Abstract
Oral mucosa is a critical protective interface between external and internal environments. Therefore, it must serve as a barrier to a huge number of microbial species present in the environment. Saliva is an important factor that provides for the environment in the oral cavity, and it is indispensable to the host defense reaction in this manner. Oral neutrophils are also important contributors to maintaining the balance between health and disease in this complex environment. These produce reactive oxygen species, nitric oxide, and several antimicrobial peptides, and enzymes. Neutrophils and saliva all contribute to the maintaining the health of the oral cavity in overlapping but independent ways. In addition to production by neutrophils and macrophage, some bacteria can also generate superoxide, hydrogen peroxide, and nitric oxide. Dietary intake of nitrate-enriched vegetables might play important roles in the protection of the oral and stomach against hazardous pathogens via the gastro-intestinal-salivary cycle of nitric oxide (NO) and related metabolites. This review will focus on defense system of the human oral cavity and metabolism of reactive oxygen and NO.
Keywords: oral cavity, neutrophils, bacteria, superoxide, nitric oxide
Introduction
The oral cavity is a unique environment. Oral mucosa is a critical protective interface between external and internal environments. Therefore, it must serve as a barrier to a huge number of microbial species present in the environment. Saliva is an important factor that provides for the environment in the oral cavity, and it is indispensable to the host defense reaction in this manner. Oral neutrophils are also important contributors to maintaining the balance between health and disease in this complex environment. These produce reactive oxygen species, nitric oxide, and several antimicrobial peptides, the α-defensins, and other enzymes. Oral neutrophils are part of the host innate immune response in this environment. Neutrophils, and saliva all contribute to the maintaining the health of the oral cavity in overlapping but independent ways. This review will focus on defense system of the human oral cavity and include 1) the function of saliva and neutrophils in the oral cavity, 2) nitric oxide and reactive oxygen species of microbacteria which has led to advances in our understanding, and 3) functional efficacy against oral nitric oxide metabolism when it is known.
Defense System of Oral Cavity
Saliva is an important factor that provides for the environment in the oral cavity, and it is indispensable to the host defense reaction in this manner. The continuous flow of saliva removes a large number of bacteria from teeth and mucosal surfaces. Saliva also contains several specific and nonspecific defense factors. Salivary IgA is the principal specific defense factor of saliva. The nonspecific defense factors include mucins, salivary glycoproteins, lactoferrin, lysozyme, peroxidase, histatins, and cystatins. The mucins form a viscous slime layer on oral mucosa that traps microorganisms and antigens, limiting their penetration into the tissues [1, 2]. Potentially harmful microorganisms are thus eliminated by the continuous renewal of the mucous layer combined with the washing action of salivary flow. Lysozyme can lyse some bacterial species by hydrolyzing glycosidic linkages in the cell wall peptidoglycan. It may also cause lysis of bacterial cells by interacting with monovalent anions, such as thiocyanate, perchlorate, iodide, bromide, bicarbonate, nitrate, and fluoride, and with proteases found in saliva. Salivary peroxidase is produced by salivary gland acinar cells and salivary myeloperoxidase is released from oral neutrophils. These enzymes are part of an antimicrobial system that involves the oxidation of salivary thiocyanate to hypothiocyanite and hypothiocyanous acid by hydrogen peroxide (1), generated by oral bacteria and neutrophils. It is known that thiocyanite anion has the strong cell toxicity. It is thought that this compound plays a major role to the host defense in oral cavity. In addition, the oxidizing agents react with sulfhydryl groups of the enzymes (2 and 3) involved in glycolysis and sugar transport [3]. Then, these enzymes were inactivated.
