Abstract
Natural killer (NK) cells have been implicated in early immune responses against certain viruses, including cytomegalovirus (CMV). CMV causes downregulation of class I major histocompatibility complex (MHC) expression in infected cells; however, it has been proposed that a class I MHC homolog encoded by CMV, UL18, may act as a surrogate ligand to prevent NK cell lysis of CMV-infected cells. In this study, we examined the role of UL18 in NK cell recognition and lysis using fibroblasts infected with either wild-type or UL18 knockout CMV virus, and by using cell lines transfected with the UL18 gene. In both systems, the expression of UL18 resulted in the enhanced killing of target cells. We also show that the enhanced killing is due to both UL18-dependent and -independent mechanisms, and that the killer cell inhibitory receptors (KIRs) and CD94/NKG2A inhibitory receptors for MHC class I do not play a role in affecting susceptibility of CMV-infected fibroblasts to NK cell–mediated cytotoxicity.
Keywords: cytomegalovirus, class I major histocompatibility complex, UL18, natural killer cell, cytotoxicity
Human cytomegalovirus (hCMV)1 is an extremely widespread infectious agent. Healthy individuals acquiring hCMV postnatally are usually asymptomatic, although the virus persists in the host for life (1). Both T and NK cells play a critical role in controlling the initial infection and the disease that follows viral reactivation in immunocompromised individuals (2, 3). The importance of NK cells is highlighted by the fact that patients with an NK cell deficiency are extremely susceptible to hCMV infection and its associated diseases (4). In addition, NK cells also play an important role in the control of mouse CMV (5). Strain-dependent mouse CMV resistance or susceptibility has been mapped to the NK complex (NKC) region of murine chromosome 6 (6). The NKC region contains genes involved in modulating murine NK cell functions and codes for molecules that can trigger (NKR-P1) or inhibit (Ly49) NK cell–mediated cytotoxicity (7). Murine Ly49 molecules and their human functional counterparts, the KIRs (killer cell inhibitory receptors) and CD94/ NKG2A receptors, inhibit NK cell cytotoxicity after cognate interaction with class I MHC molecules (8). Since many viruses downregulate host cell class I MHC expression upon infection, the hypothesis that NK cell inhibitory receptors serve as a physiological means to monitor for viral infection is compelling.
Both human (9) and mouse CMV (10) encode glycoproteins with homology to class I MHC heavy chains, designated UL18 (human) and M144 (mouse), respectively. It has been suggested that these molecules serve as surrogates for class I MHC molecules to engage inhibitory NK cell class I MHC receptors. In support of this hypothesis, Farrel et al. (10) have shown that mouse CMV lacking the M144 gene is more virulent in vivo. Transfection studies using the human CMV UL18 gene have implicated this protein in the protection of B lymphoblastoid cells from lysis by NK cells expressing CD94/NKG2A receptor (11). In addition, Cosman and coworkers (12, 13) have recently demonstrated that UL18 is specifically recognized by LIR-1, a membrane of the Ig receptor superfamily, which is predominantly expressed on monocytes, B cells, and a minor subset of NK cells (12, 13). In this study we examined the roles of endogenous class I MHC and an hCMV encoded class I homolog (UL18) in modulating NK cell–mediated cytotoxicity during hCMV infection.
Materials and Methods
Cell lines, NK Cell Lines, and Clones.
293EBV is a human kidney cell line expressing the EBNA-1 nuclear antigen (Invitrogen, Carlsbad, CA). COS-7, Chinese hamster ovary (CHO)-K1, 293EBV, and human foreskin fibroblast (HFF) cell lines were maintained in RPMI (MediaTech, Herndon, VA) supplemented with 10% FCS (GIBCO BRL, Bethesda, MD). NK cells were cloned and cultured as previously described (14, 15). HFF cell lines were prepared and cultured as previously described (16).
Constructs.
The UL18 open reading frame was subcloned into the EBV episomal expression vectors pREP10 and pCEP4 (Invitrogen). The cDNA for CD32 and CD94 were cloned into pBJneo. The hCMV gB (UL55) cDNA in the pRcCMV vector (Invitrogen) was a gift from Dr. L. Pereira (University of California San Francisco, San Francisco, CA).
Transient Transfection.
293EBV were plated at 60–80% confluence and transfected with pREP10 UL18 using Lipofectamine (GIBCO BRL). 48 h after transfection, cells were trypsinized and stained with anti-UL18 mAb (10C7), followed by PE-conjugated goat anti–mouse IgG. Viable UL18 positive cells were sorted and cultured for 48 h before use in cytotoxicity assays. COS-7 and CHO-K1 were transfected with pCEP4 UL18 and sorted as above. Flow cytometry was performed as previously described (17).
Antibodies.
10C7 (mouse IgG1) was generated by immunizing mice with soluble, partially deglycosylated UL18. The mAbs against KIRs were: DX9 (KIR3DL1); DX27 (KIR2DL2, KIR2DL3, and KIR2DS2); DX30 (KIR3DL1, KIR3DL2); DX31 (KIR3DL2); and HP-3E4 (KIR2DL1, KIR2DS1, and KIR2DS4). DX22 mAb is against CD94. Anti–class I mAbs (DX15, DX16, and DX17) have been previously described (18). Anti-CMV UL55 (gB) was purchased from the Goodwin Institute (Goodwin Institute, Plantation, FL). Anti-hCMV IE mAb was purchased from Chemicon (Temecula, CA). All other mAbs were provided by Becton Dickinson Immunocytometry Systems (San Jose, CA).
