Abstract
The effects of pyrroloquinoline quinone (PQQ) and coenzyme Q10 (Co Q10), either alone or together, on the learning ability and memory function of rats were investigated. Rats fed a PQQ-supplemented diet showed better learning ability than rats fed a CoQ10-supplemented diet at the early stage of the Morris water maze test. The combination of both compounds resulted in no significant improvement in the learning ability compared with the supplementation of PQQ alone. At the late stage of the test, rats fed PQQ-, CoQ10- and PQQ + CoQ10-supplemented diets showed similar improved learning abilities. When all the groups were subjected to hyperoxia as oxidative stress for 48 h, rats fed the PQQ- and CoQ10 supplemented diets showed better memory function than the control rats. The concurrent diet markedly improved the memory deficit of the rats caused by oxidative stress. Although the vitamin E-deficient rats fed PQQ or CoQ10 improved their learning function even when subjected to hyperoxia, their memory function was maintained by PQQ rather than by CoQ10 after the stress. These results suggest that PQQ is potentially effective for preventing neurodegeneration caused by oxidative stress, and that its effect is independent of either antioxidant’s interaction with vitamin E.
Keywords: cognitive deficit, oxidative stress, pyrroloquinoline quinone, coenzyme Q10
Introduction
Reactive oxygen species (ROS) generated through oxidative stress experienced over a long period during aging induce oxidative changes to proteins, lipids, and DNAs in living tissues [1–5]. Since the brain is more vulnerable to oxidative stress than other organs, ROS have been considered to attack neurons in the brain, thereby inducing neurodegenerative diseases including Alzheimer’s disease and Parkinsonism. In a model system of neurodegeneration caused by oxidative stress, hyperbaric oxygen significantly induces deficits of cognitive performance; that is, deficits in learning ability and memory retention, in rats accompanied by the delayed-type apoptosis of pyramidal cells and the accumulation of amyloid β-like substances in the CA 1 region of the hippocampus of the brain [6–8]. In association with these changes caused by oxidative stress, the levels of thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides, F2-isoprostane and conjugated dienes increase significantly with oxidative stress in the brain [2, 9], and the activities of antioxidative enzymes and vitamin E content in the brain decrease markedly [3]. These abnormalities are also observed in both normal old rats and vitamin E-deficient young rats not subjected to oxidative stress [6]. In contrast, young rats fed vitamin E-supplemented diet show no such abnormalities even when placed in hyperbaric atmosphere [8]. Therefore, these results suggest that the impairment in cognitive function during aging is caused by oxidative stress, and that vitamin E protects neurons from oxidative damage.
The effect of antioxidants and antioxidant-rich extracts from natural products such as coenzyme Q10 (CoQ10) [10], vitamin C and β-carotene [11], lipoic acid [12], melatonin [13], ginkgo biloba [14], apple juice [15], cocoa [16], and green tea [17], on cognitive deficit have been widely investigated. On the basis of these findings, antioxidants have been used in the treatment of various types of neurodegenerative disease. In fact, it has been revealed that long-term high dose vitamin E supplementation in the elderly significantly enhances cognitive function [18–19]. A clinical trial on vitamin E supplementation in patients with moderately severe Alzheimer’s disease showed delays in institutionalization and the onset of severe dementia [20]. Thus, it seems likely that antioxidants prevent or improve cognitive impairment.
Pyrroloquinoline quinone (PQQ), a cofactor of dehydrogenase and amine oxidase, increases the production of the nerve growth factor (NGF), and protects N-methyl-D-aspartate (NMDA) receptors by a direct oxidation of the receptor redox site [21]. PQQ also protects neurons from NMDA toxicity by suppressing peroxynitrite and stimulates NGF production [21–23]. Furthermore, PQQ acts as an antioxidant against lipid peroxidation [24]. Thus, it is likely that PQQ improves cognitive deficit caused by oxidative stress, similarly to vitamin E [7]. To test this possibility, here, we examined the effects of PQQ, CoQ10 and their combination on the cognitive deficit of rats caused by hyperoxia using a Morris water maze test, because the concurrent administration of vitamin E and CoQ10 has been shown to improve learning in mice [10].
