Abstract
Previously, we identified four metabolites of (−)-epicatechin in blood and urine: (−)-epicatechin-3'-O-glucuronide (E3'G), 4'-O-methyl-(−)-epicatechin-3'-O-glucuronide (4'ME3'G), (−)-epicatechin-7-O-glucuronide (E7G), and 3'-O-methyl-(−)-epicatechin-7-O-glucuronide (3'ME7G) (Natsume et al. Free Radical Biol. Med. 34, 840-849, 2003). The aim of the current study was to compare the antioxidative activities of these metabolites with that of their parent compound. After oral administration of (−)-epicatechin, E3'G and 4'ME3'G were isolated from human urine, and E7G and 3'ME7G isolated from rat urine. We found that these compounds inhibited peroxynitrite-mediated tyrosine nitration, in the following order of potency: E3'G > (−)-epicatechin > E7G = 3'ME7G. = 4'ME3'G. These results demonstrate that the metabolites of (−)-epicatechin retain antioxidative activity on peroxynitrite-induced oxidative damages to some extent.
Keywords: (−)-epicatechin, glucuronide, metabolites, peroxynitrite, nitrotyrosine
Introduction
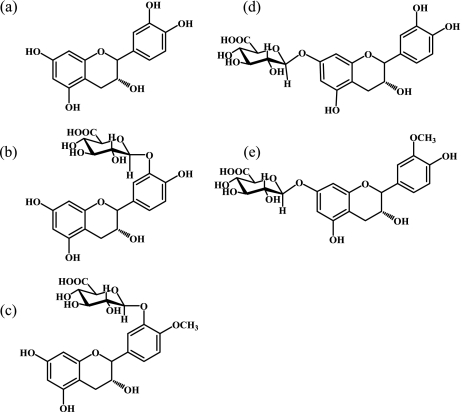
Catechins are found in high levels in a variety of foods, including black tea, apples, wine, and chocolate [1, 2]. Flavonoids including (−)-epicatechin have multiple biological functions that are thought to reduce the incidence of atherosclerosis [3]. Epidemiological studies have suggested that the intake of catechins correlates with risk reduction for coronary heart disease [4, 5]. (−)-Epicatechin has been shown to protect biological molecules from nitration by peroxynitrite [6]. Oxidation of tyrosine residues, which occurs upon the nitration of proteins, is of particular interest in the pathology of inflammation. Upon oral administration, most flavonoids are metabolized in the gastrointestinal tract and absorbed into the blood. There have been a few reports to date on antioxidant activities in these metabolites [7–9]. We have shown in humans and rats that, following the ingestion of cocoa powder, (−)-epicatechin is absorbed from the intestinal tract and distributed as a variety of conjugated and methylated forms in the blood before being excreted in the urine [10, 11]. We also isolated and identified (−)-epicatechin glucuronide metabolites from human and rat plasma. In humans, these include (−)-epicatechin-3'-O-glucuronide (E3'G) and 4'-O-methyl-(−)-epicatechin-3'-O-glucuronide (4'ME3'G), and in rat, the metabolites were (−)-epicatechin-7-O-glucuronide (E7G) and 3'-O-methyl-(−)-epicatechin-7-O-glucuronide (3'ME7G) [12]. The structures of these compounds are shown in Fig. 1.
Fig. 1.
Structures of (−)-epicatechin and its glucuronide metabolites isolated from human and rat urine. (a) (−)-epicatechin. (b) (−)-epicatechin-3'-O-glucuronide (E3'G). (c) 4'-O-mehyl-(−)-epicatechin-3'-O-glucuronide (4'ME3'G). (d) (−)-epicatechin-7-O-glucuornide (E7G), and (e) 3'-O-methyl-(−)-epicatechin-7-O-glucuonide (3'ME7G).
The aim of the current study was to examine the antioxidative activities, especially that of nitrotyrosine formation, of the glucuronide metabolites of (−)-epicatechin present in human and rat plasma.
Materials and Methods
Peroxynitrite was synthesized from sodium nitrite and H2O2 using a quenched-flow reactor [13]. H2O2 was eliminated by passage of the peroxynitrite solution over MnO2 powder with the final peroxynitrite concentration determined by its absorbance at 302 nm (ε = 1620 M−1cm−1). Protection against peroxynitrite-mediated tyrosine nitration was performed as described by Arteel et al. [14]. Briefly, peroxynitrite (0.5 mM) was added as a bolus to a 0.1 mM tyrosine solution in 0.1 M phosphate buffer (pH 7.3) containing 0.1 mM DTPA with constant vortexing. The effects of the tested compounds (0–31.5 µM) were determined by injection of 10 µl of sample, supplemented with 0.1 mM 3-hydroxy-4-nitrobenzoic acid as an internal standard, onto a C-18 reverse-phase column (ODS-UG-Deverosil 4.6 × 150 mm; Nomura Chemical, Aichi, Japan) interfaced with the autosampler from an HP1100 liquid chromatography system (Agilent Technologies, Palo Alto, CA). Separation was performed with a 50 mM potassium phosphate buffer (pH 7.0)/acetonitrile step-gradient delivered by the HP1100 system at a flow rate of 1.0 ml/min. The gradient was initiated at a ratio of 95:5 buffer/acetonitrile, followed in stepwise adjustment to a final ratio of 50:50 buffer/acetonitrile over 5 min, with a return to 95:5 buffer/acetonitrile ratio at 13 min, which was then maintained for an additional 13 min. Formation of 3-nitrotyrosine was monitored with a UV/visible detector set at 430 nm. To determine the concentration of 3-nitrotyrosine in the sample, the peak area ratio of the 3-nitrotyosine to the internal standard was calculated and compared to a standard curve. Each analysis was performed in duplicate.
