Abstract
The efficacy of thalidomide (α-phthalimido-glutarimide) therapy in leprosy patients with erythema nodosum leprosum is thought to be due to inhibition of tumor necrosis factor α. In other diseases reported to respond to thalidomide, the mechanism of action of the drug is unclear. We show that thalidomide is a potent costimulator of primary human T cells in vitro, synergizing with stimulation via the T cell receptor complex to increase interleukin 2–mediated T cell proliferation and interferon γ production. The costimulatory effect is greater on the CD8+ than the CD4+ T cell subset. The drug also increases the primary CD8+ cytotoxic T cell response induced by allogeneic dendritic cells in the absence of CD4+ T cells. Therefore, human T cell costimulation can be achieved pharmacologically with thalidomide, and preferentially in the CD8+ T cell subset.
Keywords: thalidomide, costimulation, T cells, CD8+, pharmacologic immune modulation
Thalidomide's immune-modulating effects were discovered serendipitously over 30 years ago. Administration of the drug, when prescribed as a sedative (its original indication) to leprosy patients with erythema nodosum leprosum (ENL),1 was found to heal the inflammatory skin lesions and eliminate the systemic symptoms that characterize that condition (1). We showed subsequently that the efficacy of thalidomide in ENL is associated with a rapid reduction in the circulating level of the proinflammatory cytokine TNF-α (2). In further studies, we demonstrated that thalidomide exerts a specific inhibitory effect on the production of TNF-α by lipopolysaccharide-stimulated monocytes in vitro (3). These results suggested that inhibition of this cytokine is a possible mechanism for the antiinflammatory effects of thalidomide therapy.
Recently, studies in animal models and human diseases have been initiated to explore the potential application of this agent in a range of inflammatory and immunologic disorders. Compelling data have now accumulated confirming the efficacy of thalidomide in ENL (4) and in aphthous ulceration complicating HIV disease (5, 6). Other studies have suggested a potential application in chronic GVHD (7, 8). The observation that thalidomide appears to be effective in these conditions when conventional immunosuppressive therapy (usually with corticosteroids) has failed continues to stimulate interest in identifying the possible mechanism(s) of action of this controversial agent.
There has been much interest in the possibility that thalidomide might not only affect monocyte cytokine production, but also influence the functions of T cells, since the latter have central effector and regulatory roles in immune responses. However, the results of investigations by several different groups have revealed no clear or consistent effect of thalidomide on primary T cell responses. Taken together, these observations have suggested either that any effect on T cell function is indirect, or that such an effect is elusive in conventional T cell assays.
Recent studies have shown that optimal activation of T cells requires the delivery of a second, costimulatory signal in addition to antigenic stimulation via the TCR (9). Physiologically, these costimulatory signals are effected by interactions between molecules on the surface of specialized APCs and their ligands on T cells. Thus, modifying T cell costimulation may have a profound impact on the integrity of the immune response. This is exemplified by the immunosuppressive effect of specifically blocking the interaction of one such ligand pair, B7 (on the APC) and CD28 (on the T cell) (10). Therefore, it is important to consider such accessory pathways when investigating T cell function.
We set out to examine the effects of thalidomide on primary human T cell responses in vitro. Initially, we were interested in studying the effects of thalidomide on TNF-α production by these cells, but found no effect of the drug on this response. Unexpectedly, when purified T cells were stimulated by antibodies to CD3, a component of the TCR complex, thalidomide provided an essential costimulatory signal for T cell proliferation and lymphokine production. The costimulatory effect of thalidomide was more pronounced in the CD8+ subset of T cells. This is the first example of a pharmacologic agent used in the treatment of human disease that acts as a costimulator for T cells. Moreover, thalidomide preferentially stimulates CD8+ T cells.
Materials and Methods
Preparation of Cells
PBMCs and T Cells.
PBMCs were isolated from the blood of healthy volunteers by Ficoll density gradient separation. T lymphocytes were purified by rosetting with neuraminidase-treated sheep erythrocytes and subsequent incubation of erythrocyte-rosetting (ER+) cells on a nylon wool column. Nonadherent cells were eluted from the column and were ≥97% CD3 antigen–positive by flow cytometry (FACStar®; Becton Dickinson, San Jose, CA). The latter population we refer to herein as “bulk” T cells. Primary CD4+ and CD8+ T cells were purified from PBMCs by positive selection with magnetic beads coated with mouse anti–human CD4 and anti-CD8 antibodies, respectively (Dynal A.S., Oslo, Norway), at a bead/cell ratio of 2–3:1. Positively selected cells were then dissociated from the beads by incubation with a second antibody competing for the antigen binding site on the primary anti–human antibody (Detachabead; Dynal A.S.). These cells were ≥98% CD4+CD3+ or CD8+CD3+ by flow cytometry.
APCs.
PBMCs were enriched for APCs in experiments with staphylococcal enterotoxin superantigens or in one-way allogeneic reactions by depletion of ER+ populations. For the latter experiments, these non-erythrocyte-rosetting (ER−) populations were irradiated with 2,000 rads from a cesium source. In experiments with staphylococcal superantigens, ER− populations were rendered costimulator-deficient by 0.05% glutaraldehyde fixation for 30 s, followed by the addition of 0.2 M l-lysine to stop the reaction and four washes in PBS.
These cell cultures were set up in RPMI medium (GIBCO BRL, Gaithersburg, MD) supplemented with 10% heat-inactivated FCS and penicillin/streptomycin.
Dendritic Cells.
Dendritic cells (DCs) were derived from peripheral blood progenitors as described by Bender et al. (11). In brief, ER− cells were incubated for 7 d with recombinant human (r-hu) IL-4 (Genzyme Corp., Cambridge, MA) and r-hu GM-CSF (Immunex, Seattle, WA), each at 1,000 u/ml, followed by a 4-d incubation with macrophage-conditioned medium. At the end of this period, the cells exhibited stellate morphology characteristic of DCs, were CD83+, HLA-DR+, and CD14− by flow cytometry, and were highly efficient inducers of proliferation and cytotoxicity in purified alloreactive T cells.
