Abstract
In the principal pathway of α/β T cell maturation, T cell precursors from the bone marrow migrate to the thymus and proceed through several well-characterized developmental stages into mature CD4+ and CD8+ T cells. This study demonstrates an alternative pathway in which the bone marrow microenvironment also supports the differentiation of T cell precursors into CD4+ and CD8+ T cells. The marrow pathway recapitulates developmental stages of thymic maturation including a CD4+CD8+ intermediary cell and positive and negative selection, and is strongly inhibited by the presence of mature T cells. The contribution of the marrow pathway in vivo requires further study in mice with normal and deficient thymic or immune function.
Keywords: extrathymic, bone marrow, T cell, development, thymus
Since the landmark discoveries that lymphocytes initiate cellular immune responses and that the thymus is the principal source of these immune cells, the maturation of lymphocytes in the thymus has been studied extensively, and the principal intermediary stages of development have been identified (1, 2). α/β T cell precursors emigrate from the bone marrow to the thymus and the earliest precursors in the thymus do not express the CD4, CD8, or CD3 surface receptors, and have not rearranged the TCR-α or -β chain genes (3–5). Shortly after the latter genes are rearranged during thymic maturation, the predominant intermediary CD4+CD8+ (double positive) T cells develop and give rise to CD4+ and CD8+ (single positive) α/β T cells via processes of positive and negative selection (6–9). The latter cells emigrate to the periphery and, after stimulation by antigen, generate effector cells of cell-mediated immunity or helper cells that regulate humoral immunity (10, 11). The predominance of the thymus in T cell maturation is thought to be related to its unique stromal microenvironment, which has been shown to support differentiation in vitro in thymic organ cultures or in heterogeneous thymic stromal cell cultures (12–14).
Extrathymic maturation of T cells also occurs, and contributes to the pool of T cells in the lymph nodes, spleen, marrow, liver, and intestines of athymic nude mice and thymectomized lethally irradiated mice injected with a source of hematopoietic progenitor cells (15–19). The expression of the genes encoding the recombinase enzymes recombination-activating gene (RAG)-1 and RAG-2, as well as the presence of circular DNA products of TCR-α chain gene rearrangements, indicate that T cell development occurs in several extrathymic sites, including the bone marrow, adult and fetal liver, and yolk sac (20, 21).
The nature of the extrathymic microenvironments that support T cell maturation and the intermediate stages of maturation in these sites are presently unknown. Previous studies have shown that T cell–depleted cultures of single cell suspensions of bone marrow from euthymic and athymic mice and from thymectomized, lethally irradiated mice reconstituted with stem cells are capable of generating T cells in vitro (19, 22–24). In this study, we show that these bone marrow cultures can give rise to α/β T cells with the mature CD4+CD8− and CD4−CD8+ surface phenotype via a CD4+CD8+ intermediary stage that recapitulates the principal features of thymic maturation, including negative selection of autoreactive cells. Although the marrow microenvironment is able to support the maturation of T cells, the presence of mature T cells strongly inhibits the process, and the marrow must be stringently depleted of the latter cells in order to observe the process. These results indicate that T cell production might be under complex homeostatic controls that regulate the differentiation of T cell precursors in the marrow and thymic pathways.
Materials and Methods
Animals.
C57BL/6 (Ly5.1,Thy1.2), congenic C57BL/6 Ly5.2 (Ly5.2,Thy1.1), C57BL/Ka, and BALB/c male and female mice, 6–8 wk of age, were obtained from a colony maintained in the Department of Comparative Medicine, Stanford University (Stanford, CA). SWR/J mice were purchased from The Jackson Laboratories (Bar Harbor, ME).
Antibodies.
The following monoclonal antibodies used in immunofluorescent staining were purified and conjugated to FITC as previously described (14): KT31.1 (anti-CD3); 6B2 (anti-B220, CD45R); GK1.5 (anti-CD4); 53-6.7 (anti-CD8α); Ter119 (anti-erythrocyte lineage); 136TC (anti-NK1.1); MR10-2 (anti-TCRVβ9); A20.1.7 (anti-Ly5.2, CD45.1); and M1/69 (anti–heat-stable antigen [HSA], CD24).1 Some antibodies were conjugated to PE: GK1.5, 53-6.7 (anti-CD8α); M1/70 (anti-Mac1, CD11b); and 6B2. Others were conjugated to allophycocyanin: IM781 (anti-CD44); 53-7.3 (anti-CD5); 2B8 (anti-cKit, CD117); H57-597 (anti–TCR-α/β); and 6B2; or were conjugated to biotin: KT31.1; MEL-14 (anti–L-selectin, CD62L); AL1-4A2 (anti-Ly5.1, CD45.2); E13-161 (anti-Sca1); 19XE5 (anti-Thy1.1, CD90.1); and 6B2.
Monoclonal FITC-conjugated antibodies H57-597, KJ23 (anti-TCRVβ17), 7D4 (anti-CD25), H1.2F3 (anti-CD69), MR11-1 (anti-TCRVβ12), AB20.6 (anti-TCRVβ2), KT4 (anti-TCRVβ4), MR9-4 (anti-TCRVβ5.1/5.2), RR3-15 (anti-TCRVβ11), RR4-7 (anti-TCRVβ6), TR310 (anti-TCRVβ7), and MR5-2 (anti-TCRVβ8.1/8.2) were obtained from PharMingen (San Diego, CA). Antibodies conjugated to biotin (H57-597, M1/69, KJ25 [anti-TCRVβ3], 2.4G2 [anti-CD16/32], RM2-5 [anti-CD2], 53-5.8 [anti-CD8β]), or to PE (RB6-8C3 [anti-GR1] and PK136 [anti-NK1.1]) were also obtained from PharMingen. The following additional PE-conjugated antibodies were acquired from Caltag (South San Francisco, CA): YTS191.1 (anti-CD4); CT-CD8a (anti-CD8α); and 5a-8 (anti-Thy1.2, CD90.2). The isotype control hamster IgG-FITC and rat IgG2b-PE were also purchased from Caltag. Biotinylated antibodies were counterstained with streptavidin reagents conjugated to either allophycocyanin, Texas red, or PE, all purchased from Caltag. 30H12 (anti-Thy1.2) conjugated to FITC was purchased from Becton Dickinson (Mountain View, CA).
Cell Preparation and Immunofluorescent Staining.
Single cell suspensions of spleen and bone marrow cells were obtained as previously described (22). Fresh single cell suspensions were incubated with various combinations of fluorochrome- or biotin-conjugated antibodies at saturation for 30 min on ice. In the case of biotin-conjugated reagents, counterstaining for 15 min with fluorochrome-conjugated streptavidin was performed. After staining, cells were washed and resuspended in fresh medium containing PBS without calcium or magnesium (Biowhittaker, Walkersville, MA) with 1% fetal bovine serum (FBS; Hyclone, Logan, UT) before sorting or analysis.
Cultured bone marrow samples were stained as described above, except that cells were pretreated with unconjugated anti-CD16/32 mAb at saturation to block FcγRIIIA/B receptors. Stained samples were resuspended in staining media (calcium- and magnesium-free PBS containing 1% FBS, 0.05% NaN3 [Fisher Scientific Co., Fair Lawn, NJ]) with propidium iodide at 0.5 μg/ml (Sigma Chemical Co., St. Louis, MO). In all instances, cells were gated to exclude those stained with propidium iodide (dead cells).
Cell Sorting and FACS® Analysis.
All fresh and cultured marrow samples were gated by light scatter and propidium iodide uptake to exclude dead cells and erythrocytes from the analysis of fluorochrome staining or from cell sorting. Sorting of stained bone marrow cells to stringently deplete T cells was performed using a FACStar® (Becton Dickinson, Mountain View, CA). Thresholds for TCR-α/β−, CD4−, and CD8− populations were set 10 channels below the background staining channels obtained with isotype control antibodies. To enhance purity of TCR-α/ β+ and TCR-α/β− populations, a 20-channel gap was placed between the sorted cells. Cells falling in the gap were discarded. Reanalysis was performed on all sorted samples to ensure their purity.
