Abstract
Cephalotaxus sinensis (C. sinensis) large size, evergreen tree common in China and utilized for numerous effective pharmacological applications in Chinese traditional medicine. The hepato-renal effects of C. sinensis were evaluated in vivo using Streptozotocin (STZ)-induced diabetic rats as an tentative model. Animals were orally treated with 80% EtOH extract (aq.EE), H2O extract (WtE) and ethylacetate (EaF)/butanol fractions (BtF) of C. sinensis (200 mg/kg, b.w.) for 28 days whereas control received vehicle merely. The degree of fortification was measured by using biochemical parameters like serum transaminases (ALT and AST), alkaline phosphatase (ALP), creatinine, urea and urine sugar. Meanwhile, the histopathological studies were conducted out to support the above parameters. Administration of C. sinensis aq.EE/BtF (p<0.05) and EaF (p<0.01) patently prevented STZ-induced elevation levels of serum ALT, AST, ALP, creatinine, urea, urine sugar and increase body weight respectively, which were comparable with the standard drug tolbutamide, while WtE did not show any significant effect (p>0.05). Phytochemical studies revealed the presence of saponins, terpenes, sterols and flavonoids in C. sinensis which could be responsible for the possible hepato-renal protective action. The results sustain the fact that the extract/fractions of C. sinensis have an immense potential to be developed further into a phytomedicine.
Keywords: hepato-renal effects, HPLC, biochemical parameters, STZ-induced diabetic rats, Cephalotaxus sinensis
Introduction
The use of herbal medicine is widespread, which are used by the people for the treatment of disparate diseases even at this modern era. There are diverse medicinal plants in the world, which are the impending sources of the drugs. These drugs are invariably single plant extracts or fractions or mixtures of extracts/fractions from different plants, which have been carefully standardized for their safety and efficacy [1]. Now a days, scientists and researchers are very much tiring on research of natural plant products all over the world and a large number of substantiation have shown the immense potential of medicinal plants used traditionally [2].
Liver diseases are a leading health problem after cardiovascular disease (CVD) cancer and AIDS. During chronic liver injury, hepatic progenitor cells (HPCs; oval cells) appear in periportal liver regions, then undergo proliferation, migration and differentiation to replenish hepatocytes and biliary cells. This occurs in numerous rodent models and human diseases such as Hepatitis B, C, hemochromatosis and alcoholic liver disease [3]. Herbal drugs play a major role in the treatment of hepatic disorders. A number of medicinal plants and their formulations are used to cure hepatic disorders in traditional systems of medicine [4]. In addition to these known plants, there are unexplored group of plants used by tribal and folk medical practitioners which are a promising source of effective hepatoprotective agents [5].
The genus Cephalotaxus, evergreen trees of southern Asia, composed of 10 species, eight of which are distributed in China. Cephalotaxus (C.) sinensis (Rehd et Wile) Li, large sized, evergreen tree common in China, traditionally it is used against dyspepsia, ascariasis, inflammation and cough [6]. Investigations of the chemical constituents of extracts of Cephalotaxus sps. resulted in the isolation of lactones, flavonoids and a number of alkaloids [7–9]. Recently, osteoblast differentiation stimulating activity of biflavonoids from Cephalotaxus koreana Nakai (Cephalotaxaceae) have been reported [10]. In the screening of anti-diabetic natural substances in our labortary, it was found that the ethanol extract of the leaves of C. sinensis possess hypoglycemic activity, our recent studies also revealed that the pharmacological activity of this plant may be due to flavonoids.
The intention of the present study was to scrutinize the influence of oral administration of C. sinensis extracts and fractions on the levels of biochemical parameters and to ascertain the scientific basis for the use of this plant in the management of liver and kidney injuries using streptozotocin-induced diabetic rats.
Materials and Methods
Instruments and chemicals
An Elite P230 series HPLC–DAD system consisting of binary pump and DAD detector (DAD230, Dalian Elite, PR China) was used for acquiring chromatogram, UV-VIS 2550 spectrophotometer (Shimadzu, Japan), Biofuge 22 R (Heraeus, Germany), light microscope and rotary evaporator (PR China) were used for study. Streptozotocin (STZ), hematoxylin, eosin and assay kits for serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphates (ALP), cretinine and urea were purchased from Sigma Chemical Co., St. Louis, MO. All the other chemicals and solvents were of analytical grade.