H2O2 + SCN− + H+ → HOSCN + H2O (1)
RSH + OSCN− → RS-SCN + OH− (2)
RS-SCN + H2O → RSOH + SCN− + H+ (3)
The gingival crevice of the oral cavity is almost essentially controlled by the antimicrobial factors of plasma. Cellular and humoral components of blood can reach the gingival crevice. Even in the healthy state, there is a continuous flow of small quantities of fluid and leukocytes from the gingival capillaries through the crevicular epithelium into the gingival crevice. This flow increases markedly with inflammation. The flow of gingival fluid from the crevice to the oral cavity removes nonadherent bacteria. Gingival fluid also contains antimicrobial substances including IgM, IgG, IgA, complement, and leukocytes. These factors are primarily protective against microbial invasion. Complement factors may initiate bacterial cell lysis or enhance phagocytosis of microorganisms. The IgG antibodies may enhance phagocytosis and killing of oral microorganisms through activation of complement or opsonization [4–6]. Lysozyme and peroxidase that are released from the lysosome of neutrophils during phagocytosis might also control microbial growth in the gingival crevice.
Production of Superoxide and Nitric Oxide (NO) in Oral Cavity and Defense System
Oral neutrophils
Neutrophils play crucial roles in protecting hosts against invading microbes and in the pathogenesis of inflammatory tissue injury. Although neutrophils migrate into mucosal layers of digestive and respiratory tracts, only limited information is available of their fate and function in situ. We previously reported that, unlike circulating neutrophils, neutrophils in the oral cavity spontaneously generate superoxide radical and nitric oxide in the absence of any stimuli. Large numbers of neutrophils migrate through the mucosal layers of the intestinal and respiratory tracts and appear in their luminal compartments. The leukocytes in oral cavity are composed of 90% polymorphonuclear leukocytes (PMNs, neutrophils) and 10% mononuclear cells. About 80% of neutrophils are viable and functional within the oral cavity and crevice. The circulating neutrophils have been known to be primed by various ligands, such as lipopolysaccharide (LPS), interleukin-1, and TNF-α [7, 8]. The circulating neutrophils undergo priming during the migration into the oral cavity and spontaneously release reactive oxygen species, including superoxide, hypochloride, and nitric oxide [9]. Oral neutrophils are further activated by various ligands. It was realized that components of Gram-negative bacterial cell walls, such as LPS, are able to enhance the oxidative burst of human circulating neutrophils in response to secondary stimulation without themselves initiating any measurable activity of the NADPH oxidase [10–12]. Large numbers gram-negative appear in oral cavity. Gram-negative organisms contain LPS in their outer membranes, and this may be released during infection. It has been demonstrated that LPS elicits TNF-α production [13]. It is well known that the priming of circulating neutrophils by LPS and TNF-α results in an enhanced oxidative burst and enhanced degranulation [7, 8, 14]. Since Gram-negative microorganisms predominate in the subgingival environment, and bacteremia and endotoxemia are commonly observed even during normal oral hygiene procedures, oral neutrophils always primed by these factors.
Fate of oral neutrophils
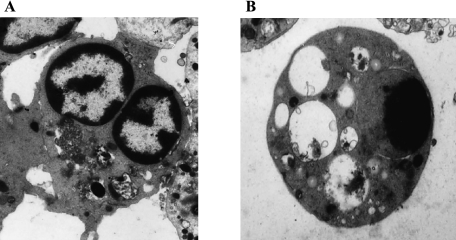
The life span of neutrophils is fairly short compared with those of other leukocytes [15]. Even under physiological conditions, neutrophils spontaneously undergo apoptosis in the absence of any stimuli, and apoptotic cells are phagocytosed by macrophages [16–18]. The life cycle of neutrophils is regulated by many factors, including granulocyte macrophage colony-stimulating factor (GM-CSF) [19, 20], nerve growth factor [21], interleukin-1 [22], interleukin-2 [23], and granulocyte colony-stimulating factor (G-CSF) [20, 24]. Unlike circulating neutrophils, oral neutrophils spontaneously generate superoxide radical and nitric oxide in the absence of any stimuli [9, 25]. The oral neutrophils perhaps remain functional at a short distance from the gingival margin by the flow of gingival fluid along the tooth surface, but once neutrophils are in saliva, they rapidly dead, due to osmotic shock [26]. Therefore, oral neutrophils immediately showed morphological changes which are characteristic of those of apoptosis (Fig. 1) [27]. In oral cavity, oxidative stress and/or redox regulation, dependent pathway(s) activated caspase-3 in oral neutrophils, thereby inducing their apoptosis.
