Abstract
Malaria male gametocytes within a newly ingested infected blood meal in the mosquito midgut emerge from erythrocytes and extrude approximately eight flagellar microgametes in a process termed exflagellation. In culture, and in blood removed from infected patients, emerging microgametes avidly adhere to neighboring uninfected and infected erythrocytes, as well as to emerged female macrogametes, creating “exflagellation centers”. The mechanism of erythrocyte adherence is not known nor has it been determined for what purpose microgametes may bind to erythrocytes. The proposition of a function underlying erythrocyte adherence is supported by the observation of species-specificity in adhesion: microgametes of the human malaria Plasmodium falciparum can bind human erythrocytes but not chicken erythrocytes, whereas avian host Plasmodium gallinaceum microgametes bind chicken but not human erythrocytes. In this study we developed a binding assay in which normal, enzyme-treated, variant or null erythrocytes are identified by a cell surface fluorescent label and assayed for adherence to exflagellating microgametes. Neuraminidase, trypsin or ficin treatment of human erythrocytes eliminated their ability to adhere to Plasmodium falciparum microgametes, suggesting a role of sialic acid and one or more glycophorins in the binding to a putative gamete receptor. Using nulls lacking glycophorin A [En(a−)], glycophorin B (S−s−U−) or a combination of glycophorin A and B (Mk/Mk) we showed that erythrocytes lacking glycophorin B retain the ability to bind but a lack of glycophorin A reduced adherence by exflagellating microgametes. We propose that either the sialic acid moiety of glycophorins, predominantly glycophorin A, or a more complex interaction involving the glycophorin peptide backbone, is the erythrocyte receptor for adhesion to microgametes.
Keywords: malaria, Plasmodium falciparum, exflagellation, microgamete, rosetting
The first evidence that Plasmodium was the etiologic agent of malaria was recognized by Charles Laveran in 1880 as he scanned a live mount of a febrile soldier's blood at Constantine Hospital in Algeria (1). Laveran noted pigment granule-containing red blood cells in various forms of elongated or crescent-shaped disfigurement. Translucent round cells with pigmented granules were also seen, and most notably, amoeboid-like cells possessing long whipping strands that dramatically interacted with, and were capable of “drawing in”, neighboring red blood cells. Laveran understood that he was looking at the malaria pathogen and this observation stands as a singular historical event in providing support for a protozoan basis of disease. Twenty years later “exflagellation”—the whipping motions of the sinuous flagella—was described by MacCallum (2, 3) to be the extrusion of male gametes after emergence from within red blood cells, a process placed by Ross to occur in the gut of the mosquito vector (4, 5). Although the emergence and formation of male gametes (termed microgametes) is now well described, it is not known why the male gametes interact with neighboring red blood cells in such a visually striking fashion. To date, the strongest argument that erythrocyte adherence serves a function, perhaps in some manner to enhance infectivity, is based upon the fact that multiple species of Plasmodium are capable of binding host erythrocytes.
Gametogenesis, the emergence of male and female gametocytes from within erythrocytes and the exflagellation of male gametes, is triggered primarily by a drop in temperature (6, 7) accompanying the transition from the warm-blooded host to the mosquito gut after the taking of a blood meal. Changes in pH, carbon-dioxide tension (8–10), and mosquito midgut factors (7, 11, 12) also significantly contribute to the induction of gametogenesis. Species-specific differences may exist in the relative importance of temperature, pH, carbon-dioxide tension, and mosquito factors in the triggering the parasite's development. Emergence occurs rapidly: within a few minutes of the switch from the host peripheral circulation to the environment of the mosquito gut, the mature gametocytes round up, then rupture and shed the surrounding erythrocyte membrane. During this time the male gametocytes undergo a process of DNA replication and nuclear segregation, progressing from an approximately haploid genome to an octaploid genome with the formation and migration of eight nuclei into the extruding gametes (13, 14). This process is completed by ∼15 min after the taking of a blood meal. Fertilization of female gametes (termed macrogametes) by free-swimming male gametes is complete within 30 min. Thus emergence, DNA replication, formation of male gametes, and fertilization takes place within 30 min in response to environmental triggers in the mosquito gut (for review see reference 15).
It is possible to inhibit the process of exflagellation with various pharmacological agents. Colchicine blocks emergence and exflagellation, presumably via inhibition of microtubule polymerization (16). Aphidicolin is an effective blocker of exflagellation and this is thought to be due to an inhibition of DNA polymerization in the male gametocyte, suggesting that genome replication is a requisite step in gamete formation (13, 14). Calcium metabolism antagonists have been found to be inhibitors of exflagellation (17), whereas phosphodiesterase inhibitors induce exflagellation (18), providing toeholds into the unraveling of signaling pathways regulating the induction of exflagellation. A blockade of erythrocyte adherence during exflagellation has not yet been described.
In this study we were interested in determining the molecular mechanism involved in erythrocyte adhesion and to investigate the role, if any, of red blood cell binding by male gametes in the infectivity of malaria parasites to the mosquito. Is the adhesion to red blood cells during exflagellation a phenomenological oddity—perhaps an in vitro artifact—or does it have a requisite function for efficient infectivity? Here we show that red blood cell adhesion is species specific, both at the level of erythrocyte ligand and at the level of microgamete receptor. Binding of human erythrocytes by Plasmodium falciparum microgametes is dependent on cell surface sialic acid and the presence of glycophorin, in particular glycophorin A.
Materials and Methods
Plasmodium Mature Gametocytes.
Culture of 3D7 (19), 7G8 (20), and HB3 (21) strains of P. falciparum gametocytes (22), and induction of mature exflagellating microgametocytes (11) were performed as previously described. All experimental laboratory animal work described herein was performed using Animal Care and Use Committee (ACUC)–approved procedures and protocols as per National Institutes of Health (NIH) guidelines. White leghorn chickens were infected with Plasmodium gallinaceum and mature gametocytes were obtained as previously described (23). For exflagellation of P. gallinaceum gametocytes, blood was drawn by cardiac puncture of anesthetized infected chickens using heparin as an anticoagulant. The blood was immediately centrifuged (2,000 g; 5 min) and the pellet was resuspended in a bicarbonate-buffered exflagellation medium containing mosquito pupae extracts as previously described (11).
Erythrocytes.
En(a−), Mk/Mk, and S−s−U− erythrocytes were from liquid nitrogen frozen stocks and were the same as used in a study by Dolan et al. (24). Null and variant erythrocytes were obtained from the Department of Transfusion Medicine, NIH, and were either from consenting volunteer blood donors or from commercially prepared panels of variant erythrocytes (Organon Teknika, Durham, NC; Gamma Biologicals, Inc., Houston, TX; Immucor, Norcross, GA). Normal erythrocytes were from O+ volunteers within the laboratory. Mouse erythrocytes were obtained by terminal exsanguination via cardiac puncture of anesthetized CAF1, DBA/2, or C57/B6 female mice. Rhesus and Aotus monkey erythrocytes were drawn from peripheral blood of anesthetized animals. Chicken blood was obtained by terminal exsanguination of anesthetized white leghorn chickens. Citrate phosphate dextrose solution (CPD) or heparin was used as anticoagulant, and lymphocytes were removed by discarding the buffy layer during several washes and centrifugation (2,000 g; 2 min) in RPMI 1640.
Enzyme Treatment of Erythrocytes.
