Abstract
Platelet-activating factor (PAF) is a potent phospholipid mediator with diverse biological activities in addition to its well-known ability to stimulate platelet aggregation. Pharmacologic studies had suggested a role for PAF in pregnancy, neuronal cell migration, anaphylaxis, and endotoxic shock. Here we show that disruption of the PAF receptor gene in mice caused a marked reduction in systemic anaphylactic symptoms. Unexpectedly, however, the PAF receptor–deficient mice developed normally, were fertile, and remained sensitive to bacterial endotoxin. These mutant mice clearly show that PAF plays a dominant role in eliciting anaphylaxis, but that it is not essential for reproduction, brain development, or endotoxic shock.
Keywords: platelet-activating factor, platelet-activating factor receptor, anaphylaxis, endotoxic shock, gene targeting
Platelet-activating factor (PAF1; 1-O-alkyl-2-acetyl- sn-glycero-3-phosphocholine) is a potent phospholipid mediator with various biological activities, including platelet activation, airway constriction, hypotension, and vascular permeation (1–4). PAF acts by binding to a G protein– coupled seven transmembrane receptor (5). Through G proteins, PAFR links to signal transduction mechanisms, including turnover of phosphatidylinositol, elevation in intracellular calcium concentration (6), and activation of many kinases, e.g., phosphatidylinositol-3 kinase (7), protein kinase C (8), and mitogen-activated protein kinase (9). We and others have cloned PAFR cDNAs of guinea pig (10), human (11–14), and rat (15) and a PAFR gene of mouse (16). A unique PAFR has been identified in each species by the molecular cloning technique, although possible existence of PAFR subtypes has been suggested by biochemical or pharmacologic methods (17, 18).
PAF is synthesized not only in various blood cells but also in many other tissues, e.g., early mammalian embryo (19), fetal lung (20), kidney (21), and brain (22) under physiological conditions. Thus, PAF, even at low concentrations, has been considered to constitutively regulate various physiological processes such as reproduction (19, 23), blood pressure (24), neural development (25), and hippocampal long-term potentiation (18). Despite extensive studies, the physiological importance of PAF in vivo remains largely unknown.
In addition to its role as a physiological mediator, PAF has been believed to be associated with the pathology of anaphylaxis and endotoxic shock. Systemic anaphylaxis is a rapid, often fatal type-I allergic reaction characterized by acute airway constriction, heart rate (HR) alteration, hypotension, and vascular leakage (26, 27); many anaphylactic symptoms can be mimicked by PAF injection in animals (4, 5). Indeed, PAF is released rapidly from antigen-stimulated mast cells (28) and basophils (29) in vitro. In studies in vivo, an increase in blood PAF level is also detected after antigen challenge to sensitized animals (30). PAFR antagonists, which inhibit the binding of PAF to the receptor, exert protective effects on anaphylaxis in animal models (30–32). However, the precise role of PAF among a lot of allergic mediators in the development of anaphylactic responses is unclear.
Endotoxic or septic shock is the result of activation of the immune system by endotoxin/LPS, a component of Gram-negative bacterial cell wall. In this state, macrophages produce large amounts of cytokines such as TNF-α and IL-6, as well as other inflammatory mediators, including PAF, that are released into the circulation (33, 34). Thus, these events lead to hypotension, tissue injury, vascular leakage, and frequently, death. As is the case in anaphylaxis, PAF per se is capable of eliciting many symptoms associated with endotoxic shock (4, 5). Further, it has been reported that the blood PAF level increased during endotoxemia (30, 35) and the PAF acetylhydrolase activity decreased in plasma of patients with sepsis (36). The administration of PAFR antagonists to animals and humans protects them from the deleterious effects of endotoxin (30, 32, 37–40). Our recent demonstration that transgenic mice overexpressing PAFR are hypersensitive to endotoxin has suggested that PAF plays an exaggerating role in the in vivo response to endotoxin (41).
To date, our ability to address the pathophysiological role of PAF has largely depended on the use of structurally diverse PAFR antagonists. However, pharmacologic studies often encounter problems related to the specificity of drugs. Indeed, several PAFR antagonists are demonstrated to have an inhibitory effect on the activity of intracellular PAF acetylhydrolase, an inactivating enzyme of PAF (42, 43). Some other antagonists inhibit histamine effects through the interaction with its G protein–coupled receptor (40, 44).
To clearly identify both the nonredundant physiological role of PAF and the pathophysiological role of PAF without the use of possibly nonspecific PAFR antagonists, we generated animals in which the PAFR signaling was congenitally declined by targeted disruption of the PAFR gene in murine embryonic stem (ES) cells. We report here that the resulting PAFR-deficient mice were fertile and developed normally. Furthermore, although these mice showed the markedly attenuated anaphylactic symptoms, the responses to endotoxin remained intact. Thus, our studies demonstrate that PAF plays a dominant role in eliciting anaphylaxis, and is not essential for reproduction, brain development, or endotoxic shock.
Materials and Methods
Reagents.
Materials and chemicals were obtained from the following sources. α-[32P]dCTP (111 TBq/mmol) and transfer membrane (Hybond-N+) were from Amersham (Tokyo, Japan); restriction enzymes and DNA-modifying enzymes were from Takara (Kyoto, Japan) or Toyobo (Kyoto, Japan); agaroses (Sea Kem and Nu Sieve) were from FMC (Rockland, ME); PAF (1- O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine) and leukotriene B4 (LTB4) were from Cayman Chemical (Ann Arbor, MI); [3H]WEB 2086 (499.5 GBq/mmol) and [3H]acetyl-PAF (370 GBq/mmol) were from Du Pont/New England Nuclear (Tokyo, Japan); fatty acid–free BSA, grade VI OVA, and endotoxin (LPS; Escherichia coli serotype O127:B8, code No. L-3129) were from Sigma Chemical Co. (St. Louis, MO); lyso-PAF was from Cascade Biochem (Berkshire, UK); pyrogen-free saline was from Otsuka Pharmaceuticals (Tokyo, Japan); RPMI 1640 medium was from Nissui (Tokyo, Japan). Other materials and reagents were of analytical grade. PAF and LTB4 were dissolved in pyrogen-free saline containing 0.25% BSA just before use. Endotoxin was suspended in saline. Unlabeled WEB 2086 was a gift from Boehringer Ingelheim (Ingelheim, Germany) and was dissolved in saline by sonication at a concentration of 0.5 mg/ml.
Targeted Disruption of Mouse PAFR Gene.