Plant material
Leaves of Cephalotaxus sinensis were collected from Anhui province, PR China and the plant were authenticated by Professor Dr. Yulin Deng (Dean, School of Life Science and Technology, Beijing Institute of Technology, Beijing, PR China) and Associate Researcher Bing Wen (Xishuanbanna Tropical Botanical Garden, Chinese Academy of Sciences, PR China). Voucher specimen (No. S20041101) were deposited at the School Herbarium for future references.
Preliminary screening
The ethanolic extract was subjected to preliminary screening, for various active phytochemical constituents such as alkaloids, carbohydrates, steroids, protein, tannins, flavonoids, mucilage, glycosides, saponins and terpenes [11].
HPLC Analysis
HPLC analysis were performed on Elite P230 series HPLC–DAD system consisting of binary pump and DAD detector (DAD-230, Dalian Elite, PR China) A Kromasil C18 analytical column (250 mm × 4.6 mm i.d., 5 µm, AKZO Nobel Inc., Sweden) coupled with a C18 guard column (10 × 8 mm i.d., 5 µm, Tianhe Inc., PR China) was used at room temperature. The optimum conditions were as: mobile phase H2O-ACN (1:1), linear gradient mode, 5–40 min, flow-rate was 0.7 ml/min, wavelength 254 nm. For HPLC analysis ethanol extract was dissolve in methanol and filtered through a 0.22 µm membrane filter and injected (10 µl) in triplicate [12].
Acute toxicity studies
Healthy adult Wistar albino rats of either sex, starved overnight were divided into four groups (n = 6) and were orally fed with the aqueous ethanolic extract of C. sinensis in escalating dose levels of 100, 500, 1000 and 3000 mg/kg body weight [13]. The rats were pragmatic continuously for 2 h for behavioral, neurological and autonomic profiles and after a period of 24 and 72 h for any lethality or death [14].
Extraction and fractionation
About 500 g of the dry leaf powder was extracted with 80% ethanol at 80˚C for 3 h. The above procedures were repeated three time. The combined ethanolic extract were concentrated in a rotary evaporator at reduced pressure to obtain about 172.5 g (34.5%, w/w) of extract. The ethanol extract was then suspended in distilled water and partitioned sequentially with petroleum ether, chloroform, ethyl acetate and n-butanol.
Animals
Male Wistar rats (270–315 g) were selected for the study and maintained at a controlled temperature of 25–28°C with a 12 h light/dark cycle and fed a standard diet and water ad libitum. Animal studies were conducted according to the Institute of Animal Ethics Committee regulations approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Induction of diabetes
Diabetes was induced in the male rats by a single intraperitoneal injection of STZ (65 mg/kg body weight) dissolved in 10 mM sodium citrate buffer (pH 4.0), while the control group was injected with the buffer only. Stable hyperglycemia was confirmed by estimating the glucose level in urine of rats.
Experimental procedure
The rats were alienated into seven groups (six in each group). Group I, normal rats + vehicle (solution of normal saline and 3.0% tween 80); Group II, untreated diabetic + vehicle; Group III, diabetic + tolbutamide (25 mg/kg body weight, p.o.); Group IV, diabetic + 80% aqueous ethanolic extract (aq.EE); Group V, diabetic + water extract (WtE); Group VI, diabetic + ethylacetate fraction (EaF) and Group VII, diabetic + Butanol fraction (BtF) (200 mg/kg body weight) daily by gastric intubations for a period of 28 days. All the extracts and fractions were dissolves in vehicle (solution of normal saline and 3.0% tween 80). All groups were sacrificed on the 28th day in fasting condition by cervical dislocation. Body weights of all the animals were recorded weakly, prior to the treatments and sacrifice.
Biological assays
Rats of all groups were anaesthetized by ether, the blood samples of each animal were taken by puncturing retro-orbital plexus and allowed to clot for 60 min at room temperature. Serum was separated by centrifugation at 3000 rpm at 25°C for 15 min and analyzed for assorted biochemical parameters. The serum ALT [15], AST [16], ALP [17] activities, cretinine [18] and urea [19] were measured using the respective spectrophotometric diagnostic kit obtained from Biosino Biotechnology Company Ltd. (Beijing, PR China). The urine glucose level of rats was measured by benedict’s qualitative test.
Histological preparation
The liver was fixed in a neutral buffered formalin solution (10%) and dehydrated in a graded series (50%, 70%, 80%, 90%, 95%, and 100%) of ethanol. The tissue was embedded in Paraplast, sectioned into approximately 4 µm thickness and stained with hematoxylin and eosin (H&E). The tissues were observed using a light microscope.