Materials and Methods
Animals
All animal experiments were performed with the permission of the Animal Protection and Ethics Committee of the Shibaura Institute of Technology. Male Wistar rats (3 months old, Japan SLC Co., Hamamatsu, Japan) and rats fed an antioxidant-supplemented diet (3 months old, fed 20 mg of PQQ, 300 mg of Co Q10, 200 mg of R,R,R-α-tocopherol or 20 mg of PQQ + 300 mg of Co Q10/kg·body weight/day for 9 weeks from 4 weeks of age) were used in this study. To assess the effect of oxidative stress on memory function, each rat was subjected to hyperoxia as oxidative stress in a 100% oxygen chamber at room temperature for 48 h, as described previously [8]. Vitamin E-deficient rats (3 months old, fed vitamin E-deficient diet for 9 weeks from 4 weeks of age; no tocopherols were detected by HPLC, Funabashi Nojyo, Chiba, Japan) were fed 20 mg of PQQ, 300 mg Co Q10, 200 mg of R,R,R-α-tocopherol or 20 mg of PQQ + 300 mg of Co Q10/kg·body weight/day for 5 weeks, and subjected to hyperoxia before the behavioral test.
Chemicals
PQQ and coenzyme Q10 were kindly supplied from Mitsubishi Gas Chemical Company, Inc. (Tokyo, Japan).
Behavioral testing
The rats were tested of their learning ability and memory using a Morris water maze apparatus (140 cm in diameter and 45 cm in height) [25]. The bottom of the pool was divided into quadrant using white lines, and the transparent platform was submerged 2 cm below the surface of the water at the center of one of the quadrants; the water was maintained at 21 ± 1°C. For pre-training, the rats were allowed to swim freely in the pool for 60 s without the platform. Daily training consisted of one trial in which the rats swam from the start point to a fixed goal; this was conducted for 20 consecutive days. Goal time and swimming distance were measured, and the rates of decreases in swimming time and distance from the start point to the platform from their values in the first trial were expressed as learning ability. After all the groups had learned the task completely, the control rats and the rats fed the antioxidant-supplemented diet were kept in 100% oxygen atmosphere as oxidative stress at 21 ± 1°C for 48 h in an oxygen chamber. The platform was removed, and the rats were placed opposite the quadrant where the platform had been located. The percentage of time spent in the quadrant where the platform had been was used as an assessment of memory retention.
Statistical analysis
The results are presented as mean ± SE. All data were assessed by ANOVA analysis and a p-value less than 0.05 was considered to be statistically significant.
Results
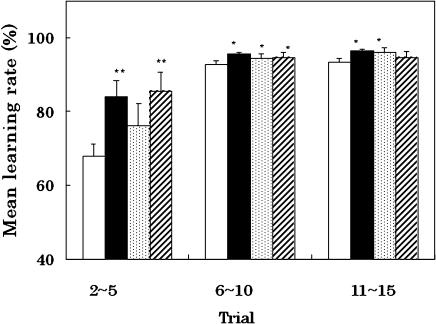
As shown in Fig. 1, although the mean learning rate of the control rats was only 67% until 5th trial, that of the rats fed a PQQ-supplemented diet was significant high at the early stage of trials. CoQ10 did not improve the learning function of the rats, so that no synergistic efficacy was observed by the concurrent supplementation of PQQ and CoQ10. At the late stage of the trials, the rats fed a PQQ-, CoQ10- and (PQQ + CoQ10)-supplemented diets showed higher learning rates than the control rats. However, the efficacies of the supplementations showed no significant difference. From these results, PQQ is likely more effective in improving the learning ability of the rats using space cognition than CoQ10 itself.
Fig. 1.
Effects of PQQ, CoQ10 and their combination on learning function of rats in a Morris water maze. Swimming frequency, once a day for 15 days for each group. Learning ability, expressed as average rate of decrease in latency time (days 2–5, 6–10 and 11–15) to find hidden platform from their values in the first trial. Open column, control rats fed normal diet; black column, rats fed PQQ-supplemented diet; dotted column, rats fed CoQ10-supplemented diet; and slashed column, rats fed concurrent diet of both antioxidants. *p<0.05 versus normal control rats at early stage, **p<0.02 versus normal control at late stage; means ± SE, n = 9 for each group of rats.