Four-color FACS® analysis was done using a highly modified dual laser (488-nm argon and 599-nm dye lasers), FACS® III (Becton Dickinson) with four-decade logarithmic amplifiers (25). One- to three-color sorting was done in the FACStar® (Becton Dickinson). Data was analyzed using FACS®/DESK software as either histograms or two-parameter 5% probability plots.
Bone Marrow Cell Cultures.
T cell–depleted bone marrow cells were cultured in RPMI-1640 (Biowhittaker) supplemented with 10% FBS (Hyclone), penicillin/streptomycin (Biowhittaker), glutamine (Biowhittaker), and 2-mercaptoethanol (GIBCO BRL, Bethesda, MD). 4–8 × 106 sorted cells were cultured at 37°C, for up to 48 h in a humidified incubator with 5% CO2 in a vented, upright T12.5 flask (Falcon, Franklin Lakes, NJ) at 2 × 106 cells/ml. For time course analysis, the culture was stopped at the indicated time. At the end of the culture period, cells were harvested and washed twice in staining medium before immunofluorescent staining. The nucleated cell yields of all cultures included in the Tables and Figures were at least 80%, but did not exceed the input cell number.
Results
Outgrowth of CD4+ and CD8+ α/β T Cells from Bone Marrow Cultures.
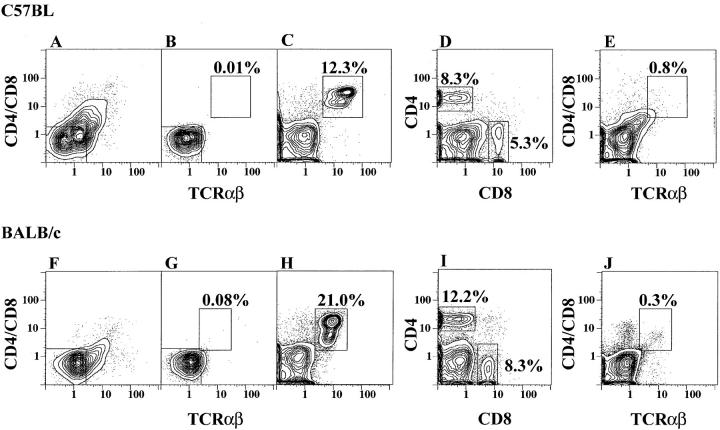
Previous studies showed that T cell–depleted cultures of bone marrow cells from a variety of euthymic and athymic mice generated CD4−CD8− α/β T cells after a 48-h incubation in tissue culture medium, supplemented with FBS but without growth factors (19, 23). In this study, use of the same culture system with an appropriate source of FBS generated CD4+ and CD8+ α/β T cells without CD4−CD8− α/β T cells as shown in Fig. 1. Fresh C57BL/Ka marrow cells were stringently depleted of cells expressing CD4, CD8, and TCR-α/β markers by immunofluorescent staining and flow cytometry. Fig. 1 A shows the staining pattern of the marrow cells using PE-conjugated anti-CD4 and anti-CD8 monoclonal antibodies, and FITC-conjugated anti–TCR-α/β antibodies. The box shows the gating thresholds used for cell sorting, and reanalysis of the sorted cells is shown in B. T cell–depleted (sorted) cells were cultured for 48 h and stained again for CD4 and CD8 versus TCR-α/β surface markers or for CD4 versus CD8 markers. C shows that a population of bright CD4+ or CD8+ TCR-α/β+ T cells, accounting for 12.3% of cells (enclosed in box), appeared in the cultures after 48 h. Less than 0.01% of cells were enclosed within the same box in B. Analysis of CD4 versus CD8 receptors in the same cultured cells as in C showed that almost all α/β T cells were either CD4+ (8.3%, enclosed in left box) or CD8+ (5.3%; Fig. 1 D). Less than 1% of cultured cells in C were TCR-α/β+ and CD4− and CD8−. In control experiments, bone marrow cells were stained as in A, and cultured for 48 h without depletion of T cells by flow cytometry. The latter cells were restained after culture for CD4 and CD8 versus TCR-α/β receptors, and the staining pattern is shown in E. Fewer than 1% of bright CD4+ or CD8+ α/β T cells were generated in these cultures.
Figure 1.
CD4, CD8, and TCR-α/β profiles of bone marrow cells before and after culture. Freshly isolated bone marrow cells from C57BL/Ka (A) or BALB/c (F) mice were depleted of cells expressing CD4, CD8, and TCR-α/β markers using the gating boxes in the lower left and reanalyzed in B and G. The depleted marrow cells were cultured for 48 h and the bright TCR-α/β+ cells that developed are enclosed in the boxes in C and H. The depleted cultured cells were analyzed for CD4 versus CD8 markers in D and I. Analyses of non–T cell–depleted marrow cells after culture are shown in E and J.
In similar cultures, marrow cells from BALB/c mice were stained for CD4, CD8, and TCR-α/β markers, and T cells were depleted by flow cytometry. Fig. 1 F shows the staining pattern of the unfractionated BALB/c marrow cells, and G shows the reanalysis of the T cell–depleted cells after cell sorting. Culture of the sorted cells for 48 h generated a population of bright CD4+ or CD8+ α/β+ T cells (21.0%) enclosed in the box in Fig. 1 H. Analysis of CD4 and CD8 receptors of the cultured cells showed that 12.2% were CD4+ and 8.3% were CD8+ (Fig. 1 I). Staining of the control culture of unsorted marrow cells is shown in J. Three different batches of fetal bovine serum from the same commercial source were tested and all supported the outgrowth of CD4+ and CD8+ T cells. However, the vigor of outgrowth varied from ∼5–30% of nucleated cells harvested.
T Cell Maturation Stages and Kinetics.
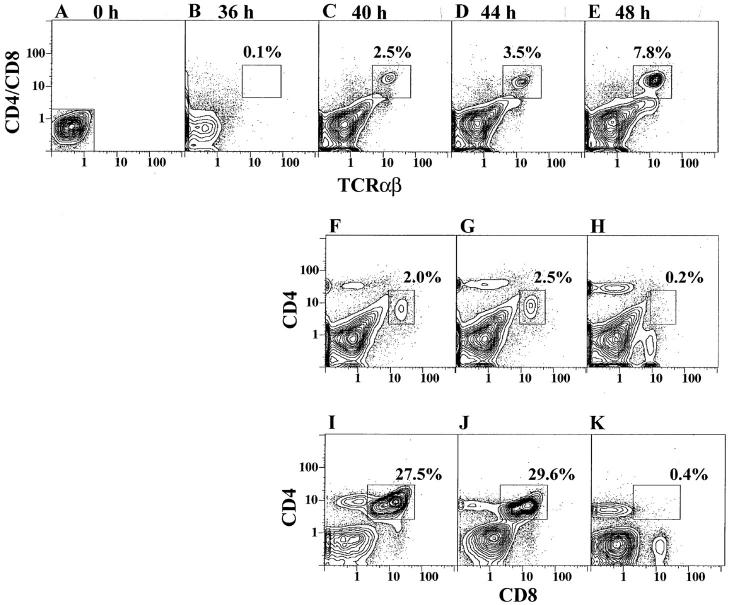
To determine the kinetics of T cell outgrowth from marrow cultures, sorted CD4−CD8− α/β− marrow cells from C57BL/Ka mice were cultured for 36, 40, 44, and 48 h, and cells were harvested and restained for CD4, CD8, and TCR-α/β markers at each time point as shown in Fig. 2. A and B show that little or no bright CD4+ and/or CD8+ α/β+ T cells developed after 36 h of culture, and 0.1% of the cultured cells were enclosed in the box in B. Between 40 and 48 h, the percentage of CD4+ and/or CD8+ α/β+ T cells increased rapidly, and the percentages of cells enclosed in the boxes in Fig. 2, C, D, and E were 2.5, 3.5, and 7.8%, respectively. Analysis of CD4 versus CD8 markers at the later time points showed that at 40 and 44 h (F and G), the majority of T cells were CD4+CD8+ (enclosed in boxes). However, at 48 h the latter cells accounted for only 0.2% of cultured cells (panel H). CD4+ T cells were observed also at 40 and 44 h, but a discrete population of CD8+ cells was not observed until 48 h (panel H). Fig. 2, I, J, and K show CD4 versus CD8 receptor expression at 40, 44, and 48 h in a repeat experiment with a more robust outgrowth of CD4+ CD8+ T cells. The transient appearance of CD4+CD8+ T cells was observed again at 40 and 44 h (27.5 and 29.6% of cells, respectively), and the marked loss of these cells at 48 h (0.4%) was associated with the appearance of a discrete population of CD8+ T cells. At 40 h (Fig. 2 I), a discrete population of CD4+CD8med cells was observed, and at 48 h the intensity of CD8 expression on CD4+ cells was reduced (Fig. 2 K) as reported in heterogeneous thymic stromal cell cultures (14). The rapid development of CD4+CD8+ T cells, which are the immediate precursors of CD4+ and CD8+ T cells, has been described also in the thymic stromal cell cultures, but the double positive T cells persisted as the single positive T cells were generated.