Cytotoxicity Assays.
Cell-mediated cytotoxicity was assessed using 4-h 51Cr–release assays. In these assays, effector cells at various concentrations were incubated with 5 × 103 target cells in U-bottomed 96-well microtiter plates at 37°C. Percentage of lysis was determined as previously described (18). Spontaneous 51Cr release was <10% of total counts. Only cells with >80% viability were used for labeling. The spontaneous release of 293EBV UL18 cells and 293EBV controls were similar.
Western Blot.
Infected HFFs were lysed in 0.5 ml of lysis buffer (Tris buffered saline, 50 mM Tris, 150 mM NaCl, pH 8.0, with 1% NP-40). Lysates were resolved by SDS-PAGE on 12% gels. Western blots were performed using 10C7 anti-UL18 or anti-hCMV IE mAbs, followed by horseradish peroxidase–conjugated second antibodies as previously described (19).
hCMV Infection.
AD169 and Δ18 (20) viruses were titered and propagated in HFFs as previously described (16). HFFs were infected at a multiplicity of infection (MOI) of 3–5. After adsorption of the virus for 1 h at 37°C, the inoculum was removed and medium containing 10% FCS was added. Viral stocks were titered using classical cytopathic effect as an end-point.
Results and Discussion
The Role of Endogenous Class I in Modulating NK Cell Killing of Infected and Uninfected HFFs.
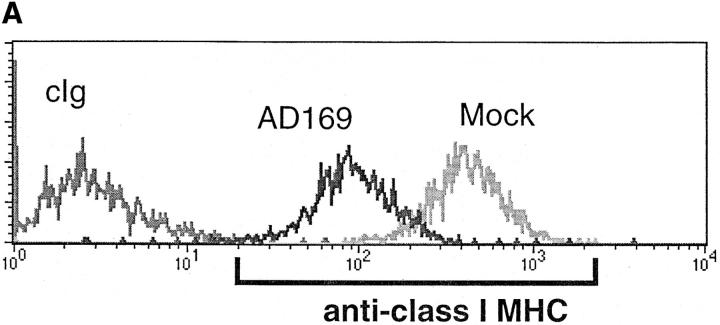
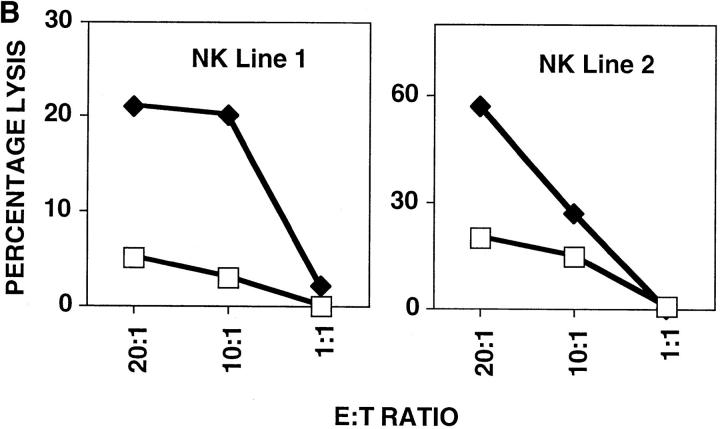
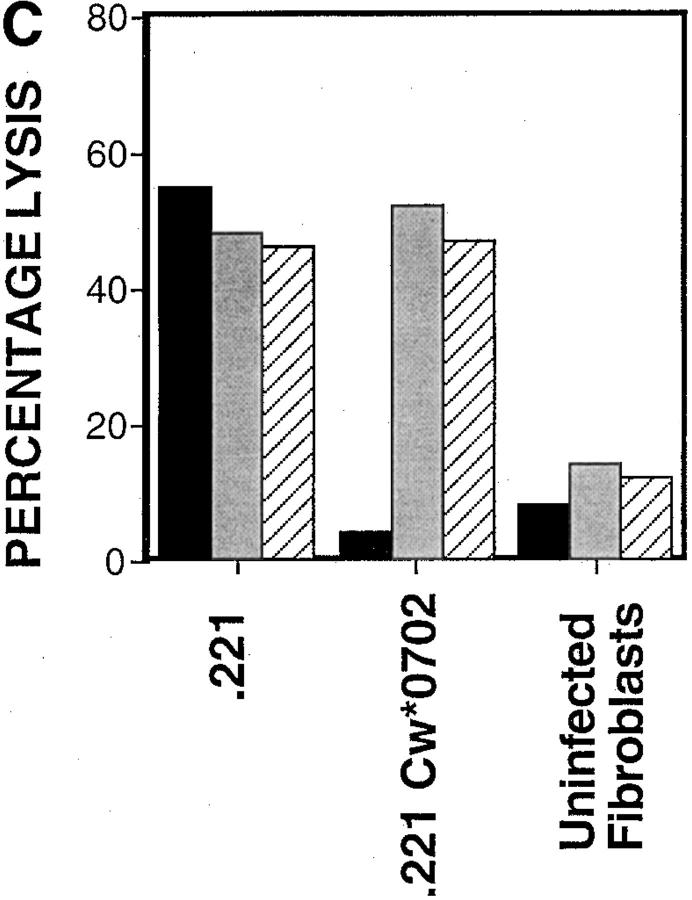
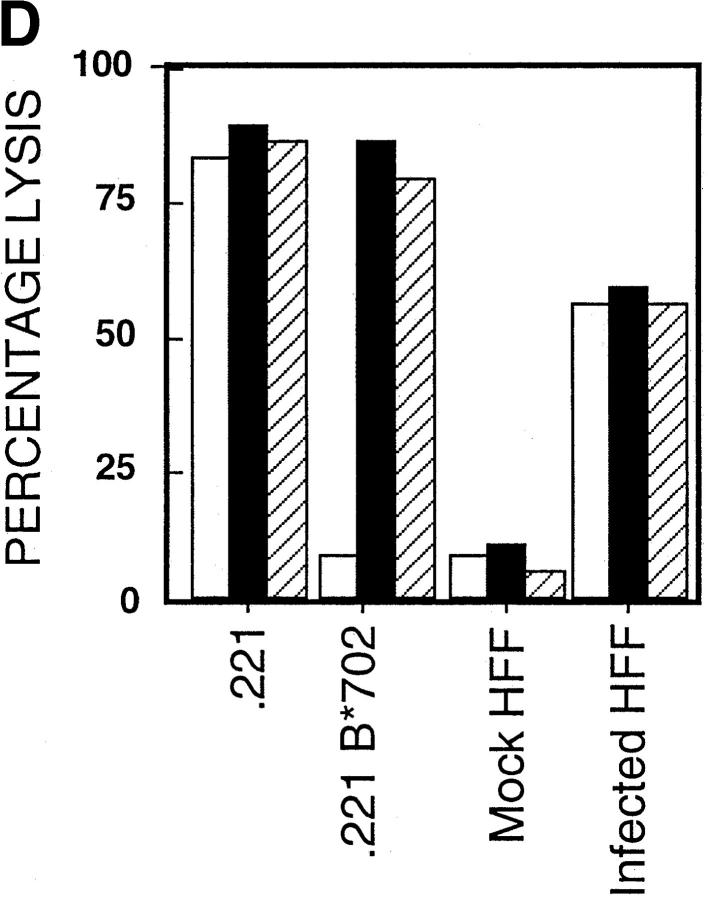
Fibroblasts downregulate class I MHC expression upon hCMV infection (21). In Fig. 1 A, we show that class I MHC is downregulated 10-fold in hCMV-infected HFFs, but a significant level of class I MHC is still present on the cell surface. This downregulation was seen by 24 h and was maintained for the duration of the studies (5 d, data not shown). The ‘missing self' hypothesis stipulates that the loss of class I MHC expression confers susceptibility to NK cell killing (22). The expectation from this hypothesis is that infected cells would become more susceptible to NK cell–mediated lysis. To address this question, we performed NK cell cytotoxicity assays using hCMV-infected and mock-infected cells. In repeated experiments, NK cells efficiently lysed hCMV-infected HFFs, but not mock-infected cells (Fig. 1 B). This inability to efficiently kill mock-infected HFFs may be due to their high level of class I MHC expression. If NK cells were restrained from killing uninfected HFFs by a class I MHC–dependent mechanism, blocking the KIRs and/or CD94/NKG2A