Statistical analysis
Results were articulated as mean ± SD and all statistical comparisons were made by means of one-way ANOVA test followed by Tukey post hoc analysis and p values less than or equal to 0.05 were considered significant.
Results
Preliminary chemical tests
Our phytochemical studies indicated that ethanolic extract of leaves of C. sinensis contains alkaloids, flavonoids, glycosides, saponins terpenes and steroids, while mucilage and proteins were absent.
HPLC analysis
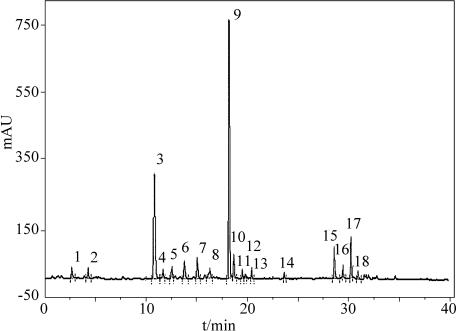
The optimized chromatogram of ethanol extract of C. sinensis was given in Fig. 1. Optimization of parameters in HPLC was done through investigating the influence of the mobile phase and detection wavelength, because these two parameters play a key role on resolution and sensitivity. In this work, a water and acetonitrile (1:1) was chosen as the mobile phase. HPLC chromatogram of ethanol extract of C. sinensis showed 18 common peaks.
Fig. 1.
HPLC chromatograph of Cephalotaxus sinensis
Acute toxicity studies
In performing preliminary tests for pharmacological activity in rats, aqueous ethanolic extract did not produce any significant changes in the auto-nomic, behavioral or neurological responses up to doses of 3000 mg/kg body wt. Acute toxicity studies revealed the non-toxic nature of the aqueous ethanolic extract of C. sinensis.
Effect on body weight and urine sugar
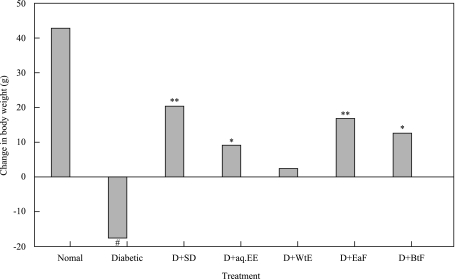
The final body weight of untreated control group was significantly increased than at the beginning of the experiment, on the contrary a significant increase urine glucose level and decrease body weight was observed in diabetic treated group (p<0.001). On the other hand, the administration of EaF (p<0.01), BtF, 80% ethanol extract (p<0.05) of C. sinensis increase body weight and decrease urine glucose levels respectively, while water extract did not show any significant effect (p>0.05). The changes in body weights and urine glucose levels in all groups of animals were given in Fig. 2 and Table 1.
Fig. 2.
Effect of treatment with 80% ethanol extract (aq.EE), water extract (WtE), ethylacetate fraction (EaF), butanol fraction (BtF) of C. sinensis and standard drug (SD) tolbutamide on changes in body weights in normal and diabetic (D) rats. (*p<0.05, **p<0.01, #p<0.001).
Table 1.
Effect of 4-week treatment with various extracts and fractions of C. sinensis on body weight (g) and urine sugar in normal and STZ induced diabetic rats.
| Group | Duration of treatment and body weight |
Urine sugar | ||
|---|---|---|---|---|
| Day 1 | Day 14 | Day 28 | ||
| Untreated (+) control | 290.12 ± 3.5 | 316.90 ± 3.7 | 332.82 ± 4.3 | — |
| Diabetic (–) control | 280.33 ± 3.2# | 265.70 ± 2.9# | 262.73 ± 3.1# | +++# |
| Diabetic + tolbutamide | 304.65 ± 4.1** | 317.20 ± 4.2*** | 324.95 ± 4.3** | +** |
| Diabetic + 80% EtOH extract | 274.35 ± 2.3* | 280.62 ± 2.4* | 283.55 ± 2.4* | +* |
| Diabetic + H2O extract | 266.20 ± 2.2 | 269.50 ± 2.3 | 272.00 ± 2.2 | ++ |
| Diabetic + EaF | 310.85 ± 4.2** | 319.75 ± 4.4** | 326.95 ± 4.3** | +** |
| Diabetic + BtF | 283.41 ± 3.1* | 293.62 ± 3.2* | 296.01 ± 3.3* | +* |
All values are expressed as mean ± S.E.M. (n = 6) except for urine sugar. Diabetic control was compared with untreated control and extracts/fractions were compared with the diabetic control. *p<0.05, **p<0.01, #p<0.001. Urine sugar (+) indicates is equal or less than 0.25%; (++) indicates more than 2% and (+++) indicates more than 5% sugar. EaF: ethylacetate fraction of C. sinensis 200 mg kg−1 day−1 p.o. for 28 days. BtF: butanol fraction of C. sinensis 200 mg kg−1 day−1 p.o. for 28 days. Tolbutamide: 25 mg kg−1 day−1 p.o. for 28 days.