Figure 2.
Kinetics of development of CD4+CD8+, CD4+, and CD8+ T cells during marrow cultures. C57BL/Ka marrow cells depleted of α/β T cells (A) were cultured, and the CD4/CD8 versus TCR-α/β profiles are shown at 36 (B), 40 (C), 44 (D), and 48 (E) h. Boxes enclose the newly generated T cells. Analyses of CD4 versus CD8 markers are shown in the same cultures for 40 (F), 44 (G), and 48 (H) h, and boxes enclose CD4+CD8+ T cells. A repeat experiment is shown at 40 (I), 44 (J), and 48 (K) h.
Positive and Negative Selection of Bone Marrow–derived T Cells.
During thymic maturation, CD4+ and CD8+ T cells expressing Vβ receptors, which are reactive to endogenous superantigens, undergo clonal deletion via a process of negative selection (9, 26). The pattern of deletion of Vβ receptors has been characterized for many mouse strains and is dependent on their expression of MHC gene haplotypes (9, 27, 28). Comparison of the C57BL (H-2b) and BALB/c (H-2d) strains have shown that the Vβ4, Vβ6, Vβ8.1/8.2, and Vβ9 receptors are not deleted in both strains, and the Vβ3, Vβ5.1, Vβ11, and Vβ12 receptors are deleted in the BALB/c but not C57BL strain (29). Vβ2 receptors are deleted in some strains of BALB/c mice, but not others (30). To determine whether the CD4+ and CD8+ T cells generated in marrow cultures undergo negative selection, the percentage of T cells expressing nondeleted and deleted Vβ receptors was compared in both strains after harvesting cells at 48 h (see Fig. 1). In each case, the percentage of T cells expressing each Vβ receptor was measured by two-color analysis after staining for TCR-α/β or CD3 versus Vβ receptors with the appropriate fluorochrome-conjugated monoclonal antibodies. Since the Vβ8.1/8.2 T cells represent the largest subset of nondeleted T cells in both strains, the ratio of T cells expressing other Vβ receptors versus Vβ8.1/8.2+ T cells was determined to normalize the variation of the levels of all T cells generated in each culture.
Table 1 compares the mean Vβn:Vβ8 ratios of T cells in the normal spleens of C57BL/Ka and BALB/c mice in three to seven experiments. The mean ratios of the nondeleted Vβ receptors (Vβ2, Vβ4, Vβ6, and Vβ9) were similar in the two strains, and the ranges were 0.13–0.45 (C57BL/ Ka) and 0.11–0.47 (BALB/c). The mean ratios of Vβ receptors deleted in BALB/c mice (Vβ3, Vβ5.1, Vβ11, and Vβ12) were uniformly reduced in BALB/c (range: 0.03– 0.08) versus C57BL/Ka (range: 0.24–0.42) spleen cells. The ranges for the nondeleted Vβ receptors in marrow culture–derived T cells from the two strains were similar, and varied from 0.10 to 0.39 in C57BL/Ka and from 0.08 to 0.38 in BALB/c. On the other hand, the mean ratios for deleted Vβ receptors were uniformly lower again in BALB/c mice (range: 0.05–0.12) versus that in C57BL/Ka mice (range: 0.19–0.39).
Table 1.
Ratios of Vβn:Vβ8 in Normal Spleen and Marrow Culture–derived T Cells
| Vβ2 | Vβ4 | Vβ6 | Vβ9 | Vβ3 | Vβ5.1 | Vβ11 | Vβ12 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C57BL Sp1* | 0.26 ± 0.08 | 0.35 ± 0.11 | 0.45 ± 0.02 | 0.13 ± 0.06 | 0.30 ± 0.04 | 0.42 ± 0.07 | 0.37 ± 0.05 | 0.24 ± 0.08 | ||||||||
| BALB/c Sp1 | 0.30 ± 0.02 | 0.28 ± 0.12 | 0.47 ± 0.03 | 0.11 ± 0.05 | 0.08 ± 0.03 | 0.05 ± 0.06 | 0.07 ± 0.05 | 0.03 ± 0.05 | ||||||||
| C57BL MCD | 0.33 ± 0.02 | 0.39 ± 0.09 | 0.36 ± 0.10 | 0.10 ± 0.01 | 0.20 ± 0.01 | 0.39 ± 0.03 | 0.37 ± 0.04 | 0.19 ± 0.03 | ||||||||
| BALB/c MCD | 0.25 ± 0.06 | 0.34 ± 0.03 | 0.38 ± 0.09 | 0.08 ± 0.03 | 0.05 ± 0.06 | 0.07 ± 0.03 | 0.12 ± 0.07 | 0.10 ± 0.07 |
Ratio = %Vβn+:%Vβ8.1/8.2+ T cells. Mean percentage of Vβ8.1/8.2+ in the Spl and MCD T cells of C57BL mice was 19 ± 2 and 16.5 ± 0.4% respectively, and for BALB/c mice was 22 ± 1 and 23 ± 5%, respectively. Bold shows Vβ ratios indicative of superantigen-mediated deletion. Mean and SD of three to seven replicate experiments are shown.
Spl, spleen; MCD, marrow culture–derived.
During thymic maturation, CD4+ and CD8+ T cells expressing certain Vβ receptors are positively selected by endogenous superantigens from their CD4+CD8+ T cell precursors. CD4+ T cells expressing Vβ17a receptors are strongly selected in I-E+ strains of mice that express the H-2q haplotype and are weakly selected in those that express the H-2b haplotype (14, 31, 32). Accordingly, the mean percentages of Vβ17a+ T cells were compared in CD4+CD8+ T cells harvested at 40 and 44 h and in CD4+ T cells harvested at 44 and 48 h in marrow cultures from SWR (H-2q) and C57BL/Ka (H-2b) mice in three experiments. Table 2 shows that the mean percentage of Vβ17a+ cells within the gated CD4+CD8+, CD4+CD8med, or CD4+CD8− subsets at 40, 44, and 48 h was <1% in cultures of C57BL/Ka marrow cells. In contrast, the mean percentage of Vβ17a+ cells rose from 2.9% in the CD4+CD8+ subset at 40 and 44 h to 14% in the CD4+CD8med subset at 44 h and 19% in the CD4+CD8− subset at 48 h in the SWR cultures.
Table 2.
Positive Selection of Marrow Culture–derived T Cells
| T cell subset | Percentage of Vβ17a+ T cells* | |||
|---|---|---|---|---|
| C57BL/Ka (H-2b) | SWR (H-2q) | |||
| CD4+CD8+ (40 h) | 0.6 ± 0.2 | 2.9 ± 0.5 | ||
| CD4+CD8+ (44 h) | 0.6 ± 0.1 | 2.9 ± 0.5 | ||
| CD4+CD8med (44 h) | 0.4 ± 0.1 | 14 ± 3 | ||
| CD4+CD8− (48 h) | 0.6 ± 0.3 | 19 ± 3 | ||
Percentage of Vβ17a+ cells after gating on T cell subset. Mean and SD of three replicate experiments are shown for each strain.