receptors on the effector cells or class I MHC on the fibroblasts should reverse the protection. Experiments performed with anti–class I or a cocktail of mAbs against CD94 and KIRs did not induce killing (Fig. 1 C). This inability to kill was evident using a variety of NK clones and lines (data not shown). The KIR and CD94/ NKG2A receptors expressed by these NK cells were functional against B lymphoblastoid target cells transfected with relevant class I MHC genes, with the target cell protection being reversed using anti–class I MHC or anti-CD94 + anti-KIR mAbs. Therefore, class I MHC expression alone by HFFs does not prevent NK cell–mediated killing. It can be argued that mock-infected HFFs lack positive signals needed to trigger NK cells, and that class I MHC may be functional in the context of infected cells, which have upregulated ligands for the ‘triggering' receptors. To address this, we repeated these experiments using hCMV-infected HFFs. Again, blocking class I MHC, KIRs, or CD94 neither augmented nor attenuated NK cell–mediated cytotoxicity (Fig. 1 D).
Figure 1.
(A) Downregulation of class I MHC in hCMV-infected HFFs. AD169 or mock-infected HFFs were stained with anti–class I (DX17) or control Ig (cIg), followed by PE-conjugated goat anti–mouse Ig. HFFs were infected with AD169 at an MOI of 3, and cells were stained at 24 h after infection. (B) NK cell cytotoxicity assay against hCMV or mock-infected HFF cells. hCMV or mock-infected HFFs were used as targets in a 4-h 51Cr–release assay. HFFs were infected at an MOI of 3 and used at 48 h after infection. Mock-infected, white squares; AD169, black diamonds. (C) Blocking class I MHC, KIR, or CD94 does not induce NK cell killing of uninfected HFFs. Normal HFFs were incubated in the presence of NK cells and with cIg or a mixture of mAb against class I MHC (DX15, DX16, and DX17 at 20 μg/ml) or a mixture of anti-KIR mAbs (DX27, DX30, DX31, and HP-3E4) and anti-CD94 mAb (DX22), each at 20 μg/ml. cIg, black bars; anti-KIR/CD94, gray bars; anti–class I, hatched bars. (D) AD169 or mock-infected HFFs were incubated in the presence of NK cells with cIg or mAb directed at class I MHC (DX15, DX16, and DX17 at 20 μg/ml) or a cocktail of mAbs against KIR and CD94. Cytotoxicity assays were performed at E/T ratios of 5:1. As controls, 721.221 class I HLA transfectants expressing HLA-B*0702 or HLA-Cw*0702 were analyzed, using NK clones expressing the relevant CD94/NKG2A or KIR, respectively. cIg, white bars; anti-KIR/CD94, black bars; anti–class I, hatched bars.