For the treatment of erythrocytes with neuraminidase or trypsin, ∼200 μl of 50% hematocrit normal human or chicken cells were washed several times with RPMI 1640 and the pellet was resuspended in 3 ml of RPMI 1640. Erythrocytes were treated for 1 h at 37°C with either 0.1 U of Vibrio cholerae or Arthrobacter ureafaciens neuraminidase (Calbiochem Corp., La Jolla, CA). For neuraminidase treatment of mature cultures, care was taken to not let the temperature drop during treatment, in an attempt to avoid triggering of emergence. Normal human serum contains antibodies recognizing a cryptic antigen, called T or Tn, present on desialylated erythrocytes (25, 26); and to avoid agglutination, neuraminidase-treated erythrocytes or cultures were not resuspended in normal human serum, but instead fetal bovine serum or T antigen–depleted human serum was used for culturing of neuraminidase-treated gametocyte cultures and for exflagellation assays. For trypsin treatment, erythrocytes were treated for 2 h at 37°C with 1 mg/ml trypsin (Sigma Chemical Co., St. Louis, MO). After enzyme treatments the cells were washed several times with RPMI 1640 and resuspended at ∼50% hematocrit in appropriate media for use in exflagellation assays or mosquito membrane feeds. Ficin-treated cells were from a commercial panel of erythrocytes (Gamma Biologicals, Inc.) and had matched untreated erythrocytes as controls.
Cell Surface Labeling of Erythrocytes.
Erythrocytes were labeled with cell surface fluorescence in a procedure modified from that previously described (27). Roughly 50–100 μl of erythrocytes at 50% hematocrit (in RPMI 1640) were pelleted, washed once with RPMI 1640, and resuspended in 200 μl of RPMI 1640. 200 μl of an RPMI 1640 solution containing 1 μl of PKH26 (PKH26-GL; Sigma Chemical Co.) was added to this suspension, mixed immediately and rapidly, and the labeling was quenched 1 min later with 400 μl of chicken serum or heat-inactivated fetal bovine serum. After 2–5 min the labeled erythrocytes were washed several times in RPMI 1640 and resuspended in a small volume for addition to exflagellating cultures.
Exflagellation Assay.
Cultures of mature P. falciparum gametocytes were either used directly in an exflagellation assay or were pretreated with neuraminidase to block the ability of microgametes to bind to erythrocytes within their culture. Approximately 1.5 ml of P. falciparum gametocyte culture at 5% hematocrit was pelleted and resuspended in 20–40 μl of heat-inactivated human or fetal bovine serum. The change in pH, pCO2, and temperature under in vitro conditions are the principle triggers initiating exflagellation. For exflagellation assays of P. gallinaceum gametocytes, ∼100 μl of infected chicken blood was pelleted and resuspended in 20–40 μl of chicken serum. Heterologous labeled red blood cells were added in a 5–15 μl volume and the ability of microgametes to bind labeled cells was assayed by fluorescence microscopy 12–20 min after the addition of serum. Depending on the experimental conditions, the ability of microgametes to bind red blood cells of their parent culture served as a convenient internal control for adhesion in assays involving heterologous erythrocytes.
Mosquito Membrane Feeds.
Mosquito infectivity assays were performed using membrane feeders as previously described (28, 29). Mature P. falciparum cultures were either used directly or were pretreated with enzymes as described above. Efficient infectivity requires addition of fresh red blood cells to the feed blood (30), and this provided an opportunity for adding heterologous erythrocytes. For example, a typical membrane-feed would entail combining in one membrane feeding apparatus: 80 μl of cultured mature gametocytes (1–3% mature gametocyte parasitemia) at a 60% hematocrit in serum; 120 μl of fresh uninfected normal or variant erythrocytes at a 60% hematocrit in serum; and 60 μl of additional serum. The level of transmission was determined by counting mercurochrome-stained oocyst numbers in the midgut wall 1 wk after feeding.
Results
Exflagellation Assay and Species-specific Interaction of Male Gametes with Red Blood Cells.
Erythrocyte rosetting during exflagellation has been observed in species of Plasmodium infecting humans, non–human primates, rodents, and birds (references 1 and 10 and our observations). A rosette of erythrocytes surrounding a P. falciparum exflagellating microgametocyte is shown in Fig. 1 A. Fig. 1 C shows an immunofluorescence-stained (α-tubulin II antisera) exflagellating microgametocyte fixed under conditions in which erythrocyte adhesion is blocked. It is at this stage, from 12 to 20 min after induction of exflagellation, that the strand-like microgametes adhere and draw in neighboring erythrocytes before breaking free from the residual body. For the following assays, heterologous or control erythrocytes were labeled with the fluorescent cell surface