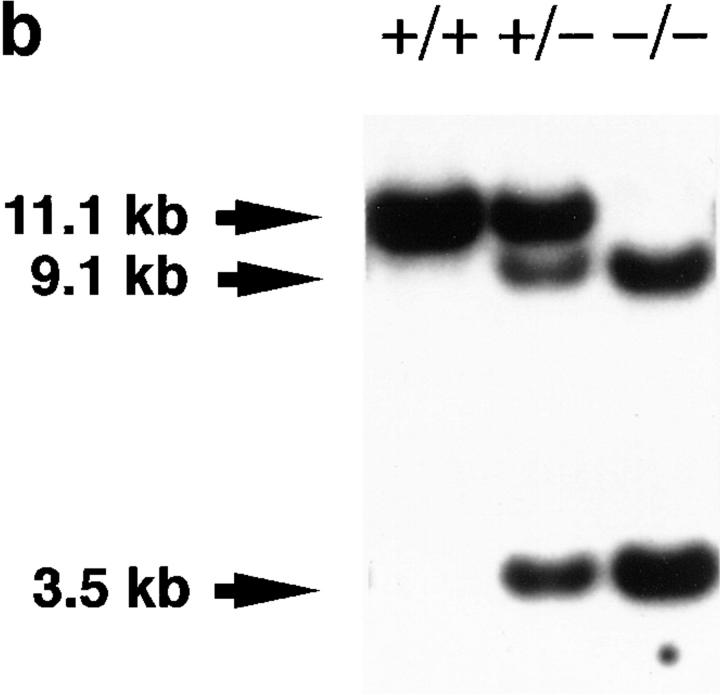
The murine PAFR genomic DNA was isolated by screening a λFixII 129/Sv genomic library (Stratagene, La Jolla, CA) (16) with guinea pig PAFR cDNA (10). The targeting vector was constructed in pBluescript vector (Stratagene) using a 10.7-kb EcoRI fragment of the genomic DNA, a PGK-neo cassette, and an MC1-tk cassette (Fig. 1 a). The PGK-neo cassette was inserted into the PstI site in the exon. E14-1 cells were electroporated with NotI-linearized targeting vector and selected with G418 (225 μg active weight/ml) and fluoroiodoadenosyluracine (FIAU; 0.2 μM). The ES clones carrying the PAFR-targeted mutation on one allele through the homologous recombination event were screened by Southern blot analysis after ScaI digestion with a 5′ flanking 0.6-kb SalI–EcoRI fragment (5′ probe shown in Fig. 1 a). This fragment is located at the margin of the murine PAFR genomic DNA clone used; the SalI site derives from the polycloning sites of λFixII vector. The 5′ probe identified a 9.1-kb mutant band in addition to a 11.1-kb wild-type band (data not shown). Results were verified by hybridization of BamHI restriction digests with a 3′ flanking 1.4-kb EcoRI–EcoRI fragment (3′ probe shown in Fig. 1 a). Hybridization of ScaI restriction digests either with a 1.2-kb Eco47III–SpeI fragment containing the entire exon (PAFR probe shown in Fig. 1 a) or with a 1.1-kb AatI–BglII fragment of a neomycin resistance gene (Neo probe shown in Fig. 1 a) was also performed to ensure a single integration site. The former probe identified 9.1- and 3.5-kb mutant bands in addition to a 11.1-kb wild-type band (see Fig. 1 b), and the latter probe identified a 9.1-kb mutant band (data not shown).
Figure 1.
Disruption of the PAFR gene in mice. (a) Gene-targeting strategy: structure and partial restriction maps of the PAFR gene, targeting construct, and predicted targeted allele, and location of the probes used for hybridization analyses. Solid boxes, exon containing the entire PAFR open reading frame (PAFR ORF); hatched boxes, PGK promoter (PGK-neo) and neomycin-resistance gene with poly(A) signal; gray boxes, MC1 promoter (MC1-tk) and herpes simplex virus thymidine kinase gene. The direction of transcription for each gene is indicated by an arrow. Restriction endonuclease sites: E, EcoRI; H, HindIII; Ba, BamHI; P, PstI; and Sc, ScaI. (b) Southern blot analysis of tail DNA from pafr +/+ (+/+), pafr +/− (+/−), and pafr −/− (−/−) mice. The wild-type (11.1-kb) and targeted (9.1- and 3.5-kb) ScaI fragments are detected using the PAFR probe shown in a. (c) Northern blot analysis of PAFR mRNA expression in pafr +/+ (+/+), pafr +/− (+/−), and pafr −/− (−/−) neutrophils. PAFR mRNA (upper blot) is identified using the PAFR probe indicated in a. The expression of GAPDH mRNA is also shown (lower blot). (d) Intracellular calcium measurements in C57BL/6 (WT) and pafr −/− (−/−) neutrophils. Changes in intracellular calcium in response to 10 nM PAF or 10 nM LTB4 were determined using Fura-2AM. Agonists were added at the times indicated by the arrows. (e) MAP measurements of pafr +/+ (+/+) and pafr −/− (−/−) mice. PAF (200 μg/kg) was administered intravenously at the times indicated by the arrows. Lyso-PAF (200 μg/kg) had no effects on MAP values in either group (data not shown). Nearly identical data were obtained with two animals in each group.
Mutant clones were injected into blastocysts of C57BL/6 mice and the resulting male chimeras were mated to C57BL/6 females to test for germline transmission of the mutation. Offspring derived from ES cells were identified by coat color and screened for the presence of the targeted allele by Southern blot analysis of ScaI-digested tail DNA using 5′ probe (Fig. 1 b). For the following experiments, homozygous mutants and wild-type littermates were obtained by intercrossing heterozygous mutant mice under specific pathogen-free conditions. The genotypes of mice were determined by Southern blot analysis of ScaI-digested tail DNA using the PAFR probe (Fig. 1 b).
Northern Hybridization.
Total RNA was prepared from 2% casein-induced neutrophils as previously described (16). The RNA (1 μg) was electrophoresed, transferred to a nylon membrane, and hybridized (16) with either the PAFR probe shown in Fig. 1 a or a human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe (Clontech, Palo Alto, CA).
Radioligand Binding Assay.