The Role of a Virus-encoded Class I MHC Homolog in NK Cell Killing.
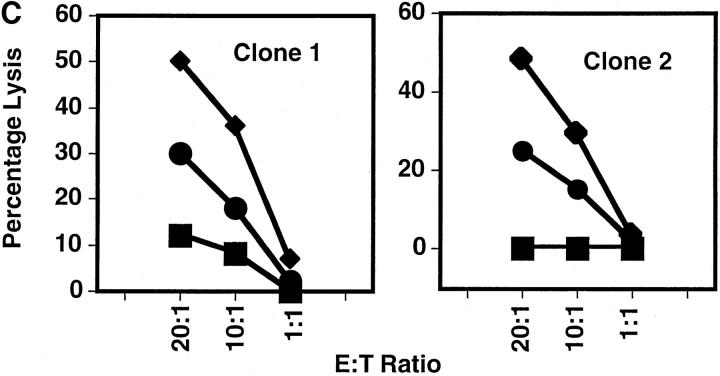
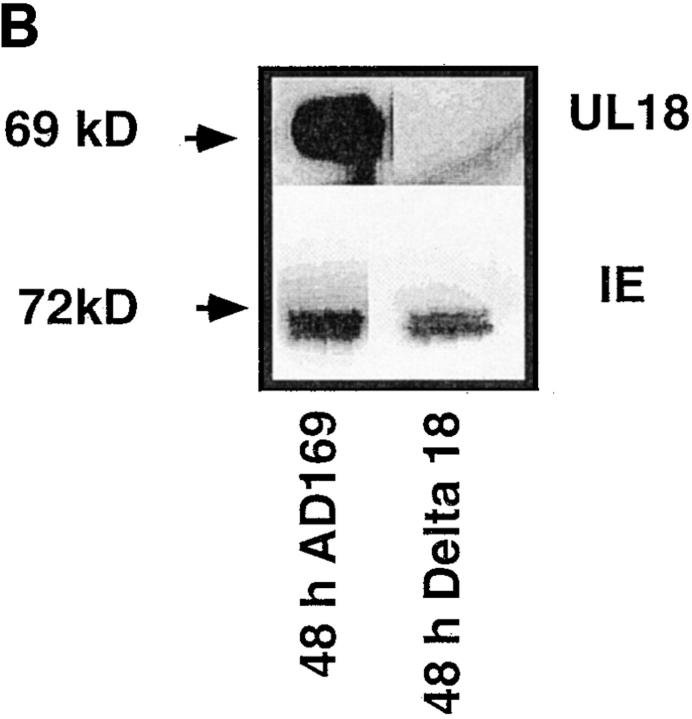
hCMV encodes a glycoprotein with homology to class I MHC (9). This molecule is able to form a complex with β2-microglobulin (β2M)(23) and contains a peptide-binding groove (24). KIR recognition of class I MHC requires the formation of a trimeric complex comprising class I MHC, β2M, and peptide (25, 26). It was therefore hypothesized that UL18 would interact with NK cell class I receptors to inhibit killing of CMV-infected cells. We examined this by infecting HFFs with wild-type AD169 CMV virus or Δ18 (a mutant virus with the UL18 gene deleted). The level of class I MHC downregulation by the two viruses was comparable (Fig. 2 A). A 69-kD UL18 protein was detectable by 24 h in AD169-infected HFFs by Western blot analysis, but not in Δ18-infected HFF lysates (Fig. 2 B). Although UL18 protein was readily detected by Western blot analysis in HFF infected with AD169 virus, analysis by flow cytometry suggested that very little UL18 was expressed on the cell surface (data not shown). The amounts of hCMV IE protein (Fig. 2 B) and class I MHC (data not shown) in lysates prepared from Δ18-infected HFFs were comparable to those detected in HFF infected with AD169 wild-type CMV. Furthermore, the titer of AD169 and Δ18 viruses were comparable, as assessed by plaque formation assays (data not shown) and the HFFs were homogeneously infected as assessed by the uniform downregulation of class I in all the cells (Fig. 2 A). Many NK cell lines and clones killed AD169-infected cells somewhat better than they did Δ18-infected cells (Fig. 2 C). However, HFFs infected with AD169 or Δ18 showed enhanced killing when compared with mock infected cells (Fig. 2 C). Therefore, UL18 confers a slight enhancement of susceptibility to NK cell killing, although UL18-independent mechanism(s) also exists.
Figure 2.