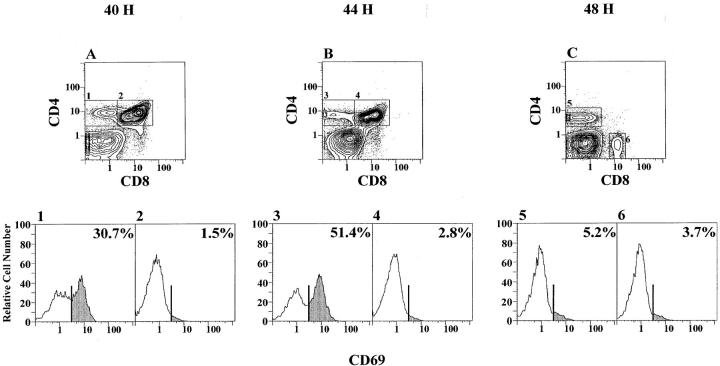
Another indication of positive selection is the transient appearance of the CD69 T cell activation marker on CD4+CD8med intermediary cells during maturation from CD4+CD8+ precursors to their CD4+CD8− progeny (33). Accordingly, the percentage of CD69+ cells was determined in the gated C57BL/Ka T cell subsets at 40, 44, and 48 h. Fig. 3 A shows that the percentage of CD69+ cells was 30.7% in the CD4+CD8med subset (box 1) and 1.5% in the CD4+CD8+ subset (box 2) at 40 h. At 44 h (Fig. 3 B), the percentage of CD69+ cells increased to 51.4% in the CD4+CD8med subset (box 3) and 2.8% in the CD4+CD8+ subset (box 4). At 48 h (Fig. 3 C), the percentage of CD69+ cells within the CD4+CD8− subset (box 5) had fallen to 5.2% and was 3.7% in the CD4−CD8+ subset (box 6). Stages of thymic development between the TCRlo double positive and the TCRhi single positive cells are CD69+ in mice, and are similar to the bone marrow T cell development described here (14).
Figure 3.
Changes in the expression of CD69 on T cell subsets during marrow cultures. C57BL/Ka marrow cells were depleted of α/β T cells and cultured for 40 (A), 44 (B), and 48 (C) h as was shown in Fig. 2, F–H. Gated CD4+CD8med or CD4+CD8− cells (boxes 1, 3, and 5) or CD4+ CD8+ cells (boxes 2 and 4) or CD4−CD8+ cells (box 6) were analyzed for the expression of CD69. The profiles and percentages of CD69+ cells in each box are shown in corresponding panels 1–6. Shaded areas show the CD69+ cells.
Other Surface Markers on CD4+ and CD8+ T Cells Cultured from Bone Marrow.
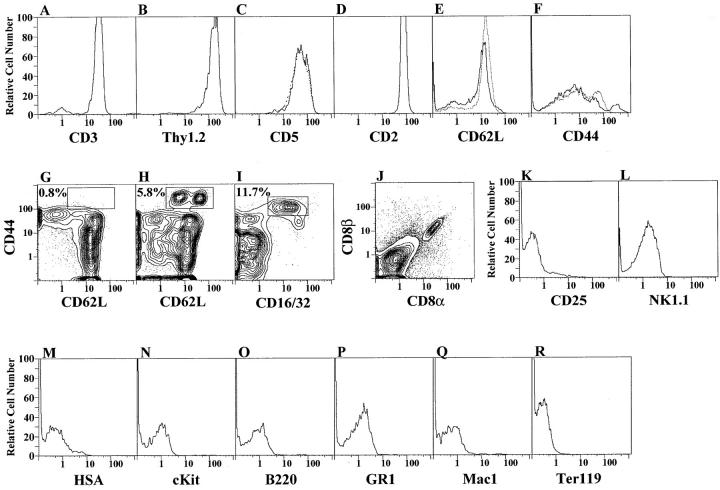
Sorted CD4−CD8− α/β− marrow cells from C57BL/Ka mice were cultured for 48 h as before, and the α/β+ T cells were counterstained for a variety of other markers as shown in Fig. 4. In panels A–L, cultures were gated for α/β+ cells and one- or two-color analysis of other markers are shown. Almost all of the α/β+ T cells expressed the other usual T cell markers CD3, CD2, CD5, and Thy1.2 at bright staining levels (A–D; solid lines). The intensity of staining of these markers, as well as CD4 and CD8, was similar to that of splenic T cells as shown in Table 3. Heterogeneous staining for both L-selectin (CD62L) and CD44 was observed in both cultured and splenic T cells (E and F). Two-color analysis resolved several discrete populations of T cells as shown in G and H. Two subsets of CD44hi cells that stained with either bright or intermediate intensity for CD62L are enclosed in the box in H, and accounted for 5.8% of T cells. Less than 1% of the normal splenic T cells were TCR-α/β+CD44hi CD62L+, and no discrete contours for this CD44hi subset were observed (G). The remaining CD62L+ cells showed a staining intensity that was similar to that of normal splenic T cells (Table 3) with a minor population of CD44int cells, and a major population of CD44dull/− cells, reported to be virgin T cells (34). Further experiments showed that the CD44hi subset in the cultured marrow cells expressed CD16+ also (I). The remaining cultured cells were CD16−. Almost all of the CD8+ α/β T cells derived from the marrow cultures expressed both the CD8α and CD8β markers, and few if any showed the CD8α/α phenotype (J). Less than 2% of the cultured T cells exceeded the background staining for the CD25 or NK1.1 markers (K and L) found on activated or NK1.1+ T cells (35, 36). Markers of immature thymocytes (HSA and cKit), which are not present on single positive CD4+ and CD8+ thymic T cells, were also not present on the cultured marrow T cells (M and N). The staining of conventional markers of B cells, granulocytes, macrophages, and erythroid cells (B220, GR-1, Mac-1, and Ter119) were not above background on the marrow-derived T cells (O–R).
Figure 4.
Surface markers on CD4+ and CD8+ T cells harvested at 48 h from C57BL/Ka marrow cultures. After gating of α/β+ cells, single color profiles for CD3, Thy1.2, CD5, CD2, CD62L, and CD44 receptors are shown in A–F (solid lines). Normal spleen T cell profiles are shown for comparison in C, E, and F (broken lines). Two-color profiles for CD44 versus CD62L receptors on gated normal spleen cells and on gated marrow-cultured cells are compared in G and H, respectively. In addition, the profile for CD44 versus CD16 on gated marrow cultured cells is shown in I. A two-color profile for CD8α versus CD8β receptors on α/β+-gated marrow cultured cells is shown in J. One-color profiles for CD25, NK1.1, HSA, cKit, B220, Gr-1, Mac-1, and Ter119 markers on α/β+-gated marrow-cultured cells are shown in K–R.
Table 3.
Mean Channel Intensity Comparison between Normal Spleen and Marrow Culture–derived T Cells
| TCR-α/β | CD2 | CD3 | CD4 | CD5 | CD8 | CD62L | Thy 1.2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C57BL Spl* | 172.2 | 144.2 | 95.3 | 125.8 | 166.3 | 130.0 | 137.1 | 200.8 | ||||||||
| C57BL MCD | 172.8 | 143.1 | 95.1 | 121.6 | 169.4 | 123.1 | 131.8 | 200.5 |
Values indicate mean channel intensity of fluorescence for each receptor. One-color analysis was perfromed after gating on TCR-α/β+ cells for all receptors other than TCR-α/β itself.
Spl, spleen, MCD, marrow culture–derived.
Inhibition of T Cell Maturation by Mature Cells.