Fig. 1.
Morphological features of oral neutrophils.Electron micrographs: A: OPMN at 0 h; B: OPMN at 4 h
Bacterial ROS Prodution and Their Defense Mechanism
The oral bacteria of humans are composed of more than 300 bacterial species. Streptococcus mutans, S. sobrinus, S. cricetus, and S. rattus and S. sanguis are found in larger numbers on teeth, while S. salivarius is isolated mainly from the tongue [28]. On teeth, microorganisms colonize in a dental plaque. The microbial composition of saliva is similar to that of the tongue. Helicobacter pylori (H. pylori) and Enterococcus faecalis (E. faecalis) are also isolated as minor bacteria in oral cavity. In addition to production by neutrophils and macrophage, bacteria can also produce superoxide anion and hydrogen peroxide. We previously reported that H. pylori generates the superoxide radical from respiratory chain and that the sensitivity of its respiration to NO is significantly lower than that of Escherichia coli (E. coli) and mammalian mitochondria [29–33]. Because superoxide is extremely reactive with NO, this reaction may contribute, at least in part, to the mechanism that allows H. pylori to be resistant to gastric NO [29]. However, peroxynitrite generated from NO and superoxide is also toxic and impairs DNA and cellular proteins [34]. Thus, oxidative stress elicited by NO, superoxide, and related metabolites may also injure H. pylori. To further elucidate the mechanism for the strong resistance of H. pylori to NO and related metabolites, we compared the effects of NO on the respiration of H. pylori and E. coli and the fate of this gaseous radical in and around these bacteria. A kinetic analysis revealed that the strong resistance of the H. pylori respiration was due, at least in part, to the low affinity of the respiratory chain terminal oxidase for NO in the membrane/lipid bilayers of intact cells [35].
E. faecalis, an intestinal commensal of humans and animals, generates large amounts of extracellular superoxide [36]. Extracellular superoxide is produced by E. faecalis through the respiratory chain due to rapid nonenzymatic reaction of semiquinone radicals of demethylmenaquinone with oxygen [37].
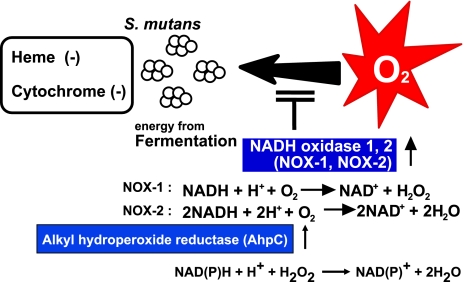
NADH oxidases are found in several microorganisms [38]. There are two types of NADH oxidase, H2O forming and hydrogen peroxide forming. The hydrogen peroxide-forming NADH oxidases found in Streptococcus mutans, which are facultatively anaerobic bacteria that lack a heme and a respiratory chain (Fig. 2) [39, 40]. The enzymes catalyze the reduction of oxygen by NADH to form hydrogen peroxide. However, in the presence of AhpC, the NADH oxidases also showed extremely high reductase activity for both hydrogen peroxide and alkyl hydroperoxides [39, 41]. These NADH oxidases are involved not only in the regeneration of NAD but also in the removal of peroxides. Thus, in spite of lacking a respiratory chain and peroxide-scavenging enzymes such as catalase and heme-containing peroxidases, Streptococcus mutans can grow as well under aerobic conditions as do conventional aerobic bacteria [39, 42].
Fig. 2.