Lung membranes were prepared and used for ligand binding assay as described (41). Peritoneal exudate macrophages were obtained using thioglycollate as described in reference 16. The cells were cultured in 6-well dishes at 106 cells/well. After incubation for 2 h, nonadherent cells were washed away with RPMI 1640 medium containing 10% heat-inactivated FCS (GIBCO BRL, Rockville, MD). Adherent macrophages were then cultured for 1 or 2 d. Binding of [3H]WEB 2086 (20 nM) or [3H]acetyl-PAF (10 nM) was competed with unlabeled WEB 2086 (20 μM) in Hepes-Tyrode's buffer (pH 7.4) containing 0.1% BSA (Hepes-BSA buffer) at 20°C for 90 min or 25°C for 60 min, respectively. The cells were washed three times with cold Hepes-BSA buffer and lysed with 5% Triton X-100. The radioactivity associated with the cells was measured by liquid scintillation counting.
Measurement of Intracellular Calcium Concentration.
Neutrophils were loaded with 3 μM Fura-2AM (Dojindo, Kumamoto, Japan) at 37°C for 1 h and resuspended in calcium-free Hepes-Tyrode's buffer (pH 7.4) at 2 × 106 cells/ml. Intracellular calcium concentrations were measured using a fluorometer (CAF-110; JASCO, Tokyo, Japan) as described previously (45).
Sensitization of Mice.
pafr +/+ and pafr −/− (18–22-wk-old) male mice were given an intraperitoneal injection of OVA (100 μg), aluminum hydroxide gel (1 mg), and pertussis toxin (300 ng; Kaken Pharmaceutical, Tokyo, Japan) (26). Elicitation of anaphylaxis or bleeding were performed 18–21 d after the immunization. Serum levels of OVA-specific IgE and IgG1 were determined by ELISA as described in the PharMingen (San Diego, CA) protocol. In brief, 96-well flat-bottomed plates (Corning Glass Works, Corning, NY) were coated with either rat anti– mouse IgE antibody (clone R35-72) or OVA. Bound IgE or IgG1 was detected with biotinylated OVA in combination with horseradish peroxidase–conjugated streptavidin or horseradish peroxidase–conjugated anti–mouse IgG1 (clone G1-6.5), respectively. 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma Chemical Co.) was used as a substrate to generate the color reaction, which was read at 405 nm. The Ig levels of the samples were estimated using serum of an OVA-immunized mouse as a standard, and were compared using the Mann-Whitney U test.
Measurement of Mean Arterial Pressure and HR.
Under urethane anesthesia (1.5 g/kg, intraperitoneal), mice were cannulated with PE-10 polyethylene tubing (Clay Adams, Parsippany, NJ) into the right femoral artery. The tubing was filled with heparinized saline and connected to a pressure transducer (TP-200T; Nihon Kohden, Tokyo, Japan). The blood pressure signals were amplified (AP-600G; Nihon Kohden) and stored into a tape recorder. HR was computed using a tachometer (AT-601G; Nihon Kohden) on the basis of an electrocardiogram. The data were fed into a computer (PC9801RX; NEC, Tokyo, Japan) to calculate the mean values. Body temperature was maintained at 37 ± 1°C with a heating pad by monitoring rectal temperature. The cannulated mice were injected with 200 μg/kg of PAF (10 ml/kg), 200 μg/kg of lyso-PAF (10 ml/kg), 15 mg/kg of endotoxin (1 ml/kg), or 150 μg of OVA (100 μl/head) via the right femoral vein.
Bronchopulmonary Responses.
Total lung resistance (RL) was measured as previously described (41, 46, 47). Mechanically ventilated mice were challenged with 500 μg OVA via the jugular vein in a 100-μl bolus. The lungs were removed intact 5 min after the challenge of OVA. For histological analysis, the lungs were frozen in liquid nitrogen, fixed in Carnoy's solution, and stained with hematoxylin and eosin (46). In other experiments, the dissected lungs were weighed and placed in a gravity convection oven at 90°C for 72 h to determine dry weights for calculation of wet weight/dry weight ratios (46).
Determination of Cytokine Levels.
Adherent peritoneal macrophages were prepared as described in reference 16 and were cultured on 48-well dishes at 2.5 × 105 cells/well in 250 μl of RPMI 1640 medium supplemented with 10% heat-inactivated FCS (Moregate, Melbourne, Australia). The cells were exposed to endotoxin (100 ng/ml) plus mouse rIFN-γ (100 U/ml; Genzyme, Cambridge, MA) for 24 h. The supernatants were harvested and stored at −80°C. Concentrations of TNF-α and IL-6 were determined in duplicate using murine ELISA kits obtained from Genzyme and Amersham, respectively. Limits of detection for TNF-α and IL-6 were 35 and 40 pg/ml, respectively. Data shown are averages from two separate experiments. Unstimulated macrophages released undetectable levels of the cytokines.
Results and Discussion
Generation of PAFR-deficient Mice.
A gene-targeting construct was generated in which an open reading frame of the G protein–coupled PAFR gene was inserted by a PGK-neo cassette (Fig. 1 a). The construct was electroporated into E14-1 ES cells. Two targeted ES clones were identified after screening 670 ES clones resistant to both G418 and fluoroiodoadenosyluracine by Southern blot analysis of ScaI-digested genomic DNA using the 5′ probe shown in Fig. 1 a (data not shown). Further Southern blot analyses with the 3′ probe after BamHI digestion, and Neo probe and PAFR probe after ScaI digestion, demonstrated the absence of rearrangements or extra insertional events in these two clones (data not shown). The targeted clones were injected into C57BL/6 blastocysts, and the resulting male chimeras bred to C57BL/6 females to produce mice heterozygous for the disrupted PAFR allele. Crossings between F1 or F2 heterozygous mice under specific pathogen-free conditions yielded 82 wild-type (pafr +/+), 140 heterozygous (pafr +/−) mutant, and 72 homozygous (pafr −/−) mutant mice, consistent with a normal 1:2:1 Mendelian distribution of offspring (χ2 test). pafr −/− mouse lines were established from both clones.
Inactivation of the PAFR gene was confirmed by genomic Southern analysis (Fig. 1 b) and by Northern analysis of RNA isolated from neutrophils (Fig. 1 c). Neither peritoneal macrophages nor lung tissues from pafr −/− mice displayed any detectable binding sites for the PAFR antagonist WEB 2086 (16, 48; data not shown), confirming the loss of surface receptors. In pafr −/− mice, challenge with PAF no longer induced an increase in intracellular calcium concentration in neutrophils (49) (Fig. 1 d) or a decrease in mean arterial pressure (MAP; reference 50; Fig. 1 e). These results provide compelling evidence that the PAFR plays an essential role in PAF-induced calcium mobilization in neutrophils and in systemic hypotension.
