Abstract
The membrane permeability transition (MPT) of mitochondria plays an important role in the mechanism of apoptotic cell death in various cells. Classic type MPT is induced by Ca2+ in the presence of inorganic phosphate and respiratory substrate, and is characterized by various events including generation of reactive oxygen species (ROS), membrane depolarization, swelling, release of Ca2+ and high sensitivity to cyclosporine A. However, the sequence of these events and the effect of antioxidants on their events remain obscure. Flow cytometry is a convenient method to investigate the order of events among various functions occurring in MPT using a limited amount of mitochondria (200 µl of 0.02 mg protein/ml) without contamination by other organelles. Flow cytometric analysis revealed that Ca2+ sequentially induced ROS generation, depolarization, swelling and Ca2+ release in mitochondria by a cyclosporine A-inhibitable mechanism. These results were supported by the finding that Ca2+-induced MPT was inhibited by antioxidants, such as glutathione and N-acetylcysteine. It was also revealed that various inhibitors of Ca2+-induced phospholipase A2 suppressed all of the events associated with Ca2+-induced MPT. These results suggested that ROS generation and phospholipase A2 activation by Ca2+ underlie the mechanism of the initiation of MPT.
Keywords: antioxidant, membrane permeability transition, flow cytometric analysis, mitochondria, phospholipase A2
Introduction
Apoptosis plays an important role in various physiological processes including embryonic development, maintenance of tissue and cell homeostasis, and in the pathogenesis of various diseases [1–3]. Among various organelles [4–7] mitochondria play the most important roles in the process of apoptosis by inducing membrane permeability transition (MPT). Opening of MPT pores releases apoptosis-related proteins including cytochrome c from mitochondria to cytosol thereby activating the caspase cascade [4, 8]. Mitochondria thus play pivotal roles in determining cell survival and death through energy transduction and release of apoptosis-related proteins, respectively.
In the presence of inorganic phosphate (Pi) and respiratory substrates, Ca2+ induces typical classic type MPT characterized by its dependency on Ca2+ and energy metabolism, mitochondrial depolarization, swelling, release of Ca2+, and high sensitivity to cyclosporine A, a specific inhibitor of MPT [4, 9, 10]. Although Ca2+ loading into mitochondria induces cytochrome c release, the molecular mechanism and sequence of events leading to cell death remain unclear.
Reactive oxygen species (ROS) produced by a variety of physiological and pathological metabolisms [11–13] function as critical second messenger in a variety of intracellular signaling pathways [14, 15]. We previously reported that mitochondria generated ROS followed by the induction of MPT [10]. Although the generation of ROS has been postulated to be one of the early events that induce MPT [15], the effects of antioxidants on Ca2+-induced mitochondrial swelling and other events leading to MPT remain obscure. Since flow cytometric analysis is an excellent method for the analysis of mitochondrial swelling, depolarization, Ca2+ release and ROS generation [16–18], we analyzed a sequence of events occurring in small amount of mitochondria using a FACScan analyzer.
Materials and Methods
Chemicals
Bromophenacyl bromide (BPB), chlorpromazine (CP), fatty acid free bovine serum albumin (BSA), N-acetylcysteine (NAC), quinacrine (QC), ruthenium red (RR), cyclosporine A (CsA) and trifluoperazine (TFP) were obtained from Sigma Co. Ltd. (Saint Louis, MO). Ca2+-dependent secretary phospholipase A2 (cPLA2) α inhibitor was obtained from Calbiochem (Darmstadt, Germany). 2',7'-Dichlorodihydrofluorescein diacetate (H2DCF-DA), hydroethidine (HE), tetramethylrhodamine-ethyl-ester (TMRE) and 10-nonyl acridine orange (NAO) were obtained from Molecular Probes (Eugene, OR). 1-[2-Amino-5-(-dimethylamino-6-dimethylammonio-9-xanthenyl)phenoxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N',N'-tetraacetic acid (Rhod 2)-tetraacetoxymetyl (AM) was obtained from Dojindo Co. Ltd. (Kumamoto, Japan). Cyanine dye, 3,3'-dipropyl-2,2'-thiodicarbocyanine iodide (diS-C3-(5)), a cyanine dye, was obtained from the Hayashibara Biochemical Laboratories (Okayama, Japan). All other chemicals were of analytical grade and obtained from Nacalai Tesque (Kyoto, Japan). NAO, TMRE, hydroethidine and CsA were dissolved in DMSO and stored at 4°C until use.
Isolation of rat liver mitochondria
After fasting Wistar rats overnight, excised rat livers were homogenized in 0.25 M sucrose containing 10 mM Tris-HCl buffer (pH 7.4) and 1 mM EDTA at 4°C. Mitochondria were isolated from the homogenates by the method of Hogeboom as described previously [19].
Assay for mitochondrial functions
Oxygen consumption and oxidative phoshorylation of mitochondria were measured by an oxygen electrode [10]. Mitochondria (0.25 mg protein/ml) were incubated in a medium consisting of 250 mM sucrose, 5 mM MgCl2, 10 mM KCl and 10 mM Tris-HCl buffer (pH 7.4) at 25°C. Mitochondria used for the experiments maintained a high respiratory control ratio (RCR of 5.0) and ADP/O ratio (1.7) in the presence of Pi and succinate.