Target Cells for CTL Assays.
To obtain target cells for the CTL 51Cr-release assays, polystyrene-adherent ER− cells from either the same donor as the DCs (allogeneic targets) or the same donor as the T cells (autologous control targets) were cultured in Teflon tissue culture dishes in RPMI containing 5% human serum at 37°C until required for the CTL assay (up to 1 wk).
Thalidomide
Thalidomide (Celgene Corp., Warren, NJ) was dissolved in DMSO to give a stock solution of 20 mg/ml, which was kept for up to 1 wk. Before stimulation, T cells were preincubated for 1–2 h at 37°C with thalidomide or DMSO (control) at the concentrations indicated in Results. Cell cultures were treated daily with the appropriate dilutions of thalidomide stock or DMSO.
T Cell Stimulation
Purified T cells were stimulated in three experimental systems:
Cross-linking of the TCR by Immobilized Monoclonal Mouse Anti– human CD3.
The anti-CD3 antibody (Orthoclone OKT3; a gift of Dr. Robert Zivin, OrthoBiotech Inc., Raritan, NJ) was diluted in PBS and coated onto flat-bottomed polystyrene tissue culture plates by overnight incubation at 4°C, at concentrations indicated in Results. In some experiments, exogenous r-hu IL-2 (Chiron Corp., Emeryville, CA) was added at a final concentration of 10 U/ml. Thalidomide- or DMSO-treated T cells were added to 96-well plates at 105 cells per well for triplicate proliferation assays, and to 24-well plates at 106 cells per ml for the measurement of cytokine production in culture supernatant fluid. In preliminary experiments, unsorted PBMCs were also stimulated in this system. In cytokine blocking experiments, cultures were treated with polyclonal rabbit anti–human IL-2 or polyclonal sheep anti–human IL-4 antibodies (Endogen, Inc., Cambridge, MA) at final concentrations of 40 and 10 μg/ml, respectively.
Presentation of the Superantigens Staphylococcal Enterotoxin A and B by Costimulator-deficient APCs.
Glutaraldehyde-fixed (GAF) APCs were coincubated with purified CD4+ or CD8+ T cells and both staphylococcal enterotoxin A and B (SEA and SEB; Toxin Technology, Inc., Sarasota, FL,) so that the final concentration of each superantigen was 10 ng/ml and the APC/T cell ratio was 1:1. Cultures were set up in triplicate in 96-well round-bottomed tissue culture plates, with 105 of each cell type per well.
Stimulation of T Cells by Allogeneic DCs.
DC/T cell cocultures were set up in round-bottomed 96-well plates. T cells were purified CD4+, CD8+, or recombined CD4+ and CD8+ (ratio of 2:1), and were treated with thalidomide or DMSO as above. Each well contained 105 T cells in a final volume of 0.2 ml. The ratio of DC to T cells was 1:30.
T Cell Proliferation Assays
T cell proliferative responses were assayed by measuring [3H]thymidine (NEN Research Products, Boston, MA) incorporation during the last 12 h of 120-h cultures. DNA was harvested onto fiber mats with an automatic cell harvester (Skatron Instruments Inc., Sterling, VA), and [3H]thymidine incorporation was measured with a betaplate liquid scintillation counter (model LKB 1205; Wallac, Gaithersburg, MD).
CTL Assays
Standard 6-h 51Cr-release assays were set up as described by Young and Steinman (12). On the day of assay, 5 × 103 51Cr-labeled monocytic target cells were added to the wells in the 96-well plates, and supernatants were collected with a multiwell harvesting frame (Skatron Instruments Inc.) after a 6-h incubation. Released radioactivity was measured with an automated γ counter (Wallac). Identical 96-well plates set up in parallel were pulsed with [3H]thymidine for 12 h to measure lymphocyte proliferation. Assays were set up in triplicate for each experimental condition. Spontaneous release in the absence of effector cells and total release after detergent lysis of targets were also measured. Specific lysis was calculated as (cpmexperimental − cpmspontaneous) ÷ (cpmtotal − cpmspontaneous) × 100%.
Cytokines
Tissue culture supernatants were harvested and frozen immediately at −70°C until assay in duplicate for IL-2, IFN-γ, IL-4, or TNF-α by enzyme-linked immunoassay (Endogen, Inc.).
Donor Blood and Experimental Design
Blood for these experiments was drawn from a total of nine healthy adult donors. Each experiment was repeated at least three times with different donors. The observations reported here were consistently present in experiments from all donors. Findings from individual representative experiments are shown to illustrate each point.
Results
Effect of Thalidomide on T Cell Proliferative Responses after Stimulation by Immobilized Anti-CD3.