Effect on biochemical parameters
In order to examine the effect of C. sinensis supplementation on the regulation of biochemical parameters of the diabetic rats, serum ALT, AST and ALP levels were determined to evaluate the hepatic functions, while creatinine and urea concentrations were studied to assess the renal functions. STZ induced significant elevations in ALT, AST, ALP, creatinine and urea serum levels (p<0.001) when compared to control values. EaF (200 mg/kg, b.w, p.o.) of C. sinensis supplementation significantly reduced the ALT, AST, ALP, creatinine and urea serum levels (p<0.01) and the activities became similar with the standard drug (tolbutamide, 25 mg/kg, b.w, p.o.) administrated group. On the other hand, the administration of BtF and 80% ethanol extract (200 mg/kg, b.w, p.o.) of C. sinensis also significantly reduced the levels of these parameters (p<0.05), while the effect of water extract was insignificant (p>0.05) as depicted in Table 2.
Table 2.
Effect of various extracts and fractions of C. sinensis on serum profile in normal and STZ induced diabetic rats after 28 days of treatment.
| Biochemical parameters | |||||
|---|---|---|---|---|---|
| Group | ALT (U/L) | AST(U/L) | ALP(U/L) | Creatinine (mg/dl) | Urea (mg/dl) |
| Untreated control | 37.60 ± 0.7 | 139.15 ± 3.6 | 120.30 ± 2.4 | 0.85 ± 0.1 | 52.50 ± 2.1 |
| Diabetic control | 68.30 ± 1.5# | 172.30 ± 4.2# | 158.35 ± 2.6# | 1.40 ± 0.3# | 84.85 ± 2.4# |
| D + tolbutamide | 44.52 ± 1.2** | 145.75 ± 3.6** | 131.25 ± 2.2** | 0.92 ± 0.1** | 59.42 ± 1.9** |
| D + 80% EE | 57.34 ± 1.3* | 155.65 ± 3.3* | 141.25 ± 2.1* | 1.16 ± 0.2* | 69.32 ± 2.2* |
| D + WtE | 64.87 ± 1.4 | 163.76 ± 3.8 | 152.72 ± 2.5 | 1.30 ± 0.3 | 79.84 ± 2.3 |
| D + EaF | 48.15 ± 1.1** | 142.89 ± 3.1** | 134.50 ± 1.9** | 0.98 ± 0.1** | 63.15 ± 2.1** |
| D + BtF | 51.84 ± 1.2* | 146.37 ± 3.4* | 137.83 ± 2.1* | 1.07 ± 0.2* | 65.70 ± 2.2* |
All values are expressed as mean ± S.E.M. (n = 6) except for urine sugar. Diabetic control was compared with untreated control and extracts/fractions were compared with the diabetic control. *p<0.05, **p<0.01, #p<0.001. D: diabetic. 80% EE: 80% ethanol extract. WtE: water extract. EaF: ethylacetate fraction of C. sinensis 200 mg kg−1 day−1 p.o. for 28 days. BtF: butanol fraction of C. sinensis 200 mg kg−1 day−1 p.o. for 28 days. Tolbutamide: 25 mg kg−1 day−1 p.o. for 28 days.