NADH oxidase of Streptococcus mutans
The Entero-Salivary Circulation of Nitric Oxide Metabolites and Their Defense System
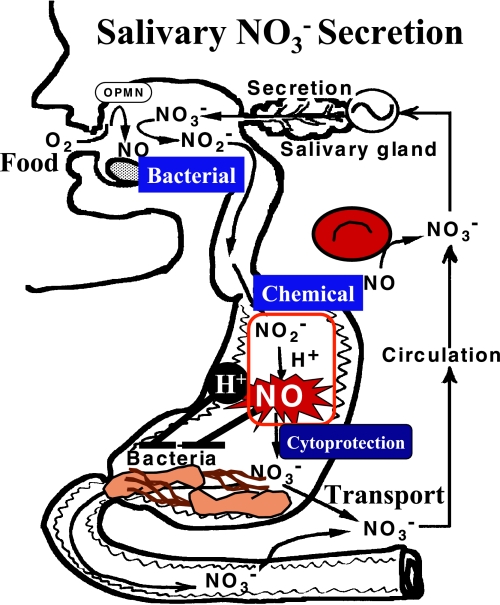
Because NO is potent bactericidal compound [43], its generation in gastrointestinal tracts is of critical importance in host defense (Fig. 3). In this context, Benjamin et al. [44] reported that Candida albicans retained their viability in acidic medium but died in the presence of nitrite. Dykhuizen et al. [45] also reported that H. pylori died in nitrite-containing acidic medium but survived in nitrite-containing neutral medium. Dietary intake of nitrate-enriched vegetables might play important roles in the protection of the oral and stomach against hazardous pathogens via the gastro-intestinal-salivary cycle of NO and related metabolites. Other environmental sources of nitrate and nitrite include cigarette smoke and car exhausts. These and other environmental pollutants contain volatile nitrogen oxides, some of which are converted to nitrate or nitrite in the body. After ingestion, nitrate is rapidly and effectively absorbed proximally from the gastrointestinal tract into the bloodstream [46]. Most nitrate is ultimately excreted in the urine but some is excreted in the saliva, sweat and possibly also the intestines. Up to 25% of plasma nitrate is actively taken up by the salivary glands and secreted with saliva, and the resulting salivary nitrate concentrations are at least 10 times higher than the concentrations in plasma. Many of oral microorganisms express enzymes that can effectively reduce nitrate. Facultative anaerobic bacteria in the oral cavity reduce salivary nitrate to nitrite and this nitrite enhances the gastric generation of NO in acidic condition. Although nitrite is converted non-enzymatically to NO at low pH (3, 5), the rate of this conversion at the physiological pH (about 6.2–7.6) in the oral cavity is fairly low and, hence, the mechanism of oral production of NO remains obscure. In this context, we previously reported that neutrophils in the oral cavity of healthy humans generated NO by inducible NO synthase [9, 25]. Production of NO in the oral cavity has been known to increase in patients with dental caries [47]. One recent study reported that some species of streptococci in human saliva also generate NO [48].
Fig. 3.
The entero-salivary circulation of nitric oxide metabolites
References
- 1.Tabak L.A., Levine M.J., Mandel I.D., Ellison S.A. Role of salivary mucins in the protection of the oral cavity. J. Oral Pathol. 1982;11:1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 2.Slomiany B.L., Murty V.L., Piotrowski J., Slomiany A. Salivary mucins in oral mucosal defense. Gen. Pharmacol. 1996;27:761–771. doi: 10.1016/0306-3623(95)02050-0. [DOI] [PubMed] [Google Scholar]
- 3.Grisham M.B., Ryan E.M. Cytotoxic properties of salivary oxidants. Am. J. Physiol. 1990;258:C115–121. doi: 10.1152/ajpcell.1990.258.1.C115. [DOI] [PubMed] [Google Scholar]
- 4.Anderson D.M., Ebersole J.L., Novak M.J. Functional properties of nonhuman primate antibody to Porphyromonas gingivalis. Infect. Immun. 1995;63:3245–3252. doi: 10.1128/iai.63.9.3245-3252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McArthur W.P., Clark W.B. Specific antibodies and their potential role in periodontal diseases. J. Periodontol. 1993;64:807–818. doi: 10.1902/jop.1993.64.8s.807. [DOI] [PubMed] [Google Scholar]