linker PKH26 and then observed for adherence by exflagellating microgametes. A and B of Fig 1 are corresponding phase-contrast and fluorescence views of a typical field containing an exflagellation rosette, one of many such fields viewed per experiment. In this instance, the inability of 3D7 strain P. falciparum microgametes to bind neuraminidase-treated human cells is demonstrated. Fig. 1 B shows that fluorescence-labeled, neuraminidase-treated cells, although abundantly present, do not participate in the centering alongside unlabeled normal erythrocytes (A). The fluorescent label itself did not interfere with adherence to microgametes, as indicated by the ability of numerous sources of fluorescent-labeled erythrocytes to rosette (see below).
Figure 1.
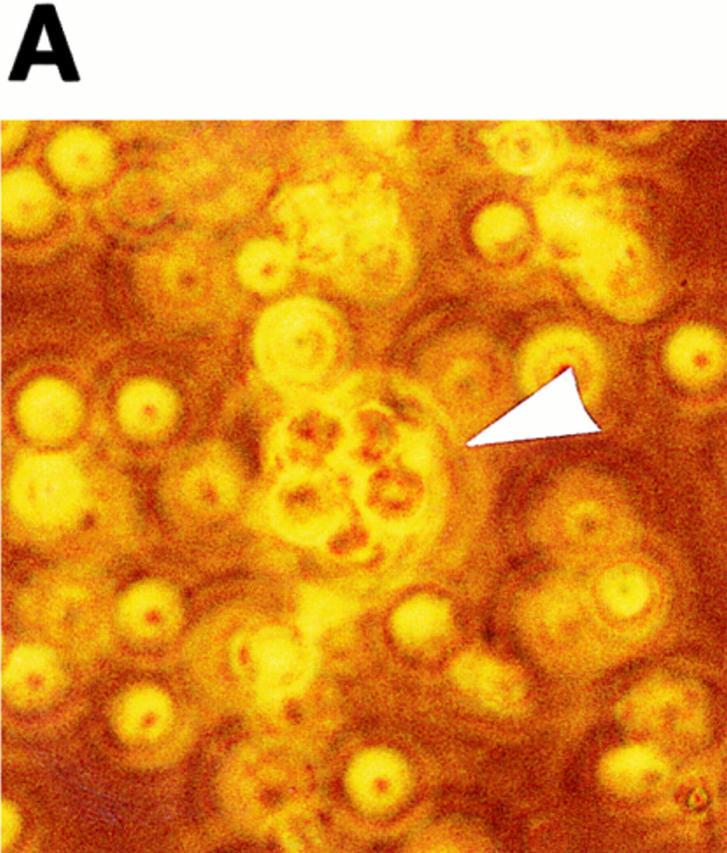
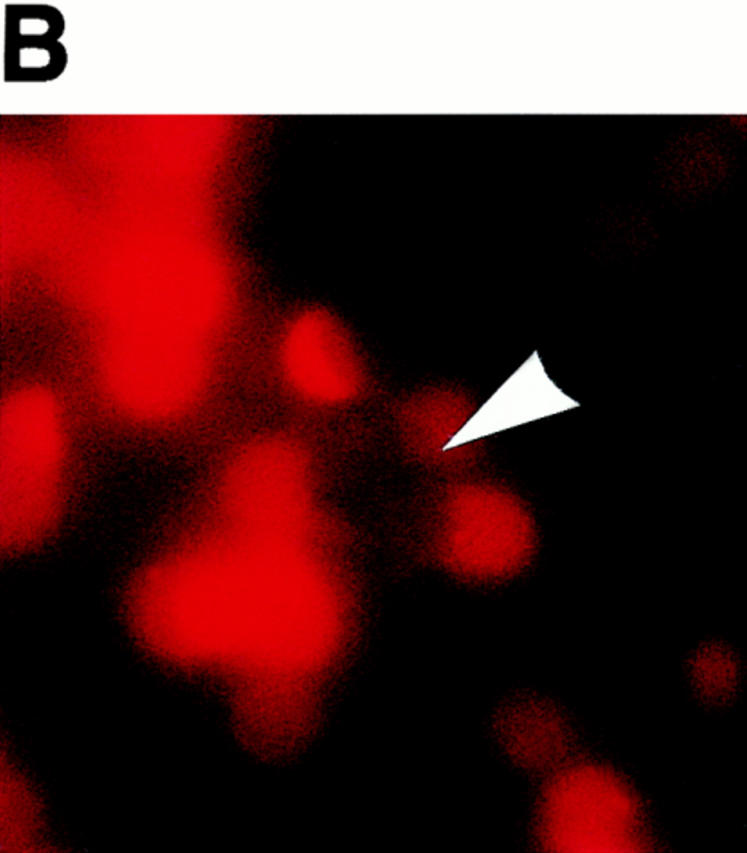
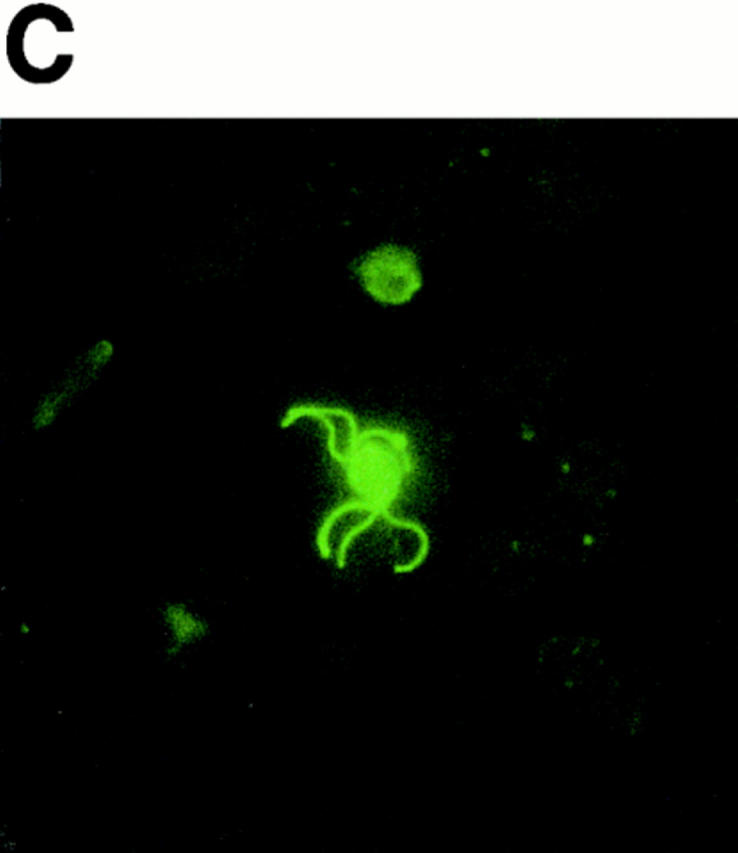
Exflagellating microgametes of P. falciparum rosette neighboring erythrocytes (A) to form a vigorously moving mass of cells, but are unable to adhere and draw in fluorescence-labeled, neuraminidase-treated erythrocytes (B) added to the culture upon induction of exflagellation. Arrows in A and B point to the same exflagellation rosette; the rosette in B is not visible because fluorescent cells do not contribute to the rosette. The cultures are photographed live, and because the exflagellation rosettes and erythrocytes are moving it is impossible to get perfectly matched sets of bright field (A) and fluorescent (B) images. The octopus-like arms are emerging microgametes (C), visible by FITC-labeling in a methanol-fixation IFA using anti–α-tubulin II sera (provided by Dr. Michal Fried, Walter Reed Army Institute of Research, Washington, DC). 10–20 min after induction of exflagellation the microgametes are still attached to the residual body of the microgametocyte and thus are capable of adhering to and drawing in neighboring erythrocytes.
Using the above assay we were first interested in determining if the binding of erythrocytes by male gametes is specific such that a given species of Plasmodium cannot bind red blood cells of unnatural hosts. Table 1 shows that P. falciparum microgametes can bind human and Rhesus erythrocytes but do not adhere to mouse, rat, chicken, or Aotus erythrocytes. Likewise, avian P. gallinaceum exflagellating microgametes readily bind chicken erythrocytes but are unable to bind to human erythrocytes (Table 2).
Table 1.
Species-specific Erythrocyte Binding of 3D7 P. falciparum Microgametes
| Erythrocyte | Binding | n * | ||
|---|---|---|---|---|
| Human | + | >10 | ||
| Rhesus | + | 4 | ||
| Aotus | − | 5 | ||
| Mouse CAF1 | − | 2 | ||
| Mouse C57/B6 | − | 2 | ||
| Mouse DBA/2 | − | 4 | ||
| Rat | − | 2 | ||
| Chickn | − | 2 |
Number of independent experiments performed.
Table 2.
Ability of P. falciparum and P. gallinaceum Microgametes to Bind Enzyme-treated Human or Chicken Erythrocytes
| Malaria species and strain | Enzyme treatment | Binding | n * | |||
|---|---|---|---|---|---|---|
| P. falciparum | ||||||
| 3D7 | Untreated human RBCs | + | >10 | |||
| Ficin | − | 4 | ||||
| Trypsin | − | 5 | ||||
| Neuraminidase (V.c.)‡ | − | >10 | ||||
| Neuraminidase (A.u.)§ | − | 2 | ||||
| 7G8 | Untreated human RBCs | + | 2 | |||
| Neuraminidase (V.c.) | − | 2 | ||||
| HB3 | Untreated human RBCs | + | 2 | |||
| Neuraminidase (V.c.) | − | 2 | ||||
| P. gallinaceum | Untreated chicken RBCs | + | >10 | |||
| Neuraminidase (V.c.) | + | 2 | ||||
| Neuraminidase (A.u.) | + | 2 | ||||
| Untreated human RBCs | − | 4 |
Number of independent experiments performed.
V. cholerae neuraminidase.
A. ureafaciens neuraminidase.
Adhesion to Enzyme-treated and Null Erythrocytes.
The interaction of exflagellating microgametes with enzyme-treated erythrocytes was examined to characterize putative erythrocyte cell surface receptors recognized by microgametes. Table 2 shows that pretreatment of erythrocytes with trypsin, neuraminidase, or ficin completely blocked adherence by exflagellating microgametes. In contrast, P. gallinaceum microgametes were capable of binding neuraminidase-treated chicken red blood cells, suggesting either that the binding is not dependent on sialic acid or that the chicken erythrocyte sialic acid is not sufficiently removed by V. cholerae neuraminidase. The trivial explanation, that V. cholerae neuraminidase does not recognize chicken erythrocyte sialic acid, can be partially excluded by the fact that neuraminidase treatment reduced, by 8- to 16-fold, the degree of erythrocyte agglutination using the sialic acid-binding lectin Maackia amurensis in a parallel assay (data not shown). Additionally, neuraminidase from A. ureafaciens was unable to appreciably reduce the ability of P. gallinaceum microgametes to bind chicken red blood cells, whereas it is effective in eliminating the ability of P. falciparum to bind to human erythrocytes (Table 2).
