normal C3H mice is discussed below. In all experiments a single dose of SEB was injected.
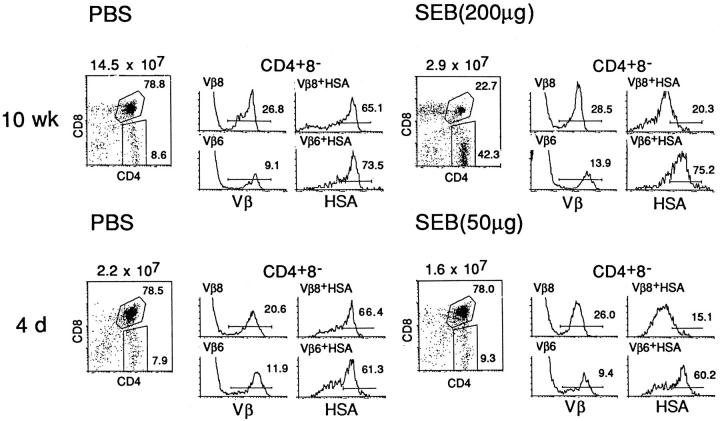
At the level of CD4+ 8+ double-positive (DP) thymocytes, the effects of SEB injection in adult (10 wk) and neonatal (4 d) C3H mice were quite different (Fig. 1). In adult mice, injection of a large dose of SEB (200 μg) caused pronounced destruction of DP cells and reduced the total cellularity of the thymus by ∼80% at 2 d after injection; the elimination of DP cells was associated with a reciprocal increase in single-positive (SP) CD4+ 8− and CD4− 8+ cells. In neonatal mice, by contrast, a large dose of SEB (50 μg) caused little or no reduction in the proportion of DP cells and led to a smaller (20–50%) reduction in total cellularity.
Figure 1.
SEB-mediated negative selection of Vβ8+ CD4+ 8− thymocytes in adult and neonatal C3H mice. Adult (10-wk-old) or newborn (4-d-old) mice were injected intraperitoneally with PBS or the indicated amount of SEB, and thymuses were harvested 2 d after injection; thymocyte suspensions were four-color stained for CD4, CD8, HSA, and Vβ8 or Vβ6 and were FACS® analyzed as described in Materials and Methods. The following FACS® plots are shown: CD4 versus CD8 on all thymocytes (left); Vβ8 and Vβ6 expression on gated CD4+ 8− cells (middle); and HSA expression on gated Vβ8+ CD4+ 8− and Vβ6+ CD4+ 8− cells (right). The average number of total thymocytes recovered from each mouse is shown at the left. The FACS® plots refer to thymocytes from individual mice and are representative of data from 2 to 3 mice per group.
In contrast to DP cells, SEB injection had a clear Vβ-specific effect on CD4+ 8− SP cells, in both adult and neonatal mice (Fig. 1). At 2 d after injection, SEB injection caused little or no alteration in the total proportion of SEB-reactive Vβ8+ CD4+ 8− cells. However, when these cells were typed for HSA expression, the Vβ8+ CD4+ 8− subset showed a marked and selective reduction of semi-mature HSAhi cells and a reciprocal increase in fully mature HSAlo cells. In control PBS-injected mice, by contrast, the majority of the Vβ8+ CD4+ 8− cells were HSAhi. The effect of SEB injection appeared to be specific for Vβ8+ cells since there was no change in the HSA phenotype of Vβ6+ cells. SEB injection thus led to substantial elimination of HSAhi Vβ8+ CD4+ 8− cells while causing apparent expansion of HSAlo cells; these findings applied in both adult and neonatal mice. Unless stated otherwise, the experiments discussed below refer to neonatal (4-d-old) C3H mice.
Kinetics.
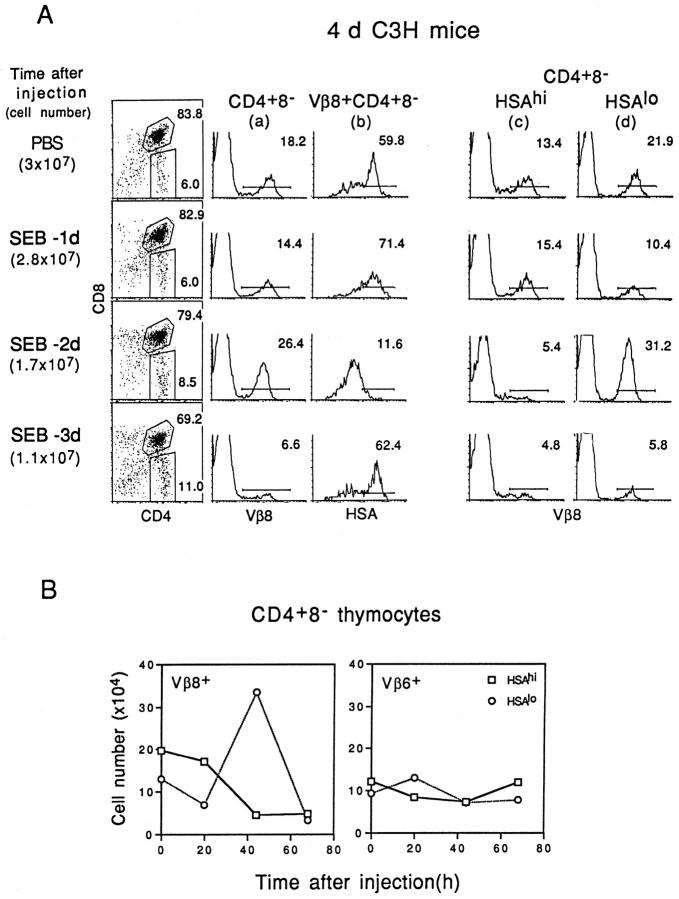
The above data apply to mice given SEB 2 d before. Other time points are shown in Fig. 2. Gating on unseparated CD4+ 8− cells (Fig. 2 A, column a) revealed that the proportion of Vβ8+ cells was slightly reduced on day 1 (20 h), increased on day 2 (44 h), and greatly reduced on day 3 (68 h). Examining HSA expression on Vβ8+ CD4+ 8− cells (Fig. 2 A, column b) showed that HSAhi cells were present on day 1, greatly reduced on day 2, but again visible on day 3. When the data were expressed as total numbers of cells/thymus (Fig. 2 B, left), numbers of Vβ8+ HSAhi CD4+ 8− cells were slightly reduced on day 1 and substantially reduced on days 2 and 3; levels of Vβ6+ cells, by contrast, remained largely unchanged (Fig. 2 B, right). Essentially similar findings applied when Vβ8 expression was examined on gated HSAhi CD4+ 8− cells (Fig. 2 A, column c). Thus, Vβ8+ cells in the HSAhi subset were present at day 1 but markedly reduced on days 2 and 3. This applied to both the proportion of cells (Fig. 2 A, column c) and total cell numbers (Fig. 2 B).