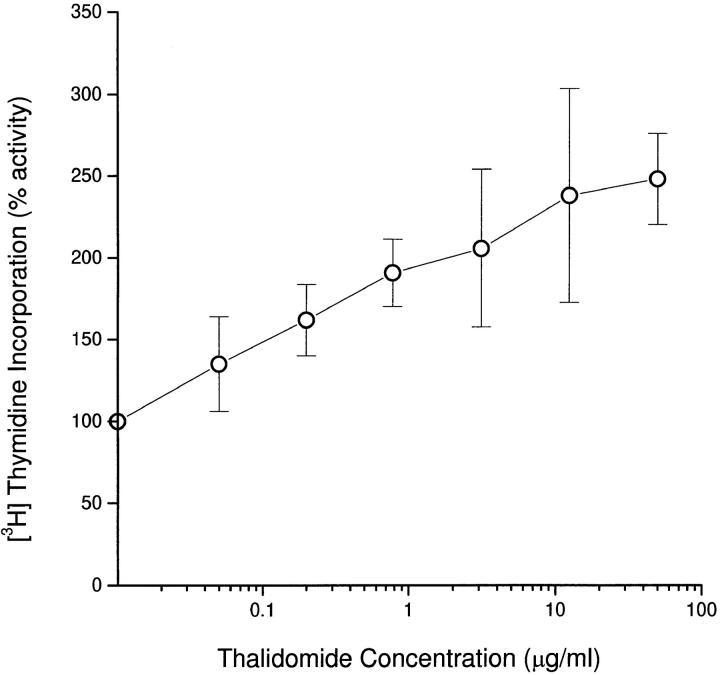
Preliminary experiments carried out on PBMCs obtained from two healthy tuberculin-sensitive donors showed no effect of thalidomide on lymphocyte proliferative responses to the mitogens PHA and PMA, the superantigens SEA and SEB, or purified protein derivative (data not shown). However, when PBMCs from three individual donors were stimulated by cross-linking the TCR with immobilized anti-CD3 mAbs alone, thalidomide induced a concentration-dependent increase in proliferative responses (Fig. 1).
Figure 1.
Effect of thalidomide on mean proliferative responses of PBMCs from three healthy donors stimulated with 10 μg/ml immobilized anti-CD3. PBMCs (105/well) were cultured in triplicate at the indicated concentrations of thalidomide. [3H]Thymidine incorporation was measured for the last 12 h of 120-h cultures. Results are expressed as mean±SD. 100% activity on the y axis represents [3H]thymidine incorporation in the absence of thalidomide (DMSO-treated control).
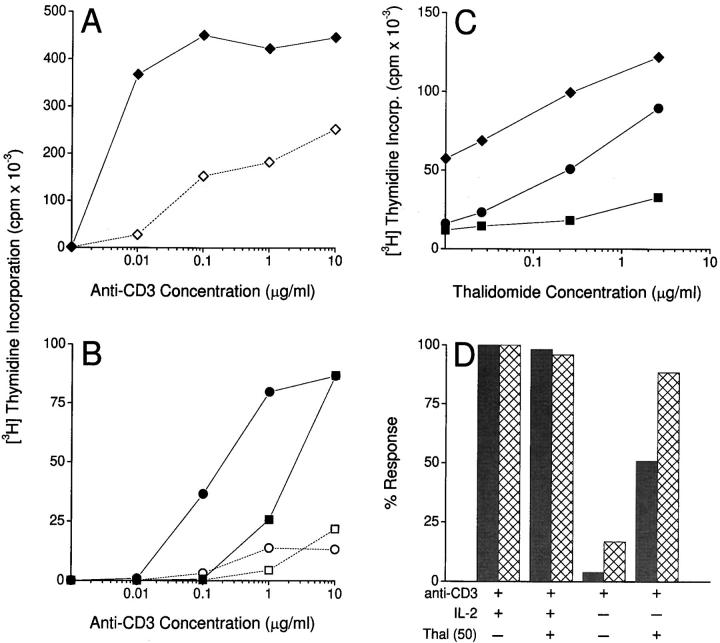
In subsequent studies, the effect of thalidomide on purified peripheral blood T cells (“bulk” T cells) was evaluated. Exposure to a constant concentration of thalidomide (10 μg/ml) caused a marked enhancement of proliferative responses to increasing concentrations of anti-CD3 (Fig. 2 A). At low concentrations of anti-CD3, the T cells failed to respond unless thalidomide was added. This effect was also evident in purified CD4+ and CD8+ T cell subsets (Fig. 2 B). Again, there was no proliferative response to thalidomide in the absence of anti-CD3, indicating that thalidomide was not mitogenic per se, but acted as a costimulator. We observed that significant augmentation of the proliferative response by thalidomide occurred at lower concentrations of anti-CD3 for bulk and CD8+ T cells than for CD4+ T cells (Fig. 2, A and B). Fig. 2 C shows that when the concentration of anti-CD3 was constant, there was a thalidomide concentration–dependent increase in proliferative responses in all three lymphocyte populations. This effect was relatively modest in the CD4+ T cell subset compared with bulk or CD8+ T cells within individual donors, although large thalidomide-induced increases in the CD4+ T cell response were seen in some individuals. To confirm that the differential effect of thalidomide on the two T cell subsets was not due to an incapacity of the CD4+ population to respond fully, experiments were carried out using PMA, a well-described chemical costimulator of T cells. Addition of PMA stimulated CD4+ and CD8+ T cell proliferation similarly, in contrast to thalidomide (data not shown).
Figure 2.
(A) Effect of thalidomide (10 μg/ml) on the proliferative response of purified (>97% CD3+) T cells in the presence of increasing concentrations of immobilized anti-CD3. Filled symbols, Thalidomide-treated cultures. Open symbols, DMSO-treated controls. (B) Effect of thalidomide (10 μg/ml) on the proliferative response of highly purified positively selected CD4+ (squares) and CD8+ (circles) T cell subsets in the presence of increasing concentrations of immobilized anti-CD3. Filled symbols, Thalidomide-treated cells. Open symbols, DMSO-treated controls. (C) Effect of thalidomide on proliferation of purified bulk T cells (diamonds), CD4+ (squares), and CD8+ (circles) subsets in the presence of anti-CD3 at 0.1 μg/ml for bulk and CD8+, 10 μg/ml for CD4+ T cells. Symbols on the y axis represent responses in the absence of thalidomide (DMSO-treated control). T cells (105/well) were cultured in triplicate at the indicated concentrations of anti-CD3. [3H]Thymidine incorporation was measured for the last 12 h of 120-h cultures. (D) Lack of effect of thalidomide (Thal; 50 μg/ml) on CD4+ and CD8+ T cell proliferative responses to immobilized anti-CD3 (10 μg/ml) in the presence of exogenous r-hu IL-2 (10 U/ml). In the absence of exogenous IL-2, the enhancement by thalidomide of proliferation of CD8+ T cells is equivalent to that observed when exogenous IL-2 is present. Data expressed as percent activity of anti-CD3 plus IL-2–induced [3H]thymidine incorporation. T cells (105/well) were cultured in triplicate, and [3H]thymidine incorporation was measured for the last 12 h of 120-h cultures.