The effect of neuraminidase indicates that sialic acid residues are required for P. falciparum binding and suggests that one or more members of the glycophorin family might be the erythrocyte surface receptor. Ficin removes glycophorins A and B (31), and thus the effect of ficin in inhibiting erythrocyte adhesion (Table 2) is consistent with a role of glycophorins in the erythrocyte–gamete interaction. If the ligand on the erythrocyte surface is present on a single type of cell surface molecule, then analysis of null erythrocytes lacking specific cell surface molecules may reveal the identity of the receptor. Erythrocytes lacking one or more cell surface molecules, for example Duffy receptor null or Rhnull (32, 33), are capable of interacting with microgametes (Table 3). Glycophorin B does not appear to be an essential microgamete receptor because a null lacking glycophorin B (S−s−U−) is capable of interacting with exflagellating microgametes. Further evidence against a requirement for glycophorin B is indicated by the fact that trypsin treatment, which leaves glycophorin B intact (34), abolishes the ability of erythrocytes to bind microgametes (Table 2). The erythrocyte variant Ge:−1,−2,−3, lacking glycophorins C and D (35), adheres to microgametes. Nulls lacking glycophorin A [En(a−)] or lacking both glycophorin A and glycophorin B (Mk/Mk) appear to have a substantially reduced capacity for being bound by exflagellating microgametes. The reduction in binding is not absolute, as observed following neuraminidase treatment, and this argues against glycophorin A as the sole receptor for microgametes. Neuraminidase-treated En(a−) erythrocytes did not appear to bind (Table 3), although only two assays were run rather than greater than ten distinct assays with untreated En(a−) cells. However, a working model remains that the glycophorin family, possibly by virtue of possessing the majority of cell surface sialic acid, is the principle ligand recognized by exflagellating microgametes.
Table 3.
Binding of P. falciparum Microgametes to Variant and Null Human Erythrocytes
| Erythrocyte | Binding | n * | ||
|---|---|---|---|---|
| Rhnull ‡ | + | 2 | ||
| Ge:-1,-2,-3§ | + | 2 | ||
| Duffy [Fy(a−b−)] | + | 2 | ||
| Yk(a−),Kn(a−),McC(a−)‖ | + | 2 | ||
| Cad¶ †† | + | 2 | ||
| Tn** †† | + | 2 | ||
| Tn, neuraminidase-treated | − | 1 | ||
| S−s−U− | + | >5 | ||
| En(a−) | −/−/+§§ | >10 | ||
| Mk/Mk | −/−/+§§ | >5 | ||
| En(a−), neuraminidase-treated | − | 2 |
Number of independent experiments performed.
Lack Rh and LW; deficient in CD47, Duffy (Fy5), and glycophorin B.
Known as “Gerbich-type”, possess truncated glycophorin C.
York, Knopps, and McCoy antigens; refer to the complement receptor 1 (CR1).
Cad erythrocytes possess a normal sialic acid content but with an extra N-acetylgalactosamine reside on the tetrasaccharide due to N-acetylgalactosaminyltransferase activity.
Tn erythrocytes lack terminal sialic acid.
Erythrocyte population is most likely mosaic for the variant structure.
Exflagellation centering assay is qualitative, occasional binding observed.
Adhesion of Microgametes to Erythrocytes Does Not Correlate with Previously Characterized Asexual Blood Stage Invasion Phenotypes.
Because erythrocyte adhesion by microgametes is dependent on sialic acid it was of interest to determine if the exflagellation-binding phenotype correlates with the erythrocyte-invasion phenotype of merozoites. Dolan et al. (24) reported that erythrocyte invasion of merozoites by a sialic acid–dependent pathway was strain dependent. Merozoite invasion of erythrocytes by many strains of P. falciparum, including the 3D7 strain used in this study, is inhibited by removal of erythrocyte sialic acid, whereas two P. falciparum strains, HB3 and 7G8, readily invade neuraminidase-treated erythrocytes in a manner that is likely to be EBA-175 independent. Gametocytes of HB3 and 7G8 strains of P. falciparum were cultured and exflagellating microgametes assayed for their ability to bind normal and neuraminidase-treated erythrocytes. Both 7G8 and HB3 microgametes readily bound untreated erythrocytes but, similarly to 3D7 microgametes, were unable to adhere to V. cholerae neuraminidase-treated erythrocytes (Table 2). This suggests that the ability of microgametes to adhere to erythrocytes is not correlated with a merozoite-invasion phenotype as defined by the neuraminidase-insensitive invasion phenotype of the 7G8 and HB3 strains. In addition, the fact that microgametes bind Rhesus but not Aotus monkey cells is in contrast to the invasion efficiencies of these cell types: Aotus erythrocytes are efficiently invaded by P. falciparum (36), whereas Rhesus erythrocytes are refractory to invasion (P. falciparum Camp strain; 37). Although invasion phenotypes do not correlate with exflagellation adhesion, it is still possible that the initial adherence of merozoites to erythrocytes, before apical reorientation and the initiation of invasion, is mediated by receptors that are similarly involved in the binding of microgametes to erythrocytes.
Pharmacological Blockade of Adhesion.
To test the role of erythrocyte adhesion on infectivity, it is desirable to block microgamete binding to erythrocytes without disrupting the process of exflagellation or the potential for fertilization. Toward this end we tested a variety of agents for their ability to inhibit erythrocyte binding without interfering with exflagellation. Table 4 shows that sulfated glycoconjugates are potent inhibitors of binding but do not appear to affect exflagellation. In the presence of 0.1 mg/ml dextran sulfate, exflagellation proceeds normally but the binding to erythrocytes is inhibited. The sulfated polysaccharide fucoidin (see reference 38) also blocks binding at 1 mg/ml, whereas mucin, a highly sialylated proteoglycan, does not block at a similar concentration. In the presence of dextran sulfate or fucoidin the whipping motions of the exflagellating erythrocytes appear to push erythrocytes away rather than drawing them in. Protamine sulfate at 1 mg/ml is able to reverse the blocking effect of dextran sulfate at a similar concentration. Under these conditions the exflagellation centers of erythrocytes appear normal. It is possible that protamine sulfate exerts its action simply by binding and neutralizing dextran sulfate, nullifying an interaction of this reagent with the microgametes or erythrocytes. Neither protamine sulfate nor dextran sulfate are able to reverse the inhibition seen after neuraminidase treatment of erythrocytes (Table 4). The ability of microgametes to bind to emerged macrogametes is not inhibited by dextran sulfate or fucoidin, and in high parasitemic cultures, exflagellating centers composed of multiple emerged macrogametes are allowed to form. This implies that a different mechanism operates for gamete–gamete interactions that is not affected by sulfated polysaccharides; or that gamete–gamete interactions are enhanced by the lack of obstruction by bound erythrocytes.
Table 4.
Effect of Sulfated Glycoconjugates and Sialylated Proteoglycans on adhesion of P. falciparum Microgametes to Normal Human Erythrocytes
| Reagent | Concentration | Binding | ||
|---|---|---|---|---|
| Normal erythrocytes | ||||
| Dextran sulfate | 0.5 mg/ml | − | ||
| 0.2 mg/ml | − | |||
| 0.07 mg/ml | − | |||
| 0.02 mg/ml | +/−≳ | |||
| 0.007 mg/ml | + | |||
| Protamine sulfate | 1 mg/ml | + | ||
| Fucoidin | 1 mg/ml | − | ||
| 0.33 mg/ml | − | |||
| Mucin | 10 mg/ml | + | ||
| 1 mg/ml | + | |||
| Dextran sulfate plus | ||||
| protamine sulfate | 0.1 mg/ml each | + | ||
| Neuraminidase-treated erythrocytes | ||||
| Dextran sulfate | 1 mg/ml | − | ||
| Protamine sulfate | 1 mg/ml | − | ||
Exflagellation binding assay is qualitative. Some microgametes adhered to RBCs and some were nonadherent, such that the overall binding was judged (+/−) at this reagent concentration.