After the rats learned the location of the platform in the pool, the effect of hyperoxia as oxidative stress on memory function was assessed. When the rats were subjected to hyperoxia as oxidative stress for 48 h, they retained their memories within four days after the oxidative stress treatment. However, their memories suddenly declined five days after the oxidative stress treatment, as previously reported [8]. The rats fed either the PQQ- or CoQ10-supplemented diet showed memory retention even after the oxidative stress treatment for 48 h. Furthermore, the rats fed the concurrent diet of PQQ and CoQ10 showed marked the memory retention (Fig. 2).
Fig. 2.
Changes in memory retention of control rats fed normal diet (closed circle), and rats fed PQQ-supplemented diet (closed square), CoQ10-supplemented diet (opened triangle), and concurrent diet of both antioxidants (opened circle). Each group was subjected to hyperoxia as oxidative stress for 48 h. The arrowhead shows the endpoint of hyperoxia, and -2 day shows the start point of such stress. *p<0.05 versus normal control rats, **p<0.01 versus normal control rats, #p<0.05 versus rats fed PQQ-supplemented diet; means ± SE, n = 9 for each group for rats.
To test the possibility that the high potential of PQQ to improve cognitive function is caused by the interaction with vitamin E, because vitamin E is known to act as an antioxidant in vivo synergistically with other antioxidants such as CoQ10 and vitamin C, the learning function of the rats was assessed after PQQ or CoQ10 was supplemented to the vitamin E-deficient rats. As shown in Fig. 3, although the vitamin E-deficient rats had poor learning ability, either PQQ or CoQ10 supplementation improved their cognitive performance. The learning rates obtained were similar to those in the rats fed either concurrent diet of PQQ and CoQ10 or the vitamin E-supplemented diet [8], so that the efficacy of the concurrent diet on the improvement of learning function was not synergistic.
Fig. 3.
Effects of PQQ, CoQ10 and their combination on impairment of learning function of vitamin E-deficient rats. Swimming frequency, once a day for 15 days for each group. Solid circle, vitamin E-deficient rats; open circle, vitamin E-deficient rats fed PQQ-supplemented diet; open square, vitamin E-deficient rats fed CoQ10-supplemented diet; and triangle, vitamin E-deficient rats fed concurrent diet of both antioxidants. *p<0.001 versus vitamin E-deficient rats; means ± SE, n = 9 for each group of rats.
The memory function of the vitamin E-deficient rats kept in normal atmosphere for 48 h declined markedly. When the vitamin E-deficient rats were subjected to hyperoxia, their memory much more deteriorated as reported previously [8]. Moreover, PQQ, CoQ10 and its concurrent diet improved the memory deficit of the vitamin E-deficient rats even after they were subjected to hyperoxia. The inhibitory effect of PQQ on the memory deficit of the vitamin E-deficient rats was more effective than that of CoQ10 (Fig. 4). However, the memory function of the vitamin E-deficient rats fed the CoQ10-supplemented diet declined after 5 days. The vitamin E-deficient rats fed the concurrent diet did not showed an improvement of their memory deficit.
Fig. 4.
Effects of PQQ, CoQ10 and its combination diets on memory deficit of vitamin E-deficient rats. Closed circle, vitamin E-deficient rats without hyperoxia; closed square, vitamin E-deficient rats subjected to hyperoxia; open circle, vitamin E-deficient rats fed PQQ-supplemented diet and subjected to hyperoxia; open triangle, vitamin E-deficient rats fed CoQ10-supplemented diet and subjected to hyperoxia; closed triangle, vitamin E-deficient rats fed the concurrent diets and subjected hyperoxia. *p<0.01, **p<0.05 versus the vitamin E-deficient rats; #p<0.005 versus rats fed CoQ10-supplemented diet. The arrowhead shows the endpoint of hyperoxia, and -2 day shows the start point of such stress; means ± SE, n = 9 for each group of rats. The arrowhead shows the endpoint of hyperoxia, and -2 day shows the start point of such stress; means ± SE, n = 9 for each group of rats.
Thus, the effect of PQQ on the improvement of cognitive deficit was independent of the synergistic effect on the interaction with vitamin E.