Mitochondrial swelling was monitored by the change in light scattering at 540 nm and recorded by a Hitachi fluorescence spectrophotometer (650-10LC) equipped with a thermostatically controlled cuvette holder and a magnetic stirrer [10]. Mitochondrial membrane potential was measured by the fluorescence intensity of diS-C3-(5) (0.2 µg/ml) at 670 nm during excitation at 622 nm by a Hitachi 650-10LC [10].
Flow cytometry
Flow cytometric analysis was carried out using a FACScan equipped with a 488-nm Argon laser (Becton Dickinson, San Jose, CA). Data from the experiments were analyzed using the CELLQuest software (Becton Dickinson) as described previously [16–18]. To exclude debris in the side scatter (SSC) and forward scatter (FSC) modes, 50,000 events per sample within this gate (R1) were collected using the “low” setting for sample flow rate. Mitochondria were selectively stained with NAO (100 nM, excitation at 488 nm and emission at 525 nm) that binds to cardiolipin in the inner mitochondrial membrane [17, 20]. TMRE (100 nM, excitation at 488 nm and emission at 590 nm), HE (10 µM, excitation at 495 and emission at 580 nm) and Rhod 2 (2.5 µM, excitation at 488 nm and emission at 576 nm) were used to measure membrane potential, ROS and release of Ca2+, respectively [16–18, 21–24]. TMRE accumulated in mitochondria in membrane potential dependent manner. Binding of Ca2+ to Rhod 2 increases its fluorescence. Thus, it has been used to monitor changes in [Ca2+] within the mitochondrial matrix [24]. HE also used to detect ROS, especially superoxide [22].
Mitochondria (0.1 mg protein/ml) were stained under dark conditions with either NAO, HE, TMRE or Rhod 2-AM in 1 ml of standard medium (3 mM HEPES buffer, pH 7.4, containing 70 mM sucrose, 230 mM mannitol, 1 µM EDTA and ∼10 µM contaminating Ca2+) in the presence of 0.5 mM Pi and 2.5 mM succinate under dark conditions at 25°C for 3 min. Then, adding various concentration of Ca2+ in the presence or absence of various reagents induced MPT. After 20 seconds∼5 min, membrane depolarization, Ca2+-release and ROS generation of NAO-positive mitochondria were analyzed by FACScan for changes in FL2-H of TMRE, Rhod 2 and HE. ROS generation and mitochondrial swelling were also analyzed by FACScan based on the changes in FL1-H of H2DCF-DA and in SSC and FSC of NAO-positive particles before and after adding Ca2+. Protein concentrations were determined by the method of Bradford using BSA as a standard [25].
Results
Analysis of light scattering properties of mitochondria
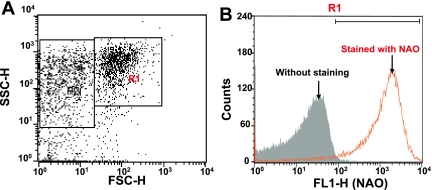
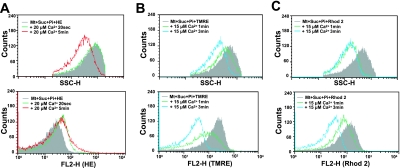
To analyze the relationship among Ca2+-induced membrane depolarization, swelling, Ca2+ release and ROS generation, mitochondria were selected from rat liver based on their light-scattering properties using a FACScan analyzer. The purity of mitochondrial preparations was determined by staining with NAO [18]. Fig. 1A shows the FSC/SSC plot of the isolated rat liver mitochondria. We gated the largest population with reasonable FSC values as R1, assuming that they are mitochondrial fraction. When the gated R1 population was analyzed for NAO fluorescence, almost all events were positive for NAO (Fig. 1B), which confirmed that they were mitochondrial fraction. Thus, the gated R1 events were analyzed in the following experiments.
Fig. 1.
Selection of mitochondria by the light scattering profile and NAO staining. A) On the FSC/SSC plot of the isolated mitochondria, we gated the largest population with reasonable FSC values as R1, assuming that they are mitochondrial fraction. B) When the gated R1 fraction was analyzed for NAO fluorescence, almost all events in the gate R1 were positive for NAO (orange line) when compared to samples without NAO staining (gray area), which confirmed that they were mitochondrial fraction.
Effect of succinate and Ca2+ on the membrane potential of isolated mitochondria
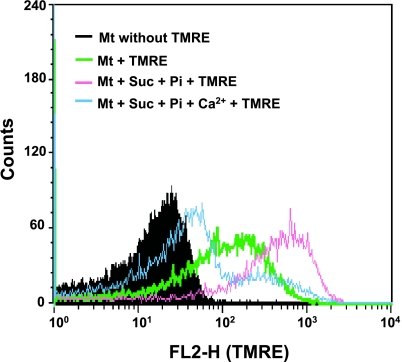
It is well-known that mitochondrial membrane depolarization occurs prior to the occurrence of MPT [4]. We tested whether the flow cytometric technique was useful for the analysis of the mitochondrial membrane depolarization, and the effect of Ca2+ on TMRE fluorescence intensity. When the mitochondria were added with only TMRE, FL2-H intensity increased (Fig. 2), probably due to the endogenous membrane potential. Adding Pi and succinate, a respiratory substrate, further increased the intensity of fluorescence. In contrast, exogenously-added Ca2+ decreased the FL2-H fluorescence and the peak shifted to the left, which indicated depolarization (Fig. 2). These results indicate that flow cytometry is useful for the analysis of the sequence of MPT in isolated mitochondria.