In further experiments, when the TCR was cross-linked by anti-CD3 in the presence of exogenous rIL-2 (10 U/ml), no effect of thalidomide on T cell proliferation was apparent (Fig. 2 D). However, when the cytokine was not added, thalidomide substituted almost completely for exogenous IL-2 in stimulating the proliferative responses of CD8+ T cells, while restoring ∼50% of the CD4+ T cell response under the same conditions (Fig. 2 D).
Effect of Thalidomide on T Cell Production of Cytokines.
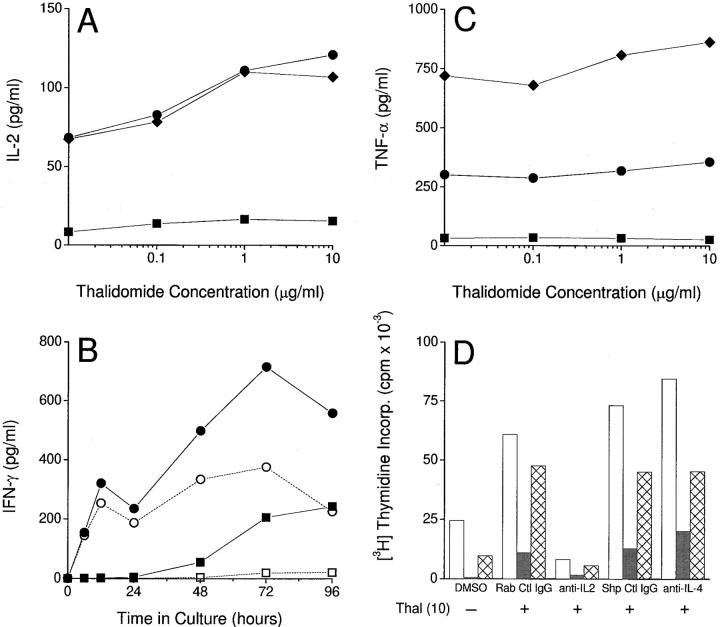
We next examined the effect of thalidomide on cytokine production by the three T cell populations when stimulated by anti-CD3 in the absence of exogenous IL-2. Thalidomide induced a consistent concentration-dependent increase in IL-2 production at 12 h (time of peak production determined in preliminary kinetic experiments), in CD8+ and in bulk T cells (Fig. 3 A). Although a trend towards a thalidomide dose response in IL-2 production by purified CD4+ T cells was apparent, production of this cytokine was modest in comparison to that of bulk and CD8+ populations. Exposure to thalidomide consistently increased IFN-γ production by all three T cell populations. Since there was no clear peak in production of the latter cytokine, the results of a kinetic experiment are shown for CD4+ and CD8+ T cells (Fig. 3 B) using a single concentration of thalidomide (10 μg/ml). Again, the drug induced relatively greater production of IFN-γ by purified CD8+ T cells. However, the thalidomide-induced augmentation of IFN-γ production by CD4+ T cells was marked in comparison to the drug's weak effect on IL-2 production by this subset. Bulk T cells displayed a similar IFN-γ profile to CD8+ T cells (data not shown). All three lymphocyte populations showed a concentration-dependent increase in IFN-γ production in response to thalidomide (data not shown). In contrast, thalidomide did not affect significantly the production of TNF-α by either bulk or CD8+ T cells, and the production of TNF-α by CD4+ cells was minimal in this system (Fig. 3 C). A critical consequence of T cell costimulation is the production of IL-2. Thus, experiments to evaluate the effect of the drug on TNF-α production were also carried out in which exogenous IL-2 was added to eliminate the costimulatory effect of thalidomide. Again, no consistent inhibitory effect of thalidomide on TNF-α production by T cells was found (data not shown). IL-4 was not detected in the supernatants of T cells stimulated with anti-CD3 in the presence or absence of thalidomide (data not shown).
Figure 3.
(A) Effect of thalidomide on IL-2 production by T cells stimulated with immobilized anti-CD3 (12 h). Diamonds, Bulk T cells. Squares, CD4+ T cells. Circles, CD8+ T cells. (B) Effect of thalidomide (10 μg/ml) on the kinetics of IFN-γ production by CD4+ (squares) and CD8+ (circles) T cells stimulated with immobilized anti-CD3. Filled symbols, Thalidomide-treated cells. Open symbols, DMSO-treated controls. (C) Lack of effect of thalidomide on TNF-α production by T cells stimulated with immobilized anti-CD3 (12 h). Symbols as in A. Cytokine concentrations were determined by ELISA of culture supernatants. No IL-4 was detected. (D) Effect of polyclonal rabbit anti– human IL-2 (anti-IL-2; 40 μg/ml) and polyclonal sheep anti–human IL-4 (anti-IL-4; 10 μg/ml) on thalidomide (Thal)-induced proliferation of bulk (white bars), CD4+ (gray bars), and CD8+ (hatched bars) T cells. Results for appropriate control antibodies (Rab Ctl IgG and Shp Ctl IgG) at the same concentrations are also shown. [3H]Thymidine incorporation was measured for the last 12 h of 120-h triplicate cultures.