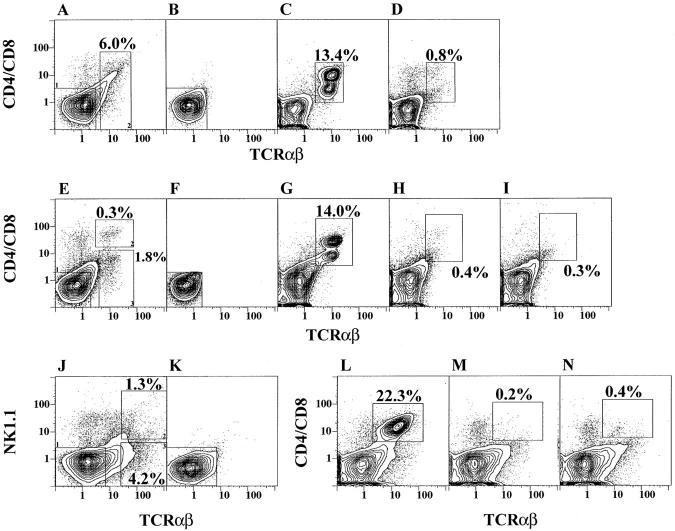
Since the outgrowth of the bright CD4+ and CD8+ T cells from cultures shown in Fig. 1 was dependent upon depletion of marrow T cells before culture, several experiments were performed to determine whether the addition of T cells to T cell–depleted cultures prevented the outgrowth of the bright T cells. Fig. 5, A–D shows an experiment in which C57BL/Ka marrow cells were stained for CD4, CD8, and TCR-α/β markers, and sorted into CD4−CD8− α/β− and α/β+ cells as shown by the gating thresholds of boxes 1 and 2 (left and right), respectively, in Fig. 5 A. Reanalysis of the cells in box 1 are shown in B, and culture of 4 × 106 of the depleted marrow cells and restaining are shown in C. Bright CD4+ or CD8+ α/β+ T cells enclosed in the box of C accounted for 13.4% of cultured cells at 48 h. Addition of 3 × 105 sorted TCR-α/β+ T cells from box 2 of A to 4 × 106 depleted marrow cells from box 1 of A at the initiation of the culture inhibited the outgrowth of bright CD4+ or CD8+ α/β T cells after the 48-h culture, and <1% of the cultured cells are enclosed in the box shown in D.
Figure 5.
Add back of marrow T cells to T cell–depleted marrow cultures. Freshly isolated marrow cells (A) were sorted using the gating boxes 1 and 2, and reanalysis of T-depleted cells in box 1 are shown in B. After 48 h of culture of cells in B, newly formed T cells are enclosed in the box in C. Culture of combined cells from boxes 1 and 2 are shown in D. Marrow cells were also sorted for CD4+ or CD8+ TCR-α/β+ (E, box 2), or CD4−CD8− TCR-α/β+ (E, box 3) T cells. F and G show T cell-depleted marrow cells before and after culture. H and I show T cell-depleted marrow cells cultured in combination with cells from E, box 2 or 3, respectively. Marrow cells were also gated for NK1.1− TCR-α/β− ( J, box 1), NK1.1+ TCR-α/β+ ( J, box 2), and NK1.1−TCR-α/β+ ( J, box 3) subsets, respectively. Sorted NK1.1−TCR-α/β+ (K) cells were cultured alone (L), or with cells from box 2 (M) or box 3 (N).
To determine whether the inhibition was mediated by CD4+ and CD8+ α/β T cells and/or CD4−CD8− α/β T cells, subsequent experiments were performed in which C57BL/Ka marrow cells were sorted using threshold gates shown in Fig. 5 E. Box 1 (left) enclosed CD4−CD8− α/β− marrow cells, box 2 (upper right) enclosed CD4+ and CD8+ α/β+ T cells, and box 3 (lower right) enclosed CD4−CD8− α/β+ T cells. Fig. 5 F shows the reanalysis of cells from box 1 before culture, and G shows the restaining after culture. Bright CD4+ and CD8+ α/β T cells accounted for 14% of cultured cells (enclosed in the box in G). In further experiments, 0.3 × 105 T cells from box 2 or 2 × 105 from box 3, (Fig. 5 E) were added to 4 × 106 depleted marrow cells in box 1, and the combined cells were cultured for 48 h. Fig. 5, H and I, show the staining of the cultured cells with CD4+ and CD8+ or CD4−CD8− T cells added back, respectively. The boxes in H and I enclosed <1% of bright cells. Thus, the CD4+ and/or CD8+ α/β+ T cells, as well as CD4−CD8− α/β+ T cells, from the marrow markedly inhibited the outgrowth of bright T cells from the marrow cultures.
NK1.1+ T cells have been reported to have an unusual cytokine secretion pattern and Vα and Vβ repertoire (20, 37, 38), and their percentage among T cells is increased in the marrow as compared to the spleen and thymus (39). We tested the ability of NK1.1+ and NK1.1− marrow T cells to inhibit the outgrowth of bright CD4+ and CD8+ T cells from the marrow cultures. Fig. 5 J shows C57BL/Ka marrow cells stained for NK1.1 and TCR-α/β surface markers. Box 1 (left) encloses TCR-α/β− NK1.1− marrow cells. Boxes 2 and 3 (upper and lower right) enclose the TCR-α/β+ NK1.1+ and TCR-α/β+ NK1.1− T cells, respectively. Reanalysis of the sorted cells within box 1 is shown in Fig. 5 K. Culture of the latter cells for 48 h, and staining for CD4, CD8, and TCR-α/β markers after culture is shown in panel L. Bright CD4+ or CD8+ α/β+ T cells (enclosed in box) represented 22.3% of the harvested cells. Addition of 0.5 × 105 of the sorted T cells from box 2 or 2.5 × 105 from box 3 from J were added to 4 × 106 cells from box 1, and the staining patterns of the cultured cells are shown in M and N, respectively. The combined cell cultures contained <1% of bright CD4+ and CD8+ α/ β+ T cells (enclosed in boxes) after 48 h.
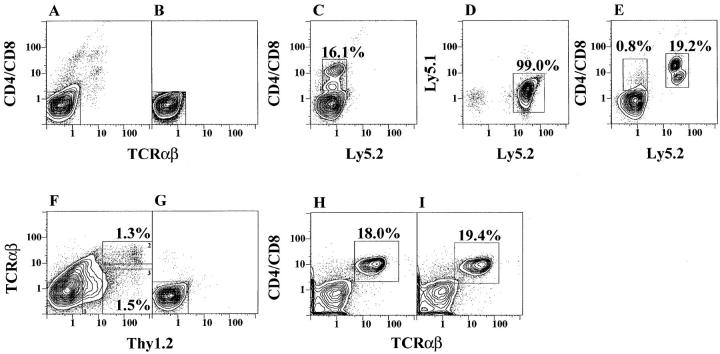
Since CD4+ and CD8+, CD4−CD8−, NK1.1+, and NK1.1− marrow T cells strongly inhibited the outgrowth of bright T cells, we determined whether the inhibitory function was a unique property of marrow T cells or a property of peripheral T cells as well. Accordingly, sorted CD4+ and CD8+ T cells from the lymph nodes of C57BL/6 mice expressing the Ly5.2 marker were added to T cell– depleted marrow cultures from C57BL/6 congenic mice expressing the Ly5.1 marker. Fig. 6 A shows the staining pattern of the marrow cells from Ly5.1 mice for CD4 and CD8 versus TCR-α/β markers, and reanalysis of sorted CD4−CD8− α/β− marrow cells is shown in B. Cells (4 × 106) in Fig. 6 B were cultured for 48 h and stained for the Ly5.2 versus CD4 and CD8 markers. Bright CD4+ and CD8+ α/β+ T cells (16.1%) were generated from these cultures, and, as expected, these cells did not express the Ly5.2 marker (see box in C).
Figure 6.
Addition of lymph node T cells or Thy1.2+α/β− marrow cells to marrow cultures. Freshly isolated marrow cells from Ly5.1 congenic mice were depleted of T cells by sorting (A and B), and profiles of CD4/CD8 versus Ly5.2 markers are shown after culture (C). The Ly5.1 versus 5.2 profile of lymph node cells from Ly5.2 congenic mice after gating on TCR-α/β+ cells is shown in D. Gated TCR-α/β+ cells from D were added to T-depleted marrow cells (B) and cultured for 48 h (E). CD4+ and CD8+ T cells that are Ly5.2− or Ly5.2+ are enclosed in left and right boxes, respectively. The profile of TCR-α/β versus Thy1.2 markers on C57BL/Ka marrow is shown in F, and sorted Thy1.2− TCR-α/β− cells were reanalyzed (G). Cells from G were cultured alone and analyzed (H) or cultured in combination with Thy1.2+ TCR-α/β− cells (I).