Fig. 2.
Effect of succinate and Ca2+ on TMRE fluorescence intensity. Membrane depolarization was analyzed by flow cytometry for changes in FL2-H in the gated R1 events. Membrane potential was detected by TMRE. Mitochondrial background fluorescence (without TMRE staining) (black area). Membrane potential in the absence of Pi and succinate (Suc) (green line) and in the presence of Pi and Suc (pink line). Induction of membrane depolarization by 40 µM Ca2+ for 5 min incubation in the presence of respiratory substrate (blue line). Similar results were obtained in 3 separate experiments.
Effect of succinate and cyclosporine A on Ca2+-induced mitochondrial events
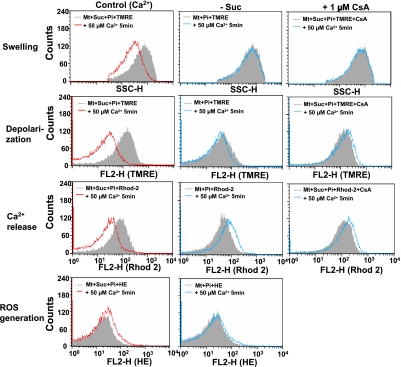
Since Ca2+-induced MPT occurred by some energy-dependent and CsA-inhibitable mechanism, we analyzed the effects of succinate and CsA on various events observed with MPT. In the presence but not in the absence of succinate, ROS generation, depolarization, swelling and Ca2+ release were observed with Ca2+-treated mitochondria (Fig. 3). All of these Ca2+-induced events associated with MPT of Ca2+-treated mitochondria were suppressed by CsA (Fig. 3). Since CsA affected the fluorescence intensity of HE, ROS generation by mitochondria could not be measured.
Fig. 3.
Effect of respiratory substrate and cyclosporine A on the Ca2+-induced swelling, depolarization, Ca2+ release and ROS generation of isolated mitochondria. Experimental conditions were the same as described in Figure 2. Membrane depolarization, Ca2+-release and ROS generation were analyzed with TMRE, Rhod 2 and hydroethidine (HE), respectively, and detected with flow cytometry for changes in FL2-H in the gated R1. Mitochondrial swelling was also analyzed simultaneously with these events by SSC. (Left, Control) MPT was induced by addition of 50 µM Ca2+ for 5 min at 25°C. Mitochondria were incubated in the standard medium containing 2.5 mM succinate and 0.5 mM Pi before addition of Ca2+. (Center, -Succ) The effect of the absence of respiratory substrate on the Ca2+-induced MPT. Mitochondria were incubated in standard medium containing 0.5 mM Pi without succinate (Suc). (Right, +CsA) The effect of cyclosporine A on the Ca2+-induced MPT. Mitochondria were incubated in the presence of 1 µM CsA in the standard medium containing 2.5 mM succinate and 0.5 mM Pi before addition of Ca2+. Similar results were obtained in 3 separate experiments.
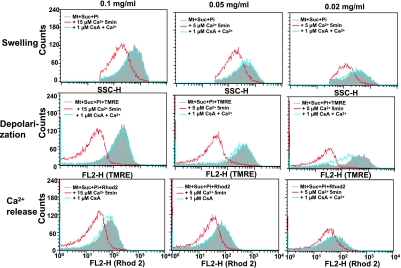
Although most experiments were carried out using 0.1∼1 mg mitochondrial protein/ml, the extent of the Ca2+-induced events in mitochondria occurred independently from these concentrations. At protein concentrations between 0.02 to 0.1 mg/ml, mitochondria showed typical patterns of depolarization, Ca2+ release and swelling 5 min after the treatment with 5∼15 µM Ca2+ (Fig. 4). These results indicate that various functions can be analyzed with a relatively small amount of mitochondria.
Fig. 4.
Effect of mitochondrial concentration on the flow cytometric analysis of mitochondrial functions. Experimental conditions were the same as described in Fig. 3. Used mitochondrial concentrations were 0.02 to 0.1 mg/ml. Used Ca2+ concentrations were 5∼15 µM. Similar results were obtained in 3 separate experiments.
Sequence of events occurring with Ca2+-induced membrane permeability transition
To determine the sequence of events occurring with Ca2+-induced mitochondrial MPT, flow cytometric analysis of swelling, depolarization, Ca2+ release, and ROS generation was carried out with isolated mitochondria which were stained with either TMRE, Rhod 2, or HE for analysis. The mitochondrial swelling decrease in SSC of Argon laser at 488 nm was comparable to the decrease in light scattering at 540 nm in a fluorescence spectrophotometer (data not described) [16]. As shown in Fig. 5, Ca2+-induced ROS generation was detectable from the increase in FL2-H fluorescence (green line) of HE. The increased HE fluorescence (green line) was detectable before the onset of mitochondrial swelling detected in SSC-H (Fig. 5A). The decrease of TMRE fluorescence in FL2-H (green line) reflecting Ca2+-induced depolarization occurred more rapidly than mitochondrial swelling (Fig. 5B). In contrast, mitochondrial swelling occurred more rapidly than the release of Ca2+ detected by the decrease of Rhod 2 fluorescence in FL2-H (green line in Fig. 5C). ROS generation was also detectable from the increase in the fluorescence of H2DCF-DA (data not shown).