Histological appearance of the liver
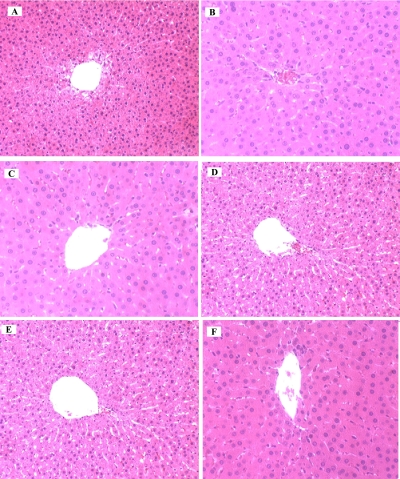
In order to obtain more information on liver damage as a result of the induction of type 1 diabetes and the possible recovery effect of C. sinensis on the hepatic injury, the histological studies of liver was carried out which were sacrificed after 28 days of the experiment (Table 3). Histology of the liver sections of normal control animals showed normal liver architecture with well brought out central vein, well-preserved cytoplasm and prominent nucleus and nucleolus (Fig. 3A). On the contrary, the STZ-induced diabetic rat, displayed feathery degeneration, micro and macro cellular fatty changes and inflammatory cells around portal tract (Fig. 3B). The diabetic + tolbutamide (standard drug, 25 mg/kg b.w. p.o.)) treated animals showed an excellent protection from STZ-induced changes in the liver (Fig. 3C). The diabetic + 80 % ethanolic extract and butanol fraction treated animals also showed a good protection from STZ-induced changes in the liver (Fig. 3D, 3F). The histopathological examination of ethylacetate fraction of C. sinensis (200 mg/kg b.w. p.o.) group clearly indicated that the hepatic cells, central vein, and portal triad were comparable to standard drug administrated group (Fig. 3E).
Table 3.
Effect of C. sinensis on histopathological damages in STZ-induced liver damage in rats.
| Microscopic observation | |||
|---|---|---|---|
| Groups | Fatty changes | Infiltration of lymphocyte | Deformation in hepatocyte |
| Untreated control | — | — | — |
| Diabetic control | +++ | +++ | +++ |
| D + tolbutamide | + | + | + |
| D + 80% EE | ++ | ++ | ++ |
| D + WtE | +++ | +++ | +++ |
| D + EaF | + | + | + |
| D + BtF | ++ | ++ | ++ |
All values are expressed as mean ± S.E.M. (n = 6). —: absent; +: mild; ++: moderate; +++: severe. D: diabetic. 80% EE: 80% ethanol extract. WtE: water extract. EaF: ethylacetate fraction of C. sinensis 200 mg kg−1 day−1 p.o. for 28 days. BtF: butanol fraction of C. sinensis 200 mg kg−1 day−1 p.o. for 28 days. Tolbutamide: 25 mg kg−1 day−1 p.o. for 28 days.
Fig. 3.
Photomicrographs of rats liver stained by haematoxylin and eosin of untreated control (A), STZ-induced diabetic (B), diabetic + tolbutamide (C), diabetic + aq. ethanolic extract (D), diabetic + ethylacetate fraction (E), diabetic + butanol fraction of C. sinensis. Microscope magnification (×400).
Discussion
Traditional Chinese medicines have been used by over one-fifth of the world population since ancient times and is a potential source of pharmaceutical remedies [20–22]. Now a major challenge to curing liver and kidney injuries are to find novel chemical entities with less toxicity and greater effectiveness than those used in current chemotherapy. In this study, we attempted to use scientific methods to elucidate the hepato-renal effects of C. sinensis to justify its use in folk medicine. In order to obtain a large amount of detectable and stronger peaks in the HPLC chromatogram, the spectra of all peaks in the chromatogram of sample was investigated with photodiode array detection. The programmed wavelength gave the best absorbance for target compounds within the chromatographic windows: 0–40 min, 254 nm. The optimization strategy consisted on obtaining as many peaks as possible in only one run in accordance with well-known optimization strategies [23]. There were total eighteen common peaks obtained in the chromatograph of C. sinensis, most of them were flavonoids by HPLC–DAD analysis as given in Fig. 1.
In respect to LD50 values and maximum non-fatal doses studies revealed the non-toxic nature of the aqueous ethanol extract of this plant. There was no lethality or any toxic reactions found at any of the doses selected until the end of the study period. According to a toxicity classification [24], the ethanol extract of C. sinensis was non toxic. Toxicity studies showed that the hematological and biochemical parameters were within normal range. The body weight was significantly decreased in STZ-diabetic rats [25], the administration of C. sinensis extract and fractions increases the body weight in STZ-diabetic rats as described in Fig. 2. The ability of C. sinensis to protect massive body weight loss seems to be due to its ability to reduce hepato-renal dysfunction. It might enhance glucose utilization and improve diabetes associated disorders because it significantly decreased the urine glucose level as depicted in Table 1.