In subsequent experiments, 1.2 × 105 sorted Ly5.2+ α/β+ T cells from the lymph nodes of congenic Ly5.2 mice were added to 4 × 106 T cell–depleted marrow cells from Ly5.1 mice and cultured for 48 h. Fig. 6 D shows the staining pattern of the lymph node cells for Ly5.1 versus Ly5.2 markers after gating on TCR-α/β+ cells. As expected, most all of these cells expressed the Ly5.2 marker (enclosed in box). Sorted α/β+ T cells from D were added to those from B, and the cultured cells were stained for Ly5.2 versus CD4 and CD8 markers as shown in E. The latter cells contained <1% of CD4+ and CD8+ cells (enclosed in left box) derived from the Ly5.1+ marrow cells, but included a discrete population of CD4+ and CD8+ Ly5.2+ T cells (19.2% enclosed in right box) derived from the added lymph node cells. Comparison of Fig. 6, C with E, indicated that the addition of the lymph node T cells to marrow cultures markedly inhibited the outgrowth of Ly5.2− T cells derived from the marrow.
The experimental results showed that all of the sorted mature T cells from the marrow and lymph nodes that expressed the TCR-α/β were able to inhibit the outgrowth of bright CD4+ and CD8+ T cells from the marrow cultures. Bone marrow cells which express the Thy1.2+ TCR-α/β− phenotype of the earliest T cell precursors in the thymus (40, 41) also were tested for the ability to inhibit the marrow cultures. C57BL/Ka marrow cells were stained for Thy1.2 versus TCR-α/β markers. Fig. 6 F showed that bright Thy1.2+ cells that are bright α/β+ T cells (enclosed in box 2, upper right) account for 1.3% of cells, and bright Thy1.2+ cells that are dull or negative for the TCR-α/β marker (enclosed in box 3, lower right) account for 1.5% of cells. The Thy1.2+ α/β− cells were added to C57BL/Ka marrow cells, which were stringently depleted of Thy1.1+ and TCR-α/β+ cells as shown in Fig. 6 G. The latter cells (4 × 106) were cultured for 48 h as in previous experiments. Staining of the cultured cells from G for CD4, CD8, and TCR-α/β receptors showed that a population of bright CD4+ and/or CD8+ α/β+ T cells were present and accounted for 18% of cells (enclosed in box) in H. Fig. 6 I shows that the addition of 3 × 105 Thy1.2+ α/β− cells (Fig. 6 F, box 3) to the cultures did not inhibit the outgrowth of the bright CD4+ and/or CD8+ α/β+ T cells at 48 h, and 19.4% of the cultured cells are enclosed in the box. Thus, inhibition was associated with the expression of the TCR-α/β, but not the Thy1.2 marker.
Discussion
Several lines of evidence have supported the notion that α/β T cells can develop in the absence of the thymus microenvironment, including studies of in vivo T cell development in nontransgenic and TCR-α/β transgenic athymic nude mice, and in irradiated thymectomized fetal liver, bone marrow, or hematopoietic stem cell–reconstituted mice (15, 18, 19, 23, 24). In vitro studies of T cell development have demonstrated that single cell agar gel cultures of marrow precursors, or dispersed cultures of hematopoietic progenitor cells, can give rise to T cells (42, 43). Our previous reports of the generation of T cells from dispersed bone marrow cultures showed that mature T cells must be stringently depleted from nontransgenic or TCR-α/β transgenic marrow cells before culture to allow for in vitro T cell maturation (19, 24). In the latter studies, almost all the T cells generated within 48 h expressed the CD4−CD8− α/β+ surface phenotype, and few if any CD4+ or CD8+ T cells were observed (19, 24). In this study, almost all the T cells generated in the marrow cultures were CD4+ or CD8+ at 48 h, using a different source of FBS to supplement the culture medium. The nature of the substances in the different sources of serum that dramatically alter the maturation of T cells is unknown. Candidate molecules include thymic hormones that have been shown to facilitate T cell maturation (44).
Our experiments show that few if any α/β+ T cells were generated in the T cell–depleted marrow cultures during the first 36 h, and a rapid maturation of CD4+ and CD8+ (single positive) T cells occurred during the ensuing 12 h. The first expression of bright TCR expression occurred at 40 h, and was predominantly on CD4+CD8+ (double positive) T cells and on a minority of CD4+CD8med T cells. The transition from the predominant double positive T cells at 40 h to the almost complete predominance of single positive T cells at 48 h recapitulates the transition from double positive to single positive T cells in the thymus (1, 2). In the heterogeneous thymic stromal cell culture system, the transition from CD4−CD8−CD3− thymus precursor cells to single positive cells also occurs in 48 h, and, as in the bone marrow cultures, the transition from double positive to single positive cells occurs within 12 h. In both the thymic stromal and bone marrow cultures, the appearance of CD4+CD8med T cells preceded that of the CD8+ T cells (14). It is likely that rearrangements of the TCR genes occurred in the marrow cultures before TCR expression at 40 h, since previous studies showed that no functional rearrangements of the TCR-β chain genes were detected in T cell–depleted marrow cultures at time zero due to the presence of stop codons in the junctions or out of frame reading of the Jβ segments. However, functional rearrangements were plentiful at 48 h (19). Thus, the outgrowth of T cells in these cultures does not represent the upregulation of expression of previously rearranged TCR genes. A similar robust outgrowth of extrathymically derived CD4+ CD8+ T cells in the peripheral lymphoid tissues has been observed in athymic mice reconstituted with bone marrow obtained from mice expressing the bovine oncostatin-M transgene under the control of the p56lck proximal promoter (45). The alternate pathway described here is a likely source for the development of the transgenic T cells.
As in the thymic stromal cultures (14), evidence for positive selection of the CD4+CD8− T cells was obtained by comparing their expression of Vβ17a to that of the CD4+CD8+ T cells in the cultures from SWR (H-2q) and C57BL/Ka (H-2b) strains. As expected, a marked increase in the percentage of Vβ17a+ cells within the CD4+CD8− as compared to the CD4+CD8+ subset was observed in the SWR but not in the C57BL cultures. Additional evidence for positive selection was the transient expression of the CD69 activation antigen on CD4+CD8med T cells at 40 and 44 h, but not on CD4+CD8− at 48 h. Comparison of superantigen-deleted and nondeleted Vβ receptors on the single positive T cells in 48-h cultures of C57BL/Ka and BALB/c marrow cells showed that the latter cells had undergone negative selection, and the pattern of Vβ receptor expression was similar to that of T cells in the spleen of both strains.
The surface phenotype of single positive T cells generated in the marrow cultures was similar to that of mature (HSA−) single positive T cells in the thymus and peripheral lymphoid tissues, since the typical T cell markers Thy1.2, CD5, CD2, and CD3 were expressed in the absence of markers of B cells, NK cells, or other hematopoietic cells. The marrow-derived T cells contained subsets usually associated with virgin (CD44−CD62L+) and memory (CD44+CD62L−) T cells in normal spleen (34, 46). An unusual minor subset of CD44hiCD16+CD62L+ marrow-derived T cells was not found in the spleen, and more closely resembles the immature CD44hi subsets of T cells in thymus (47–49).
The marrow-derived CD8+ T cells expressed both the CD8α and CD8β chains, and few if any expressed only CD8α/α homodimers. The expression of the latter homodimers has been found on extrathymically derived CD8+TCR-α/β+ intraepithelial lymphocytes in the intestines (16, 50). Since there were no surface markers on most marrow-derived single positive T cells that clearly distinguished them from thymus-derived single positive T cells in the periphery, the contribution of marrow-derived T cells to the peripheral T cell pool in vivo is unclear. The almost complete absence of single positive T cells in the spleen and lymph nodes of nude mice in the first 3 mo of life and the slow accumulation thereafter (15) suggest that the marrow contribution to the peripheral pool is minimal. However, the thymus may augment the marrow production of T cells by the secretion of humoral factors that promote T cell maturation (44). Thus, the rate of production of single positive T cells by the marrow and their contribution to the peripheral pool of euthymic mice may not be accurately determined by studies of athymic mice.