Discussion
ROS generated by oxidative stress have been proposed to be one of the causes of neurodegenerative diseases [26]. An oxidatively damaged nervous system caused by ROS implies the induction of cognitive deficit by dysfunction in neurotransmission. Vitamin E is a potential antioxidant and can be used as a complementary intervention substance for patients with cognitive dysfunction [20, 27–28], in the same way, learning and memory deficit in rats can be prevented by antioxidants, such as vitamin E, β-carotene, and vitamin C [8, 11, 29].
In the present study, we investigated the inhibitory effects of the antioxidants PQQ and CoQ10, and their combination on the effect elicited by oxidative stress on rat cognitive performance, as assessed using a Morris water maze. From the results in this study, it is evident that the rats fed the PQQ-supplemented diet had a greater improvement in learning ability than the control rats fed normal diet, and that the rats fed the CoQ10-supplemented diet had less improvement in learning ability at early stage of the trial. The combination of PQQ and CoQ10 showed no synergistic effect throughout the trial, as shown in Fig. 1. These results suggest that although both compounds have a potent effect of improving the learning ability of the rats, PQQ does not interact synergistically with CoQ10 to induce an antioxidative event in vivo. It has been reported that vitamin E acts as a potent radical scavenger synergistically with CoQ10 for its regeneration back to reduced vitamin E, and that it improves cognitive deficit [26]. To elucidate whether PQQ and CoQ10 interact synergistically with native vitamin E in tissue to improve the learning function, the learning ability of the vitamin E-deficient rats fed PQQ or CoQ10 or their concurrent diet was assessed. Although vitamin E deficiency reduced the maximum learning ability similarly to that in the case of aged rats [8], the vitamin E-deficient young rats fed PQQ or CoQ10 or their combination showed a marked improvement in their learning ability, as shown in Fig. 3. The rats fed the vitamin E-supplemented diet revealed a similar learning ability to the respective vitamin E-deficient young rats fed the PQQ or CoQ10 or their concurrent diet [8]. These results suggest that the efficacies of PQQ and CoQ10 in improving learning ability are independent of the interaction of either antioxidant with vitamin E, and that either PQQ or CoQ10 acts individually as an effective substance for improving learning ability without interacting with each other.
To assess the change in another cognitive function, that is, memory function, the rats were kept in 100% oxygen for 48 h as a model of oxidative stress. Although it has been criticized questionably that hyperoxia induces ROS in living tissues, there are several reports on ROS being generated by hyperoxia in the lungs, heart muscle, brain and erythrocytes of several animals, and on the rate of ROS generation being proportional to the concentration of oxygen inhaled [30–31]. Thus, it is reasonable to consider that the hyperoxia used in this study induced ROS in the rat brain. It has also been reported that the activity of antioxidative enzymes for scavenging ROS changes in the brain through hyperoxia; hence, the level of lipid peroxides produced by ROS increases in the brain [20, 21, 29].
As shown in Fig. 2, the decline in the memory function of the control rats caused by the oxidative damage of the nervous system has been observed 5 days after hyperoxia as the oxidative stress. This impairment in memory function was inhibited by PQQ and CoQ10 supplementations. Interestingly, although the rats did not improved their learning ability with a concurrent diet of PQQ and CoQ10 (Fig. 1), their memory function was maintained by this combination even after they were subjected to hyperoxia. These results suggest that PQQ suppresses the impairment in memory function caused by oxidative stress synergistically with CoQ10 through its antioxidant effect. Accordingly, it seems likely that PQQ antioxidatively prevents the oxidative damage of the nervous system, and hence maintain its cognitive function. This possibility is supported by previous reports that PQQ acts as a neuroprotector that suppresses peroxynitrite formation and the redox modulation of NMDA receptor [21, 23]. However, it is impossible at present to define the mechanism of the improvement in the learning ability of rats induced by PQQ, because such improvement in learning function is implied to be independent of the antioxidant/radical scavenging ability. To determine the essential contribution of PQQ to improvement in cognitive deficit, further studies are necessary.
Acknowledgements
This study has been supported, in part, by MEXT HAITEKU (2004), a subsidy for Project Research from Shibaura Institute of Technology, and Grants-in-Aid from Mitsubishi Gas Chemical Company, Inc.