Figure 2.
The kinetics of negative selection of Vβ8+ CD4+ 8− cells induced by injection of SEB. Neonatal (4-d-old) C3H mice were injected intraperitoneally with SEB (25 μg) at 1, 2, or 3 d before mice were killed. Thymocytes from SEB-injected mice were then stained with mAbs specific for Vβ8 or Vβ6, CD4, CD8, and HSA. (A) Left: Total cell numbers (mean data from 5–8 mice per group) and CD4 versus CD8 expression (data from one representative mouse). Middle and right: (a) Vβ8 expression on gated CD4+ 8− cells; (b) HSA expression on gated Vβ8+ CD4+ 8− cells; (c) Vβ8 expression on gated HSAhi CD4+ 8− cells; (d) Vβ8 expression on gated HSAlo CD4+ 8− cells. (B) Total numbers of CD4+ 8− thymocyte subsets per mouse recovered from SEB-injected mice at the time points shown. Total numbers of Vβ8+ HSAhi versus Vβ8+ HSAlo cells (left) and Vβ6+ HSAhi versus Vβ6+ HSAlo cells (right) are shown. The data represent the mean values obtained from two different experiments involving 5–8 mice per group.
The data on Vβ8+ HSAlo CD4+ 8− cells were more complex. Thus, total numbers of these fully mature cells were moderately reduced on day 1, elevated on day 2, and greatly reduced on day 3 (Fig. 2 B, left). Similar findings applied to the proportion of Vβ8+ cells in gated HSAlo CD4+ 8− cells (Fig. 2 A, column d).
Other Thymocyte Subsets.
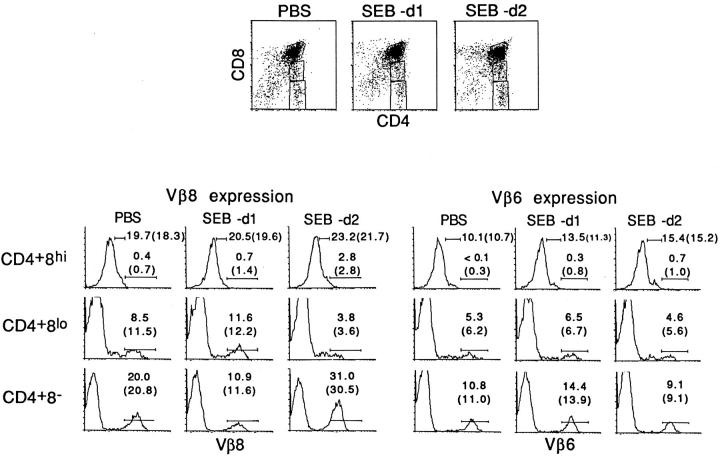
Since CD4+ 8− thymocytes arise from CD4+ 8+ precursors through progressive downregulation of CD8, FACS-gated CD4+ 8− thymocytes include a proportion of CD4+ 8lo cells, which are presumably less mature than CD4+ 8− cells. The tolerance susceptibility of CD4+ 8+ (CD4+ 8hi), CD4+ 8lo, and CD4+ 8− cells to SEB is shown in Fig. 3 (note the “sharper” FACS® gate for CD4+ 8− cells in Fig. 3 than in Figs. 1 and 2). At day 2 after SEB injection, it can be seen that the proportion of Vβ8+ cells (the data in parentheses refer to mean values) was considerably reduced in the CD4+ 8lo subset but markedly elevated in the CD4+ 8− subset; proportions of Vβ6+ cells remained relatively unchanged. For CD4+ 8hi cells, Vβ8+ cells consisted mostly of TCRlo cells with very few TCRhi cells. Significantly, SEB injection failed to deplete either Vβ8hi or Vβ8lo cells from the CD4+ 8hi subset.
Figure 3.
SEB-induced negative selection of CD4+ 8hi, CD4+ 8lo, and CD4+ 8− thymocytes. The data show Vβ8 (bottom left) and Vβ6 (bottom right) expression on gated CD4+ 8hi, CD4+ 8lo, and CD4+ 8− thymocytes from neonatal C3H mice injected with PBS or SEB 1 or 2 d earlier. The FACS® gates used to define CD4/CD8 expression are shown at the top. For Vβ expression, the percentages shown refer to Vβlo and Vβhi cells for CD4+ 8hi cells and to Vβhi cells for CD4+ 8lo and CD4+ 8− cells. The data illustrated are from a single representative from each group; the numbers in parentheses refer to the mean values obtained from three mice per group.
The above data suggest that, for the FACS® gates used, SEB-induced elimination of thymocytes was restricted to CD4+ 8lo cells and did not include either CD4+ 8hi or CD4+ 8− cells. Here two points should be made. First, before injection, nearly all of the Vβ8+ CD4+ 8lo cells were HSAhi (90%), attesting to their relative immaturity. However, after SEB injection the depleted population of Vβ8+ cells found on day 2 was enriched for HSAlo cells (50%), implying that SEB selectively deleted the HSAhi Vβ8+ cells and caused expansion of the small subset of Vβ8+ HSAlo cells (data not shown). Second, HSAhi cells also accounted for a considerable proportion of CD4+ 8− cells (60%). However, at 2 d after SEB injection the expanded population of Vβ8+ cells consisted almost entirely of HSAlo cells (>90%) (data not shown).