Fig. 5.
Causal sequence of various events occurring in Ca2+-induced MPT. Experimental conditions were the same as described in Fig. 3. A) Effect of Ca2+ on the ROS generation of mitochondria. B) Effect of Ca2+ on the depolarization of mitochondrial membrane. C) Effect of Ca2+ on the release of loaded Ca2+ from mitochondria. The experiments B and C were performed using different mitochondrial preparations. Thus, the time course of the mitochondrial swelling was slightly different. Similar results were obtained in 3 separate experiments.
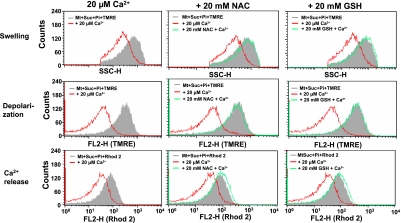
Effect of antioxidants on the Ca2+-induced changes in mitochondrial functions
Since ROS generation is one of the early events in Ca2+-induced MPT, we tested the effect of antioxidants on the Ca2+-induced depolarization, swelling and Ca2+ release of mitochondria. Reduced GSH, a typical hydrophilic antioxidant, suppressed the Ca2+-induced swelling, depolarization and release of Ca2+ in isolated mitochondria in a concentration dependent manner (Fig. 6). Similar effects were also observed with NAC, a hydrophilic antioxidant. We also studied ROS production by HE and, contrary to our expectation, found that HE fluorescence increased in the presence of GSH and NAC (data not shown). Additional experiment showed that even without mitochondria, GSH increased the HE fluorescence in xanthine-xanthine oxidase system. Thus, HE may not be a suitable ROS detector when GSH and NAC were present in the system.
Fig. 6.
Effects of NAC and GSH on the Ca2+-induced MPT in isolated mitochondria analyzed by flow cytometry. Experimental conditions were the same as described for Fig. 3. 20 mM NAC or GSH were added in the standard medium containing 2.5 mM succinate and 0.5 mM Pi before addition of Ca2+.
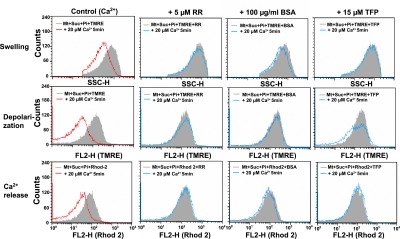
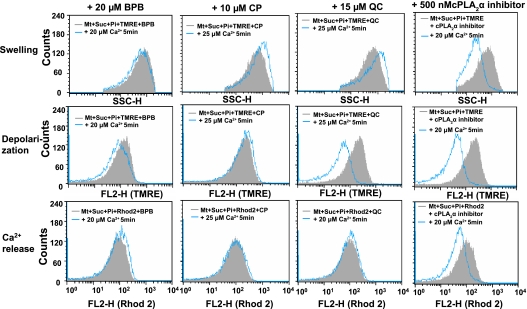
Effect of RR and inhibitors of phospholipase A2 on Ca2+-induced swelling, depolarization, and Ca2+-release of mitochondria
To elucidate the mechanism of Ca2+-induced MPT, we tested the effect of RR, an inhibitor of Ca2+ uniporter [26] that inhibits Ca2+ influx into mitochondrial matrix, on mitochondrial swelling, depolarization and release of loaded Ca2+. Analysis using SSC-H, FL2-H (TMRE), and FL2-H (Rhod 2) revealed that Ca2+-induced mitochondrial swelling, depolarization and Ca2+ release were suppressed by RR (Fig. 7). Similar inhibition was observed with BSA that binds free fatty acid. Furthermore, various inhibitors of phospholipase A2 (PLA2), such as TFP, BPB, CP and QC suppressed the Ca2+-induced mitochondrial swelling, depolarization and Ca2+ release [27–32] (Fig. 8). In contrast, inhibitor of cytosolic cPLA2 [32] failed to suppress these changes induced by Ca2+. These results suggested that both Ca2+ uniporter and PLA2 play important role in the mechanism of Ca2+-induced MPT. Furthermore, PLA2 inhibitors did not affect significantly the ROS generation demonstrated by HE fluorescence (data not shown).
Fig. 7.
Effect of RR, BSA and TFP on the various Ca2+-induced events in isolated mitochondria. Experimental conditions were the same as described for Fig. 3. Various reagents were added in the standard medium before addition of Ca2+. The concentrations of RR, BSA and TFP were 5 µM, 100 µg/ml and 15 µM, respectively.
Fig. 8.
Effect of PLA2 inhibitors on the Ca2+-induced depolarization, swelling and Ca2+-release of isolated mitochondria. Experimental conditions were the same as described for Fig. 7. The concentration used of BPB, CP, QC and cPLA2α inhibitor were 20 µM, 10 µM, 15 µM and 0.5 µM, respectively. Similar results were obtained in 3 separate experiments.