Development, Reproduction, and Cardiac Homeostasis.
pafr −/− mice were healthy in appearance and grew normally up to 1 yr of age. There were no changes in blood cell populations, differential leukocyte counts, or serum biochemical parameters (data not shown). Light microscopic examination of major organs (brain, heart, lung, liver, kidney, pituitary, thyroid, adrenal, thymus, spleen, gastrointestinal tract, bone, bone marrow, and skin) revealed no differences in morphology between pafr +/+ and pafr −/− mice at 13–16 wk old (data not shown). A requirement for PAF in neural cell migration had previously been proposed based on the finding that mutation in the LIS1 gene encoding a subunit of brain PAF acetylhydrolase causes Miller-Dieker lissencephaly (25). The apparent lack of brain malformation in pafr −/− mice suggests, however, that the LIS1 subunit may have other function(s) relevant to brain development that are unrelated to PAF. This is consistent with a recent report that LIS1 reduces microtubule catastrophe events by interacting with tubulin (51).
PAF has also been proposed to play significant roles in reproductive functions such as implantation and parturition in various species (52–54). In fact, human preembryos produced by in vitro fertilization show an increased rate of implantation after exposure to PAF (53) and transgenic mice that overproduce PAFR display reproductive disorders (41). Hence, there existed a possibility that pafr −/− mice might be infertile. Contrary to this expectation, however, the homozygous pafr −/− mice were fertile and produced healthy litters of normal size (6.2 ± 1.2 [pafr −/− × pafr −/−; n = 5] versus 8.0 ± 0.7 [pafr +/− × pafr +/−; n = 7]; mean litter size ± SEM, P ≈ 0.2, the unpaired t test [two-tailed]). These results suggest that PAF is not essential for normal development and reproduction of mice, although we cannot rule out the possible existence of additional PAFR subtypes as previously suggested by biochemical and pharmacologic experiments (17, 18). Furthermore, it is also possible that there may be receptor-independent actions of PAF.
MAP was measured through a catheter placed in the femoral artery. There were no significant differences in the baseline of both parameters between pafr +/+ and pafr −/− mice (101 ± 5 versus 107 ± 4 mmHg; mean ± SEM, n = 5 each, P ≈ 0.4, the unpaired t test [two-tailed]). Although intravenously administered PAF has a potent hypotensive effect, as shown in Fig. 1 e, the lack of difference in MAP between pafr +/+ and pafr −/− mice suggests that PAF may not be a mediator involved in setting the basal tone of vascular smooth muscle. HR of pafr +/+ and pafr −/− mice were also indistinguishable (586 ± 22 versus 560 ± 28 beats/ min; mean ± SEM, n = 5 each, P ≈ 0.5, the unpaired t test [two-tailed]). However, on the contrary, we observed in OVA-sensitized mice (see below) that HR of pafr −/− mice (648 ± 7 beats/min; mean ± SEM, n = 10) was significantly higher than that of pafr +/+ mice (570 ± 18 beats/ min; n = 12, P = 0.003, the Mann-Whitney U test), whereas MAPs were comparable between pafr +/+ and pafr −/− mice. Sensitization for active anaphylaxis may be associated with many changes, e.g., in levels of antigen-specific Igs of different isotypes, numbers of various effector cells, and local concentrations of immunomodulatory cytokines. It is possible that some of these changes cause the observed increase in the basal HR.
Active Systemic Anaphylaxis.
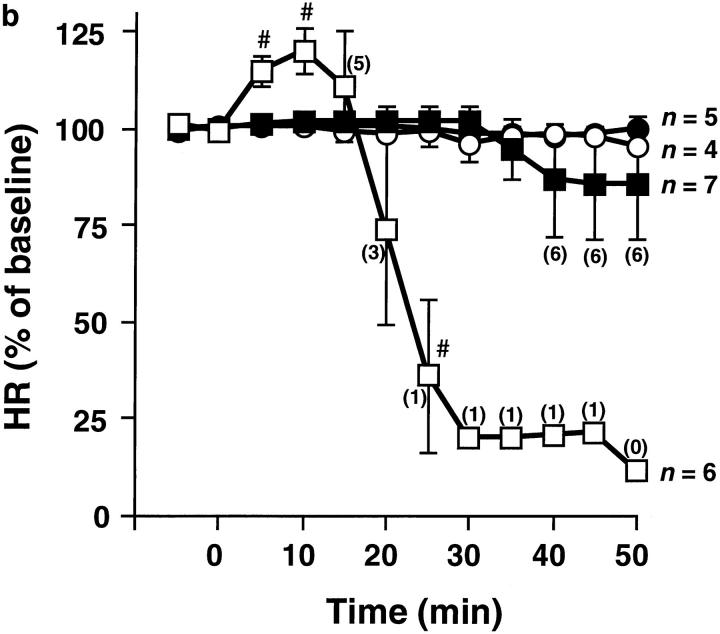
To directly evaluate the pathophysiological role of PAF in systemic anaphylaxis, pafr +/+ and pafr −/− mice were sensitized with OVA in the presence of adjuvants (26). This treatment caused mice of both genotypes to develop comparable and significant levels of OVA-specific IgE and IgG1 (data not shown), which mediate systemic anaphylaxis (27). The sensitized animals were injected intravenously with OVA to elicit systemic anaphylaxis. Cardiovascular or bronchopulmonary parameters were measured to assess the severity of the shock. Soon after challenge with OVA, all pafr +/+ mice developed severe and irreversible hypotension (Fig. 2 a). In this shock state, HR response to OVA was biphasic. OVA caused a rapid onset of tachycardia that reached a maximal increase of ∼20% after 10 min, followed by bradycardia (Fig. 2 b). The tachycardia may be a compensatory response due to baroreceptor reflex. Five of six pafr +/+ mice died within 25 min (Fig. 2), and the remaining mouse died after 48 min. In contrast, the fall in MAP was much milder in pafr −/− mice compared with pafr +/+ mice (Fig. 2 a). Furthermore, HR remained stable upon the administration of OVA (Fig. 2 b). Only one of seven pafr −/− mice died after 36 min. Lethality among pafr −/− mice monitored 50 min after the antigen challenge was significantly lower than that of pafr +/+ mice (P <0.005, as determined by Fisher's exact test).
Figure 2.