Collectively, these data appear to indicate that the Vβ8+ cells eliminated by SEB injection were predominantly HSAhi CD4+ 8lo cells. However, whether deletion also affected the slightly more mature population of HSAhi cells found in the CD4+ 8− subset could not be accurately established because of the high levels of fully mature HSAlo cells in the CD4+ 8− subset. To seek direct information on this issue, we prepared purified populations of HSAhi CD4+ 8− cells by positive panning (for HSA+ cells) and negative selection with anti-CD8 mAb + C. This treatment removed both CD4+ 8hi and CD4+ 8lo cells. The HSAhi cells surviving this procedure were thus CD4+ 8− rather than CD4+ 8lo. The susceptibility of these purified HSAhi CD4+ 8− cells to SEB-mediated deletion in vitro is discussed below. For the in vivo experiments that follow, it should be pointed out that the FACS® gate used to define CD4/CD8 expression was the same as in Figs. 1 and 2 (rather than in Fig. 3); with this gate the cells defined as being CD4+ 8− consisted predominantly of CD4+ 8− cells plus a small proportion of CD4+ 8lo cells (∼30%). For convenience, the HSAhi cells in this mixed population will be referred to hereafter as HSAhi CD4+ 8− cells.
Disappearance of HSAhi Cells Reflects Elimination.
In interpreting the above data, a key issue is whether the disappearance of HSAhi CD4+ 8− cells on day 2–3 after SEB injection (Fig. 2) simply reflected a switch to HSAlo cells. This possibility is unlikely because previous studies showed that culturing purified HSAhi CD4+ 8− cells with a combination of cross-linked anti-TCR + anti-CD28 mAb in vitro caused many of the cells to undergo apoptosis (27) but failed to downregulate HSA expression on the surviving cells (our unpublished data). Similar findings applied when purified HSAhi CD4+ 8− cells (depleted of 8lo cells; see above) were cultured overnight with SEB plus spleen APC in vitro (Fig. 4). As manifested by TUNEL staining, this treatment induced substantial apoptosis of Vβ8+ HSAhi cells (but not Vβ6+ cells) relative to cells cultured with APCs without SEB (Fig. 4, A and B). However, there was no reduction of HSA expression on the surviving (TUNEL−) cells (Fig. 4 C). With mature HSAlo cells, exposure to SEB failed to cause apoptosis of Vβ8+ cells (Fig. 4 D); since the culture period was brief (20 h), expansion of Vβ8+ HSAlo cells was not apparent. These findings applied to normal cells stimulated with SEB in vitro. If the disappearance of Vβ8+ HSAhi CD4+ 8− cells from the thymus between day 1 and 2 after SEB injection (Fig. 2) reflected death of these cells (rather than a switch to HSAlo cells), onset of apoptosis would be expected if the cells were harvested on day 1 after in vivo SEB injection and then cultured in vitro without further stimulus. This was indeed the case. Thus, when HSAhi versus HSAlo CD4+ 8− thymocytes were purified from mice given SEB 1 d before and cultured in vitro overnight without APCs, there was significant apoptosis of Vβ8+ HSAhi cells but no apoptosis of Vβ6+ HSAhi cells (relative to the background apoptosis in total HSAhi CD4+ 8− cells) (Fig. 5). For the HSAlo cells, there was slight apoptosis of Vβ8+ cells and no apoptosis of Vβ6+ cells.
Figure 4.
SEB-induced negative selection of CD4+ 8− thymocytes in vitro. Purified HSAhi or HSAlo CD4+ 8− thymocytes from neonatal C3H mice were cultured in vitro for 20 h with or without SEB (10 μg/ml) in the presence of splenic APCs. After culture, cells were surface stained for HSA, Vβ8, and Vβ6 expression; after washing, cells were fixed and stained by TUNEL for apoptotic cells. (A) Proportion of TUNEL+ (apoptotic) cells in gated Vβ8+ cells (left) and Vβ6+ cells (right) in HSAhi versus HSAlo subsets; TUNEL staining is plotted against forward scatter (FSC). (B) Representation of the data shown in A after subtraction of the background values for apoptosis found for cells cultured with APCs without SEB. (C) HSA expression on viable (TUNEL−) Vβ8+ cells (left) and Vβ6+ cells (right) after culture with APCs ± SEB. (D) Proportion of Vβ8+ cells in the viable (TUNEL−) subsets of HSAhi versus HSAlo cells after culture with APCs ± SEB. The data shown are representative of three separate experiments.
Figure 5.
Susceptibility of HSAhi versus HSAlo subsets of CD4+ 8− thymocytes to apoptosis in vitro after exposure to SEB in vivo. HSAhi versus HSAlo CD4+ 8− thymocytes from neonatal C3H mice injected with PBS or SEB (50 μg/mouse) 28 h earlier were purified by panning and mAb + C (see Materials and Methods). The purified cells were cultured in normal tissue culture medium in vitro for 20 h at 37°C without SEB or APCs and then stained for Vβ8 or Vβ6 followed by TUNEL staining after fixation. Levels of apoptosis found for comparable cultured populations of thymocytes prepared from uninjected control mice have been subtracted from the data shown. The data represent the mean values obtained from three separate experiments.
BrdU Incorporation.
Collectively, these data suggest that the disappearance of Vβ8+ HSAhi CD4+ 8− cells after SEB injection reflected elimination of these cells rather than a switch to HSAlo cells. For the HSAlo cells, the transient reduction in Vβ8+ cells at 1 day after SEB injection (Fig. 2) suggested that a small subset of these cells was eliminated at day 1. The minor degree of apoptosis seen when the cells were cultured in vitro at 1 day after SEB injection (Fig. 5) is consistent with this possibility. The expansion of Vβ8+ HSAlo cells at day 2 after SEB injection presumably indicated proliferation of mature T cells to SEB.
To examine this possibility, groups of neonatal C3H mice were injected with SEB and thymocytes were prepared 1, 2, or 3 d later; BrdU (2 injections 12 h apart) was administered for the last 20 h before preparation of thymocytes. The data in Fig. 6 make three points. First, in agreement with the known high turnover of CD4+ 8− cells in the neonatal thymus (34), 20 h of exposure to BrdU labeled a high proportion of the Vβ8+ and Vβ6+ CD4+ 8− thymocytes in PBS-injected control mice; labeling was higher for HSAhi cells than for HSAlo cells and, for Vβ6+ HSAhi cells of the SEB-injected groups, labeling declined progressively as the 4-d-old mice aged over the 3-d period studied. Second, relative to Vβ6+ cells, SEB injection caused no obvious change in BrdU labeling of Vβ8+ cells at 1 d after injection; this applied to both HSAhi and HSAlo Vβ8+ cells. Third, labeling of Vβ8+ HSAlo cells increased markedly at day 2 after SEB injection and then declined abruptly on day 3. Labeling of Vβ8+ HSAhi cells, by contrast, was below normal on both day 2 and 3. Collectively, these data indicate that SEB injection caused Vβ8+ HSAlo cells to proliferate for a brief period (evident only on day 2) and then disappear. This transient proliferative response did not affect Vβ8+ HSAhi CD4+ 8− cells.