Discussion
The present work describes the sequence of events that elicited Ca2+-induced MPT in isolated mitochondria without being affected by cytosol and other organelles. Kinetic analysis using FACScan equipment revealed that the sequence of events occurring during the process of Ca2+-induced MPT were Ca2+-uptake into mitochondria, which was followed by ROS generation and activation PLA2, depolarization, swelling, and then efflux of the loaded Ca2+.
In this experiment we measured mitochondrial swelling by SSC, a parameter for the complexity of the target object, and not by FSC, a parameter for the object size. This was because the actual measurement showed that SSC was more sensitive than FSC in the detection of mitochondrial swelling. Probably the mitochondrial swelling result in the simplification of inner membrane structure and SSC decreases more sensitively than the increase in FSC [33, 34].
Recent studies using newly developed multi channel analyzers have revealed that selective ion leaks occur prior to the onset of permeability transition [35]. Although Ca2+ plays important roles in cell signaling for cell survival, it accumulates in mitochondria by an energy-dependent mechanism and triggers the reaction causing MPT, a prerequisite to cell death. We previously described that CsA inhibited the ROS generation from mitochondria [10]. The present work demonstrates that ROS generation is an initial step of the sequence of events triggering Ca2+-induced MPT of mitochondria.
In the present experiments, it was found that inhibitors of Ca2+-induced PLA2, RR and BSA suppressed the various events in MPT. These results suggested that PLA2 might affect the early events in MPT. Several investigators have reported that Ca2+ accumulated in mitochondria stimulated PLA2 and released free fatty acid and lysophosphatides, which activate the caspase cascade [36–39]. RR suppressed the uniport channel found in the inner mitochondrial membrane [26, 40]. It is known that free fatty acids elicit CsA-sensitive MPT and induce mitochondrial swelling [41–43] by a mechanism that is suppressed by fatty acid binding BSA [27, 28]. In this context, ischemia/reperfusion increased free fatty acids in rat brain, and CsA and TFP effectively suppressed the reperfusion-induced release of fatty acids [37]. These results indicate that MPT seems to involve the uncoupling effect of fatty acids generated by activated PLA2 [43]. However, it was reported that mitochondrial Ca2+ could be released spontaneously by MPT without generating free fatty acid although long-term incubation of mitochondria significantly increased the products of PLA2 [32]. This result suggests that the accumulation of fatty acids in mitochondria might be the consequence rather than the cause of MPT, and that free fatty acid generated in mitochondria might sustain the permeable state.
It is well known that PLA2 consists of a wide variety of enzymes [44]. Thus, it is very important to identify the isoform involving in MPT mechanism. The PLA2 isoform that is localized within mitochondria is different from the cytosolic isoform of PLA2 (cPLA2), and is most likely to be a low-molecular-mass PLA2 [45], which belong to group IIA PLA2s [46]. It is interesting to note that TFP, BPB, CP and QC, but not cPLA2α inhibitor, are potent inhibitors for group IIA PLA2 [45, 47–49]. This finding indicates that the group IIA PLA2s are involved in the Ca2+-induced mitochondrial swelling. In addition, recent report showed that Ca2+-independent PLA2γ (iPLA2γ) is also localized in mitochondria, and is involved in Ca2+-induced mitochondrial MPT [50]. Thus, the possible involvement of this isoform should be studied further.
Since Ca2+-induced MPT is associated with ROS generation, some antioxidants were expected to inhibit the occurrence of mitochondrial swelling and depolarization [12, 51–53]. However, only limited information is available for the inhibitory effect of antioxidant on mitochondrial swelling [12, 54]. Recent studies showed that some peptide having strong antioxidant activity accumulated in mitochondria and inhibited the Ca2+-induced swelling [55, 56]. Since thiol-specific antioxidants suppressed the Ca2+-induced swelling of mitochondria, Ca2+-induced reactions might involve the oxidation of the critical thiol groups such as those in adenine nucleotide translocator (ANT) [10, 52, 53]. In this context, Ca2+ has been postulated to modify the reactivity of mitochondrial membrane protein thiols with N-ethylmaleimide and mersalyl [57]. The present work showed that antioxidant NAC and GSH inhibited the occurrence of Ca2+-induced mitochondrial swelling and depolarization. These observations are consistent with the hypothesis that oxidation of critical thiol underlies the mechanisms for the induction of Ca2+-induced MPT and mitochondrial dysfunction [10].
Acknowledgement
This work was supported in part by grants from the Ministry of Education, Science and Culture of Japan, and the Eisai Co.