Cardiovascular responses during anaphylaxis. Data points show the mean MAP (a) and the mean HR (b) of pafr +/+ (open symbols) and pafr −/− (closed symbols) male mice for the 5-min period preceding each time point. Results are expressed as percentage of baseline ± SEM. The baseline corresponded to the mean value over a 10-min period before intravenous injection of OVA (squares) or saline (circles) at time 0. Numbers in parentheses are the numbers of animals alive at the indicated times. MAP and HR of dead mice were taken to be zero. The basal MAP of pafr +/+ and pafr −/− mice were 89 ± 4 and 93 ± 3 mmHg, and the basal HR were 570 ± 18 and 648 ± 7 beats/min (mean ± SEM; n = 10 and 12, respectively). *P <0.005, #P <0.05 versus both the pafr −/− OVA group and the pafr +/+ saline group, ‡P <0.005, †P <0.05 versus the pafr −/− saline group, as determined by analysis of variance (ANOVA) with Scheffe's test for pair-wise comparisons. OVA had no effects on MAP or HR in unsensitized mice (data not shown).
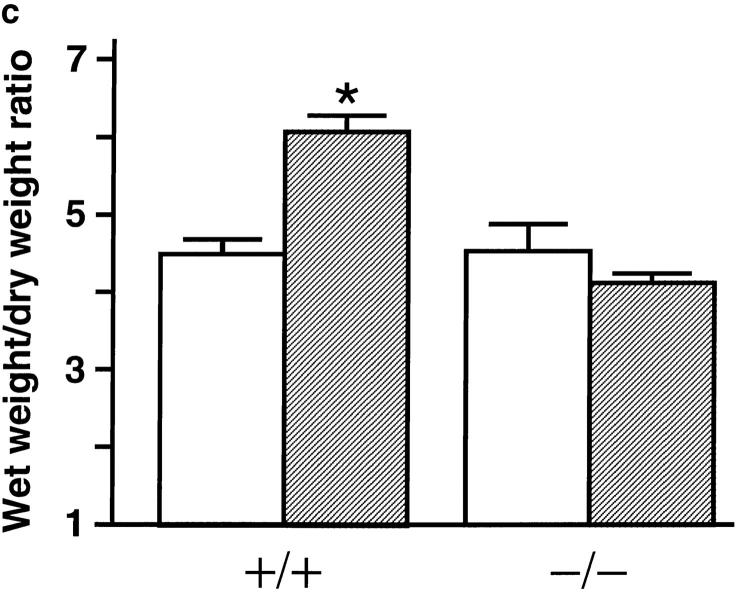
Next, RL was measured in tracheostomized, mechanically ventilated mice. All pafr +/+ mice showed a rapid increase in RL after the intravenous injection of OVA (Fig. 3 a). In comparison to the response in pafr +/+ mice, antigen challenge of pafr −/− mice produced a significantly smaller increase in RL (Fig. 3 a). Lung tissues in pafr +/+ mice given OVA were characterized by airway constriction associated with folding of airway epithelium, alveolar wall thickening, alveolar distortion, and edema (Fig. 3 b). Pulmonary edema due to increased vascular permeability was further assessed by measuring wet weight/dry weight ratios (Fig. 3 c). In marked contrast to pafr +/+ mice, pafr −/− mice displayed no response to the antigen challenge, either in the wet weight/dry weight ratios (Fig. 3 c) or in lung histology (Fig. 3 b).
Figure 3.

Bronchopulmonary responses during anaphylaxis. (a) Comparison of change in RL between pafr +/+ (open symbols) and pafr −/− (closed symbols) mice. Data from male mice are expressed as mean percentage of baseline ± SEM. The baseline RL corresponded to the mean values over a 5-min period before the intravenous injection of OVA. (squares; n = 8 each) or saline (circles; n = 3 each) at time 0. The baselines of pafr +/+ and pafr −/− mice were 0.41 ± 0.02 and 0.41 ± 0.01 cmH2O/ml/s (mean ± SEM; n = 11), respectively. No mice died during the recordings. *P < 0.05 versus the pafr −/− OVA group, †P <0.05 versus the pafr +/+ saline group, ‡P <0.03 versus both the pafr −/− OVA group and the pafr +/+ saline group, as determined by ANOVA with Scheffe's test for pair-wise comparisons. No statistically significant response was detected in the OVA-challenged pafr −/− mice compared with the saline-challenged mice. (b) Histology of the fixed lung tissue from a pafr +/+ (top) and a pafr −/− (bottom) mouse. Hematoxylin and eosin staining. Scale bar: 100 μm. (c) Determination of wet weight/dry weight ratios of the lungs of pafr +/+ (+/+) and pafr −/− (−/−) mice given OVA (hatched bars; n = 6 each) or saline (open bars; n = 3 each). Data are presented as the mean ± SEM. *P = 0.0001 versus the pafr −/− OVA group, as determined by ANOVA with Scheffe's test for pair-wise comparisons.
These results clearly demonstrate a dominant role for PAF in the anaphylactic responses. Because pafr −/− mice still show milder symptoms of anaphylaxis, chemical mediators other than PAF, such as eicosanoids (55), histamine, or serotonin, probably also contribute to the anaphylactic responses, albeit to a much lesser extent.
The results of this study elucidated a large contribution of PAF to allergen-induced airway obstruction. As for the effects of PAF on the bronchopulmonary system, we reported that PAFR overexpressing mice show a remarkable airway hypersensitivity to methacholine (41). Thus, PAF is likely to play an important role in the pathophysiology of the bronchopulmonary system. However, the murine airways are somehow unresponsive to the intravenously administered PAF (41, 50, 56), whereas PAF given in the same manner elicited severe hypotension in mice (Fig. 1 e; reference 50). This is in contrast to our present data from immunologically stimulated mice which shows that PAF is deeply involved in both hypotension and bronchoconstriction. The murine airways, therefore, do not appear to respond to the blood-released PAF, which exerts deleterious effects on the cardiovascular system. Only PAF produced in the lung may act on the airways in a paracrine fashion.
Endotoxic Shock.