Figure 6.

BrdU incorporation by CD4+ 8− thymocyte subsets after SEB injection. Neonatal C3H mice were injected with PBS or SEB and killed 1, 2, or 3 d later (1 d later for PBS); starting at 20 h before death, the mice received two intraperitoneal injections of BrdU 8 h apart (−20 h and −12 h). Purified CD8− cells (cells treated with anti-CD8 mAb + C) were surface stained for Vβ8 or Vβ6, CD4, and CD8, then fixed and stained for BrdU. The data show BrdU versus HSA expression on gated Vβ8+ CD4+ 8− (left) and Vβ6+ CD4+ 8− (right) cells. The data are representative of three separate experiments.
Dose of SEB and Role of Fas.
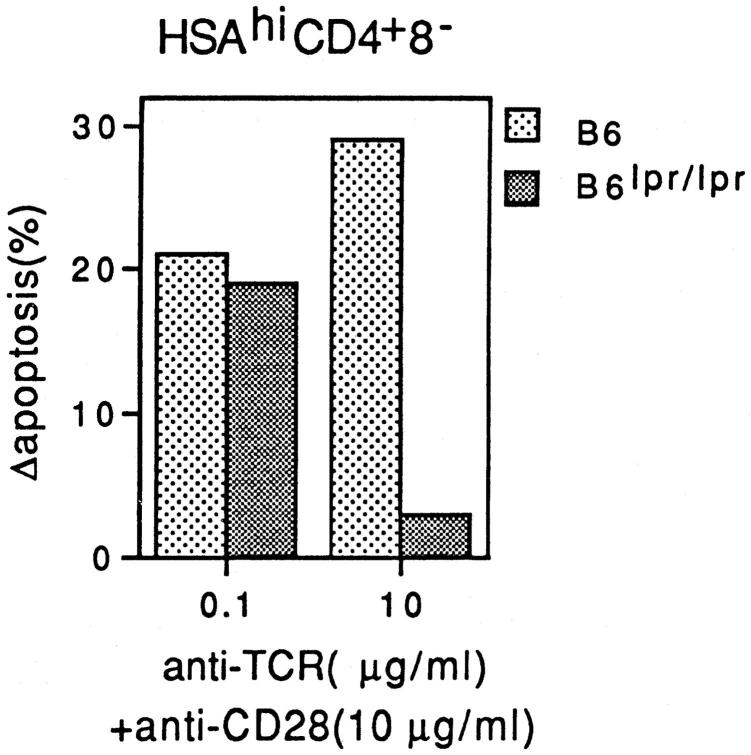
As discussed earlier (see Introduction), apoptosis of purified HSAhi CD4+ 8− thymocytes in response to combined TCR/CD28 ligation in vitro was found to be Fas independent with a low concentration of cross-linked anti-TCR mAb, but was completely Fas dependent with a high dose of this mAb (27). This finding is illustrated in Fig. 7, where it can be seen that TCR/CD28 ligation of Fas-deficient B6lpr/lpr HSAhi CD4+ 8− cells in culture caused significant apoptosis at a low concentration of anti-TCR mAb (0.1 μg/ml) but not at a high concentration (10 μg/ml). By contrast, apoptosis of HSAhi CD4+ 8− cells from normal B6 mice was higher with a high concentration of anti-TCR mAb than with a lower concentration (Fig. 7). Similar findings applied when Fas expression on normal B6 thymocytes was blocked with Fas–Ig fusion protein (27).
Figure 7.
Relative sensitivity of normal B6 and B6lpr/lpr HSAhi CD4+ 8− thymocytes to SEB-induced negative selection in vitro. Purified HSAhi CD4+ 8− thymocytes prepared from adult (6-wk-old) mice were cultured in vitro for 20 h in wells coated with a fixed concentration of anti-CD28 mAb (10 μg/ml) plus a low or high concentration of anti-TCR mAb (0.1 μg/ml or 10 μg/ml). After culture, cells were stained for TUNEL to detect apoptotic cells. The data show percentage of apoptotic (TUNEL+) cells after subtraction of the background levels for cells cultured alone. The data represent the mean of four separate experiments.
To examine whether this role of Fas in negative selection also applied in vivo, we tested the effects of injecting normal versus lpr/lpr neonatal mice with graded doses of SEB. Based on the results considered above, the expectation was that a moderate-to-low dose of SEB would induce negative selection of HSAhi Vβ8+ CD4+ 8− cells via a Fas-independent pathway and thus cause cell deletion in both normal and lpr/lpr mice. By contrast, with a high dose of antigen, negative selection would be Fas dependent and thus occur only in normal and not lpr/lpr mice.
In accordance with this prediction, injecting graded doses of SEB into normal neonatal C3H or MRL mice induced strong deletion of HSAhi Vβ8+ CD4+ 8− cells at doses ranging from 1 to 100 μg SEB/mouse (Fig. 8 A and data not shown). When injected into either MRLlpr/lpr or C3Hlpr/lpr hosts, by contrast, SEB induced strong deletion at up to 1 μg but caused little or no deletion at higher doses, e.g., 100 μg. This difference between normal and lpr/lpr mice was restricted to HSAhi cells and did not apply to control Vβ6+ cells (Fig. 8). Similar findings applied when SEB was injected into adult mice (Fig. 8 B) and also when anti-TCR mAb was injected into neonatal mice (Fig. 8 C). In each situation, a moderate dose of antigen induced strong deletion of HSAhi CD4+ 8− cells in both normal and lpr/lpr mice, whereas a high dose of antigen induced deletion only in normal and not lpr/lpr mice.
Figure 8.