One of the most sensitive and dramatic indicators of kidney injury is to increase the creatinine and urea level in serum and in hepatocyte injury is the release of intracellular enzymes, such as transaminases and serum alkaline phosphatase in the circulation after STZ administration. The enzyme ALP is located in the cytoplasm and will be released into circulation after cellular damage [26]. In addition, the soluble enzymes ALT/AST are released when injury involves organelles such as mitochondria [27]. As a result, the establishment of liver injury in this study caused both plasma membrane and organelle membrane damage. Rajesh and Latha [28] also reported that the elevated activities of these enzymes were indicative of cellular leakage and loss of the functional integrity of the cell membranes. In the present study, STZ caused histopathological damages to the rat liver (Fig. 3B) and increased the serum level of hepatic enzymes AST, ALT and ALP (Table 2). The diabetic hyperglycemia induces elevation of the serum levels of urea and creatinine which were considered as significant markers of renal dysfunction [29]. The results in Table 2 showed significant (p<0.001) increase in the level of serum urea and creatinine in the diabetic groups. After the treatment of STZ-diabetic rats with the C. sinensis extract and fractions the level of urea was significantly decreased with the mean value of diabetic group. Similarly, the elevation of creatinine level caused by diabetes was declined after the administration of the C. sinensis extract and fractions (p<0.05; p<0.01), compared with the diabetic group. The stabilization of these renal parameters and enzyme levels by the ethylacetate fraction, butanol fraction and aq. ethanolic extract of C. sinensis was a clear indication of the improvement of the functional status of the liver and kidney. These findings can be further corroborated with histopathological studies (Table 3). The histopathological examination clearly demonstrated that the hepatic cells, central vein and portal triad were almost normal in EaF of C. sinensis group as compared to the group treated with STZ alone (Fig. 3E).
In phytochemical screening of this plant, alkaloids, saponins, terpenes, sterols, and flavonoids were identified. The extract might be containing β-sitosterol, a component reported as a hepatoprotective agent [30] and flavonoids as antioxidant compounds [31]. The identified class of components in single or in combination with other components present in the extract/fractions show its hepato-renal protective effects against STZ by different mechanisms, such as: enhancing the enzymes responsible for antioxidant activity; scavenging free radicals responsible for cell damage; or induction of regeneration of the liver cells. Furthermore, CYP2E1-dependent oxidative stress following the induction of CYP2E1 by STZ is believed to be one of the main causes of hepatotoxicity [32]. Therefore, it was possible that C. sinensis may reduce the level of oxidative stress-related cellular toxicity produced by CYP2E1 induction in type 1 diabetes. The increase in ROS production, lipid peroxidation and the decrease in antioxidant defense are common in said disease [33, 34]. These results showed that, among the tissues (liver and kidney) tested; C. sinensis administration to a diabetic rat may cause the protection against CYP2E1-related oxidative stress in the liver. It was also shown that the histopathological change in the liver tissue by STZ-diabetes was recovered by the C. sinensis treatment. This suggested the possibility of a curative effect of the antioxidant on diabetic liver damage.
A few literature is available on genera Cephalotaxus, Takano et al. isolated drupangtonine, a novel antileukemic alkaloid from Cephalotaxus harringtonia var. drupacea [35], Delfel & Rothfus isolated antitumor alkaloids in callus cultures of Cephalotaxus harringtonia [36] and Morita et al. also reported cytotoxic alkaloids from C. harringtonia [37] According to Jui Han Cephalotaxus hainanesis and Camptotheca accuminata showed antineoplastic activity [38]. Clinical studies of harringtonine from Cephalotaxus harringtonia in China showed that it had a certain effect on various types of acute leukemia by intravenous administration. Furthermore, patients who had become resistant to treatment with other chemotherapeutic drugs had been reported to respond to treatment with the cephalotaxine esters [39]. Based on literature review, we concluded that there is no sufficient information available on any significant activity of Cephalotaxus sinensis. All above mentioned results are novel and reported first time.
Conclusion
In conclusion, the present study demonstrated that the treatment of diabetic rats with Cephalotaxus sinensis could ameliorate the impaired renal function and inhibit liver damage associated with STZ-induced diabetes. Based on experimentally demonstrated properties, it is a valuable candidate for developing new phytotherapeutics agent. The isolation and testing of constituents likely to be responsible for the hepato-renal protective activities of C. sinensis is under progress in our lab.
Acknowledgments
The authors thank to Nusrat Khalid and M. Azan for assistance with the preparation of figures in this manuscript and authors are very grateful to professor Dr. Yulin Deng for his nice cooperation and guides. This work was supported by Higher Education Commission Pakistan (HEC) and Beijing Institute of Technology (BIT), Beijing 100081, China.