Abbreviations
- ANT
adenine nucleotide translocator
- BPB
bromophenacyl bromide
- BSA
bovine serum albumin
- diS-C3-(5)
3,3'-dipropyl-2,2'-thiodicarbocyanine iodide
- CP
chlorpromazine
- HE
hydroethidine
- H2DCF-DA
2',7'-dichlorodihydrofluorescein diacetate
- CsA
cyclosporine A
- MPT
membrane permeability transition
- NAC
N-acetylcysteine
- NAO
10-nonyl acridine orange
- PLA2
phospholipase A2
- QC
quinacrine
- Rhod 2
1-[2-Amino-5-(-dimethylamino-6-dimethylammonio-9-xanthenyl)phenoxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N',N'-tetraacetic acid
- RR
ruthenium red
- ROS
reactive oxygen species
- SSC
side scatter
- FSC
forward scatter
- TFP
trifluoperazine
- TMRE
tetramethylrhodamine-ethyl-ester
References
- 1.Shi L., Kraut R.P., Aebersold R., Greenberg A.H. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J. Exp. Med. 1992;175:553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi L., Kam C.M., Powers J.C., Aebersold R., Greenberg A.H. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J. Exp. Med. 1992;176:1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi L., Mai S., Israels S., Browne K., Trapani J.A., Greenberg A.H. Granzyme B (GraB) autonomously crosses the cell membrane and perforin Initiates apoptosis and GraB nuclear localization. J. Exp. Med. 1997;185:855–866. doi: 10.1084/jem.185.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoratti M., Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 5.Kristal B.S., Brown A.M. Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J. Biol. Chem. 1999;274:23169–23175. doi: 10.1074/jbc.274.33.23169. [DOI] [PubMed] [Google Scholar]
- 6.Oyadomari S., Araki E., Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002;7:335–345. doi: 10.1023/a:1016175429877. [DOI] [PubMed] [Google Scholar]
- 7.Ishisaka R., Utsumi T., Yabuki M., Kanno T., Furuno T., Inoue M., Utsumi K. Activation of caspase-3-like protease by digitonin-treated lysosomes. FEBS Lett. 1998;435:233–236. doi: 10.1016/s0014-5793(98)01080-1. [DOI] [PubMed] [Google Scholar]
- 8.Mancini M., Nicholson D.W., Roy S., Thornberry N.A., Peterson E.P., Casciola-Rosen L.A., Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J. Cell Biol. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushnareva Y., Haley L., Sokolove P. The role of low (<or = 1 mM) phosphate concentrations in regulation of mitochondrial permeability: modulation of matrix free Ca2+ concentration. Arch. Biochem. Biophys. 1999;363:155–162. doi: 10.1006/abbi.1998.1039. [DOI] [PubMed] [Google Scholar]
- 10.Kanno T., Sato E.F., Utsumi T., Yoshioka T., Inoue M., Utsumi K. Oxidative stress underlies the mechanism for Ca2+-induced permeability transition of mitochondria. Free Rad. Res. 2004;38:27–35. doi: 10.1080/10715760310001626266. [DOI] [PubMed] [Google Scholar]
- 11.Kowaltowski A.J., Castilho R.F., Grijalba M.T., Bechara E.J., Vercesi A. Effect of inorganic phosphate concentration on the nature of inner mitochondrial membrane alterations mediated by Ca2+ ions. A proposed model for phosphate-stimulated lipid peroxidation. J. Biol. Chem. 1996;271:2929–2934. doi: 10.1074/jbc.271.6.2929. [DOI] [PubMed] [Google Scholar]
- 12.Kowaltowski A.J., Naia-da-Silva E.S., Castilho R.F., Vercesi A.E. Ca2+-stimulated mitochondrial reactive oxygen species generation and permeability transition are inhibited by dibucaine or Mg2+ Arch. Biochem. Biophys. 1998;359:77–81. doi: 10.1006/abbi.1998.0870. [DOI] [PubMed] [Google Scholar]
- 13.Kowaltowsk A.J., Castilho R.F., Vercesi A.E. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- 14.Le Bras M., Clement M.V., Pervaiz S., Brenner C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol. Histopathol. 2005;20:205–219. doi: 10.14670/HH-20.205. [DOI] [PubMed] [Google Scholar]
- 15.Kim J.S., Jin Y., Lemasters J.J. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2024–2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 16.Mattiasson G. Analysis of mitochondrial generation and release of reactive oxygen species. Cytometry A. 2004;62:89–96. doi: 10.1002/cyto.a.20089. [DOI] [PubMed] [Google Scholar]
- 17.Mattiasson G. Flow cytometric analysis of isolated liver mitochondria to detect changes relevant to cell death. Cytometry A. 2004;60:145–154. doi: 10.1002/cyto.a.20024. [DOI] [PubMed] [Google Scholar]
- 18.Mattiasson G., Friberg H., Hansson M., Wieloch T. Flow cytometric analysis of mitochondria from CA1 and CA3 regions of rat hippocampus reveals differences in permeability transition pore activation. J. Neurochem. 2003;87:532–544. doi: 10.1046/j.1471-4159.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 19.Hogeboom G.H. In: Colowick, S.P., Kaplan, N.O., eds., Methods Enzymol., vol. 1. Academic Press; New York: 1995. Fractionation cell components and animal tissues; pp. 16–19. [Google Scholar]