Erythrocyte Binding and Infectivity to Mosquitoes.
To determine if erythrocyte binding is necessary or enhances infectivity of malaria parasites to mosquitoes, infected blood containing dextran sulfate, mucin or fucoidin was fed to mosquitoes. Table 5 shows that dextran sulfate completely blocks infectivity at a concentration of 0.5 mg/ml and reduces infectivity by 90% at a concentration of 0.1 mg/ml. Fucoidin was active, but less so, inhibiting infectivity 76% at a concentration of 0.33 mg/ml. Mucin did not affect infectivity at 1 mg/ml. From in vitro studies it was shown that dextran sulfate still has an appreciable inhibitory effect on adhesion at a concentration of 0.02 mg/ml, but below that concentration the effect becomes unnoticeable (Table 4). It is possible that the infectivity inhibitory effect of dextran sulfate is not via disruption of binding to erythrocytes but is instead exerting a nonspecific toxic effect on the health of the developing parasite. To test for a general toxic effect of dextran sulfate, fertilization was allowed to proceed in vitro and dextran sulfate was added at various time points after the initiation of in vitro fertilization. The mixture was fed to mosquitoes after 60 min, a time point past the period of exflagellation activity. Table 5 shows that addition of dextran sulfate as late as 60 min after the initiation of fertilization reduces infectivity by 84%, suggesting that dextran sulfate is most likely not exerting its action solely at the level of erythrocyte adherence to microgametes but instead has additional toxicity or specifically blocks receptor interactions at some later time point (e.g., during mosquito midgut invasion).
Table 5.
Effects of Dextran Sulfate and Fucoidin on the Infectivity of P. falciparum to Anopheles freeborni by Membrane-feeding Assays
| Reagent and condition | Concentration | Infectivity: Geometric mean | Infectivity: percentage of control* | Infectivity: infected/dissected‡ | ||||
|---|---|---|---|---|---|---|---|---|
| Experiment 1 | ||||||||
| No addition | – | 9.1 | – | 20/20 | ||||
| Dextran sulfate | 2.5 mg/ml | 0.0 | 0.0 | 0/20 | ||||
| 0.5 mg/ml | 0.0 | 0.0 | 1/20 | |||||
| 0.1 mg/ml | 0.6 | 6.4 | 9/20 | |||||
| Experiment 2 | ||||||||
| No addition | – | 3.2 | – | 11/15 | ||||
| Fucoidin | 1.0 mg/ml | 0.6 | 20 | 11/20 | ||||
| 0.3 mg/ml | 1.2 | 38 | 15/20 | |||||
| Experiment 3: | ||||||||
| in vitro fertilization | ||||||||
| No addition | – | 5.1 | – | 13/13 | ||||
| Dextran sulfate | ||||||||
| t = 0 | 0.1 mg/ml | 0.26 | 5.1 | 4/12 | ||||
| t = 15 | 0.1 mg/ml | 0.22 | 4.3 | 4/16 | ||||
| t = 30 | 0.1 mg/ml | 0.62 | 12 | 8/14 | ||||
| t = 60 | 0.1 mg/ml | 0.60 | 12 | 6/14 |
Geometric mean oocytst number as a percentage of control geometric mean oocyst number.
Number of infected mosquitoes with one or more oocysts per total number of mosquitoes dissected.
The inability of male gametes to bind neuraminidase-treated erythrocytes afforded an additional opportunity to test the role of erythrocyte binding on infectivity. Mosquitoes were fed neuraminidase-treated 3D7 cultures (to prevent the possibility of adherence to culture erythrocytes) in which uninfected normal or neuraminidase-treated erythrocytes were added in excess just before membrane feeding. If binding to erythrocytes is required for efficient infectivity, then mosquitoes fed with treated erythrocytes would be expected to have lower total oocyst numbers in the membrane-feeding assay. Table 6 shows the summary of four successful (in that oocyst development was seen) mosquito membrane feeds, all of which gave a significant reduction in the geometric mean oocyst number per gut with neuraminidase treatment of erythrocytes in the feed. However, by the nature of the experiment, it is not possible to determine if the effect of neuraminidase treatment is occurring at the exflagellation step or during a subsequent stage in development within the midgut. Often mosquito feed experiments fail for technical reasons and numerous experiments (data not shown) were unsuccessful, in that development to oocysts was not seen; in particular, experiments designed to address whether the effect of neuraminidase was occurring before or after fertilization failed. It is likely that the multiple manipulations involved in neuraminidase or sham treatment, as part of the experimental designs, were detrimental to the viability of the gametocytes.
Table 6.
Summary of Effect of Neuraminidase on Infectivity of P. falciparum to Anopheles freeborni by Membrane-feeding Assays
| Erythrocytes | Infectivity: geometric mean | Infectivity: percentage of control* | P value‡ | Infectivity: infected/dissected§ | ||||
|---|---|---|---|---|---|---|---|---|
| Experiment 1 | ||||||||
| Normal | 5.4 | – | 21/21 | |||||
| Na-treated | 0.4 | 7.5 | <0.001 | 7/20 | ||||
| Experiment 2 | ||||||||
| Normal | 11.2 | – | 26/28 | |||||
| Na-treated | 4.5 | 40 | <0.001 | 21/23 | ||||
| Experiment 3 | ||||||||
| Normal | 1.9 | – | 22/24 | |||||
| Na-treated | 0.6 | 31 | 0.001 | 13/24 | ||||
| Experiment 4 | ||||||||
| Normal | 9.0 | – | 20/22 | |||||
| Na-treated | 2.8 | 31 | 0.001 | 18/22 |
Na, neuraminidase.
Geometric mean oocytst number as a percentage of control geometric mean oocyst number.
P value refers to staistical significance of comparison of the two groups by Mann-Whitney analysis.
Number of infected mosquitoes with one or more oocysts per total number of mosquitoes dissected.
Discussion
Although the binding of erythrocytes to microgametes during exflagellation has been observed for more than a century, the molecular nature of this interaction has not been elucidated. It has not been determined if the binding is via a specific receptor–ligand interaction or is nonspecific, such as an electrostatic attraction or perhaps a mechanical consequence of the whipping motions of the microgametes, a hydrodynamic “vortex” phenomenon. In this study, a specificity in the microgamete–erythrocyte interaction is demonstrated, exemplified by the species-specificity of adherence: the inability of P. falciparum microgametes to bind chicken erythrocytes and, conversely, the inability of P. gallinaceum microgametes to bind human erythrocytes. In addition, the fact that P. falciparum is unable to bind mouse, rat, chicken, or Aotus erythrocytes argues that the whipping motion of the microgametes does not pull in erythrocytes by a simple whirlpool effect, otherwise the whirlpool would be such that human but not mouse or chicken cells are affected.