Taken together, these observations suggested that thalidomide-induced T cell proliferation was dependent on the autocrine production of IL-2 but not IL-4. To confirm these findings, neutralization of IL-2 by a polyclonal antibody was carried out. Anti–IL-2 abrogated the proliferative effect of thalidomide, whereas the addition of anti–IL-4 antibody had no effect. This observation was consistent in the three T cell populations under study (Fig. 3 D), and confirmed that the stimulatory effect of thalidomide was dependent on the induction of endogenous IL-2 production. Together, these experiments suggest that thalidomide is acting as a costimulus to T cells, which have received a primary signal through the cross-linked TCR.
Effect of Thalidomide on Lymphocyte Proliferative Responses to Staphylococcal Superantigens Presented by Autologous GAF APCs.
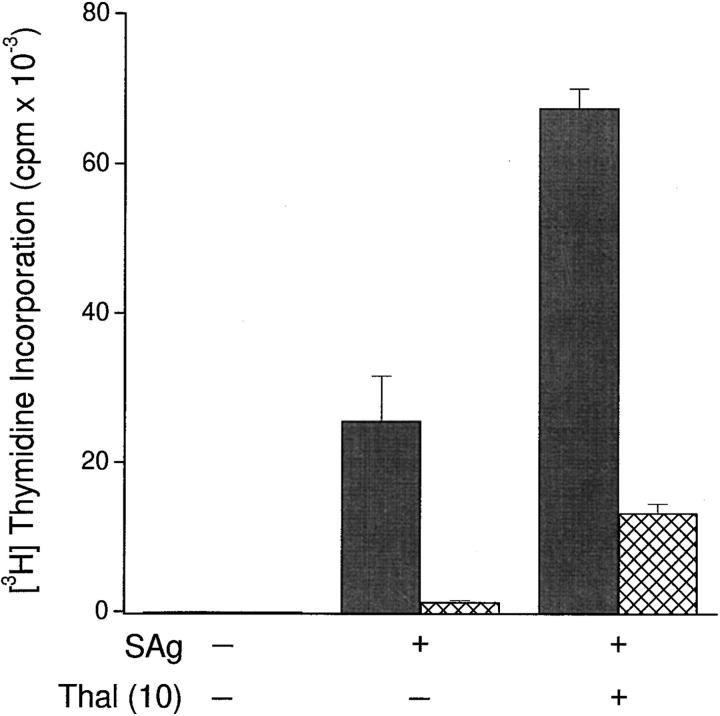
Next, we sought to determine whether thalidomide could costimulate T cell responses after superantigen presentation by APCs. PBMCs enriched for APCs were fixed in 0.05% glutaraldehyde. This chemical modification rendered the APCs deficient in costimulatory function (13, 14) while preserving their capacity to present staphylococcal superantigens to purified autologous CD4+ and CD8+ T cells. When T cells were exposed to thalidomide in this system, there was a marked increase in CD4+ and CD8+ T cell proliferative responses (Fig. 4). The absolute response of CD8+ T cells was modest in comparison with that of CD4+ T cells, since characteristically, CD4+ T cells respond more vigorously to superantigens. However, the fold increase in response to thalidomide relative to cells treated with DMSO was consistently greater in CD8+ than in CD4+ T cells if expressed as a “stimulation ratio” (thalidomide cpm ÷ DMSO cpm). Accordingly, the mean (±SD) stimulation ratio was 7.14 (±3.55) for CD8+ T cells in three experiments versus 3.88 (±1.04) for CD4+ T cells in six experiments.
Figure 4.
Effect of thalidomide (Thal; 50 μg/ml) on CD4+ (gray bars) and CD8+ (hatched bars) T cell proliferative responses to SEA and SEB (combined, SAg: 10 ng/ml each) presented by autologous GAF ER− cells. Cultures were set up in triplicate in 96-well U-bottomed plates, with 105 T cells/well. ER−/T cell ratio was 1:1. [3H]Thymidine incorporation was measured for the last 12 h of 120-h cultures.
Effect of Thalidomide on the Proliferative and Cytotoxic Responses of Purified CD8+ T Cells in a DC/T Cell Primary Allogeneic Reaction.
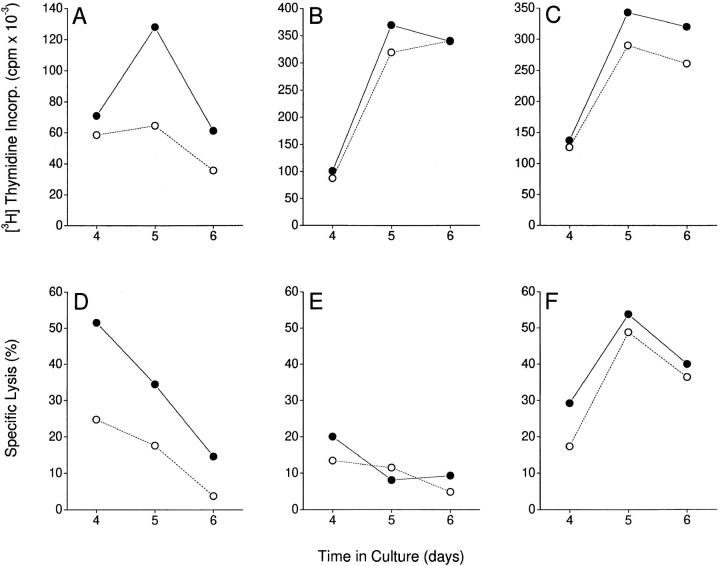
To evaluate the effect of thalidomide on T cells receiving a more physiologic stimulus, we set up cocultures of purified T cells with blood-derived HLA-mismatched DCs and studied primary CTL killing of allogeneic targets. A standard 6-h microassay was used. Monocytes (from the same donor as the DCs) labeled with 51Cr were used as targets. No significant cytotoxic activity was observed before day 4 of DC/T cell coculture. Maximum CTL activity by purified CD8+ T cells was observed on days 4–5 (Fig. 5 D), whereas recombined CD4+ and CD8+ T cells showed peak CTL activity on days 5–6 (Fig. 5 F). Purified CD4+ T cells stimulated with allogeneic DCs were not cytotoxic (Fig. 5 E). Thalidomide increased both proliferative and cytotoxic responses of purified CD8+ T cells in the absence of CD4+ T cells (Fig. 5, A and D). However, when a mixed CD4+/CD8+ T cell population was reconstituted at a physiologic ratio of 2:1, no significant enhancing effect of thalidomide was evident (Fig. 5, C and F). Similarly, thalidomide did not enhance the proliferative response of a purified CD4+ T cell population (Fig. 5 B). Thus, CD8+ T cell functional responses to allogeneic DCs can be further enhanced by thalidomide, but only in the absence of CD4+ T cell “help.”