References
- 1.Lu C.Y., Lee H.C., Fahn J.J., Wei Y.H. Oxidative damage elicited by imbalance of free radical scavenging enzymes associated with large-scale mtDNA deletions in aging human skin. Mutat. Res. 1999;423:11–21. doi: 10.1016/s0027-5107(98)00220-6. [DOI] [PubMed] [Google Scholar]
- 2.Nishio T., Miyadera R., Sakai R., Abe K., Kanazawa H., Fukui K., Urano S. Increased F2-isoprostane levels in the rat brain and plasma caused by oxidative stress and aging, and inhibitory effect of vitamin E. J. Clin. Biochem. Nutr. 2006;38:161–166. [Google Scholar]
- 3.Onodera K., Omoi N., Fukui K., Hayasaka T., Shinkai T., Suzuki S., Urano S. Oxidative damage of rat cerebral cortex and hippocampus, and changes in antioxidative defense systems caused by hyperoxia. Free Rad. Res. 2003;37:367–372. doi: 10.1080/1071576031000090019. [DOI] [PubMed] [Google Scholar]
- 4.Stadtman E.R. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 5.Urano S., Sato Y., Otonari T., Makabe S., Suzuki S., Ogata M., Endo T. Aging and oxidative stress in neurodegeneration. BioFactors. 1998;7:103–112. doi: 10.1002/biof.5520070114. [DOI] [PubMed] [Google Scholar]
- 6.Fukui K., Hayasaka T., Shinkai T., Suzuki S., Abe K., Urano S. Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Annal. N.Y. Acad. Sci. 2001;928:168–175. doi: 10.1111/j.1749-6632.2001.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukui K., Omoi N., Hayasaka T., Shinkai T., Suzuki S., Abe K., Urano S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Annal. N.Y. Acad. Sci. 2002;959:275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 8.Fukui K., Takatsu H., Shinkai T., Suzuki T., Abe K., Urano S. Appearance of amyloid β-like substances and delayed-type apoptosis in rat hippocampus CA1 region through aging and oxidative stress. J. Alzheimer’s Dis. 2005;8:299–309. doi: 10.3233/jad-2005-8309. [DOI] [PubMed] [Google Scholar]
- 9.Urano S., Asai Y., Makabe S., Matsuo M., Izumiyama N., Ohtsubo K., Endo T. Oxidative injury of synapse and alteration of antioxidative defense systems in rats, and its prevention by vitamin E. Eur. J. Biochem. 1997;245:64–70. doi: 10.1111/j.1432-1033.1997.00064.x. [DOI] [PubMed] [Google Scholar]
- 10.McDonald S.R., Sohal R.S., Forster M.J. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free. Radic. Biol. Med. 2005;38:729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Bickford P.C., Gould T., Briederick L., Chadman K., Pollock D., Young D., Shukitt-Hale B., Joseph J. Antioxidant-rich diets improve cerebellar physiology and moter learning in aged rats. Brain Res. 2000;866:211–217. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- 12.Sharma M., Gupta Y.K. Effect of alpha lipoic acid on intracerebro-ventricular streptozotocin model of cognitive impairment in rat. Eur. Neuropsychopharmacol. 2003;13:241–247. doi: 10.1016/s0924-977x(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 13.Gonenc S., Uysal N., Acikgoz O.B.M., Kayatekin B.M., Sonmez A., Kiray M., Aksu I., Gulecer B., Topcu A. Effects of melatonin on oxidative stress and spatial memory impairment induced by acute ethanol treatment in rats. Physiol. Res. 2005;54:341–348. [PubMed] [Google Scholar]
- 14.Ahmad M., Saleem S., Ahmad A.S., Yousuf S., Ansari M.A., Khan M.B., Ishrat T., Chaturvedi R.K. Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced parkinsonism in rats: neurobehavioural, neurochemical and immunohisto chemical evidences. J. Neurochem. 2005;93:94–104. doi: 10.1111/j.1471-4159.2005.03000.x. [DOI] [PubMed] [Google Scholar]
- 15.Tchantchou F., Chan A., Kifle L., Ortiz D., Shea T.B. Apple juice concentrate prevents oxidative damage and impaired maze performance in aged mice. J. Alzheimer’s Dis. 2005;8:283–287. doi: 10.3233/jad-2005-8306. [DOI] [PubMed] [Google Scholar]