The asexual cycle of malaria is propagated in the bloodstream by rupture of schizont-infected erythrocytes followed by merozoite invasion of fresh erythrocytes. In P. falciparum, the recognition of erythrocytes by merozoites has been found to involve erythrocyte sialic acid and glycophorin A (37, 39–42; for review see 43). Thus, at this level the inability of P. falciparum microgametes to adhere to neuraminidase-treated erythrocytes suggests that this system has a similarity to merozoite recognition of erythrocytes. Recognition of erythrocytes by microgametes could be due to either a molecular interaction with sialic acid as a specific ligand or an electrostatic interaction involving the negatively charged sialic acid coat of the erythrocyte. One observation may support the hypothesis that microgametes bind via an electrostatic interaction: highly charged sulfated molecules such as dextran sulfate and fucoidin block binding at concentrations as low as 0.1 mg/ml. Numerous studies have shown an inhibitory effect of sulfated glycoconjugates on merozoite invasion of erythrocytes and sporozoite invasion of liver cells (44–47), but it is not known if these inhibitory effects are electrostatic or represent more specific interactions. However, the fact that P. falciparum is unable to adhere to erythrocytes from a number of species (mouse, rat, Aotus, and chicken) implies that the interaction is not via charge alone, as the erythrocyte surface of these species would also be expected to possess a substantial negative charge.
On human erythrocytes, the majority of the sialic acid, and the bulk of the surface charge, is present on glycophorins A and B. Ficin treatment removes both glycophorin A and B from the erythrocyte surface and this treatment blocks adherence to microgametes. Trypsin-treated erythrocytes also can not bind, and because they lack glycophorin A but retain glycophorin B, glycophorin A is thereby implied to be the principle receptor. In addition, microgametes are capable of binding Rhnull erythrocytes that reportedly have a 60–70% reduction in levels of glycophorin B (48), consistent with a minor role of glycophorin B in adhesion by microgametes. A null lacking glycophorin B (S−s−U−) is capable of binding to exflagellating microgametes, whereas nulls lacking glycophorin A [En(a−)] or a combination of glycophorin A and glycophorin B (Mk/Mk) have a greatly reduced capacity to bind, implying that glycophorin A is a principle receptor, although not absolutely required for binding. Binding to glycophorin A could be via its sialic acid moiety alone or via a more complex interaction requiring the glycophorin A peptide backbone. This second explanation would be similar to the requirement of both sialic acid and peptide backbone in the binding of EBA-175 to glycophorin A (49). It is plausible that sialic acid alone is responsible, since glycophorin A possesses the majority of the sialic acid coat of erythrocytes. In this model the absence of glycophorin B, which constitutes only 10–15% of the glycophorin surface coat, would not affect adherence to microgametes, whereas the absence of glycophorin A, possessing the majority of the glycophorin coat, drops the sialic acid content below a level necessary for binding. The sialic acid content of En(a−) and Mk/Mk nulls is still ∼30% of normal erythrocytes and may possess increased sialylation of other surface molecules such as Band 3 (50). Thus, if binding were due to sialic acid alone, then these cells might be expected to possess sufficient sialic acid for binding. The fact that En(a−) and Mk/Mk cells are occasionally observed to adhere exflagellating microgametes, whereas neuraminidase-treated erythrocytes are essentially unable to bind, fits with a model in which surface sialic acid is the relevant ligand and binding is affected by the abundance of surface ligand.
Because both microgametes and merozoites appear to recognize erythrocyte cell surface glycophorin A, is there a conservation of the mechanism between the two life-cycle stages? The binding data presented here suggest that the receptor(s) involved in adherence to erythrocytes during exflagellation is distinct from binding during invasion of erythrocytes by merozoites. First, P. falciparum merozoites are capable of invading mouse erythrocytes (51, 52) but cannot invade Rhesus erythrocytes. The opposite is true of exflagellation; 3D7 microgametes can adhere to Rhesus erythrocytes but are incapable of binding to mouse erythrocytes. Second, the P. falciparum strains HB3 and 7G8 do not bind to neuraminidase-treated erythrocytes during exflagellation, in contrast to the ability of merozoites of these strains to invade neuraminidase-treated erythrocytes (24). However, because invasion assays do not necessarily take into account the initial adhesion of merozoites to erythrocytes, before apical reorientation and invasion, it is still plausible that initial interactions with erythrocytes in the two systems are similar.
The binding of erythrocytes during exflagellation is also most likely distinct from the erythrocyte binding observed in rosetting—the binding of uninfected erythrocytes by late stage asexual-infected erythrocytes. The argument for this reasoning is indirect: in general, protein-trafficking considerations would preclude the possibility that parasite-encoded proteins on the infected erythrocyte cell surface would be the same as receptors on the parasite outer membrane. For example, all members of the var (variant antigenic receptor; see 53–55) family isolated to date lack recognizable signal peptide sequences, and it can be conjectured that transport to the erythrocyte surface of an infected cell is via a mechanism independent of the endoplasmic reticulum pathway. Therefore, a putative receptor involved in microgamete adhesion to erythrocytes would be on the parasite membrane and facilitation of its localization would be by a classical protein trafficking pathway, and therefore by a gene distinct from a putative erythrocyte cell surface protein involved in rosetting.
Why do exflagellating microgametes bind neighboring erythrocytes? An obvious possibility is that the microgametes grab hold of erythrocytes to aid in breaking free from the residual body of the microgametocyte. In other words, the microgametes “gain purchase” in their efforts to break loose. Under the microscope, free swimming microgametes appear to also interact with the surface of erythrocytes. Is it possible that it is advantageous for the microgametes to move along the surface of erythrocytes rather than swim through the fluid-phase between erythrocytes? If it can be assumed that the role of the microgamete is to cover as much territory as possible in search of a macrogamete, then it is tempting to speculate that it is more efficient for the microgamete to whip past erythrocytes by briefly adhering to them, rather than forcing themselves between erythrocytes without any interaction. In this model the rosetting observed during exflagellation might be simply a consequence of an interaction whose importance lies in the freely motile gamete stage. A third hypothesis for the interaction with erythrocytes derives from the fact that microgametes bind erythrocyte surface sialic acid—are the microgametes binding in conjunction with a sialidase or trans-sialidase enzyme activity? This is not without precedence; the protozoan Trypanosoma is known to possess sialidase and trans-sialidase activity (56, 57), and sialidase activity has been well-described in bacteria and viruses. However, it would be speculation to suggest a reason why microgametes would either remove erythrocyte sialic acid with a sialidase or coat themselves in sialic acid with a sialyltransferase.
In an attempt to determine if erythrocyte adhesion influences levels of transmission we performed several P. falciparum mosquito feeds in which the adherence of erythrocytes by exflagellating microgametes was blocked by pretreatment with neuraminidase. In general, it should be noted that the mosquito feeds were conducted in the absence of complement and were done at the high gametocytemia levels typical of laboratory membrane feeds. In the field, transmission levels are much lower, on average less than two total oocysts per infected mosquito midgut (58–60), as opposed to up to 30 oocysts seen with laboratory membrane feeds with gametocyte cultures. Possibly the adhesion of erythrocytes confers an advantage for transmission in the field, but this difference might not be amenable to experimentation with laboratory mosquito feeds. Of many mosquito transmission experiments attempted, only four gave development to oocysts, but in these experiments a significant reduction in transmission was observed after neuraminidase treatment relative to control, nontreated erythrocytes. Although a significant effect was observed with neuraminidase treatment, it is not yet known at which point the neuraminidase treatment was exhibiting an effect on the parasite's development in the midgut. It is possible that stripping erythrocyte surface sialic acid alters the character of the blood meal in some fashion such that ookinete maturation or movement is impaired. To get around this question, current research aims at development of an assay to assess in vitro fertilization of P. falciparum, in hopes of correlating erythrocyte adhesion more directly with fertilization efficiency.