- 6.Scully C.M., Lehner T. Opsonization, phagocytosis and killing of Streptococcus mutans by polymorphonuclear leukocytes, in relation to dental caries in the rhesus monkey (Macaca mulatta) Arch. Oral Biol. 1979;24:307–312. doi: 10.1016/0003-9969(79)90093-1. [DOI] [PubMed] [Google Scholar]
- 7.Utsumi T., Klostergaard J., Akimaru K., Edashige K., Sato E.F., Utsumi K. Modulation of TNF-alpha-priming and stimulation-dependent superoxide generation in human neutrophils by protein kinase inhibitors. Arch. Biochem. Biophys. 1992;294:271–278. doi: 10.1016/0003-9861(92)90168-v. [DOI] [PubMed] [Google Scholar]
- 8.Akimaru K., Utsumi T., Sato E.F., Klostergaard J., Inoue M., Utsumi K. Role of tyrosyl phosphorylation in neutrophil priming by tumor necrosis factor-alpha and granulocyte colony stimulating factor. Arch. Biochem. Biophys. 1992;298:703–709. doi: 10.1016/0003-9861(92)90469-d. [DOI] [PubMed] [Google Scholar]
- 9.Sato E.F., Utsumi K., Inoue M. Human oral neutrophils: isolation and characterization. Methods Enzymol. 1996;268:503–509. doi: 10.1016/s0076-6879(96)68052-5. [DOI] [PubMed] [Google Scholar]
- 10.Forehand J.R., Pabst M.J., Phillips W.A., Johnston R.B., Jr. Lipopolysaccharide priming of human neutrophils for an enhanced respiratory burst. Role of intracellular free calcium. J. Clin. Invest. 1989;83:74–83. doi: 10.1172/JCI113887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guthrie L.A., McPhail L.C., Henson P.M., Johnston R.B., Jr. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J. Exp. Med. 1984;160:1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaku M., Yagawa K., Nagao S., Tanaka A. Enhanced superoxide anion release from phagocytes by muramyl dipeptide or lipopolysaccharide. Infect. Immun. 1983;39:559–564. doi: 10.1128/iai.39.2.559-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beutler B.A., Milsark I.W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J. Immunol. 1985;135:3972–3977. [PubMed] [Google Scholar]
- 14.Steinbeck M.J., Roth J.A. Neutrophil activation by recombinant cytokines. Rev. Infect. Dis. 1989;11:549–568. doi: 10.1093/clinids/11.4.549. [DOI] [PubMed] [Google Scholar]
- 15.Squier M.K., Sehnert A.J., Cohen J.J. Apoptosis in leukocytes. J. Leukoc. Biol. 1995;57:2–10. doi: 10.1002/jlb.57.1.2. [DOI] [PubMed] [Google Scholar]
- 16.Savill J., Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin. Cell Biol. 1995;6:385–393. doi: 10.1016/s1043-4682(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 17.Savill J.S., Henson P.M., Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “charge-sensitive” recognition mechanism. J. Clin. Invest. 1989;84:1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savill J.S., Wyllie A.H., Henson J.E., Walport M.J., Henson P.M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brach M.A., deVos S., Gruss H.J., Herrmann F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood. 1992;80:2920–2924. [PubMed] [Google Scholar]
- 20.Cox G., Gauldie J., Jordana M. Bronchial epithelial cell-derived cytokines (G-CSF and GM-CSF) promote the survival of peripheral blood neutrophils in vitro. Am. J. Respir. Cell Mol. Biol. 1992;7:507–513. doi: 10.1165/ajrcmb/7.5.507. [DOI] [PubMed] [Google Scholar]
- 21.Kannan Y., Usami K., Okada M., Shimizu S., Matsuda H. Nerve growth factor suppresses apoptosis of murine neutrophils. Biochem. Biophys. Res. Commun. 1992;186:1050–1056. doi: 10.1016/0006-291x(92)90853-d. [DOI] [PubMed] [Google Scholar]