- 20.Jacobson J., Duchen M.R., Heales S.J. Intracellular distribution of the fluorescent dye nonyl acridine orange responds to the mitochondrial membrane potential: implications for assays of cardiolipin and mitochondrial mass. J. Neurochem. 2002;82:224–233. doi: 10.1046/j.1471-4159.2002.00945.x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson K.M., Monette J.S., Ross M.F., Hagen T.M., Murphy M.P., Beckman J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Nat. Acad. Sci. U S A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao H., Kalivendi S., Zhang H., Joseph J., Nithipatikom K., Vasquez-Vivar J., Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 23.Boitier E., Rea R., Duchen M.R. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J. Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond R.M., Mix T.C., Tuft R.A., Walsh J.V., Jr., Fay F.S. Mitochondrial Ca2+ homeostasis during Ca2+ influx and Ca2+ release in gastric myocytes from Bufo marinus. J. Physiol. 2000;522 (Pt 3):375–390. doi: 10.1111/j.1469-7793.2000.t01-2-00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 26.Bae J.H., Park J.W., Kwon T.K. Ruthenium red, inhibitor of mitochondrial Ca2+ uniporter, inhibits curcumin-induced apoptosis via the prevention of intracellular Ca2+ depletion and cytochrome c release. Biochem. Biophys. Res. Commun. 2003;303:1073–1079. doi: 10.1016/s0006-291x(03)00479-0. [DOI] [PubMed] [Google Scholar]
- 27.Birkett D.J., Myers S.P., Sudlow G. The fatty acid content and drug binding characteristics of commercial albumin preparations. Clin. Chim. Acta. 1978;85:253–258. doi: 10.1016/0009-8981(78)90302-9. [DOI] [PubMed] [Google Scholar]
- 28.Furuno T., Kanno T., Arita K., Asami M., Utsumi T., Doi Y., Inoue M., Utsumi K. Roles of long chain fatty acids and carnitine in mitochondrial membrane permeability transition. Biochem, Pharmacol. 2001;62:1037–1046. doi: 10.1016/s0006-2952(01)00745-6. [DOI] [PubMed] [Google Scholar]
- 29.Broekemeier K.M., Schmid P.C., Schmid H.H., Pfeiffer D.R. Effects of phospholipase A2 inhibitors on ruthenium red-induced Ca2+ release from mitochondria. J. Biol. Chem. 1985;260:105–113. [PubMed] [Google Scholar]
- 30.Gogvadze V.G., Brustovetsky N.N., Zhukova A.A. The role of phospholipase A2 in lipid peroxidation-induced fall of membrane potential of rat liver mitochondria. FEBS Lett. 1990;264:168–170. doi: 10.1016/0014-5793(90)80240-j. [DOI] [PubMed] [Google Scholar]
- 31.Ono T., Yamada K., Chikazawa Y., Ueno M., Nakamoto S., Okuno T., Seno K. Characterization of a novel inhibitor of cytosolic phospholipase A2alpha, pyrrophenone. Biochem. J. 2002;363 (Pt 3):727–735. doi: 10.1042/0264-6021:3630727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rustenbeck I., Munster W., Lenzen S. Relation between accumulation of phospholipase A2 reaction products and Ca2+ release in isolated liver mitochondria. Biochim. Biophys. Acta. 1996;1304:129–138. doi: 10.1016/s0005-2760(96)00113-0. [DOI] [PubMed] [Google Scholar]
- 33.Deamer D.W., Utsumi K., Packer L. Oscillatory states of mitochondria. 3. Ultrastructure of trapped conformational states. Arch. Biochem. Biophys. 1967;121:641–651. doi: 10.1016/0003-9861(67)90049-5. [DOI] [PubMed] [Google Scholar]
- 34.Shalbuyeva N., Brustovetsky T., Bolshakov A., Brustovetsky N. Calcium-dependent spontaneously reversible remodeling of brain mitochondria. J. Biol. Chem. 2006;281:37547–37558. doi: 10.1074/jbc.M607263200. [DOI] [PubMed] [Google Scholar]
- 35.Krasnikov B.F., Zorov D.B., Antonenko Y.N., Zaspa A.A., Kulikov I.V., Kristal B.S., Brown A.M. Comparative kinetic analysis reveals that inducer-specific ion release precedes the mitochondrial permeability transition. Biochim. Biophys. Acta. 2005;1708 (3):375–392. doi: 10.1016/j.bbabio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Adibhatla R.M., Hatcher J.F. Phospholipase A2. Phydroxyl radicals, and lipid peroxidation in cerebral ischemia. Free Rad. Biol. Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 37.Phillis J.W., Diaz F.G., O’Regan M.H., Pilitsis J.G. Effects of immunosuppressants, calcineurin inhibition, and blockade of endoplasmic reticulum calcium channels on free fatty acid efflux from the ischemic/reperfused rat cerebral cortex. Brain Res. 2002;957:12–24. doi: 10.1016/s0006-8993(02)03578-3. [DOI] [PubMed] [Google Scholar]