Several subsets of mature T cells inhibited the outgrowth of CD4+CD8+, CD4+, and CD8+ T cells from the marrow cultures. Adding back sorted subsets of marrow T cells showed that marked inhibition was achieved with CD4+ and CD8+, CD4−CD8−, NK1.1+, and NK1.1− α/β+ T cells. These α/β+ T cells prevented T cell maturation at a ratio as low as 1:100 of sorted T cells to T cell–depleted marrow cells. A similar low ratio of mature α/β+ T cells obtained from the lymph nodes prevented the outgrowth of T cells from marrow cultures. Although marrow-derived and peripheral T cells expressing the TCR-α/β were inhibitory, sorted Thy1.2+α/β− marrow cells failed to inhibit T cell maturation. Bright Thy1.2+ cells that do not express the TCR are found in the thymus also, and are able to differentiate into the more mature Thy1.2+α/β+ thymic T cells (49, 51, 52). The differences in the inhibitory activity of the Thy1.2+α/β− and Thy1.2+α/β+ marrow cells indicates that only mature T cells prevent marrow T cell maturation by a feedback inhibition process. Maturation is blocked before the double positive stage, since neither double positive nor single positive T cells were detected at 36, 40, 44, or 48 h in the nondepleted marrow cultures (data not shown). Feedback inhibition may contribute to the disappearance of double positive cells at 48 h in marrow cultures, whereas these cells persist in thymic stromal cell cultures (14). Alternatively, the in vitro lifespan of double positive cells, most of which are postselected cells and are destined to die, may be longer in heterogeneous thymic stromal cell cultures than those in bone marrow cultures for reasons other than feedback inhibition, e.g., the presence of survival factors. Bone marrow and thymic stromal cell culture mixing experiments should help resolve these alternatives.
Our results suggest that a microenvironment that supports CD4+ and CD8+ α/β+ T cell maturation from precursors is not unique to the thymus, and that the marrow microenvironment is capable of carrying out this function, presumably by specialized stromal cells. The predominance of the thymus as the source of T cells in the lymphoid tissues may be explained better by the differences in the regulation of T cell maturation in different generative sites than by differences in the capacities of microenvironments in these generative sites to support maturation. In particular, the presence of mature T cells may strongly inhibit maturation in the marrow but not in the thymus. T cell maturation in the marrow may be regulated in part by thymus-derived emigrants such that the rate of marrow T cell production and export is increased in disease states in which mature T cells are depleted, such as acquired immunodeficiency syndrome in humans, or after myeloablation and stem cell transplantation in hosts with poor thymic function.
Is T cell feedback inhibition of T cell development a process limited to bone marrow cultures? The heterogeneous thymic stromal cell cultures (13, 14) were always initiated in the absence of mature T cells, and feedback inhibition was not tested. This process might be operative also in the thymus in situ; thymic CD4−CD8−CD3− cells are located in the subcapsular cortex (1, 10, 51), whereas single positive CD4+ and CD8+ T cells are in the juxtamedullary cortex and the medulla. Thus, the separation of immature and mature cells in the thymus could prevent short-range inhibitory effects. However, in some circumstances activated T cells recirculate to the thymic cortex (53–55) and may participate in negative feedback. These potential feedback loops in the thymus and the bone marrow in situ have yet to be tested.
In conclusion, the microenvironment of the bone marrow provides necessary and sufficient signals for the maturation of single positive T cells from T cell precursors in the marrow. The process is tightly regulated by the presence of mature T cells in the microenvironment, and appears to recapitulate the main elements of thymic maturation through a double positive intermediary T cell. Differences in the functional capacities of marrow-derived and thymus-derived T cells remain to be elucidated.
Acknowledgments
We thank V. Braunstein for antibody preparation, A. Mukhopadhyay for technical assistance, M. Amano for FACS® assistance, T. Knack for FACS® support, and V. Cleaver for clerical assistance.
This work was supported by grants HL-57443 and HL-58250 from the National Institutes of Health and grant CA-42551 from the United States Public Health Service. M.E. García-Ojeda is supported by minority grant supplement AI-37683 from the National Institutes of Health.
Abbreviations used in this paper
- FBS
fetal bovine serum
- HSA
heat-stable antigen
Footnotes
M.E. García-Ojeda and S. Dejbakhsh-Jones contributed equally to the research described in this paper.
References
- 1.Adkins B, Mueller C, Okada CY, Reichert RA, Weissman IL, Spangrude GJ. Early events in T-cell maturation. Annu Rev Immunol. 1987;5:325–365. doi: 10.1146/annurev.iy.05.040187.001545. [DOI] [PubMed] [Google Scholar]
- 2.Weissman IL. Developmental switches in the immune system. Cell. 1994;76:207–218. doi: 10.1016/0092-8674(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K. Cellular aspects of early T-cell development. Curr Opin Immunol. 1992;4:140–146. doi: 10.1016/0952-7915(92)90003-w. [DOI] [PubMed] [Google Scholar]
- 4.Antica M, Wu L, Shortman K, Scollay R. Intrathymic lymphoid precursor cells during fetal thymus development. J Immunol. 1993;151:5887–5895. [PubMed] [Google Scholar]
- 5.Ismaili J, Antica M, Wu L. CD4 and CD8 expression and T cell antigen receptor gene rearrangement in early intrathymic precursor cells. Eur J Immunol. 1996;26:731–737. doi: 10.1002/eji.1830260402. [DOI] [PubMed] [Google Scholar]
- 6.von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 7.Fowlkes BJ, Schweighoffer E. Positive selection of T cells. Curr Opin Immunol. 1995;7:188–195. doi: 10.1016/0952-7915(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 8.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 9.Marrack P, Lo D, Brinster R, Palmiter R, Burkly L, Flavell RH, Kappler J. The effect of thymus environment on T cell development and tolerance. Cell. 1988;53:627–634. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- 10.Weissman IL. Thymus cell maturation. Studies on the origin of cortisone-resistant thymic lymphocytes. J Exp Med. 1973;137:504–510. doi: 10.1084/jem.137.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 12.Jenkinson EJ, Franchi LL, Kingston R, Owen JJ. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982;12:583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 13.Sen-Majumdar A, Lieberman M, Alpert S, Weissman IL, Small M. Differentiation of CD3−4−8−thymocytes in short-term thymic stromal cell culture. J Exp Med. 1992;176:543–551. doi: 10.1084/jem.176.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akashi K, Weissman IL. The c-kit+ maturation pathway in mouse thymic T cell development: lineages and selection. Immunity. 1996;5:147–161. doi: 10.1016/s1074-7613(00)80491-4. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald HR, Lees RK, Sordat B, Zaech P, Maryanski JL, Bron C. Age-associated increase in expression of the T cell surface markers Thy-1, Lyt-1, and Lyt-2 in congenitally athymic (nu/nu) mice: analysis by flow microfluorometry. J Immunol. 1981;126:865–870. [PubMed] [Google Scholar]
- 16.Rocha B, Vassalli P, Guy-Grand D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J Exp Med. 1994;180:681–686. doi: 10.1084/jem.180.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benveniste P, Chadwick BS, Miller RG, Reimann J. Characterization of cells with T cell markers in athymic nude bone marrow and of their in vitro–derived clonal progeny. Comparison with euthymic bone marrow. J Immunol. 1990;144:411–419. [PubMed] [Google Scholar]