Acknowledgments
The authors would like to thank Alex Rowe and Louis Miller for their inspiration in this work. Osamu Kaneko (Laboratory of Parasitic Diseases, NIAID, NIH) is thanked for providing trypsin-treated erythrocytes (his own) and for helpful discussions concerning merozoite invasion. Cherise Fenton (NIAID, NIH) and Andrea Egan (Laboratory of Parasitic Diseases, NIAID, NIH) are thanked for providing Rhesus and Aotus erythrocytes. We would like to thank Andre Laughinghouse for his expert help with mosquito rearing and with P. gallinaceum infections, and Joseph Vinetz (Laboratory of Parasitic Diseases, NIAID, NIH) for providing P. gallinaceum gametocytes.
Footnotes
T.J. Templeton was the recipient of an Intramural Research Training Award.
References
- 1.Lavaran, A. 1893. Paludism. J.W. Martin, trans. The New Sydenham Society, London. 197 pp.
- 2.MacCallum WG. On the flagellated form of the malarial parasite. Lancet. 1897;2:1240–1241. [Google Scholar]
- 3.MacCallum WG. On the haematozoan infections of birds. J Exp Med. 1897;3:117–136. doi: 10.1084/jem.3.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R. On some peculiar pigmented cells found in two mosquitoes fed on malarial blood. Br Med J. 1897;2:1786–1788. doi: 10.1136/bmj.2.1929.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross, R. 1905. Researches on malaria. J. R. Army Med. Corps. 4:450–474; 541–579; 705–740.
- 6.Ogwan'g RA, Mwangi JK, Githure J, Were JBO, Roberts CR, Martin SK. Factors affecting exflagellation of in vitro–cultivated Plasmodium falciparumgametocytes. Am J Trop Med Hyg. 1993;49:25–29. doi: 10.4269/ajtmh.1993.49.25. [DOI] [PubMed] [Google Scholar]
- 7.Billker O, Shaw MK, Margos G, Sinden RE. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium bergheiin vitro. Parasitology. 1997;115:1–7. doi: 10.1017/s0031182097008895. [DOI] [PubMed] [Google Scholar]
- 8.Bishop A, McConnachie EW. A study of the factors affecting the emergence of the gametocytes of Plasmodium gallinaceumfrom the erythrocytes and the exflagellation of the male gametocytes. Parasitology. 1956;46:192–215. doi: 10.1017/s0031182000026433. [DOI] [PubMed] [Google Scholar]
- 9.Carter R, Nijhout MM. Control of gamete formation (exflagellation) in malaria parasites. Science. 1977;195:407–409. doi: 10.1126/science.12566. [DOI] [PubMed] [Google Scholar]
- 10.Nijhout MM, Carter R. Gamete development in malaria parasites. Bicarbonate-dependent stimulation by pH in vitro. . Parasitology. 1978;76:39–53. doi: 10.1017/s0031182000047375. [DOI] [PubMed] [Google Scholar]
- 11.Nijhout MM. Plasmodium gallinaceum: exflagellation stimulated by a mosquito factor. Exp Parasitol. 1977;48:75–80. doi: 10.1016/0014-4894(79)90056-0. [DOI] [PubMed] [Google Scholar]
- 12.Garcia GE, Wirtz RA, Rosenberg R. Isolation of a substance from the mosquito that activates Plasmodiumfertilization. Mol Biochem Parasitol. 1997;88:127–135. doi: 10.1016/s0166-6851(97)00086-8. [DOI] [PubMed] [Google Scholar]
- 13.Janse CJ, van der Klooster PFJ, van der Kaay HJ, van der Ploeg M, Overdulve JP. DNA synthesis in Plasmodium bergheiduring asexual and sexual development. Mol Biochem Parasitol. 1986;20:172–182. doi: 10.1016/0166-6851(86)90029-0. [DOI] [PubMed] [Google Scholar]
- 14.Janse CJ, van der Klooster PF, van der Kaay HJ, van der Ploeg M, Overdulve JP. Rapid repeated DNA replication during microgametogenesis and DNA synthesis in young zygotes of Plasmodium berghei. . Trans R Soc Trop Med Hyg. 1986;80:154–157. doi: 10.1016/0035-9203(86)90219-1. [DOI] [PubMed] [Google Scholar]
- 15.Carter, R., and P.M. Graves. 1988. Gametocytes. In Malaria: Principles and Practice of Malariology. Vol. 1. W.H. Wernsdorfer and I. McGregor, editors. Churchill Livingston, Edinburgh. 253–306.
- 16.Sinden RE, Hartley RH, King NJ. Gametogenesis in Plasmodium; the inhibitory effects of anticytoskeletal agents. Int J Parasitol. 1985;15:211–217. doi: 10.1016/0020-7519(85)90089-x. [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto F, Alejo-Blanco R, Fleck SL, Kawamoto Y, Sinden RE. Possible roles of Ca2+ and cGMP as mediators of the exflagellation of Plasmodium berghei and Plasmodium falciparum. . Mol Biochem Parasitol. 1990;42:101–108. doi: 10.1016/0166-6851(90)90117-5. [DOI] [PubMed] [Google Scholar]
- 18.Martin SK, Miller LH, Nijhout MM, Carter R. Plasmodium gallinaceum: induction of male gametocyte exflagellation by phosphodiesterase inhibitors. Exp Parasitol. 1978;44:239–242. doi: 10.1016/0014-4894(78)90104-2. [DOI] [PubMed] [Google Scholar]
- 19.Walliker D, Quakyi IA, Wellems TE, McCutchan TF, Szarfman A, London WT, Corcoran LM, Burkot TR, Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. . Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- 20.Burkot TR, Williams JL, Schneider I. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitrofrom the same isolate. Trans R Soc Trop Med Hyg. 1984;78:339–341. doi: 10.1016/0035-9203(84)90114-7. [DOI] [PubMed] [Google Scholar]
- 21.Bhasin VK, Trager W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. . Am J Trop Med Hyg. 1984;33:534–537. doi: 10.4269/ajtmh.1984.33.534. [DOI] [PubMed] [Google Scholar]
- 22.Ifediba T, Vanderberg JP. Complete in vitro maturation of P. falciparumgametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 23.Kaushal DC, Carter R. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum. II. Comparison of surface antigens of male and female gametes and zygotes. Mol Biochem Parasitol. 1984;11:145–156. doi: 10.1016/0166-6851(84)90061-6. [DOI] [PubMed] [Google Scholar]
- 24.Dolan SA, Procter JL, Alling DW, Okubo Y, Wellems TE, Miller LH. Glycophorin B as an EBA-175 independent Plasmodium falciparumreceptor of human erythrocytes. Mol Biochem Parasitol. 1994;64:55–63. doi: 10.1016/0166-6851(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 25.Springer GF, Desai PR. Human blood-group MN and precursor specificities: structural and biological aspects. Carbohydr Res. 1975;40:183–192. doi: 10.1016/s0008-6215(00)82680-4. [DOI] [PubMed] [Google Scholar]
- 26.Daniel G. Effect of enzymes on and chemical modifications of high-frequency red cell antigens. Immunohematology. 1992;8:53–57. [PubMed] [Google Scholar]
- 27.Ward GE, Miller LH, Dvorak JA. The origin of the parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J Cell Sci. 1993;106:237–248. doi: 10.1242/jcs.106.1.237. [DOI] [PubMed] [Google Scholar]
- 28.Bishop A, Gilchrist BM. Experiments upon the feeding of Aedes aegyptithrough animal membranes with a view to applying this method to the chemotherapy of malaria. Parasitology. 1946;37:85–100. doi: 10.1017/s0031182000013202. [DOI] [PubMed] [Google Scholar]
- 29.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparumis also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–4217. [PubMed] [Google Scholar]