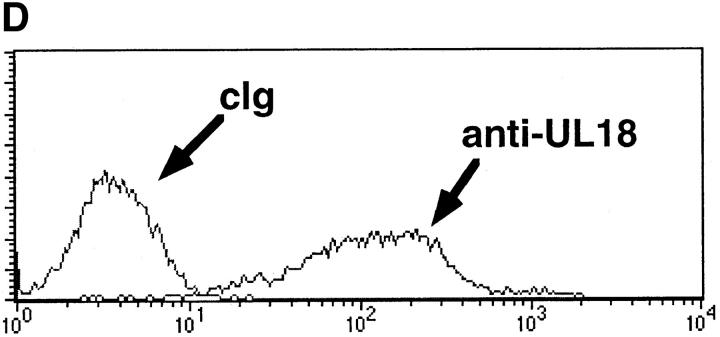
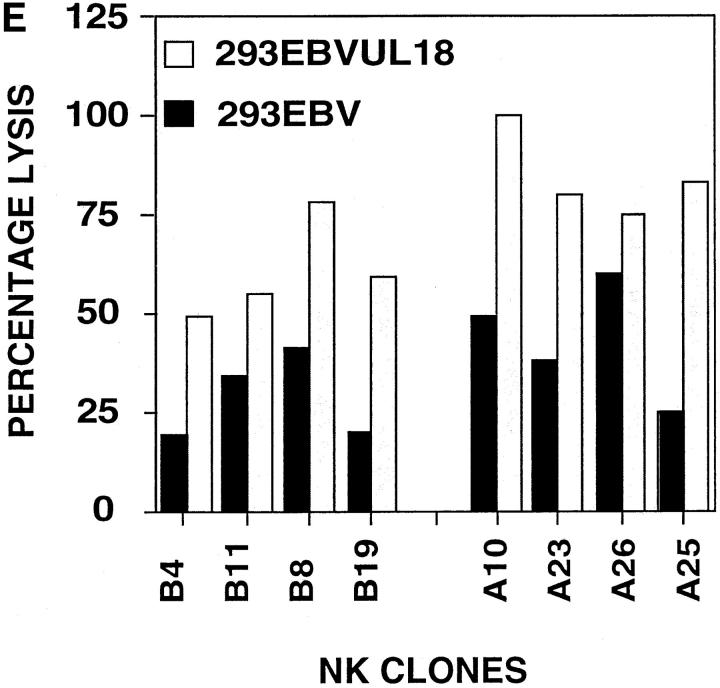
(A) Downregulation of class I MHC after Δ18 and AD169 hCMV infection of HFFs. HFFs were infected with Δ18 or AD169 at an MOI of 5. At 48 h after infection cells were stained with anti–class I MHC or cIg, followed by PE-conjugated goat anti–mouse second step. (B) Expression of UL18 in AD169 but not Δ18 cell lysates. Lysates were prepared from HFF-infected with Δ18 or AD169 at 48 h after infection and blotted with anti-UL18 or anti-hCMV IE mAb, followed by horseradish peroxidase–conjugated second step. (C) AD169-infected HFFs were lysed more efficiently than were Δ18-infected HFFs. HFFs were infected with AD169 or Δ18 at an MOI of 5, and used as targets at 48 h after infection in 4-h 51Cr–release assays. Assays were performed at an E/T ratio of 20:1, 10:1, and 1:1. Standard deviation between triplicates was <10%. Δ18, black circles; AD169, black diamonds; mock-infected, black squares. (D) Surface UL18 expression on 293EBV transfectants. 293EBV cells were transfected with pREP10 UL18. Cells were cultured for 48 h before use in cytotoxicity assays. Histograms of sorted UL18-positive cells. Cells were stained with anti-UL18 mAb 10C7 followed by PE-conjugated goat anti–mouse Ig. (E) 293EBV transfectant expressing UL18 were lysed at an enhanced level compared with parental controls. UL18-transfected 293EBV cells were used as targets in NK cell cytotoxicity assays. Experiments were performed at an E:T ratio of 5:1. All transfectants other than vector only were positively sorted using mAb directed against the protein encoded by the transfected cDNA. Cells were cultured for 48 h and used in 4-h cytotoxicity assays as described above.
To examine directly the ability of UL18 to confer increased susceptibility to NK cell killing in the absence of viral infection, we transfected 293EBV cells with a UL18 expression vector. Fig. 2 D shows the expression of UL18 on the surface of transfectants using a UL18-specific mAb. In a series of experiments, 293EBV UL18 cells consistently showed enhanced lysis compared with 293EBV (Fig. 2 E). In addition, UL18-dependent enhancement of NK cell killing could also be demonstrated in transfected COS-7 and CHO-K1 cells (data not shown) using several NK clones and lines. These clones included those expressing functional KIRs and CD94 (Table 1). Inhibition of NK cell killing by UL18-expressing target cells was never observed in any of these experiments. The enhanced lysis of 293EBV cells transfected with UL18 was not affected by mAbs against CD94 or KIR (data not shown), although it should be noted that not all KIR isoforms are recognized by the available mAbs. The enhanced killing of 293EBV UL18 transfectants versus 293EBV was not due to changes in the level of class I MHC or adhesion molecules (data not shown). As controls, cells were transfected with expression constructs encoding CD32, CD94, an hCMV surface glycoprotein (gB), or vector only; none of these transfectants showed enhanced susceptibility to NK cell lysis (data not shown).
Table 1.