Figure 5.
Effect of thalidomide on proliferative (top) and cytotoxic (bottom) T cell responses to allogeneic stimulation by blood-derived DCs. DC/T cell cocultures were set up at a ratio of 1: 30 for the durations indicated. Triplicate cultures of 105 purified CD8+ (A and D), CD4+ (B and E), and recombined CD4+/CD8+ (ratio 2:1; C and F) T cells were set up on day 0. For proliferation assays, 1 μCi [3H]thymidine was added for the last 12 h of culture. For cytotoxicity assays, 5 × 103 51Cr-labeled allogeneic monocytes (from the same donor as the DCs) were added to the cultures on the day of assay, and 51Cr release over 6 h was measured. Spontaneous release of 51Cr was <25% of the total. There was no detectable cytotoxicity against autologous (control) targets. Filled symbols, Thalidomide-treated cultures. Open symbols, DMSO-treated controls.
Discussion
Several different immunomodulating and/or antiinflammatory mechanisms of action have been suggested for thalidomide, including the specific inhibition of TNF-α both in vitro and in vivo (2, 3, 15). In addition, in vitro investigations have shown that thalidomide can modify surface adhesion molecules on leucocytes (16), cause a shift from Th1 to Th2 type T cell responses (17), and inhibit production of monocyte IL-12 (18), as well as inhibit angiogenesis (in an animal model of corneal neovascularization [19]). Reports of thalidomide's efficacy in GVHD in animal models (20–22) and in clinical studies (7, 8) suggested that the drug might modify T cell responses. However, several investigations seeking to demonstrate an inhibitory action of thalidomide on T cell responses have resulted in inconsistent findings (23–25).
In this study, we report that thalidomide can consistently boost primary human T cell responses in vitro by acting as a costimulatory molecule. The optimal demonstration of this effect required that the T cells be highly purified, and that a single stimulus be delivered to the T cell via the TCR. Experiments with sorted cells revealed that for every donor, the relative response to thalidomide was more pronounced in the CD8+ than the CD4+ T cell subset, although substantial costimulation of CD4+ T cells was observed occasionally. This differential effect contrasted with the effect of PMA, a reagent used in vitro to costimulate T cells, which exerted a similar effect on both CD4+ and CD8+ T cells. Pharmacokinetic data indicate that the costimulatory effects of thalidomide could be achieved at concentrations as low as the 1–2 μg/ml attained in the plasma of human subjects after thalidomide treatment (26).
In contrast to systems in which we tested for costimulation of T cells stimulated with anti-CD3, no effect of the drug on proliferative responses was seen when unsorted PBMCs were stimulated by recall antigens or mitogens. Therefore, the costimulatory effect of thalidomide is apt to be overlooked in in vitro systems where the dominant response is by CD4+ T cells, and in experiments designed to reveal inhibition rather than stimulation. Interestingly, two recent studies showed a stimulatory effect of thalidomide (27) and thalidomide metabolites (28) on IL-2 production by mitogen-stimulated PBMCs, but found no effect on T cell proliferation.
The differential costimulatory effect of thalidomide on CD8+ T cell proliferation is intriguing. To examine the effect of thalidomide on a well-characterized functional CD8+ response in a more physiologically relevant system, we induced primary cytotoxic T cell responses by stimulation with allogeneic DCs. The latter experiments confirmed the differential effect of thalidomide in stimulating proliferative responses in CD8+ but not CD4+ T cells, and in increasing CD8+ CTL responses. Moreover, the costimulatory effect on CD8+ T cells was not observed in the presence of CD4+ T cells, suggesting that in the presence of physiologic CD4+ T cells, no further help is required, and that the effects of thalidomide on CD8+ T cells are superfluous. Similarly, any costimulatory effect of the drug on CD4+ T cells is inapparent in the presence of a full complement of physiologic costimulatory signals, as delivered by DCs. Thus, the costimulatory effects of thalidomide on CD8+ T cell function may have particular relevance in clinical settings where CD4+ T cell function is defective, such as in HIV disease, or in situations where there is a defect in T cell costimulation.
These in vitro observations of differential costimulation of T cells by thalidomide may have an in vivo counterpart. We reported recently a differential increase in circulating CD8+ over CD4+ T cell numbers in 13 patients with HIV infection treated with thalidomide, resulting in a drop in the CD4+/CD8+ ratio (29). A consistent drug-induced increase in plasma levels of soluble IL-2 receptor, a marker of T cell activation, was also seen in this cohort. Furthermore, we have observed the restoration of delayed-type cutaneous hypersensitivity to purified protein derivative of tuberculin during thalidomide treatment in five previously anergic patients with HIV infection (reference 15, and our unpublished findings), which was lost after stopping the drug. These findings are consistent with T cell stimulation by thalidomide in patients in vivo, and raise the interesting possibility that this agent may have a novel application as an immunologic adjuvant in HIV disease.