- 18.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–767. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dejbakhsh-Jones S, Okazaki H, Strober S. Similar rates of production of T and B lymphocytes in the bone marrow. J Exp Med. 1995b;181:2201–2211. doi: 10.1084/jem.181.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino Y, Koseki H, Adachi Y, Akasaka T, Tsuchida K, Taniguchi M. Extrathymic differentiation of a T cell bearing invariant V alpha 14J alpha 281 TCR. Int Rev Immunol. 1994;11:31–46. doi: 10.3109/08830189409061715. [DOI] [PubMed] [Google Scholar]
- 21.Collins C, Norris S, McEntee G, Traynor O, Bruno L, von Boehmer H, Hegarty J, O'Farrelly C. RAG1, RAG2 and pre-T cell receptor alpha chain expression by adult human hepatic T cells: evidence for extrathymic T cell maturation. Eur J Immunol. 1996;26:3114–3118. doi: 10.1002/eji.1830261243. [DOI] [PubMed] [Google Scholar]
- 22.Palathumpat V, Dejbakhsh-Jones S, Holm B, Wang H, Liang O, Strober S. Studies of CD4− CD8−alpha beta bone marrow T cells with suppressor activity. J Immunol. 1992;148:373–380. [PubMed] [Google Scholar]
- 23.Dejbakhsh-Jones S, Jerabek L, Weissman IL, Strober S. Extrathymic maturation of alpha beta T cells from hemopoietic stem cells. J Immunol. 1995;155:3338–3344. [PubMed] [Google Scholar]
- 24.Cheng L, Dejbakhsh-Jones S, Liblau R, Zeng D, Strober S. Different patterns of TCR transgene expression in single-positive and double-negative T cells. Evidence for separate pathways of T cell maturation. J Immunol. 1996;156:3591–3601. [PubMed] [Google Scholar]
- 25.Parks DR, Herzenberg LA. Fluorescence-activated cell sorting: theory, experimental optimization, and applications in lymphoid cell biology. Methods Enzymol. 1984;108:197–241. doi: 10.1016/s0076-6879(84)08086-1. [DOI] [PubMed] [Google Scholar]
- 26.Nossal GJ. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 27.Kappler JW, Pullen A, Callahan J, Choi Y, Herman A, White J, Potts W, Wakeland E, Marrack P. Consequences of self and foreign superantigen interaction with specific V beta elements of the murine TCR alpha beta. Cold Spring Harbor Symp Quant Biol. 1989;54:401–407. doi: 10.1101/sqb.1989.054.01.049. [DOI] [PubMed] [Google Scholar]
- 28.Scherer MT, Ignatowicz L, Pullen A, Kappler J, Marrack P. The use of mammary tumor virus (Mtv)-negative and single-Mtv mice to evaluate the effects of endogenous viral superantigens on the T cell repertoire. J Exp Med. 1995;182:1493–1504. doi: 10.1084/jem.182.5.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodes, R.J., and R. Abe. 1993. Mouse endogenous superantigen: Mls and Mls-like determinants encoded by mouse retroviruses. In Current Protocols in Immunology, volume 3. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. John Wiley & Sons, Inc., New York. A.1.F1–A.1.F5. [DOI] [PubMed]
- 30.Hodes RJ, Novick MB, Palmer LD, Knepper JE. Association of a V beta 2–specific superantigen with a tumorigenic milk-borne mouse mammary tumor virus. J Immunol. 1993;150:1422–1428. [PubMed] [Google Scholar]
- 31.Kappler JW, Kushnir E, Marrack P. Analysis of V beta 17a expression in new mouse strains bearing the V beta a haplotype. J Exp Med. 1989;169:1533–1541. doi: 10.1084/jem.169.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackman MA, Marrack P, Kappler J. Influence of the major histocompatibility complex on positive thymic selection of V beta 17a+ T cells. Science. 1989;244:214–217. doi: 10.1126/science.2784868. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita I, Nagata T, Tada T, Nakayama T. CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor–mediated positive selection. Int Immunol. 1993;5:1139–1150. doi: 10.1093/intimm/5.9.1139. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald HR, Budd RC, Cerottini JC. Pgp-1 (Ly 24) as a marker of murine memory T lymphocytes. Curr Top Microbiol Immunol. 1990;159:97–109. doi: 10.1007/978-3-642-75244-5_6. [DOI] [PubMed] [Google Scholar]
- 35.Cerdan C, Martin Y, Courcoul M, Brailly H, Mawas C, Birg F, Olive D. Prolonged IL-2 receptor alpha/ CD25 expression after T cell activation via the adhesion molecules CD2 and CD28. Demonstration of combined transcriptional and post-transcriptional regulation. J Immunol. 1992;149:2255–2261. [PubMed] [Google Scholar]
- 36.Koyasu S. CD3+CD16+NK1.1+B220+ large granular lymphocytes arise from both α-β TCR+CD4−CD8− and γ-δ TCR+CD4−CD8−cells. J Exp Med. 1994;179:1957–1972. doi: 10.1084/jem.179.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arase H, Arase N, Nakagawa K, Good RA, Onoe K. NK1.1+ CD4+ CD8−thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307–310. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 38.Ohteki T, MacDonald HR. Stringent Vβ requirement for the development of NK1.1+ T cell receptor– α/β+cells in mouse liver. J Exp Med. 1996;183:1277–1282. doi: 10.1084/jem.183.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanage T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–2983. [PubMed] [Google Scholar]
- 40.I LY. Day 11 mouse fetal thymus: phenotype and search for the point of commitment. Differentiation. 1996;61:53–65. doi: 10.1046/j.1432-0436.1996.6110053.x. [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Scollay R, Egerton M, Pearse M, Spangrude GJ, Shortman K. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature. 1991;349:71–74. doi: 10.1038/349071a0. [DOI] [PubMed] [Google Scholar]
- 42.Hattori M, Sudo T, Izawa H, Kano S, Minato N. Developmental regulation of the extrathymic differentiation potential of the progenitor cells for T cell lineage. Int Immunol. 1989;1:151–159. doi: 10.1093/intimm/1.2.151. [DOI] [PubMed] [Google Scholar]
- 43.Minato N, Hattori M, Sudo T, Kano S, Miura Y, Suda J, Suda T. Differentiation in vitro of T3+ large granular lymphocytes with characteristic cytotoxic activity from an isolated hematopoietic progenitor colony. J Exp Med. 1988;167:762–776. doi: 10.1084/jem.167.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hadden JW. Thymic endocrinology. Int J Immunopharmacol. 1992;14:345–352. doi: 10.1016/0192-0561(92)90163-f. [DOI] [PubMed] [Google Scholar]
- 45.Clegg CH, Rulffes JT, Wallace PM, Haugen HS. Regulation of an extrathymic T-cell development pathway by oncostatin M. Nature. 1996;384:261–263. doi: 10.1038/384261a0. [DOI] [PubMed] [Google Scholar]
- 46.Croft M, Duncan DD, Swain SL. Response of naive antigen-specific CD4+ T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992;176:1431–1437. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scollay R, Wilson A, D'Amico A, Kelly K, Egerton M, Pearse M, Wu L, Shortman K. Developmental status and reconstitution potential of subpopulations of murine thymocytes. Immunol Rev. 1988;104:81–120. doi: 10.1111/j.1600-065x.1988.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 48.Lesley J, Schulte R, Trotter J, Hyman R. Qualitative and quantitative heterogeneity in Pgp-1 expression among murine thymocytes. Cell Immunol. 1988;112:40–54. doi: 10.1016/0008-8749(88)90274-2. [DOI] [PubMed] [Google Scholar]
- 49.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 50.Lin T, Matsuzaki G, Umesue M, Omoto K, Yoshida H, Harada M, Singaram C, Hiromatsu K, Nomoto K. Development of TCR-gamma delta CD4−CD8+ alpha alpha but not TCR-alpha beta CD4−CD8+ alpha alpha i-IEL is resistant to cyclosporin A. J Immunol. 1995;155:4224–4230. [PubMed] [Google Scholar]
- 51.Guidos CJ, Weissman IL, Adkins B. Developmental potential of CD4−8− thymocytes. Peripheral progeny include mature CD4−8− T cells bearing alpha beta T cell receptor. J Immunol. 1989;142:3773–3780. [PubMed] [Google Scholar]
- 52.Rodewald HR, Moingeon P, Lucich JL, Dosiou C, Lopez P, Reinherz EL. A population of early fetal thymocytes expressing Fc gamma RII/III contains precursors of T lymphocytes and natural killer cells. Cell. 1992;69:139–150. doi: 10.1016/0092-8674(92)90125-v. [DOI] [PubMed] [Google Scholar]
- 53.Michie SA, Kirkpatrick EA, Rouse RV. Rare peripheral T cells migrate to and persist in normal mouse thymus. J Exp Med. 1988;168:1929–1934. doi: 10.1084/jem.168.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naparstek Y, Holoshitz J, Eisenstein S, Reshef T, Rappaport S, Chemke J, Ben-Nun A, Cohen IR. Effector T lymphocyte line cells migrate to the thymus and persist there. Nature. 1982;300:262–264. doi: 10.1038/300262a0. [DOI] [PubMed] [Google Scholar]