Results
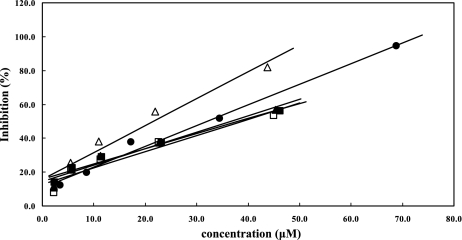
Figure 2 shows the ability of the test compounds to protect free tyrosine from the nitration by peroxynitrite. The concentration of metabolites required to yield a 50% inhibition of tyrosine nitration increased in the following order: E3'G > (−)-epicatechin > E7G = 3'ME7G. = 4'ME3'G.
Fig. 2.
Protective effect by (−)-epicatechin and its metabolites against nitration of tyrosine residues by peroxynitrite (−)-epicatechin (closed circles), E3'G (open triangles), 4'ME3'G (open squares), E7G (closed triangles) and 3'ME7G (closed squares). Each analysis was performed in duplicate.
Discussion
A variety of foods, particularly green tea, chocolate, cocoa, and red wine have high levels of the flavonoid (−)-epicatechin [1]. Flavonoids have multiple biological functions, including the suppression of LDL oxidation [3, 15], a process thought to reduce the incidence of atherosclerosis [16, 17]. In the present study, we compared the peroxynitrite scavenging activities of (−)-epicatechin and its four major metabolites: E3'G and 4'ME3'G, which are found in human plasma, and E7G and 3'ME7G, which are found in rat plasma [12]. Peroxynitrite may be generated in vivo under pathological conditions, especially in the inflammatory response, due to the reaction of superoxide radicals with nitric oxide. Peroxynitrite causes injury and degeneration of endothelial cells and erythrocytes [18]. Moreover, (−)-epicatechin strongly suppresses nitration of tyrosine residues of LDL by Myeloperoxidase (MPO)/nitrite or peroxynitrite [19]. Flavonoids protect biological molecules against peroxynitrite-mediated nitration by neutralizing peroxynitrite and its reaction intermediate, the tyrosyl radical [6, 20–24]. In this study, we found that both (−)-epicatechin and its metabolites inhibited peroxynitrite-mediated nitration. E3'G was more potent than (−)-epicatechin and the other metabolites at the concentrations between 2.1 and 42.8 µM. (−)-Epicatechin glucuronide and methylated forms of epicatechin were similar in their ability to inhibit tyrosine nitration. In the presence of 0.5 mM peroxynitrite, the IC50 values of (−)-epicatechin and E3'G were 32.1 and 21.6 µM, as calculated by linear regression analyses, respectively. Wipple et al. [6] reported that one equivalent of (−)-epicatechin prevents nitration by 15–30 equivalents of peroxynitrite.
An alternative action mechanism for epicatechin was proposed by Schroeder et al. [21], who hypothesized that epicatechin acts at the interference with tyrosyl radicals rather than by direct interaction with peroxynitrite. Furthermore, the plasma metabolites of epicatechin may contribute to the antioxidant activity on peroxynitrite attack in blood plasma after administration of catechin rich foods.