Negative selection of HSAhi CD4+ 8− thymocytes from C3H versus MRLlpr/lpr mice injected with graded doses of SEB or anti-TCR mAb. Thymocytes were prepared at 2 d after intraperitoneal injection of various doses of SEB or anti-TCR mAb and stained. (A) Neonatal (4-d-old) C3H mice injected with SEB. The data represent mean values from four separate experiments with two to three mice per group. (B) Adult (7-wk-old) mice injected with SEB. The data represent mean values from four separate experiments with two mice/group. (C) Neonatal (4-d-old) mice injected with anti-TCR mAb. The data show total numbers of CD4+ 8− subsets per thymus. The data represent mean values from two separate experiments with two mice per group.
Negative Selection in lpr/lpr TCR Transgenic Mice.
To seek further information on the role of Fas in negative selection, we examined the effects of injecting specific peptide into neonatal (4-d-old) normal versus Fas-deficient D011 TCR transgenic mice; mature CD4+ 8− cells from this line are strongly reactive to an ova peptide, ova 323–339, presented by IAd (30).
The features of thymocytes from uninjected neonatal D011 mice are shown in Fig. 9 A. Gating on the subset of CD4+ 8− thymocytes revealed that nearly all of these cells were clonotype-positive (Id+). In contrast to the discrete subsets of HSAhi and HSAlo CD4+ 8− thymocytes found in normal nontransgenic mice (Fig. 9 A, bottom, thick line), the vast majority of CD4+ 8− thymocytes from D011 mice were HSAhi (∼90%) and therefore are potentially tolerance susceptible. For this reason, detecting negative selection of D011 thymocytes after ova peptide injection was relatively easy and merely entailed examining CD4/CD8 expression (rather than enumerating HSAhi CD4+ 8− cells).
Figure 9.
Negative selection of HSAhi CD4+ 8− thymocytes from neonatal (4-d-old) Fas+/− versus Fas−/− D011 TCR transgenic mice injected with graded doses of ova 323–339 peptide. Thymocytes were prepared at 2 d after intraperitoneal injection of peptide or PBS as a control and stained for CD4, CD8, HSA, and idiotypic (Id) TCR expression. (A) Phenotype of normal (uninjected) Fas+/− D011 thymocytes showing CD4 versus CD8 expression (top), TCR expression on CD4+ 8− cells detected by the anticlonotypic mAb KJ1-26 (middle), and HSA expression on Id+ CD4+ 8− cells (bottom); Id and HSA expression on normal nontransgenic CD4+ 8− thymocytes is shown in the heavy lines. (B) Representative data on CD4 versus CD8 expression on thymocytes from Fas+/− versus Fas−/− D011 mice injected with PBS and 1 or 100 μg of ova peptide. (C) Total numbers of HSAhi CD4+ 8−, HSAlo CD4+ 8−, and CD4+ 8+ thymocytes/mouse from Fas+/− versus Fas−/− D011 mice injected with various doses of ova peptide; the data represent mean values from four separate experiments with one to two mice per group.
The effects of injecting various doses of ova peptide into neonatal D011 mice are shown in Fig. 9 B; thymocytes were examined at day 2 after injection. For normal Fas+ D011 mice, injecting either a moderate dose (1 μg) or a high dose (100 μg) of ova peptide caused a marked depletion of (total) CD4+ 8− thymocytes. With Fas-deficient D011lpr/lpr mice, by contrast, depletion of CD4+ 8− thymocytes was apparent only with the lower dose of peptide; with a high dose of peptide deletion of CD4+ 8− thymocytes was minimal. Similar findings applied when the data were expressed as total numbers of HSAhi CD4+ 8− thymocytes (Fig. 9 C, left). These data apply to thymocytes examined at day 2 after injection. As for SEB, elimination of HSAhi CD4+ 8− cells at day 1 after ova peptide injection was quite limited (data not shown).
Injecting D011 mice with ova peptide caused little if any expansion of the residual population of HSAlo CD4+ 8− thymocytes. Indeed, especially in Fas+ D011 mice, peptide injection appeared to delete both HSAhi and HSAlo CD4+ 8− cells (Fig. 9 C, middle). In interpreting this finding it should be noted that, in contrast to normal thymocytes, D011 thymocytes lacked a discrete subset of HSAlo CD4+ 8− cells (Fig. 9 A, bottom): HSA expression was relatively homogeneous and the few HSAlo cells detected expressed intermediate levels of HSA and formed a continuum with the HSAhi cells. Hence it is not surprising that the HSAlo cells resembled HSAhi cells in being tolerance susceptible.
Unlike CD4+ 8− thymocytes, D011 CD4+ 8+ thymocytes were comparatively resistant to negative selection (Fig. 9, B and C, right). Thus, even large doses of peptide caused only minimal (30%) depletion of CD4+ 8+ cells.
Discussion
The data in this paper make two main points. First, injecting mice with antigen (either SEB or ova peptide) caused marked antigen-specific elimination of the semi-mature population of HSAhi CD4+ 8− thymocytes within 2 d. Second, based on studies with normal versus lpr/lpr mice, the disappearance of these cells was Fas independent at low doses of antigen but Fas dependent at high doses. Before discussing the role of Fas, several features of the elimination of HSAhi CD4+ 8− cells require comment.
The finding that the disappearance of Vβ8+ HSAhi CD4+ 8− cells after SEB injection was associated with a reciprocal increase in HSAlo cells raised the possibility that the HSAhi cells were not deleted but simply switched to HSAlo cells. This possibility is unlikely because exposing HSAhi cells to SEB plus APCs in vitro caused rapid onset of apoptosis of Vβ8+ cells and failed to reduce HSA expression on the surviving cells. In addition, when purified HSAhi CD4+ 8− cells were prepared from SEB-injected mice at 1 d after injection, the Vβ8+ subset of these cells underwent apoptosis in vitro without further contact with SEB, implying that the prior in vivo exposure to SEB had already signaled the cells to die. In light of these findings, the disappearance of Vβ8+ HSAhi CD4+ 8− cells after SEB injection appeared to reflect clonal deletion of these cells rather than differentiation into HSAlo cells. For D011 mice, the paucity of HSAlo CD4+ 8− thymocytes in these mice makes it most unlikely that the deletion of HSAhi cells reflected a switch to HSAlo cells. Thus, in this situation, peptide injection caused extensive deletion of total CD4+ 8− thymocytes.