Phenotype of NK Cell Lines and Clones
| Clones | KIRs | CD94 | ||
|---|---|---|---|---|
| A10 | DX9−, DX24+ | CD94 | ||
| A23 | DX9−, DX24+ | CD94 | ||
| A26 | DX9+, DX24+ | ND | ||
| A25 | DX9+, DX24+ | ND | ||
| BR | KIR− | CD94/NKG2A | ||
| B8 | KIR− | CD94/NKG2A | ||
| B11 | KIR− | CD94/NKG2A | ||
| B19 | KIR− | CD94/NKG2A |
Hence, the hypothesis that UL18 replaces class I MHC molecules to prevent NK cell lysis does not seem to apply to hCMV-infected HFFs and transfected epithelial and ovary cells (293, COS-7, and CHO-K1). It was recently reported that UL18 inhibits NK cell lysis of 721.221 B lymphoblastoid targets, mediated through CD94 (11). In repeated studies, we never observed inhibition of NK cell killing against UL18 expressing targets using clones with functional CD94 or KIR. In view of this discrepancy, several aspects of the previous report should be highlighted. First, in the prior study UL18 was transfected into 721.221 targets; however, transfectants were isolated on the basis of surface β2M expression, not UL18. Second, we have failed to generate stable UL18 transfectants in 721.221 or in 15 other human or mouse lines, because it seems that prolonged expression (>2 wk) of UL18 results in cell death. We could only generate UL18 transfectants in high efficiency transient transfection systems such as 293, COS-7, and CHO-K1. Notably, 721.221 cells express low levels of HLA-E (27) and -F (28). Since Reyburn et al. (11) sorted UL18-transfected 721.221 on the basis of β2M surface expression, rather than UL18, cells expressing HLA-E or -F may have been inadvertently enriched and could be responsible for the protection against NK cell lysis. In fact, a recent study demonstrated that the endogenous HLA-E expressed by 721.221 cells can protect these target cells from lysis by NK cells expressing the inhibitory CD94/NKG2A receptor (29). Although HLA-E molecules bind peptides derived from the leader segments of certain other MHC class I proteins (27), the leader of UL18 does not conform to the preferred peptide bound by HLA-E. Therefore, it is possible that Reyburn et al. (11) simply selected for a variant of 721.221 that expressed higher levels of the endogenous HLA-E protein.
Recent studies by Cosman and coworkers (12, 13) have shown that the human CMV UL18 protein interacts with a membrane receptor designated ILT2 (30) or LIR-1 (12, 13), which is expressed predominantly on monocytes, B cells, and a subset of NK cells. Binding of a UL18–Ig Fc fusion protein to NK cells and monocytes was completely blocked using an anti-ILT2/LIR-1 mAb, suggesting that ILT2/LIR-1 is the predominant, if not exclusive, receptor for UL18 expressed on leukocytes (12). It is possible that ILT2/LIR-1 on NK cells might interact with UL18 on CMV-infected cells, preventing NK cell–mediated cytotoxicity. However, it should be appreciated that ILT2/ LIR-1 is expressed on only a minor subset of NK cells (12, 31); therefore, the physiological significance of ILT2/LIR-1 on NK cells during a CMV infection is uncertain. Although prior studies of UL18 and its mouse homolog M144 have focused on a potential role for these proteins in NK cell–mediated immunity (10, 11), an alternative possibility is that these molecules may be more important in affecting monocyte and dendritic cell function during CMV infection. For example, interactions between UL18 and ILT2/LIR-1 on monocytes or dendritic cells during a CMV infection may suppress IL-12 production, which would in turn limit IFN-γ secretion by NK cells and thus alter the early host immune response. This scenario could potentially explain the increased virulence of mouse CMV virus lacking M144 (10). This is of particular interest given the recent finding that dendritic cells may serve as the reservoir for latent CMV infection (32).