To assess the importance of PAF and PAFR in the development of endotoxic shock, PAFR-deficient mice were examined for their sensitivity to endotoxin using three kinds of assays. First, pafr +/+ and pafr −/− mice were treated with endotoxin given intravenously at a dose range of 7.5–30 mg/kg. As shown in Table 1, there was no significant difference in lethality in this model between pafr +/+ and pafr −/− mice. At each dose, mice of both genotypes exhibited apparently equivalent symptoms of endotoxic shock: lethargy, piloerection, and diarrhea. We next measured MAP of anesthetized mice in endotoxemia for 100 min as shown in Fig. 2 a. pafr −/− mice showed hypotensive responses to endotoxin that were indistinguishable from those of pafr +/+ mice (Fig. 4). The drop in MAP during endotoxic shock was slow but progressive, in contrast to the rapid drop observed during anaphylactic shock (Fig. 2 a). Finally, the ability of pafr −/− macrophages to respond to endotoxin was evaluated. Thioglycollate-stimulated peritoneal macrophages were exposed to endotoxin to induce expression of TNF-α and IL-6. Levels of these cytokines in the culture medium were measured by ELISA. In pafr +/+ and pafr −/− macrophages, endotoxin induced equivalent levels of TNF-α (10,250 and 10,544 pg/ml, respectively) and IL-6 (4,352 and 4,314 pg/ml, respectively).
Table 1.
Lethal Toxicity of Bacterial Endotoxin
| Dose of endotoxin | Lethality (deaths/total) | |||
|---|---|---|---|---|
| pafr +/+ | pafr −/− | |||
| mg/kg | ||||
| 7.5 | 0/3 | 0/3 | ||
| 15.0 | 3/8 | 3/8 | ||
| 22.5 | 5/8 | 4/8 | ||
| 30.0 | 7/8 | 7/8 | ||
Sex- and age-matched pafr −/− and pafr +/+ mice (unanesthetized, 14–27 wk old) were injected with endotoxin in a volume of 10 ml/kg via tail vein. Survival was monitored for 3 d. There were no deaths after 3 d. No statistical significance was observed at any doses in pafr −/− mice compared with pafr +/+ mice (Fisher's exact test).
Figure 4.
Change in MAP during endotoxemia. pafr +/+ and pafr −/− mice are represented by the open and closed squares, respectively. Data from female mice are shown as in Fig. 2 a. The baselines of pafr +/+ and pafr −/− mice were 101 ± 5 and 107 ± 4 mmHg (mean ± SEM; n = 5 each). No mice died during the recordings.
During endotoxic shock, a strong hypotensive factor, nitric oxide (NO), is synthesized. A PAFR antagonist is reported to inhibit the endotoxin-stimulated NO production in cultured murine macrophages (57), suggesting that endotoxin-induced hypotension is mediated by PAF and NO in this order. However, our results demonstrate that endotoxic shock can be elicited without the action of PAF. Contrary to our expectation based on the previous pharmacologic reports, PAFR-deficient mice failed to acquire resistance to endotoxin. The intact susceptibility to endotoxin is consistent with our present data that macrophages from PAFR-deficient mice produced TNF-α and IL-6, the inflammatory cytokines induced by endotoxin, to the same extent as those from wild-type mice. Since we have previously reported an increased susceptibility to endotoxin in PAFR overexpressing mice (41), it is very intriguing that PAFR-deficient mice show normal sensitivity to endotoxin. It may be that PAF functions in vivo not as an essential, but as an exaggerating factor for endotoxic shock. There are noteworthy pharmacologic reports exhibiting that ID50 values of various PAF antagonists for death due to anaphylaxis are nearly equal to those for PAF-induced death (32), whereas the doses of PAFR antagonists for inhibiting endotoxin-induced death are usually >10 times higher than those for inhibiting PAF-induced death (39). These data may suggest that PAFR antagonists attenuate the symptoms of anaphylaxis and endotoxic shock in different ways. The latter might be PAFR independent.
Concluding Remarks.
Although PAF has pleiotropic biological activities in vitro, the effects of deleting the PAFR gene were restricted to anaphylactic responses, i.e., PAFR-deficient mice were fertile and grew normally and were as sensitive to endotoxin as the wild-type mice. Thus, we conclude that although PAF is a dominant anaphylactic mediator, it may not be required for reproduction, neural development, or endotoxic shock. PAFR antagonists should, therefore, be useful in preventing anaphylactic responses in humans without causing serious mechanism-based side effects. Recent mouse models demonstrated that neither IgE (26) nor its high-affinity receptor, FcεRI (27), is required for active systemic anaphylaxis, further supporting the validity of PAF as a target mediator for preventing and treating the disorder.
Acknowledgments
We thank I. Suganuma, M. Kumai (Japan Tobacco), Y. Hara, Y. Matsumoto, H. Sakamoto, Y. Suzuki, C. Suzuki, and M. Ito (The University of Tokyo) for technical assistance, D. Saffen (The University of Tokyo), H. Bito (Kyoto University), and M. Ohara for critical reading of the manuscript, A. Takaoka (Taisho Pharmaceutical) for instruction in determining the serum Ig levels, and N. Oigawa for secretarial assistance. We are also grateful to T. Izumi (The University of Tokyo) for valuable suggestions and M. Noma, H. Sasai, Y. Nishi, and T. Matsumoto (Japan Tobacco) for their support.
This work was supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture and the Ministry of Health and Welfare of Japan, by grants from Human Science Foundation, Senri Life Science Foundation and the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan, and by Toyota Physical and Chemical Research Institute (to S. Ishii).