References
- 1.Dahiru D., Sini J.M., John-Africa L. Antidiarrhoeal activity of Ziziphus mauritiana root extract in rodents. Afr. J. Biotechnol. 2006;5:941–945. [Google Scholar]
- 2.Habib M.Y., Islam M.S., Awal M.A., Khan M.A. Herbal Products: A Novel Approach for Diabetic Patients. Pak. J. Nutri. 2005;4:17–21. [Google Scholar]
- 3.Knight B., Akhurst B., Matthews V.B., Ruddell R.G., Ramm G.A., Abraham L.J., Olynyk J.K., Yeoh G.C. Attenuated liver progenitor (oval) cell and fibrogenic responses to the choline deficient, ethionine supplemented diet in the BALB/c inbred strain of mice. J. Hepato. 2007;46:134–141. doi: 10.1016/j.jhep.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Rao G.M., Rao C.V., Pushpangadan P., Shirwaikar A. Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J. Ethno. 2006;103:484–490. doi: 10.1016/j.jep.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 5.Wills P.J., Asha V.V. Protective effect of Lygodium flexuosum (L.) Sw. extract against carbon tetrachloride-induced acute liver injury in rats. J. Ethno. 2006;108:320–326. doi: 10.1016/j.jep.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Editorial Board of Zhonghua Bencao. “Zhonghua Bencao”. Shanghai Scientific and Technical Press; Shanghai: 1999. pp. 341–342. [Google Scholar]
- 7.Wang L.-W., Su H.-J., Yang S.-Z., Won S.-J., Lin C.-N. New alkaloids and a tetraflavonoid from Cephalotaxus wilsoniana. J. Nat. Prod. 2004;67:1182–1185. doi: 10.1021/np0498657. [DOI] [PubMed] [Google Scholar]
- 8.Bocar M., Jossang A., Bodo B. New Alkaloids from Cephalotaxus fortunei. J. Nat. Prod. 2003;66:152–154. doi: 10.1021/np0203178. [DOI] [PubMed] [Google Scholar]
- 9.Du J., Chiu M.-H., Nie R.-L. Two new lactones from Cephalotaxus fortunei var. alpnia J. Nat. Prod. 1999;62:1664–1665. [Google Scholar]
- 10.Lee M.K., Lim S.W., Yang H., Sung S.H., Lee H.S., Park M.J., Kim Y.C. Osteoblast differentiation stimulating activity of biflavonoids from Cephalotaxus koreana. Bio. Med. Chem. Lett. 2006;16:2850–2854. doi: 10.1016/j.bmcl.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Evans W.C. Trease and Evan’s Pharmacognosy. 13th ed. Balliere Tindal; London: 1989. pp. 419–420. [Google Scholar]
- 12.Escarpa A., Morales M.D., Gonzalez M.C. Analytical performance of commercially available and unavailable phenolic compounds using real samples by high-performance liquid chromatography–diode-array detection. Ana. Chim. Acta. 2002;460:61–72. [Google Scholar]
- 13.Ghosh M.N. Fundamentals of Experimental Pharmacology. Scientific Book Agency; Calcutta: 1984. p. 153. [Google Scholar]
- 14.Turner M.A. Screening Methods in Pharmacology. Academic Press; New York: 1965. p. 26. [Google Scholar]
- 15.Bergmeyer H.U. IFCC methods for the measurement of catalytic concentrations of enzymes. Part 3. IFCC method for alanine aminotransferase (l-alanine: 2-oxoglutarate aminotransferase) Clin. Chem. Acta. 1980;105:147–154. [PubMed] [Google Scholar]
- 16.Bergmeyer H.U., Bowes G.N., Jr., Horder M., Jr., Moss D.W. Provisional recommendations on IFCC methods for the measurement of catalytic concentrations of enzymes. Part 2. IFCC method for aspartate aminotransferase. Clin. Chem. Acta. 1976;70:F19–F42. [PubMed] [Google Scholar]
- 17.Belfield A., Goldberg D.M. Colourimetric determination of alkaline phosphatase activity. Enzyme. 1971;12:561–568. doi: 10.1159/000459586. [DOI] [PubMed] [Google Scholar]
- 18.Bartels H., Boehmer M. Microdetermination of creatinine. Clin. Chim. Acta. 1971;32:81–85. doi: 10.1016/0009-8981(71)90467-0. [DOI] [PubMed] [Google Scholar]
- 19.Palton C.J., Crouch S.R. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Ana. Chem. 1977;49:464–469. [Google Scholar]