- 38.Virmani A., Gaetani F., Imam S., Binienda Z., Ali S. Possible mechanism for the neuroprotective effects of L-carnitine on methamphetamine-evoked neurotoxicity. Ann. N. Y. Acad. Sci. 2003;993:197–207. doi: 10.1111/j.1749-6632.2003.tb07530.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoyt K.R., Sharma T.A., Reynolds I.J. Trifluoperazine and dibucaine-induced inhibition of glutamate-induced mitochondrial depolarization in rat cultured forebrain neurones. Br. J. Pharmacol. 1997;122:803–808. doi: 10.1038/sj.bjp.0701442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreau B., Nelson C., Parekh A.B. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr. Biol. 2006;16:1672–1677. doi: 10.1016/j.cub.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 41.Arita K., Yamamoto Y., Takehara Y., Utsumi T., Kanno T., Miyaguchi C., Akiyama J., Yoshioka T., Utsumi K. Mechanisms of enhanced apoptosis in HL-60 cells by UV-irradiated n-3 and n-6 polyunsaturated fatty acids. Free Rad. Biol. Med. 2003;35:189–199. doi: 10.1016/s0891-5849(03)00310-1. [DOI] [PubMed] [Google Scholar]
- 42.Pastorino J.G., Snyder J.W., Serroni A., Hoek J.B., Farber J.L. Cyclosporin and carnitine prevent the anoxic death of cultured hepatocytes by inhibiting the mitochondrial permeability transition. J. Biol. Chem. 1993;268:13791–11378. [PubMed] [Google Scholar]
- 43.Garcia N., Correa F., Chavez E. On the role of the respiratory complex I on membrane permeability transition. J. Bioenerg. Biomembr. 2005;37:17–23. doi: 10.1007/s10863-005-4119-9. [DOI] [PubMed] [Google Scholar]
- 44.Six D.A., Dennis E.A. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim. Biophys. Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 45.Guidarelli A., Cantoni O. Pivotal role of superoxides generated in the mitochondrial respiratory chain in peroxynitrite-dependent activation of phospholipase A2. Biochem. J. 2002;366 (Pt 1):307–314. doi: 10.1042/BJ20020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tischfield J.A. A reassessment of the low molecular weight phospholipase A2 gene family in mammals. J. Biol. Chem. 1997;272:17247–17250. doi: 10.1074/jbc.272.28.17247. [DOI] [PubMed] [Google Scholar]
- 47.Madesh M., Balasubramanian K.A. Activation of liver mitochondrial phospholipase A2 by superoxide. Arch. Biochem. Biophys. 1997;346:187–192. doi: 10.1006/abbi.1997.0288. [DOI] [PubMed] [Google Scholar]
- 48.Baek S.H., Takayama K., Kudo I., Inoue K., Lee H.W., Do J.Y., Chang H.W. Detection and characterization of extracellular phospholipase A2 in pleural effusion of patients with tuberculosis. Life Sci. 1991;49:1095–1102. doi: 10.1016/0024-3205(91)90597-5. [DOI] [PubMed] [Google Scholar]
- 49.Lindahl M., Tagesson C. Selective inhibition of group II phospholipase A2 by quercetin. Inflammation. 1993;17:573–582. doi: 10.1007/BF00914195. [DOI] [PubMed] [Google Scholar]
- 50.Kinsey G.R., McHowat J., Patrick K.S., Schnellmann R.G. Role of Ca2+-independent phospholipase A2gamma in Ca2+-induced mitochondrial permeability transition. Pharmacol. Exp. Ther. 2007;321:707–715. doi: 10.1124/jpet.107.119545. [DOI] [PubMed] [Google Scholar]
- 51.Kowaltowski A.J., Castilho R.F., Vercesi A.E. Ca2+-induced mitochondrial membrane permeabilization: role of coenzyme Q redox state. Am. J. Physiol. 1995;269 (1 Pt 1):C141–147. doi: 10.1152/ajpcell.1995.269.1.C141. [DOI] [PubMed] [Google Scholar]
- 52.Kowaltowski A.J., Netto L.E., Vercesi A.E. The thiol-specific antioxidant enzyme prevents mitochondrial permeability transition. Evidence for the participation of reactive oxygen species in this mechanism. J. Biol. Chem. 1998;273:12766–12769. doi: 10.1074/jbc.273.21.12766. [DOI] [PubMed] [Google Scholar]
- 53.Kowaltowski A.J., Castilho R.F., Vercesi A.E. Opening of the mitochondrial permeability transition pore by uncoupling or inorganic phosphate in the presence of Ca2+ is dependent on mitochondrial-generated reactive oxygen species. FEBS Lett. 1996;378:150–152. doi: 10.1016/0014-5793(95)01449-7. [DOI] [PubMed] [Google Scholar]
- 54.Oliveira P.J., Esteves T., Rolo A.P., Monteiro P., Goncalves L., Palmeira C.M., Moreno A.J. Carvedilol: relation between antioxidant activity and inhibition of the mitochondrial permeability transition. Rev. Port. Cardiol. 2003;22:55–62. [PubMed] [Google Scholar]
- 55.Zhao K., Luo G., Giannelli S., Szeto H.H. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem. Pharmacol. 2005;70:1796–1806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 56.Zhao K., Zhao G.M., Wu D., Soong Y., Birk A.V., Schiller P.W., Szeto H.H. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J. Biol. Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 57.Kowaltowski A.J., Vercesi A.E., Castilho R.F. Mitochondrial membrane protein thiol reactivity with N-ethylmaleimide or mersalyl is modified by Ca2+: correlation with mitochondrial permeability transition. Biochim. Biophys. Acta. 1997;1318:395–402. doi: 10.1016/s0005-2728(96)00111-9. [DOI] [PubMed] [Google Scholar]