Abbreviations used in this paper
- ANOVA
analysis of variance; ES, embryonic stem
- HR
heart rate
- LTB4
leukotriene B4
- MAP
mean arterial pressure
- NO
nitric oxide
- PAF
platelet-activating factor
- RL
total lung resistance
References
- 1.Hanahan DJ. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- 2.Prescott SM, Zimmerman GA, McIntyre TM. Platelet-activating factor. J Biol Chem. 1990;265:17381–17384. [PubMed] [Google Scholar]
- 3.Barnes PJ. Platelet activating factor and asthma. Ann NY Acad Sci. 1991;629:193–204. doi: 10.1111/j.1749-6632.1991.tb37976.x. [DOI] [PubMed] [Google Scholar]
- 4.Imaizumi T-a, Stafforini DM, Yamada Y, McIntyre TM, Prescott SM, Zimmerman GA. Platelet-activating factor: a mediator for clinicians. J Intern Med. 1995;238:5–20. doi: 10.1111/j.1365-2796.1995.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 5.Izumi T, Shimizu T. Platelet-activating factor receptor: gene expression and signal transduction. Biochim Biophys Acta. 1995;1259:317–333. doi: 10.1016/0005-2760(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 6.Prpic, V., R.J. Uhing, J.E. Weiel, L. Jakoi, G. Gawdi, B. Herman, and D.O. Adams. 1988. Biochemical and functional responses stimulated by platelet-activating factor in murine peritoneal macrophages. J. Cell Biol. 107:363–372 (see published erratum 107:1260). [DOI] [PMC free article] [PubMed]
- 7.Stephens L, Jackson T, Hawkins PT. Synthesis of phosphatidylinositol 3,4,5-trisphosphate in permeabilized neutrophils regulated by receptors and G-proteins. J Biol Chem. 1993;268:17162–17172. [PubMed] [Google Scholar]
- 8.Liu B, Nakashima S, Takano T, Shimizu T, Nozawa Y. Implication of protein kinase C alpha in PAF-stimulated phospholipase D activation in Chinese hamster ovary (CHO) cells expressing PAF receptor. Biochem Biophys Res Commun. 1995;214:418–423. doi: 10.1006/bbrc.1995.2303. [DOI] [PubMed] [Google Scholar]
- 9.Honda Z-i, Takano T, Gotoh Y, Nishida E, Ito K, Shimizu T. Transfected platelet-activating factor receptor activates mitogen-activated protein (MAP) kinase and MAP kinase kinase in Chinese hamster ovary cells. J Biol Chem. 1994;269:2307–2315. [PubMed] [Google Scholar]
- 10.Honda Z-i, Nakamura M, Miki I, Minami M, Watanabe T, Seyama Y, Okado H, Toh H, Ito K, Miyamoto T, Shimizu T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991;349:342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Honda Z-i, Izumi T, Sakanaka C, Mutoh H, Minami M, Bito H, Seyama Y, Matsumoto T, Noma M, Shimizu T. Molecular cloning and expression of platelet-activating factor receptor from human leukocytes. J Biol Chem. 1991;266:20400–20405. [PubMed] [Google Scholar]
- 12.Ye RD, Prossnitz ER, Zou AH, Cochrane CG. Characterization of a human cDNA that encodes a functional receptor for platelet activating factor. Biochem Biophys Res Commun. 1991;180:105–111. doi: 10.1016/s0006-291x(05)81261-6. [DOI] [PubMed] [Google Scholar]
- 13.Kunz D, Gerard NP, Gerard C. The human leukocyte platelet-activating factor receptor. cDNA cloning, cell surface expression, and construction of a novel epitope-bearing analog. J Biol Chem. 1992;267:9101–9106. [PubMed] [Google Scholar]
- 14.Sugimoto T, Tsuchimochi H, McGregor CG, Mutoh H, Shimizu T, Kurachi Y. Molecular cloning and characterization of the platelet-activating factor receptor gene expressed in the human heart. Biochem Biophys Res Commun. 1992;189:617–624. doi: 10.1016/0006-291x(92)92245-s. [DOI] [PubMed] [Google Scholar]
- 15.Bito H, Honda Z-i, Nakamura M, Shimizu T. Cloning, expression and tissue distribution of rat platelet-activating-factor–receptor cDNA. Eur J Biochem. 1994;221:211–218. doi: 10.1111/j.1432-1033.1994.tb18731.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishii S, Matsuda Y, Nakamura M, Waga I, Kume K, Izumi T, Shimizu T. A murine platelet-activating factor receptor gene: cloning, chromosomal localization and up-regulation of expression by lipopolysaccharide in peritoneal resident macrophages. Biochem J. 1996;314:671–678. doi: 10.1042/bj3140671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang SB. High affinity receptor binding of platelet-activating factor in rat peritoneal polymorphonuclear leukocytes. Eur J Pharmacol. 1991;196:169–175. doi: 10.1016/0014-2999(91)90424-o. [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Clark GD, Bazan NG, Zorumski CF. Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. Nature. 1994;367:175–179. doi: 10.1038/367175a0. [DOI] [PubMed] [Google Scholar]
- 19.O'Neill C. Embryo-derived platelet activating factor. Reprod Fertil Dev. 1992;4:283–288. doi: 10.1071/rd9920283. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman, D.R., C.T. Truong, and J.M. Johnston. 1986. Metabolism and function of platelet-activating factor in fetal rabbit lung development. Biochim. Biophys. Acta. 879:88–96 (see published erratum 919:311). [DOI] [PubMed]
- 21.Blank ML, Snyder F, Byers LW, Brooks B, Muirhead EE. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979;90:1194–1200. doi: 10.1016/0006-291x(79)91163-x. [DOI] [PubMed] [Google Scholar]
- 22.Kunievsky B, Yavin E. Production and metabolism of platelet-activating factor in the normal and ischemic fetal rat brain. J Neurochem. 1994;63:2144–2151. doi: 10.1046/j.1471-4159.1994.63062144.x. [DOI] [PubMed] [Google Scholar]
- 23.Toyoshima K, Narahara H, Furukawa M, Frenkel RA, Johnston JM. Platelet-activating factor. Role in fetal lung development and relationship to normal and premature labor. Clin Perinatol. 1995;22:263–280. [PubMed] [Google Scholar]
- 24.Masugi F, Ogihara T, Saeki S, Otsuka A, Kumahara Y. Role of acetyl glyceryl ether phosphorylcholine in blood pressure regulation in rats. Hypertension (Dallas) 1985;7:742–746. doi: 10.1161/01.hyp.7.5.742. [DOI] [PubMed] [Google Scholar]
- 25.Hattori M, Adachi H, Tsujimoto M, Arai H, Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase. Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- 26.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 27.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and FcγRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mencia-Huerta J-M, Lewis RA, Razin E, Austen KF. Antigen-initiated release of platelet-activating factor (PAF-acether) from mouse bone marrow–derived mast cells sensitized with monoclonal IgE. J Immunol. 1983;131:2958–2964. [PubMed] [Google Scholar]