- 20.Gui S.-Y., Wei W., Wang H., Wua L., Suna W.-Y., Chen W.-B., Wu C.-Y. Effects and mechanisms of crude astragalosides fraction on liver fibrosis in rats. J. Ethno. 2006;103:154–159. doi: 10.1016/j.jep.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Wu J., Danielsson A. Inhibition of hepatic fibrogenesis: a review of pharmacologic candidates. Scan. J. Gastro. 1994;29:385–391. doi: 10.3109/00365529409096827. [DOI] [PubMed] [Google Scholar]
- 22.Chojkier M., Brenner D.A. Therapeutic strategies for hepatic fibrosis. Hepatology. 1988;8:176–182. doi: 10.1002/hep.1840080132. [DOI] [PubMed] [Google Scholar]
- 23.Snyder L.R., Glajch J.L., Kirkland J.J. Practical HPLC Method Development. Wiley; New York: 1998. [Google Scholar]
- 24.Loomis T.A. Essential of Toxicology. Phil. Le. 1968;6:67–78. [Google Scholar]
- 25.Ahn T., Yun C.-H., Oh D.-B. Tisue specific effect of ascorbic acid supplementation on the expression of cytochrom P450 2EI and oxidative stress in Streptozotocin-induced diabetic rats. Toxi. Let. 2006;166:27–36. doi: 10.1016/j.toxlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Sallie R., Tredger J.M., William R. Drugs and the liver. Biopharm Drug Dispos. 1991;12:251–259. doi: 10.1002/bdd.2510120403. [DOI] [PubMed] [Google Scholar]
- 27.Senthil K.R., Ponmozhi M., Viswanathan P., Nalini N. Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J. Nutr. Biochem. 2003;14:452–458. doi: 10.1016/s0955-2863(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 28.Rajesh M.G., Latha M.S. Preliminary evaluation of the antihepatotoxic effect of Kamilari, a polyherbal formulation. J. Ethno. 2004;91:99–104. doi: 10.1016/j.jep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Almdal T.P., Vilstrup H. Strict insulin treatment normalizes the organic nitrogen contents and the capacity of urea–N synthesis in experimental diabetes in rats. Diab. 1988;31:114–118. doi: 10.1007/BF00395558. [DOI] [PubMed] [Google Scholar]
- 30.Wills P.J., Asha V.V. Protective effect of Lygodium flexuosum (L.) Sw. extract against carbon tetrachloride-induced acute liver injury in rats. J. Ethno. 2006;108:320–326. doi: 10.1016/j.jep.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki E., Inagaki M., Kurita O., Inoue T. Antioxidant activity of Japanese pepper (Zanthoxylum piperitum DC.) fruit. Food Chem. 2007;100:171–177. [Google Scholar]
- 32.Jaeschke H., Gores G.J., Cederbaum A.I., Hinson J.A., Pessayre D., Lemasters J.J. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 33.Desco M.C., Asensi M., Marquez R., Martinez-Valls J., Vento M., Pallardo F.V., Sastre J., Vina J. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diab. 2002;51:1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 34.Wolff S.P. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the etiology of diabetes mellitus and complications. Br. Med. Bull. 1993;49:642–652. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- 35.Takano l., Yasuda l., Nishijima M., Hitotsuyanagi Y., Takeya K., Itokawa H. Drupangtonine, a novel antileukemic alkaloid from Cephalotaxus harringtonia var. Drupacea. Bio. Med. Chem. Let. 1996;6:1689–1690. [Google Scholar]
- 36.Delfel N.E., Rothfus J.A. Antitumor alkaloids in callus cultures of Cephalotaxus harringtonia. Phytochem. 1977;16:1595–1598. [Google Scholar]
- 37.Morita H., Arisaka M., Yoshida N., Kobayashi J. Cephalezomines A-F, potent cytotoxic alkaloids from Cephalotaxus harringtonia var. nana. Tetra. 2000;56:2929–2934. [Google Scholar]
- 38.Han J. Traditional Chinese medicine and the search for new antineoplastic drugs. J. Ethno. 1988;24:1–17. doi: 10.1016/0378-8741(88)90135-3. [DOI] [PubMed] [Google Scholar]
- 39.Delfel N.E. Alkaloid distribution and catabolism in Cephalotaxus harringtonia. Phytochm. 1980;19:403–408. [Google Scholar]