- 30.Rosenberg R, Koontz LC, Alston K, Friedman FK. Plasmodium gallinaceum: erythrocyte factor essential for zygote infection of Aedes aegypti. . Exp Parasitol. 1984;57:158–164. doi: 10.1016/0014-4894(84)90075-4. [DOI] [PubMed] [Google Scholar]
- 31.Judd WJ, Issitt PD, Pavone BG, Anderson J, Aminoff D. Antibodies that define NANA-independent MN-system antigens. Transfusion. 1979;19:12–18. doi: 10.1046/j.1537-2995.1979.19179160260.x. [DOI] [PubMed] [Google Scholar]
- 32.Issitt PD. Null red blood cell phenotypes: associated biological changes. Transfus Med Rev. 1993;7:139–155. doi: 10.1016/s0887-7963(93)70134-0. [DOI] [PubMed] [Google Scholar]
- 33.Cartron JP. Defining the Rh blood group antigens. Blood Rev. 1994;8:199–212. doi: 10.1016/0268-960x(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 34.Anstee DJ. The blood group MNSs-active sialoglycoproteins. Semin Hematol. 1981;18:13–31. [PubMed] [Google Scholar]
- 35.Cartron JP, Rahuel C. Human erythrocyte glycophorins: protein and gene structure analyses. Transfus Med Rev. 1992;6:63–92. doi: 10.1016/s0887-7963(92)70158-8. [DOI] [PubMed] [Google Scholar]
- 36.Trigg PI. Invasion of erythrocytes by Plasmodium falciparumin vitro. Parasitology. 1975;71:433–436. doi: 10.1017/s0031182000047193. [DOI] [PubMed] [Google Scholar]
- 37.Miller LH, Haynes JD, Mcauliffe FM, Shiroishi T, Durocher JR, McGinniss MH. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. . J Exp Med. 1977;146:277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patankar MS, Oehninger S, Barnett T, Williams RL, Clark GF. A revised structure for fucoidan may explain some of its biological activities. J Biol Chem. 1993;268:21770–21776. [PubMed] [Google Scholar]
- 39.Perkins M. Inhibitory effects of erythrocyte membrane proteins on the in vitro invasion of the human malarial parasite (Plasmodium falciparum)into its host cell. J Cell Biol. 1981;90:563–567. doi: 10.1083/jcb.90.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasvol G, Wainscoat JS, Weatherall DJ. Erythrocytes deficient in glycophorin resist invasion by the malarial parasite Plasmodium falciparum. . Nature. 1982;297:64–66. doi: 10.1038/297064a0. [DOI] [PubMed] [Google Scholar]
- 41.Cartron JP, Prou O, Luilier M, Soulier JP. Susceptibility to invasion by Plasmodium falciparumof some human erythrocytes carrying rare blood group antigens. Br J Haematol. 1983;55:639–647. doi: 10.1111/j.1365-2141.1983.tb02846.x. [DOI] [PubMed] [Google Scholar]
- 42.Hadley TJ, Klotz FW, Pasvol G, Haynes JD, McGinniss MH, Okubo Y, Miller LH. Falciparum malaria parasites invade erythrocytes that lack Glycophorin A and B (MkMk) J Clin Invest. 1987;80:1190–1193. doi: 10.1172/JCI113178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadley TJ, Klotz FW, Miller LH. Invasion of erythrocytes by malaria parasites: a cellular and molecular overview. Ann Rev Microbiol. 1986;40:451–477. doi: 10.1146/annurev.mi.40.100186.002315. [DOI] [PubMed] [Google Scholar]
- 44.Dalton JP, Hudson D, Adams JH, Miller LH. Blocking of the receptor-mediated invasion of erythrocytes by Plasmodium knowlesimalaria with sulfated polysaccharides and glycosaminoglycans. Eur J Biochem. 1991;195:789–794. doi: 10.1111/j.1432-1033.1991.tb15767.x. [DOI] [PubMed] [Google Scholar]
- 45.Pancake SJ, Holt GD, Mellouk S, Hoffman SL. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J Cell Biol. 1992;117:1351–1357. doi: 10.1083/jcb.117.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller H-M, Reckmann I, Hollingdale MR, Bujard H, Robson KJH, Crisanti A. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparumbinds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO (Eur Mol Biol Organ) J. 1993;12:2881–2889. doi: 10.1002/j.1460-2075.1993.tb05950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao L, Yang C, Patterson PS, Udhayakumar V, Lal AA. Sulfated polyanions inhibit invasion of erythrocytes by Plasmodialmerozoites and cytoadherence of endothelial cells to parasitized erythrocytes. Infect Immun. 1996;64:1373–1378. doi: 10.1128/iai.64.4.1373-1378.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahr W, Kordowicz M, Moulds J, Gielen W, Lebeck L, Kruger J. Characterization of the Ss sialoglycoprotein and its antigens in Rhnullerythrocytes. Blut. 1987;54:13–24. doi: 10.1007/BF00326022. [DOI] [PubMed] [Google Scholar]
- 49.Sim BK L, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. . Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 50.Gahmberg CG, Myllyla G, Leikola J, Pirkola A, Nordling S. Absence of the major sialoglycoprotein in the membrane of human En(a−) erythrocytes and increased glycosylation of Band 3. J Biol Chem. 1976;251:6108–6116. [PubMed] [Google Scholar]
- 51.Klotz FW, Chulay JD, Daniel W, Miller LH. Invasion of mouse erythrocytes by the human malaria parasite Plasmodium falciparum. . J Exp Med. 1987;165:1713–1718. doi: 10.1084/jem.165.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klotz FW, Orlandi PA, Reuter G, Cohen SJ, Haynes JD, Schauer R, Howard RJ, Palese P, Miller LH. Binding of Plasmodium falciparum 175-kilodalton erythrocyte binding antigen and invasion of murine erythrocytes requires N-acetylneuraminic but not its O-acetylated form. Mol Biochem Parasitol. 1992;51:49–54. doi: 10.1016/0166-6851(92)90199-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. Cloning the P. falciparumgene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 54.Rowe JA, Moulds JM, Newbold CI, Miller LH. P. falciparumrosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 55.Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. The large diverse gene family var encodes proteins involved in cytoadherence an antigenic variation of Plasmodium falciparum–infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 56.Cross GAM, Takle GB. The surface trans-sialidase family of Trypanosoma cruzi. . Annu Rev Microbiol. 1993;47:385–411. doi: 10.1146/annurev.mi.47.100193.002125. [DOI] [PubMed] [Google Scholar]
- 57.Schenkman S, Eichinger D, Pereira MEA, Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 58.Muirhead-Thomson RC. Factors determining the true reservoir of infection of Plasmodium falciparum and Wuchereria bancroftiin a west African village. Trans R Soc Trop Med Hyg. 1954;48:208–225. doi: 10.1016/0035-9203(54)90067-x. [DOI] [PubMed] [Google Scholar]
- 59.Muirhead-Thomson RC. The malarial infectivity of an African village population to mosquitoes (Anopheles gambiae). A random xenodiagnostic survey. Am J Trop Med Hyg. 1957;6:971–977. doi: 10.4269/ajtmh.1957.6.971. [DOI] [PubMed] [Google Scholar]
- 60.Githeko AK, Brandling-Bennett AD, Beier M, Atieli F, Owaga M, Collins FH. The reservoir of Plasmodium falciparummalaria in a holoendemic area of western Kenya. Trans R Soc Trop Med Hyg. 1992;86:355–358. doi: 10.1016/0035-9203(92)90216-y. [DOI] [PubMed] [Google Scholar]