References
- 1.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 2.Natsume M., Osakabe N., Yamagishi M., Takizawa T., Nakamura T., Miyatake H., Hatano T., Yoshida T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal- phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000;64:2581–2587. doi: 10.1271/bbb.64.2581. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg F.M., Holt R.R., Schmitz H.H., Keen C.L. Cocoa procyanidin chain length does not determine ability to protect LDL from oxidation when monomer units are controlled. J. Nutr. Biochem. 2002;13:645–652. doi: 10.1016/s0955-2863(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 4.Arts I.C.W., Hollman P.C.H., Feskens E.J. M., Bueno de Mesquita H.B., Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am. J. Clin. Nutr. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 5.Arts I.C.W., Jacobs D.R., Jr, Harnack L.J., Gross M., Folsom A.R. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–675. doi: 10.1097/00001648-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Wippel R., Rehn M., Gorren A.C., Schmidt K., Mayer B. Interference of the polyphenol epicatechin with the biological chemistry of nitric oxide- and peroxynitrite-mediated reactions. Biochem. Pharmacol. 2004;67:1285–1295. doi: 10.1016/j.bcp.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Harada M., Kan Y., Naoki H., Fukui Y., Kageyama N., Nakai M., Miki W., Kiso Y. Identification of the major antioxidative metabolites in biological fluids of the rat with ingested (+)-catechin and (−)-epicatechin. Biosci. Biotechnol. Biochem. 1999;63:973–977. doi: 10.1271/bbb.63.973. [DOI] [PubMed] [Google Scholar]
- 8.Moon J., Tsushida T., Nakahara K., Terao J. Identification of quercetin 3-O-β-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 9.Natsume M., Osakabe N., Yasuda A., Baba S., Tokunaga T., Kondo K., Osawa T., Terao J. In vitro antioxidative activity of (–)-epicatechin glucuronide metabolites present in human and rat plasma. Free Radic. Res. 2004;38:1341–1348. doi: 10.1080/10715760400022087. [DOI] [PubMed] [Google Scholar]
- 10.Baba S., Osakabe N., Natsume M., Yasuda A., Takizawa T., Nakamura T., Terao J. Cocoa powder enhances the level of antioxidative activity in rat plasma. Br. J. Nutr. 2000;84:673–680. [PubMed] [Google Scholar]
- 11.Baba S., Osakabe N., Yasuda A., Natsume M., Takizawa T., Nakamura T., Terao J. Bioavailability of (−)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic. Res. 2000;33:635–641. doi: 10.1080/10715760000301151. [DOI] [PubMed] [Google Scholar]
- 12.Natsume M., Osakabe N., Oyama M., Sasaki M., Baba S., Nakamura Y., Osawa T., Terao J. Structures of (−)-epicatechin glucuronide identified from plasma and urine after oral ingestion of (−)-epicatechin: differences between human and rat. Free Radic. Biol. Med. 2003;34:840–849. doi: 10.1016/s0891-5849(02)01434-x. [DOI] [PubMed] [Google Scholar]
- 13.Koppenol W.H., Kissner R., Beckman J.S. Syntheses of peroxynitrite: to go with the flow or on solid grounds? Methods Enzymol. 1996;269:296–302. doi: 10.1016/s0076-6879(96)69030-2. [DOI] [PubMed] [Google Scholar]
- 14.Arteel G.E., Sies H. Protection against peroxynitrite by cocoa polyphenol oligomers. FEBS Lett. 1999;462:167–170. doi: 10.1016/s0014-5793(99)01498-2. [DOI] [PubMed] [Google Scholar]
- 15.Osakabe N., Baba S., Yasuda A., Iwamoto T., Kamiyama M., Takizawa T., Itakura H., Kondo K. Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radic. Res. 2001;34:93–99. doi: 10.1080/10715760100300091. [DOI] [PubMed] [Google Scholar]
- 16.Kurosawa T., Itoh F., Nozaki A., Nakano Y., Katsuda S., Osakabe N., Tsubone H., Kondo K., Itakura H. Suppressive effects of cacao liquor polyphenols (CLP) on LDL oxidation and the development of atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. Atherosclerosis. 2005;179:237–246. doi: 10.1016/j.atherosclerosis.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Yamakoshi J., Kataoka S., Koga T., Ariga T. Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 1999;142:139–149. doi: 10.1016/s0021-9150(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 18.Hippeli S., Elstner E.F. OH-radical-type reactive oxygen species: a short review on the mechanisms of OH-radical- and peroxynitrite toxicity. Z. Naturforsch [C] 1997;52:555–563. [PubMed] [Google Scholar]
- 19.Schewe T., Sies H. Myeloperoxidase-induced lipid peroxidation of LDL in the presence of nitrite. Protection by cocoa flavanols. Biofactors. 2005;24:49–58. doi: 10.1002/biof.5520240106. [DOI] [PubMed] [Google Scholar]
- 20.Pannala A.S., Rice-Evans C.A., Halliwell B., Singh S. Inhibition of peroxynitrite-mediated tyrosine nitration by catechin polyphenols. Biochem. Biophys. Res. Commun. 1997;232:164–168. doi: 10.1006/bbrc.1997.6254. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder P., Klotz L.O., Buchczyk D.P., Sadik C.D., Schewe T., Sies H. Epicatechin selectively prevents nitration but not oxidation reactions of peroxynitrite. Biochem. Biophys. Res. Commun. 2001;285:782–787. doi: 10.1006/bbrc.2001.5210. [DOI] [PubMed] [Google Scholar]
- 22.Heijnen C.G., Haenen G.R., van Acker F.A., van der Vijgh W.J., Bast A. Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol. In Vitro. 2001;15:3–6. doi: 10.1016/s0887-2333(00)00053-9. [DOI] [PubMed] [Google Scholar]
- 23.Ketsawatsakul U., Whiteman M., Halliwell B. A reevaluation of the peroxynitrite scavenging activity of some dietary phenolics. Biochem. Biophys. Res. Commun. 2000;279:692–699. doi: 10.1006/bbrc.2000.4014. [DOI] [PubMed] [Google Scholar]
- 24.Choi J.S., Chung H.Y., Kang S.S., Jung M.J., Kim J.W., No J.K., Jung H.A. The structure-activity relationship of flavonoids as scavengers of peroxynitrite. Phytother. Res. 2002;16:232–235. doi: 10.1002/ptr.828. [DOI] [PubMed] [Google Scholar]