In tissue culture, deletion of HSAhi CD4+ 8− thymocytes occurred quite rapidly. Thus, when HSAhi CD4+ 8− cells were exposed to SEB plus APCs in vitro, apoptosis of Vβ8+ cells was clearly apparent after overnight culture; whether these rapid kinetics also apply to ova peptide was not tested. However, under in vivo conditions the elimination of HSAhi CD4+ 8− cells was limited on day 1 but prominent at days 2 and 3 both for SEB and ova peptide. Why the disappearance of HSAhi CD4+ 8− cells was delayed in vivo is unclear. One possibility is that the intraperitoneal route of injection impeded entry of antigen into the bloodstream, and thus led to relatively slow accumulation in the thymus. In support of this idea, injecting SEB intravenously rather than intraperitoneally accelerated the onset of Vβ8+ HSAhi cell elimination by ∼10 h (our unpublished data). For ova peptide, we have yet to examine the effects of peptide injection beyond day 2. For SEB, the maximal deletion of Vβ8+ HSAhi CD4+ 8− cells after intraperitoneal injection of SEB was at days 2 and 3. These cells began to reappear in the thymus at about day 5 after injection and reached normal levels by day 7 (our unpublished data). The implication is that SEB was cleared from the thymus within a few days, thus allowing rapid replacement of the deleted cells by a new wave of HSAhi CD4+ 8− cells derived from DP precursors in the cortex.
With regard to the site of negative selection, many workers consider that the deletion of immature T cells occurs largely in the cortex (3). In favor of this idea, it is well documented that injecting adult mice with antigen causes massive apoptosis in the cortex (30). However, in this situation cortical apoptosis could be a reflection of stress induced by stimulation of mature T cells in the periphery (37). Such nonantigen-specific destruction of cortical thymocytes is less of a problem in neonatal mice, which have few peripheral T cells. Here, it is of interest that, for both SEB and ova peptide, injecting neonatal hosts with antigen caused marked elimination of HSAhi CD4+ 8− cells but minimal deletion of CD4+ 8+ cells. We considered the possibility that injection of antigen caused deletion of a small subset of CD4+ 8+ cells, e.g., TCRhi cells. However, gating on typical CD4+ 8+ (8hi) cells after SEB injection showed no detectable deletion of either Vβ8lo or Vβ8hi cells (Fig. 3). Similarly, we failed to see deletion of either TCRlo or TCRhi CD4+ 8+ cells in D011 mice after ova peptide injection (our unpublished data). Hence, for neonatal hosts and the two antigens studied here, negative selection of typical cortical CD4+ 8+ cells appeared to be very limited. It should be emphasized that these data do not exclude the possibility that the cortex is an important site of negative selection in other systems. Nevertheless, it is notable that, even in adult mice, SEB-induced deletion of Vβ8+ thymocytes is reported to be undetectable in mice expressing MHC class II molecules only in the cortex (on cortical epithelium) but not in the medulla (38).
In contrast to typical cortical CD4+ 8+ cells, the immediate progeny of these cells, namely TCRhi HSAhi CD4+ 8lo cells, were strongly deleted after antigen injection (Fig. 3). Since the anatomical localization of CD4+ 8lo cells in the thymus is unclear, it is possible that some of these cells were deleted in the cortex before entering the medulla. However, it is important to emphasize that, for both SEB and ova peptide, the elimination of SP thymocytes applied not only to HSAhi CD4+ 8lo cells but also to the larger population of HSAhi CD4+ 8− cells, which are presumably slightly more mature than HSAhi CD4+ 8lo cells. In fact most of the experiments with SEB concerned CD4+ 8− cells rather than CD4+ 8lo cells. Similarly, for D011 mice, injecting peptide deleted both HSAhi CD4+ 8lo and HSAhi CD4+ 8− thymocytes (Fig. 9 B).
Since the vast majority of lymphoid cells in the medulla are typical SP cells (39), most HSAhi CD4+ 8− thymocytes are probably situated primarily in the medulla rather than the cortex. In support of this idea, the medulla contains considerable numbers of HSA+ cells in tissue sections (our unpublished data), suggesting that many medullary T cells are HSAhi. In addition, TUNEL staining has revealed that SEB injection leads to increased numbers of apoptotic cells in the medulla at day 2 after injection (our unpublished data). For these reasons, it would seem highly likely that the deletion of HSAhi CD4+ 8− cells takes place mainly in the medulla (although the possibility that these cells initially receive a death signal in the cortex cannot be excluded [40]).
The elimination of HSAhi CD4+ 8− cells after antigen injection did not appear to involve cell division. Thus, cell cycle analysis of HSAhi CD4+ 8− cells has shown that the rapid death of these cells after TCR ligation in vitro does not involve entry into cell cycle (our unpublished data). Likewise, the elimination of HSAhi CD4+ 8− cells in vivo was not preceded by an increase in BrdU incorporation (Fig. 6). This finding contrasts with fully-mature T cells where TCR-mediated apoptosis (AICD) generally occurs slowly and is preceded by an overt proliferative response (41). Hence, for the fully mature subset of HSAlo CD4+ 8− thymocytes, we expected initial contact with SEB to be directly immunogenic for these cells. In line with this prediction, SEB injection caused Vβ8+ HSAlo CD4+ 8− cells to proliferate (incorporate BrdU) at 2 days after injection and undergo considerable expansion. Surprisingly, however, the expansion of Vβ8+ HSAlo cells was preceded by a transient decrease in numbers of these cells at day 1 after injection, probably via deletion. Why a proportion of these mature T cells succumbed to early deletion is unclear, although a similar finding has been reported for peripheral T cells after SEB injection (10, 42). It is of interest that the expansion of Vβ8+ HSAlo thymocytes was evident only at day 2 after injection and was followed by a marked reduction in numbers of these cells on day 3; the disappearance of Vβ8+ HSAlo cells was also prominent on day 5 and levels of these cells did not return to normal until after day 7, i.e., ∼2 d later than for HSAhi cells (our unpublished data). At face value the rapid elimination of proliferating Vβ8+ HSAlo thymocytes after day 2 could reflect AICD and be the counterpart of the rapid sequence of expansion followed by deletion reported for peripheral Vβ8+ cells after SEB injection (10). However, whether the Vβ8+ HSAlo cells in the thymus died in situ or migrated to the periphery is still unclear.