- 22.Colotta F., Re F., Polentarutti N., Sozzani S., Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 23.Pericle F., Liu J.H., Diaz J.I., Blanchard D.K., Wei S., Forni G., Djeu J.Y. Interleukin-2 prevention of apoptosis in human neutrophils. Eur. J. Immunol. 1994;24:440–444. doi: 10.1002/eji.1830240226. [DOI] [PubMed] [Google Scholar]
- 24.Adachi S., Kubota M., Lin Y.W., Okuda A., Matsubara K., Wakazono Y., Hirota H., Kuwakado K., Akiyama Y. In vivo administration of granulocyte colony-stimulating factor promotes neutrophil survival in vitro. Eur. J. Haematol. 1994;53:129–134. doi: 10.1111/j.1600-0609.1994.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakahara H., Sato E.F., Ishisaka R., Kanno T., Yoshioka T., Yasuda T., Inoue M., Utsumi K. Biochemical properties of human oral polymorphonuclear leukocytes. Free Radic. Res. 1998;28:485–495. doi: 10.3109/10715769809066886. [DOI] [PubMed] [Google Scholar]
- 26.Edashige K., Watanabe Y., Sato E.F., Takehara Y., Utsumi K. Reversible priming and protein-tyrosyl phosphorylation in human peripheral neutrophils under hypotonic conditions. Arch. Biochem. Biophys. 1993;302:343–347. doi: 10.1006/abbi.1993.1221. [DOI] [PubMed] [Google Scholar]
- 27.Sato E.F., Higashino M., Ikeda K., Wake R., Matsuo M., Utsumi K., Inoue M. Oxidative stress-induced cell death of human oral neutrophils. Am. J. Physiol. Cell Physiol. 2003;284:C1048–1053. doi: 10.1152/ajpcell.00016.2002. [DOI] [PubMed] [Google Scholar]
- 28.Smith D.J., Anderson J.M., King W.F., van Houte J., Taubman M.A. Oral streptococcal colonization of infants. Oral Microbiol. Immunol. 1993;8:1–4. doi: 10.1111/j.1399-302x.1993.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 29.Nagata K., Yu H., Nishikawa M., Kashiba M., Nakamura A., Sato E.F., Tamura T., Inoue M. Helicobacter pylori generates superoxide radicals and modulates nitric oxide metabolism. J. Biol. Chem. 1998;273:14071–14073. doi: 10.1074/jbc.273.23.14071. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa M., Sato E.F., Utsumi K., Inoue M. Oxygen-dependent regulation of energy metabolism in ascites tumor cells by nitric oxide. Cancer Res. 1996;56:4535–4540. [PubMed] [Google Scholar]
- 31.Takehara Y., Kanno T., Yoshioka T., Inoue M., Utsumi K. Oxygen-dependent regulation of mitochondrial energy metabolism by nitric oxide. Arch. Biochem. Biophys. 1995;323:27–32. doi: 10.1006/abbi.1995.0005. [DOI] [PubMed] [Google Scholar]
- 32.Takehara Y., Nakahara H., Inai Y., Yabuki M., Hamazaki K., Yoshioka T., Inoue M., Horton A.A., Utsumi K. Oxygen-dependent reversible inhibition of mitochondrial respiration by nitric oxide. Cell Struct. Funct. 1996;21:251–258. doi: 10.1247/csf.21.251. [DOI] [PubMed] [Google Scholar]
- 33.Okada S., Takehara Y., Yabuki M., Yoshioka T., Yasuda T., Inoue M., Utsumi K. Nitric oxide, a physiological modulator of mitochondrial function. Physiol. Chem. Phys. Med. NMR. 1996;28:69–82. [PubMed] [Google Scholar]
- 34.Nakamura A., Park A., Nagata K., Sato E.F., Kashiba M., Tamura T., Inoue M. Oxidative cellular damage associated with transformation of Helicobacter pylori from a bacillary to a coccoid form. Free Radic. Biol. Med. 2000;28:1611–1618. doi: 10.1016/s0891-5849(00)00284-7. [DOI] [PubMed] [Google Scholar]
- 35.Park A.M., Nagata K., Sato E.F., Tamura T., Shimono K., Inoue M. Mechanism of strong resistance of Helicobacter pylori respiration to nitric oxide. Arch. Biochem. Biophys. 2003;411:129–135. doi: 10.1016/s0003-9861(02)00691-4. [DOI] [PubMed] [Google Scholar]