Although thalidomide is the drug of choice in ENL (4), corticosteroids are often used in the management of this condition when thalidomide is unavailable. A recent controlled trial has established the efficacy of thalidomide in the treatment of refractory HIV-associated oral aphthous ulceration (5). This agent has also been used in the management of chronic GVHD when alternatives, notably corticosteroids, have failed. The ability of thalidomide to act in lieu of such immunosuppressive agents has contributed to a perception that thalidomide is also an immunosuppressive drug (17, 23). However, on a clinical level, the classical infectious complications of standard immunosuppressive therapies (glucocorticoids and cyclosporin A) have never been reported with thalidomide treatment, arguing powerfully against indiscriminate suppression of immunity by this agent. Together with these observations, our data suggest that immune modulation by thalidomide may occur through the inhibition of TNF-α production and/or the promotion of T cell responses, without inhibiting normal immunity.
We suggest that the differential CD8+ T cell stimulating effects of thalidomide may contribute to the clinical immune-modulating effects of the drug, including its antiinflammatory properties. Indeed, it has been demonstrated that CD8+ T cells can regulate immune responses by a variety of mechanisms (30–32). Inflammatory bowel disease represents one clinical setting where there is evidence that the immunopathology may be due in part to a failure of active immune regulation by mucosal CD8+ T lymphocytes (33, 34). Therefore, it is interesting in the context of the findings presented here to note anecdotal reports of the efficacy of thalidomide in treating refractory inflammatory bowel disease (35, 36). However, inhibition of TNF-α by thalidomide may also play an important role in this setting (37). These considerations suggest that inflammatory bowel disease might be an appropriate clinical scenario in which to explore the clinical correlates of our in vitro findings.
In conclusion, we have shown a novel and unique property of thalidomide in differentially costimulating CD8+ T cells. Further clinical and laboratory-based studies are necessary to test the hypothesis that the costimulatory or adjuvant effects of thalidomide treatment on T cell responses contribute to its efficacy in a wide range of clinical disorders.
Acknowledgments
The authors wish to thank Judy Adams for help in preparing the figures, Marguerite Nulty for help with typing, and Drs. Ralph Steinman, Victoria H. Freedman, and Chau-ching Liu for critical discussions during the preparation of the manuscript.
This work was supported by Public Health Service grants A1-22616 and A1-33124. We also acknowledge support from Celgene Corp. (Warren, NJ) and the “Direct Effect” HIV research support organization.
Abbreviations used in this paper
- DC
dendritic cell
- ENL
erythema nodosum leprosum
- ER
erythrocyte-rosetting
- GAF
glutaraldehyde-fixed
- r-hu
recombinant human
- SEA and SEB
staphylococcal enterotoxin A and B
References
- 1.Sheskin J. Thalidomide in the treatment of lepra reactions. Clin Pharmacol Ther. 1965;6:303–306. doi: 10.1002/cpt196563303. [DOI] [PubMed] [Google Scholar]
- 2.Sampaio EP, Kaplan G, Miranda A, Nery JA, Miguel CP, Viana SM, Sarno EN. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J Infect Dis. 1993;168:408–414. doi: 10.1093/infdis/168.2.408. [DOI] [PubMed] [Google Scholar]
- 3.Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor α production by stimulated human monocytes. J Exp Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters MFR. An internally controlled double-blind trial of thalidomide in severe erythema nodosum leprosum. Lepr Rev. 1971;42:26–42. doi: 10.5935/0305-7518.19710004. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson JM, Greenspan JS, Spritzler J, Ketter N, Fahey JL, Jackson JB, Fox L, Chernoff M, Wu AW, MacPhail LA, et al. Thalidomide for the treatment of oral aphthous ulcers in patients with human immunodeficiency virus infection. N Engl J Med. 1991;336:1487–1493. doi: 10.1056/NEJM199705223362103. [DOI] [PubMed] [Google Scholar]
- 6.Alexander LN, Wilcox CM. A prospective trial of thalidomide for the treatment of HIV-associated idiopathic esophageal ulcers. AIDS Res Hum Retroviruses. 1997;13:301–304. doi: 10.1089/aid.1997.13.301. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy DM, Kanfer EJ, Barrett AJ. Thalidomide for the therapy of chronic graft-versus-host disease following allogeneic bone marrow transplantation. Biomed Pharmacother. 1989;43:693–697. doi: 10.1016/0753-3322(89)90089-9. [DOI] [PubMed] [Google Scholar]
- 8.Vogelsang GB, Farmer ER, Hess AD, Altamonte V, Beschorner WE, Jabs DA, Corio RL, Levin LS, Colvin OM, Wingard JR, et al. Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med. 1992;326:1055–1058. doi: 10.1056/NEJM199204163261604. [DOI] [PubMed] [Google Scholar]
- 9.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 10.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 11.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 12.Young JW, Steinman RM. Dendritic cells stimulate primary human cytolytic lymphocyte responses in the absence of CD4+ helper T cells. J Exp Med. 1990;171:1315–1332. doi: 10.1084/jem.171.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins MK, Ashwell JD, Schwartz R. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988;140:3324–3330. [PubMed] [Google Scholar]