For SEB, it is worth noting that the near-complete elimination of Vβ8+ cells, including mature HSAlo cells, seen at days 3–5 after a single injection of SEB, is in line with the original report that multiple injections of SEB caused marked depletion of Vβ8+ SP thymocytes 8 d after the last injection (29). Since earlier time points were not examined, the authors may have missed the prior expansion of Vβ8+ cells reported here for HSAlo SP cells.
With regard to Fas, the main finding in this paper is that in lpr/lpr mice the strong elimination of HSAhi CD4+ 8− thymocytes induced by SEB, ova peptide, and anti-TCR mAb injection failed to occur when the dose of Ag/mAb was increased to a high level. The implication therefore is that negative selection is critically dependent upon Fas, but only for antigens expressed at a high level. This finding would seem to disagree with reports that injecting large doses of specific peptide into TCR transgenic mice reduced the cellularity of the thymus by >10-fold, in both normal and lpr/lpr mice (11, 25). But because adult mice were used in these experiments, the possibility remains that thymocytes were not eliminated via negative selection but destroyed nonspecifically by stress (37). In another study, giving multiple injections of SEB to neonatal mice over a 2-wk period caused equivalent deletion of Vβ8+ cells in both normal and lpr/lpr mice (43). Since the effects of varying the dose of injected SEB was not tested, the relevance of this finding to our data is unclear.
Much of the evidence that Fas is not involved in negative selection has come from the finding that lpr/lpr mice show relatively normal thymocyte deletion in response to endogenous SAgs and the male (HY) antigen (8, 12, 15, 17–20, 22–24). A corollary of the present data is that Fas is irrelevant for negative selection to endogenous self-antigens unless the concentration of these antigens in the thymus is unusually high. Hence if endogenous SAgs and the male antigen are expressed at only an “average” level (equivalent to a low-to-moderate dose of SEB or ova peptide), there is no discrepancy with the present data.
Although the range of self-antigens causing negative selection is unknown, one can envisage that some antigens, e.g., peptides derived from histones or other common intracellular proteins, are expressed at a relatively high level. Hence, based on our findings with SEB and ova peptide, the elimination of T cells specific for these common self-proteins might be Fas dependent and thus allow the reactive T cells in lpr/lpr mice to escape central tolerance induction in the thymus. Upon maturation and exit from the thymus, these nontolerant T cells would continue to see these antigens at a high concentration, but now in immunogenic rather than tolerogenic form. Accordingly, the onset of lymphadenopathy and autoimmune disease in lpr/lpr mice (8, 17) might be a reflection of an ongoing immune response of nontolerant T cells directed to ubiquitous self-antigens expressed at a high level.
This scenario rests on two assumptions. First, one has to argue that the peripheral T cells in lpr/lpr mice are indeed responding to self-antigens. Such a possibility has yet to be proved, although the finding that all subsets of T cells in lpr/lpr mice, including CD4+ 8− and CD4− 8+ cells, have an abnormally high turnover in the periphery is consistent with this idea (24, 44). The second assumption is that if T cells in lpr/lpr mice are indeed autoreactive, the antigens recognized by these cells are expressed at a high level relative to other antigens. Assessing this idea will hinge on defining the peptide specificity of autoreactive T cells from lpr/lpr mice.
As a final comment, it is important to emphasize that our data suggest that Fas regulates negative selection at a relatively late stage of thymocyte differentiation. The data are thus in contrast with the report that Fas controls the early onset of cortical apoptosis after peptide injection in TCR transgenic models (26). Since these findings applied to adult mice, cortical apoptosis may have been stress related. If so, it is of interest that, for mature T cells, Fas ligand can act as a costimulatory molecule (45), and that cytokine production by lpr/lpr T cells in vitro is lower than for normal T cells (11). Hence, stress-induced thymic atrophy after antigen injection could be less marked in lpr/lpr mice.
In conclusion, the data in this paper indicate that clonal elimination of antigen-specific T cells in the thymus after antigen injection occurs at a relatively late stage of differentiation and does not involve Fas when the dose of antigen is kept to a low-to-moderate level. However, with a high dose of antigen the presence of Fas becomes crucial for negative selection. In light of these findings, the prior failure to find a clear role for Fas in central tolerance may reflect that the antigens studied were expressed at a relatively low level.
Acknowledgments
We thank Ms. Barbara Marchand for typing the manuscript and Dr. Z. Cai for preparing ova peptides.
This work was supported by grants CA38355, CA25803, AI21487, AI32068, AI38385 and AG01743 from the United States Public Health Service. Dr. Kishimoto is a recipient of a fellowship from the Cancer Research Institute.
Abbreviations used in this paper
- AICD
activation-induced cell death
- BrdU
bromodeoxyuridine
- DP
double positive
- guinea pig C
guinea pig complement
- HSA
heat-stable antigen
- ova
ovalbumin
- SEB
staphylococcal enterotoxin B
- SP
single positive
- TUNEL
TdT-mediated dUTP-biotin nick end labeling
Footnotes
This is publication no. 10869-IMM from the Scripps Research Institute.