Upregulation of Cell Surface Adhesion Molecules in hCMV-infected HFFs.
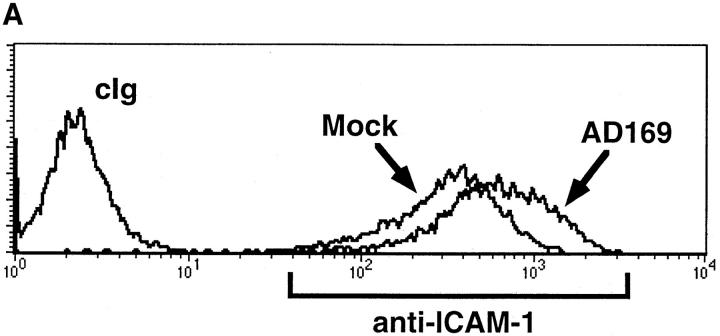
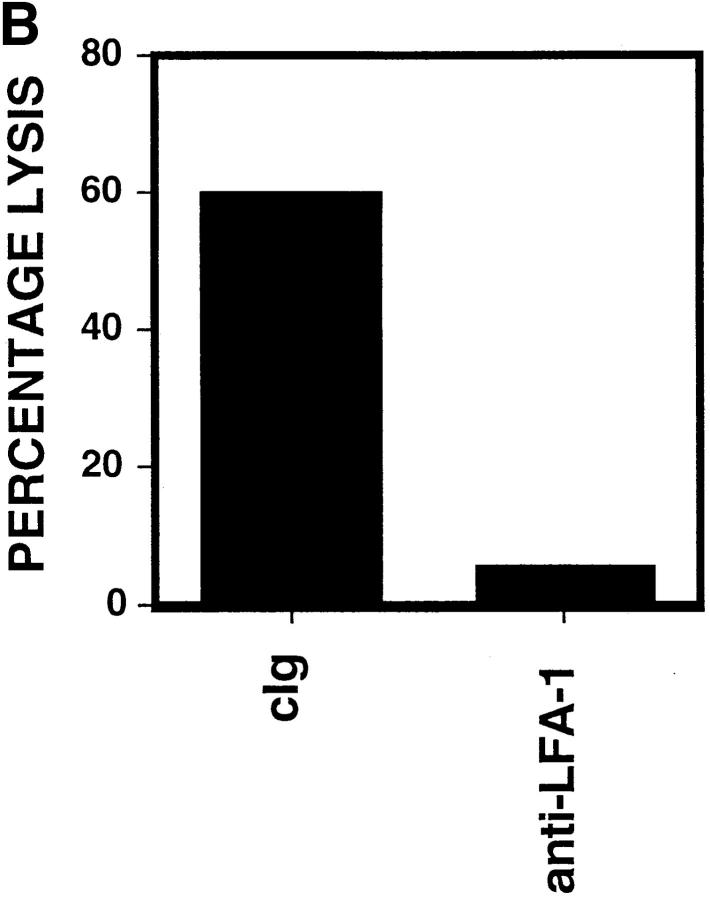
Since enhanced killing was observed in HFF infected with both AD169 and Δ18, other molecules in addition to UL18 were also playing a role. To examine what molecules were upregulated after infection, we stained mock-infected and hCMV-infected HFFs with a panel of mAb against cell surface adhesion/costimulatory molecules. The only molecule examined that was consistently upregulated in the infected cells was intracellular adhesion molecule 1 (ICAM-1; CD54) (Fig. 3 A). Blocking of ICAM-1 interaction with its ligand using anti–LFA-1β (CD18) was able to prevent the killing of infected HFF (Fig. 3 B). These data suggest that ICAM-1 is a crucial component in NK cell–mediated killing of hCMV-infected cells, but does not exclude the existence of other molecules capable of triggering NK cell cytotoxicity.
Figure 3.
(A) Upregulation of ICAM-1 on hCMV-infected cells. AD169 or mock-infected HFFs were stained (24 h after infection) with FITC-conjugated anti–ICAM-1 (LB2 mAb) or FITC-conjugated cIg. Mean fluorescence intensity of ICAM-1 increased from 351 to 705. (B) Enhanced cytotoxicity against hCMV-infected HFFs was reversed with anti–LFA-1β (CD18). hCMV or mock-infected HFFs were incubated with cIg or anti– LFA-1β (20 μg/ml). 4-h 51Cr– release assays were performed at an E/T ratio of 5:1.
Based on our observations, the hypothesis that KIRs and CD94/NKG2A class I MHC inhibitory receptors are a physiological surveillance mechanism for viral infection may not be universally applicable. Similarly, we have previously shown that in certain target cells (e.g., Jurkat and K562), the expression of an appropriate class I MHC allele recognized by KIR3DL1 (i.e., HLA-B*5801) was insufficient to prevent killing of these transfected target cells (14).
hCMV has evolved many strategies that allow its highly successful dissemination throughout the population. Although infected individuals are generally clinically asymptomatic (1), further viral transmission still occurs. In cases where the infection is not effectively controlled, death often results, thereby limiting the opportunity for further viral dissemination. Therefore, the UL18-dependent and independent mechanisms may serve to limit the severity of CMV-induced disease by rendering infected cells more susceptible to immune destruction.
Acknowledgments
We thank the members of the DNAX FACS facility for flow cytometry, and Dr. Lenore Pereira, Debra Liggett, Sasha Lazetic, and Brian Corliss for advice and reagents.
Abbreviations used in this paper
- β2M
β2-microglobulin
- CHO
Chinese hamster ovary
- cIg
control Ig
- hCMV
human cytomegalovirus
- HFF
human foreskin fibroblasts
- ICAM-1
intracellular adhesion molecule 1
- KIR
killer cell inhibitory receptor
- MOI
multiplicity of infection
Footnotes
DNAX Research Institute is supported by the Schering Plough Corporation. T.L Chapman is supported by a National Defense Science and Engineering Pre-Doctoral Fellowship.
References
- 1.Ho, M. 1982. Epidemiology of cytomegalovirus in man. In Cytomegalovirus, Biology and Infection: Current Topics in Infectious Disease. W.B. Greenough III and T.C. Merigan, editors. Plenum Medical Book Co., New York. 77–104.