- 29.Hanahan DJ, Demopoulos CA, Liehr J, Pinckard RN. Identification of platelet activating factor isolated from rabbit basophils as acetyl glyceryl ether phosphocholine. J Biol Chem. 1980;255:5514–5516. [PubMed] [Google Scholar]
- 30.Nagaoka, J., K. Harada, A. Kimura, S. Kobayashi, M. Murakami, T. Yoshimura, K. Yamada, O. Asano, K. Katayama, and I. Yamatsu. 1991. Inhibitory effects of the novel platelet activating factor receptor antagonist, 1-ethyl-2-[N-(2-methoxy) benzoyl-N-[(2R)-2-methoxy-3-(4-octadecylcarbamoyloxy) piperidinocarbonyloxypropyloxy]carbonyl] aminomethyl-pyridinium chloride, in several experimentally induced shock models. Arzneim.-Forsch./Drug Res. 41:719–724. [PubMed]
- 31.Casals-Stenzel J. Effects of WEB 2086, a novel antagonist of platelet activating factor, in active and passive anaphylaxis. Immunopharmacology. 1987;13:117–124. doi: 10.1016/0162-3109(87)90048-8. [DOI] [PubMed] [Google Scholar]
- 32.Terashita Z, Kawamura M, Takatani M, Tsushima S, Imura Y, Nishikawa K. Beneficial effects of TCV-309, a novel potent and selective platelet activating factor antagonist in endotoxin and anaphylactic shock in rodents. J Pharmacol Exp Ther. 1992;260:748–755. [PubMed] [Google Scholar]
- 33.Morrison DC, Ryan JL. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 34.Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 35.Chang TW, Wu MH, Yang YJ. Blood levels of platelet-activating factor in endotoxin-sensitive and endotoxin-resistant mice during endotoxemia. J Formosan Med Assoc. 1992;91:1133–1137. [PubMed] [Google Scholar]
- 36.Graham RM, Stephens CJ, Silvester W, Leong LL, Sturm MJ, Taylor RR. Plasma degradation of platelet-activating factor in severely ill patients with clinical sepsis. Crit Care Med. 1994;22:204–212. doi: 10.1097/00003246-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Casals-Stenzel J. Protective effect of WEB 2086, a novel antagonist of platelet activating factor, in endotoxin shock. Eur J Pharmacol. 1987;135:117–122. doi: 10.1016/0014-2999(87)90602-9. [DOI] [PubMed] [Google Scholar]
- 38.Guinot P, Braquet P. Effects of the PAF antagonists, ginkgolides (BN-52063, BN-52021), in various clinical indications. J Lipid Mediat Cell Signal. 1994;10:144–146. [Google Scholar]
- 39.Giral M, Balsa D, Ferrando R, Merlos M, Garcia RJ, Forn J. Effects of UR-12633, a new antagonist of platelet-activating factor, in rodent models of endotoxic shock. Br J Pharmacol. 1996;118:1223–1231. doi: 10.1111/j.1476-5381.1996.tb15527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merlos M, Giral M, Balsa D, Ferrando R, Queralt M, Puigdemont A, Garcia RJ, Forn J. Rupatadine, a new potent, orally active dual antagonist of histamine and platelet-activating factor (PAF) J Pharmacol Exp Ther. 1997;280:114–121. [PubMed] [Google Scholar]
- 41.Ishii S, Nagase T, Tashiro F, Ikuta K, Sato S, Waga I, Kume K, Miyazaki J-i, Shimizu T. Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO (Eur Mol Biol Organ) J. 1997;16:133–142. doi: 10.1093/emboj/16.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svetlov SI, Howard KM, Miwa M, Flickinger BD, Olson MS. Interaction of platelet-activating factor with rat hepatocytes: uptake, translocation, metabolism, and effects on PAF-acetylhydrolase secretion and protein tyrosine phosphorylation. Arch Biochem Biophys. 1996;327:113–122. doi: 10.1006/abbi.1996.0099. [DOI] [PubMed] [Google Scholar]
- 43.Adachi T, Aoki J, Manya H, Asou H, Arai H, Inoue K. PAF analogues capable of inhibiting PAF acetylhydrolase activity suppress migration of isolated rat cerebellar granule cells. Neurosci Lett. 1997;235:133–136. doi: 10.1016/s0304-3940(97)00742-8. [DOI] [PubMed] [Google Scholar]
- 44.Carceller E, Merlos M, Giral M, Balsa D, Almansa C, Bartroli J, Garcia RJ, Forn J. [(3-Pyridylalkyl)piperidylidene]benzocycloheptapyridine derivatives as dual antagonists of PAF and histamine. J Med Chem. 1994;37:2697–2703. doi: 10.1021/jm00043a009. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura M, Honda Z-i, Waga I, Matsumoto T, Noma M, Shimizu T. Endotoxin transduces Ca2+signaling via platelet-activating factor receptor. FEBS Lett. 1992;314:125–129. doi: 10.1016/0014-5793(92)80957-i. [DOI] [PubMed] [Google Scholar]
- 46.Nagase T, Ohga E, Sudo E, Katayama H, Uejima Y, Matsuse T, Fukuchi Y. Intercellular adhesion molecule-1 mediates acid aspiration–induced lung injury. Am J Respir Crit Care Med. 1996;154:504–510. doi: 10.1164/ajrccm.154.2.8756829. [DOI] [PubMed] [Google Scholar]
- 47.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, et al. Role of cytosolic phospholipase A2in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 48.Casals-Stenzel J, Muacevic G, Weber K. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharmacol Exp Ther. 1987;241:974–981. [PubMed] [Google Scholar]
- 49.Ferby IM, Waga I, Sakanaka C, Kume K, Shimizu T. Wortmannin inhibits mitogen-activated protein kinase activation induced by platelet-activating factor in guinea pig neutrophils. J Biol Chem. 1994;269:30485–30488. [PubMed] [Google Scholar]
- 50.Chen X-S, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 51.Sapir T, Elbaum M, Reiner O. Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit. EMBO (Eur Mol Biol Organ) J. 1997;16:6977–6984. doi: 10.1093/emboj/16.23.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maki N, Hoffman DR, Johnston JM. Platelet-activating factor acetylhydrolase activity in maternal, fetal, and newborn rabbit plasma during pregnancy and lactation. Proc Natl Acad Sci USA. 1988;85:728–732. doi: 10.1073/pnas.85.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Neill C, Ryan JP, Collier M, Saunders DM, Ammit AJ, Pike IL. Supplementation of in-vitro fertilisation culture medium with platelet activating factor. Lancet. 1989;2:769–772. doi: 10.1016/s0140-6736(89)90831-3. [DOI] [PubMed] [Google Scholar]
- 54.Ryan JP, Spinks NR, O'Neill C, Wales RG. Implantation potential and fetal viability of mouse embryos cultured in media supplemented with platelet-activating factor. J Reprod Fertil. 1990;89:309–315. doi: 10.1530/jrf.0.0890309. [DOI] [PubMed] [Google Scholar]
- 55.Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 56.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 57.Szabo C, Wu CC, Mitchell JA, Gross SS, Thiemermann C, Vane JR. Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ Res. 1993;73:991–999. doi: 10.1161/01.res.73.6.991. [DOI] [PubMed] [Google Scholar]