References
- 1.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 2.Nossal GJV. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 3.Sprent J, Webb SR. Intrathymic and extrathymic clonal deletion of T cells. Curr Opin Immunol. 1995;7:196–205. doi: 10.1016/0952-7915(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 4.Robey E, Fowlkes BJ. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 5.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/ Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 6.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 7.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 8.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 9.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 10.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+ CD4+T cells in mice tolerant to Staphylococcal aureus enterotoxin B. Nature. 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 11.Singer GG, Abbas AK. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 12.Adachi M, Suematsu S, Suda T, Watanabe D, Fukuyama H, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Enhanced and accelerated lymphoproliferation in Fas-null mice. Proc Natl Acad Sci USA. 1996;93:2131–2136. doi: 10.1073/pnas.93.5.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crispe IN. Fatal interactions: Fas-induced apoptosis of mature T cells. Immunity. 1994;1:347–349. doi: 10.1016/1074-7613(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 14.Scott DE, Kisch WJ, Sternberg AD. Studies of T cell deletion and T cell anergy following in vivo administration of SEB to normal and lupus prone mice. J Immunol. 1993;150:664–672. [PubMed] [Google Scholar]
- 15.Musette P, Pannetier C, Gachelin G, Kourilsky P. The expansion of a CD4+ T cell population bearing a distinctive β chain in MRL lpr/lprmice suggests a role for the Fas protein in peripheral T cell selection. Eur J Immunol. 1994;24:2761–2766. doi: 10.1002/eji.1830241128. [DOI] [PubMed] [Google Scholar]
- 16.Mogil RJ, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos AN, Green DR. Fas(CD95) participates in peripheral T cell deletion and associated apoptosis in vivo. Int Immunol. 1995;7:1451–1458. doi: 10.1093/intimm/7.9.1451. [DOI] [PubMed] [Google Scholar]
- 17.Theofilopoulos AN. The basis of autoimmunity. Part I. Mechanisms of aberrant self-recognition. Immunol Today. 1995;16:90–98. doi: 10.1016/0167-5699(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 18.Kotzin BL, Babcock SL, Herron LR. Deletion of potentially self-reactive T cell receptor specificities in L3T4−, Ly-2− T cells of lprmice. J Exp Med. 1988;168:2221–2229. doi: 10.1084/jem.168.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer PA, Balderas RS, McEvilly RJ, Bobardt M, Theofilopoulos AN. Tolerance-related Vβ clonal deletions in normal CD4−8−, TCR-α/β+, and abnormal lprand gld cell populations. J Exp Med. 1989;170:1869–1877. doi: 10.1084/jem.170.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mountz JD, Smith TM, Toth KS. Altered expression of self-reactive T cell receptor Vβ regions in autoimmune mice. J Immunol. 1990;6:2159–2166. [PubMed] [Google Scholar]
- 21.Giese J, Davidson WF. Evidence for early onset polyclonal activation of T cell subsets in mice homozygous for lpr. J Immunol. 1992;149:3097–3101. [PubMed] [Google Scholar]
- 22.Sidman CL, Marshall JD, von Boehmer H. Transgenic T cell receptor interactions in the lymphoproliferative and autoimmune syndromes of lpr and gld mutant mice. Eur J Immunol. 1992;22:499–504. doi: 10.1002/eji.1830220231. [DOI] [PubMed] [Google Scholar]
- 23.Herron LR, Eisenberg RA, Roper RE, Kakkanaiah VN, Cohen PL, Kotzin BL. Selection of the T cell receptor repertoire in lpr mice. J Immunol. 1993;151:3450–3459. [PubMed] [Google Scholar]
- 24.Mountz JD, Bluethmann H, Zhou T, Wu J. Defective clonal deletion and anergy induction in TCR transgenic lpr/lprmice. Semin Immunol. 1994;6:27–37. doi: 10.1006/smim.1994.1005. [DOI] [PubMed] [Google Scholar]
- 25.Sytwu HK, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 26.Castro JE, Listman JA, Jacobson BA, Wang Y, Lopez PA, Ju S, Finn PW, Perkins DL. Fas modulation of apoptosis during negative selection of thymocytes. Immunity. 1996;5:617–627. doi: 10.1016/s1074-7613(00)80275-7. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto H, Sprent J. Negative selection in the thymus includes semi-mature T cells. J Exp Med. 1997;185:263–272. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrack P, Kappler JW. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 29.White J, Herman A, Pullen AM, Kubo RT, Kappler JW, Marrack P. The Vβ-specific superantigen Staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- 30.Murphy KM, Heimberger AB, Loh DH. Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCRlothymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 31.Kosaka H, Surh CD, Sprent J. Stimulation of mature unprimed CD8+T cells by semiprofessional antigen-presenting cells in vivo. J Exp Med. 1992;176:1291–1302. doi: 10.1084/jem.176.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine αβ T cell receptors. J Immunol. 1989;142:2736–2742. [Google Scholar]
- 33.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 34.Ernst B, Surh CD, Sprent J. Thymic selection and cell division. J Exp Med. 1995;182:961–971. doi: 10.1084/jem.182.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishimoto H, Surh CD, Sprent J. Upregulation of surface markers on dying thymocytes. J Exp Med. 1995;181:649–655. doi: 10.1084/jem.181.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin S, Bevan MJ. Antigen-specific and nonspecific deletion of immature cortical thymocytes caused by antigen injection. Eur J Immunol. 1997;27:2726–2736. doi: 10.1002/eji.1830271037. [DOI] [PubMed] [Google Scholar]
- 38.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 39.Shortman, K., and R. Scollay. 1985. Cortical and medullary thymocytes. In Recognition and Regulation in Cell-mediated Immunity. J.D. Watson and J. Marbrook, editors. Marcell Dekker, New York. 31–60.
- 40.Punt JA, Havran W, Abe R, Sarin A, Singer A. T cell receptor (TCR)-induced death of immature CD4+ CD8+thymocytes by two distinct mechanisms differing in their requirement for CD28 costimulation: implications for negative selection in the thymus. J Exp Med. 1997;186:1911–1922. doi: 10.1084/jem.186.11.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green DR, Scott DW. Activation-induced apoptosis in lymphocytes. Curr Opin Immunol. 1994;6:476–487. doi: 10.1016/0952-7915(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 42.Miethke T, Wahl C, Gaus H, Heeg K, Wagner H. Exogenous superantigens acutely trigger distinct levels of peripheral T cell tolerance/immunosuppression: dose-response relationship. Eur J Immunol. 1994;24:1893–1902. doi: 10.1002/eji.1830240827. [DOI] [PubMed] [Google Scholar]
- 43.Zhou T, Bluethmann H, Zhang J, Edwards CK, III, Mountz JD. Defective maintenance of T cell tolerance to a superantigen in MRL-lpr/lprmice. J Exp Med. 1992;176:1063–1072. doi: 10.1084/jem.176.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balomenos D, Rumold R, Theofilopoulos AN. The proliferative in vivo activities of lpr double-negative T cells and the primary role of p59fynin their activation and expansion. J Immunol. 1997;159:2265–2273. [PubMed] [Google Scholar]
- 45.Suzuki I, Fink P. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187:123–128. doi: 10.1084/jem.187.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]