- 2.Quinnan GV, Kirmani N, Rook AH, Manishewitz JF, Jackson L, Moreschi G, Santos GW, Saral R, Burns WH. Cytotoxic T cells in cytomegalovirus infection. N Engl J Med. 1982;307:6–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- 3.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 4.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 5.Bukowski J, Warner J, Dennert G, Welsh R. Adoptive transfer studies demonstrating the anti-viral effect of natural killer cells in vivo. J Exp Med. 1985;161:40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scalzo AA, Fitzgerald NA, Wallace C, Gibbons AE, Smart YC, Burton RC, Shellam GR. The effect of the CMV-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killers. J Immunol. 1992;149:581–589. [PubMed] [Google Scholar]
- 7.Brown MG, Scalzo AA, Matsumoto K, Yokoyama WM. The natural killer gene complex: a genetic basis for understanding natural killer cell function and innate immunity. Immunol Rev. 1997;155:53–65. doi: 10.1111/j.1600-065x.1997.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL, Phillips JH. NK cell recognition of major histocompatibility complex class I molecules. Semin Immunol. 1995;7:75–82. doi: 10.1006/smim.1995.0011. [DOI] [PubMed] [Google Scholar]
- 9.Beck S, Barrell BG. Human cytomegalovirus encodes a glycoprotein homologous to MHC class I antigens. Nature. 1988;331:269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- 10.Farrel H, Vally H, Lynch D, Fleming P, Shellam G, Scalzo A, Davis-Poynter N. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature. 1997;386:446–447. doi: 10.1038/386510a0. [DOI] [PubMed] [Google Scholar]
- 11.Reyburn HT, Mandelbolm O, Vales-Gomez M, Davis D, Pazmany L, Strominger J. The class I MHC homologue of human cytomegalovirus inhibits attack by natural killer cells. Nature. 1997;386:514–517. doi: 10.1038/386514a0. [DOI] [PubMed] [Google Scholar]
- 12.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, Hsu M-L. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 13.Borges L, Hsu M-L, Fanger N, Kubin M, Cosman D. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J Immunol. 1997;159:5192–5196. [PubMed] [Google Scholar]
- 14.Litwin V, Gumperz J, Parham P, Phillips JH, Lanier LL. Specificity of HLA class I antigen recognition by human NK clones: evidence for clonal heterogeneity, protection by self and non-self alleles, and influence of the target cell type. J Exp Med. 1993;178:1321–1336. doi: 10.1084/jem.178.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yssel H, Vries JD, Koken M, van Blittersijk W, Spits H. Serum-free medium for the generation and the propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984;72:219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- 16.Mocarski ES, Bonyhadi M, Salimi S, McCune JM, Kaneshima H. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc Natl Acad Sci USA. 1993;90:104–108. doi: 10.1073/pnas.90.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanier, L.L., and D.J. Recktenwald. 1991. Multicolor immunofluorescence and flow cytometry. Methods: A Companion to Methods in Enzymology 2:192–199.
- 18.Phillips J, Gumperz J, Parham P, Lanier L. Superantigen-dependent, cell-mediated cytotoxicity inhibited by MHC class I receptors on T lymphocytes. Science. 1995;268:403–405. doi: 10.1126/science.7716542. [DOI] [PubMed] [Google Scholar]
- 19.Phillips J, Chang C, Mattson J, Gumperz J, Parham P, Lanier LL. Human C-type lectins and MHC class I recognition: evidence for involvement of CD94-associated proteins (CD94AP) in recognition of HLA-A, -B, and -C. Immunity. 1996;5:163–172. doi: 10.1016/s1074-7613(00)80492-6. [DOI] [PubMed] [Google Scholar]
- 20.Browne H, Churcher M, Minson T. Construction and characterisation of a human cytomegalovirus mutant with the UL18 (class I homolog) gene deleted. J Virol. 1992;66:6784–6787. doi: 10.1128/jvi.66.11.6784-6787.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beersma MF, Bijlmakers MJ, Ploegh HL. Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I heavy chains. J Immunol. 1993;151:4455–4464. [PubMed] [Google Scholar]
- 22.Karre K, Ljunggren HG, Plontek G, Kiessling R. Selective rejection of H-2–deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 23.Browne H, Smith G, Beck S, Minson T. A complex between the MHC class I homologue encoded by human cytomegalovirus and β2-microglobulin. Nature. 1990;347:770–772. doi: 10.1038/347770a0. [DOI] [PubMed] [Google Scholar]
- 24.Fahnestock ML, Johnson JL, Feldman RMR, Neveu JM, Lane WS, Bjorkman PJ. The MHC class I homolog encoded by human cytomegalovirus binds endogenous peptides. Immunity. 1995;3:583–590. doi: 10.1016/1074-7613(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 25.Malnati MS, Peruzzi M, Parker KC, Biddison WE, Ciccone E, Moretta A, Long EO. Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science. 1995;267:1016–1018. doi: 10.1126/science.7863326. [DOI] [PubMed] [Google Scholar]
- 26.Ciccone E, Pende D, Nanni L, Di Donato C, Viale O, Beretta A, Vitale M, Sivori S, Moretta A, Moretta L. General role of HLA class I molecules in the protection of target cells from lysis by natural killer cells: evidence that the free heavy chains of class I molecules are not sufficient to mediate the protective effect. Int Immunol. 1995;7:393–400. doi: 10.1093/intimm/7.3.393. [DOI] [PubMed] [Google Scholar]
- 27.Braud V, Jones EY, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with anchor residues at positions 2 and 9. Eur J Immunol. 1997;27:1164–1169. doi: 10.1002/eji.1830270517. [DOI] [PubMed] [Google Scholar]
- 28.Tzeng C-M, Adams EJ, Gumperz JE, Percival L, Barber LD. Peptides bound endogenously by HLA-Cw*0304 expressed in LCL 721.221 cells include a peptide derived from HLA-E. Tissue Antigens. 1996;48:325–328. doi: 10.1111/j.1399-0039.1996.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 29.Braud VM, Allan DSJ, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B, and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 30.Samaridis J, Colonna M. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur J Immunol. 1997;27:660–665. doi: 10.1002/eji.1830270313. [DOI] [PubMed] [Google Scholar]
- 31.Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, Lopez-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186:1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]