- 16.Francis S.T., Head K., Morris P., Macdonard I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006;47:21–23. doi: 10.1097/00005344-200606001-00018. [DOI] [PubMed] [Google Scholar]
- 17.Haque A.M., Hashimoto M., Katakura M., Tanabe Y., Hara Y., Shido O. Long-term administration of green tea catechins improves special cognition learning ability in Rats. J. Nutr. 2006;136:1043–1047. doi: 10.1093/jn/136.4.1043. [DOI] [PubMed] [Google Scholar]
- 18.Clausen J., Nielsen S.A., Kristensen M. Biochemical and clinical effects of an antioxidative supplementation of geriatric patients. A double blind study. Biol. Trace Elem. Res. 1989;20:135–151. doi: 10.1007/BF02919106. [DOI] [PubMed] [Google Scholar]
- 19.Grodstein F., Chen J., Willett W.C. High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am. J. Clin. Nutr. 2003;77:975–984. doi: 10.1093/ajcn/77.4.975. [DOI] [PubMed] [Google Scholar]
- 20.Sano M., Ernesto C., Thomas R.G., Klausber M.R., Schfer K., Grundman M., Woodbury P., Growdon J., Cotman C.W., Pfeitter E., Schneider L.S., Thal L.J. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s disease cooparative study. New Engl. J. Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 21.Aizenman E., Hartnett K.A., Zhong C., Gallop P.M., Rosenberg P.A. Interaction of the putative essential nutrient pyrroloquinoline quinine with the N-methyl-D-aspartate receptor redox modulatory site. J. Neurosci. 1992;12:2362–2369. doi: 10.1523/JNEUROSCI.12-06-02362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi K., Sasano A., Urakami T., Tsuji T., Kondo K. Stimulation of nerve growth factor production by pyrroloquinoline quinine and its derivatives in vitro and in vivo. Biosci. Biotechnol. Biochem. 1993;57:1231–1233. doi: 10.1271/bbb.57.1231. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Rosenberg P.A. The essential nutrient pyrroloquinoline quinine may act as a neuroprotectant by suppressing peroxynitrite formation. Eur J. Neurosci. 2002;16:1015–1024. doi: 10.1046/j.1460-9568.2002.02169.x. [DOI] [PubMed] [Google Scholar]
- 24.Miyauchi K., Urakami T., Abeta H., Shi H., Noguchi N., Niki E. Action of pyrroloquinoline quinine as an antioxidant against lipid peroxidation in solution. Antioxid. Redox Signal. 1999;1:547–554. doi: 10.1089/ars.1999.1.4-547. [DOI] [PubMed] [Google Scholar]
- 25.Morris R.G.M. Development of water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Harman D. A hypothesis on the pathogenesis of Alzheimer’s disease. Annals N Y Acad Sci. 1992;786:152–168. doi: 10.1111/j.1749-6632.1996.tb39059.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan A.S., Cheung M.C., Law S.C., Chan J.H., Phase H. Phase II study of alpha-tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer. 2004;100:398–404. doi: 10.1002/cncr.11885. [DOI] [PubMed] [Google Scholar]
- 28.Mecocci P., Mariani E., Comacchiola V., Polidori M.C. Antioxidants for the treatment of mild cognitive impairment. Neurol. Res. 2004;26:598–602. doi: 10.1179/016164104225017659. [DOI] [PubMed] [Google Scholar]
- 29.Delwing D., Bavaresco C.S., Monteiro S.C., Matte C., Netto C.A., Wyse T.S. α-Tocopherol and ascorbic acid prevent memory deficits provoked by chronic hyperprolinemia in rats. Behavioural Brain Res. 2006;168:185–189. doi: 10.1016/j.bbr.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Turrens J.F., Freeman B.A., Crapo J.D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch. Bichem. Biophys. 1982;217:411–121. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- 31.Yusa T., Crapo J.D., Freeman B.A. Hyperoxia enhances lung and liver nuclear superoxide generation. Biochim. Biophys. Acta. 1984;798:167–174. doi: 10.1016/0304-4165(84)90299-x. [DOI] [PubMed] [Google Scholar]