- 36.Huycke M.M., Joyce W., Wack M.F. Augmented production of extracellular superoxide by blood isolates of Enterococcus faecalis. J. Infect. Dis. 1996;173:743–746. doi: 10.1093/infdis/173.3.743. [DOI] [PubMed] [Google Scholar]
- 37.Huycke M.M., Moore D., Joyce W., Wise P., Shepard L., Kotake Y., Gilmore M.S. Extracellular superoxide production by Enterococcus faecalis requires demethylmenaquinone and is attenuated by functional terminal quinol oxidases. Mol. Microbiol. 2001;42:729–740. doi: 10.1046/j.1365-2958.2001.02638.x. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama Y., Massey V., Takeda K., Kawasaki S., Sato J., Watanabe T., Niimura Y. Hydrogen peroxide-forming NADH oxidase belonging to the peroxiredoxin oxidoreductase family: existence and physiological role in bacteria. J. Bacteriol. 2001;183:2431–2438. doi: 10.1128/JB.183.8.2431-2438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higuchi M., Yamamoto Y., Poole L.B., Shimada M., Sato Y., Takahashi N., Kamio Y. Functions of two types of NADH oxidases in energy metabolism and oxidative stress of Streptococcus mutans. J. Bacteriol. 1999;181:5940–5947. doi: 10.1128/jb.181.19.5940-5947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higuchi M., Shimada M., Yamamoto Y., Hayashi T., Koga T., Kamio Y. Identification of two distinct NADH oxidases corresponding to H2O2-forming oxidase and H2O-forming oxidase induced in Streptococcus mutans. J. Gen. Microbiol. 1993;139:2343–2351. doi: 10.1099/00221287-139-10-2343. [DOI] [PubMed] [Google Scholar]
- 41.Niimura Y., Nishiyama Y., Saito D., Tsuji H., Hidaka M., Miyaji T., Watanabe T., Massey V. A hydrogen peroxide-forming NADH oxidase that functions as an alkyl hydroperoxide reductase in Amphibacillus xylanus. J. Bacteriol. 2000;182:5046–5051. doi: 10.1128/jb.182.18.5046-5051.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higuchi M., Yamamoto Y., Kamio Y. Molecular biology of oxygen tolerance in lactic acid bacteria: Functions of NADH oxidases and Dpr in oxidative stress. J. Biosci. Bioeng. 2000;90:484–493. [PubMed] [Google Scholar]
- 43.Yu H., Sato E.F., Nagata K., Nishikawa M., Kashiba M., Arakawa T., Kobayashi K., Tamura T., Inoue M. Oxygen-dependent regulation of the respiration and growth of Escherichia coli by nitric oxide. FEBS Lett. 1997;409:161–165. doi: 10.1016/s0014-5793(97)00494-8. [DOI] [PubMed] [Google Scholar]
- 44.Benjamin N., O’Driscoll F., Dougall H., Duncan C., Smith L., Golden M., McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 45.Dykhuizen R.S., Fraser A., McKenzie H., Golden M., Leifert C., Benjamin N. Helicobacter pylori is killed by nitrite under acidic conditions. Gut. 1998;42:334–337. doi: 10.1136/gut.42.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyoshi M., Kasahara E., Park A.M., Hiramoto K., Minamiyama Y., Takemura S., Sato E.F., Inoue M. Dietary nitrate inhibits stress-induced gastric mucosal injury in the rat. Free Radic. Res. 2003;37:85–90. doi: 10.1080/1071576021000086632. [DOI] [PubMed] [Google Scholar]
- 47.Bayindir Y.Z., Polat M.F., Seven N. Nitric oxide concentrations in saliva and dental plaque in relation to caries experience and oral hygiene. Caries. Res. 2005;39:130–133. doi: 10.1159/000083158. [DOI] [PubMed] [Google Scholar]
- 48.Palmerini C.A., Palombari R., Perito S., Arienti G. NO synthesis in human saliva. Free Radic. Res. 2003;37:29–31. doi: 10.1080/1071576021000028398. [DOI] [PubMed] [Google Scholar]