- 14.Roska AK, Lipsky PE. Dissection of the functions of antigen-presenting cells in the induction of T cell activation. J Immunol. 1985;135:2953–2961. [PubMed] [Google Scholar]
- 15.Tramontana JM, Utaipat U, Molloy A, Akarasewi P, Burroughs M, Makonkawkeyoon S, Johnson B, Klausner JD, Rom W, Kaplan G. Thalidomide treatment reduces tumor necrosis factor α production and enhances weight gain in patients with pulmonary tuberculosis. Mol Med. 1995;1:384–397. [PMC free article] [PubMed] [Google Scholar]
- 16.Nogueira AC, Neubert R, Helge H, Neubert D. Thalidomide and the immune response. 3. Simultaneous up- and down-regulation of different integrin receptors on human white blood cells. Life Sci. 1994;55:77–92. doi: 10.1016/0024-3205(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 17.McHugh SM, Rifkin IR, Deighton J, Wilson AB, Lachmann PJ, Lockwood CM, Ewan PW. The immunosuppressive drug thalidomide induces T helper cell type 2 (Th2) and concomitantly inhibits Th1 cytokine production in mitogen- and antigen-stimulated human peripheral blood mononuclear cell cultures. Exp Immunol. 1995;99:160–167. doi: 10.1111/j.1365-2249.1995.tb05527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moller DR, Wysocka M, Greenlee BM, Ma X, Wahl L, Flockhart DA, Trinchieri G, Karp CL. Inhibition of IL-12 production by thalidomide. J Immunol. 1997;159:5157–5161. [PubMed] [Google Scholar]
- 19.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field EO, Gibbs JE, Tucker DF, Hellman K. Effect of thalidomide on the graft versus host reaction. Nature. 1966;211:1308–1310. doi: 10.1038/2111308a0. [DOI] [PubMed] [Google Scholar]
- 21.Vogelsang GB, Hess A, Gordon G, Santos GW. Treatment and prevention of acute graft-versus-host disease with thalidomide in a rat model. Transplantation (Baltimore) 1986;41:644–647. doi: 10.1097/00007890-198605000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Vogelsang GB, Hess AD, Gordon G, Brundrette R, Santos GW. Thalidomide induction of bone marrow transplantation tolerance. Transplant Proc. 1987;19:2658–2661. [PubMed] [Google Scholar]
- 23.Keenan RJ, Eiras G, Burckart GJ, Stuart RS, Hardesty RL, Vogelsang G, Griffith BP, Zeevi A. Immunosuppressive properties of thalidomide. Transplantation (Baltimore) 1991;52:908–910. [PubMed] [Google Scholar]
- 24.Coulson AS, Summers LJ, Lindahl-Kiessling K, Tucker D, Hellman K. The effects of two soluble thalidomide derivatives on lymphocyte stimulation. Clin Exp Immunol. 1970;7:241–247. [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez LP, Schlegel PG, Baker J, Chen Y, Chao NJ. Does thalidomide affect IL-2 response and production? . Exp Hematol. 1995;23:978–985. [PubMed] [Google Scholar]
- 26.Chen T-L, Vogelsang GB, Petty BG, Brundrett RB, Noe DA, Santos GW, Colvin OM. Plasma pharmacokinetics and urinary excretion of thalidomide after oral dosing in healthy male volunteers. Drug Metab Dispos. 1989;17:402–405. [PubMed] [Google Scholar]
- 27.Shannon EJ, Sandoval F. Thalidomide increases the synthesis of IL-2 in cultures of human mononuclear cells stimulated with Concanavalin-A, Staphylococcal enterotoxin A, and purified protein derivative. Immunopharmacology. 1995;31:109–116. doi: 10.1016/0162-3109(95)00039-7. [DOI] [PubMed] [Google Scholar]
- 28.Östraat O, Riesbeck K, Qi Z, Eriksson T, Schatz H, Ekberg H. Thalidomide prolonged graft survival in a rat cardiac transplant model but had no inhibitory effect on lymphocyte function in vitro. Transplant Immunol. 1996;4:117–125. doi: 10.1016/s0966-3274(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 29.Haslett P, Hempstead M, Seidman C, Diakun J, Vasquez D, Freedman VH, Kaplan G. The metabolic and immunologic effects of short-term thalidomide treatment of patients infected with the human immunodeficiency virus. AIDS Res Hum Retroviruses. 1997;13:1047–1053. doi: 10.1089/aid.1997.13.1047. [DOI] [PubMed] [Google Scholar]
- 30.Bloom BR, Modlin RL, Salgame P. Stigma variations: observations on suppressor T cells and leprosy. Annu Rev Immunol. 1992;10:453–488. doi: 10.1146/annurev.iy.10.040192.002321. [DOI] [PubMed] [Google Scholar]
- 31.Koh DR, Fung-Leung WP, Ho A, Gray D, Acha-Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 32.Ware R, Jiang H, Braunstein N, Kent J, Wiener E, Pernis B, Chess L. Human CD8+ T lymphocyte clones specific for T cell receptor Vβ families expressed on autologous CD4+ T cells. Immunity. 1995;2:177–184. doi: 10.1016/s1074-7613(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 33.Hoang P, Dalton HR, Jewell DP. Human colonic intra-epithelial cells are suppressor cells. Clin Exp Immunol. 1991;85:498–503. doi: 10.1111/j.1365-2249.1991.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer L, Eisenhardt D. Lack of induction of suppressor T cells by intestinal epithelial cells from patients with inflammatory bowel disease. J Clin Invest. 1990;86:1255–1260. doi: 10.1172/JCI114832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waters MFR, Laing ABC, Ambikapathy A, Lennard-Jones JE. Treatment of ulcerative colitis with thalidomide. Brit Med J. 1979;1:792. doi: 10.1136/bmj.1.6166.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wettstein AR, Meagher AP. Thalidomide in Crohn's disease. Lancet. 1997;350:1445–1446. doi: 10.1016/s0140-6736(05)64206-7. [DOI] [PubMed] [Google Scholar]
- 37.Stack WA, Mann SD, Roy AJ, Heath P, Sopwith M, Freeman J, Holmes G, Long R, Forbes A, Kamm MA. Randomised controlled trial of CDP571 antibody to tumour necrosis factor-α in Crohn's disease. Lancet. 1997;349:521–524. doi: 10.1016/s0140-6736(97)80083-9. [DOI] [PubMed] [Google Scholar]