Abstract
The aim of this study was to investigate the effects of a diabetic meal delivery system on glycemic control over a 12 month period in patients with type 2 diabetes. A total of 77 patients with type 2 diabetes were assigned randomly into three dietary intervention groups: group M, diabetic meal delivery; group D, individual dietary counseling; and group C, conventional dietary education. In group M, HbA1c levels decreased significantly from 8.2 ± 1.2% to 7.4 ± 0.8% after 12 months (p<0.05), while in group D, HbA1c levels decreased significantly throughout the entire 12 month period, from 8.5 ± 1.7% at baseline to 7.4 ± 1.1% at the endpoint. Similarly, fasting blood glucose (FBG) levels decreased significantly between 1 and 12 months in group M (p<0.05), and decreased significantly during the entire 12 month period in group D (p<0.01). There were no significant changes in either HbA1c or FBG levels in group C. This study provides evidence that intervention with delivery of diabetic meals to patients with type 2 diabetes can be equally effective for achieving glycemic control as individual dietary counselling by a dietitian. Diabetic meal delivery can therefore be used successfully to provide diabetes education to outpatients.
Keywords: type 2 diabetes, glycemic control, diabetic meal delivery, self-management, diet counseling
Introduction
Numerous studies have demonstrated the beneficial effects of dietary intervention and increased physical activity on hyperglycemia and other cardiovascular risk factors such as high cholesterol and hypertension [1–4]. However, it often proves very difficult for patients with diabetes to change their behavior or their lifestyle, especially their diet. Even if patients succeed in making these changes, many have a tendency to revert to their previous lifestyle and dietary habits [5, 6]. In Japan, patients with diabetes often stay in hospital for 1 or 2 weeks for diabetes education, primarily to learn about diet. However, this is not a convenient method for many patients due to high costs and suspension of work and daily life. Therefore, various other methods for managing diabetes have been designed with the aim of improving the quality and efficiency of self-care in patients. Studies have been carried out to establish educational programs that encourage patients to follow and adhere to practical guidelines. Diabetes care involves managing patients with a chronic disease consistently in outpatient settings. To establish a more efficient system for diabetes care, we devised a diabetic meal delivery system for patients with type 2 diabetes. Using this system, the patients were able to learn about diabetes dietary requirements, with this knowledge making it easier from them to diet without the need for a stay in hospital. The aim of this study was to investigate the effects of our diabetic meal delivery system on glycemic control in patients with type 2 diabetes.
Subjects and Methods
Patients and dietary intervention
The study protocol was approved by the Ethics Committee of the School of Comprehensive Rehabilitation at Osaka Prefecture University and informed consent was obtained from all patients. Participants diagnosed with type 2 diabetes were recruited from outpatients attending the Kajiyama Clinic between 2004 and 2005. Patients were excluded if they had any significant diseases that were likely to affect the outcome or compliance with this study. Such diseases or conditions included heart failure, hepatic dysfunction, renal failure or serious physical and mental conditions. A total of 77 adults with type 2 diabetes who attended the clinic (mean age 63.6 ± 10.8 years (means ± SD), range 36–80 years) were assigned into three dietary groups by the stratified randomization method that considered age, gender, and duration of diabetes. Each group received one of the following diabetic dietary interventions.
The diabetic meal delivery group (group M) had diabetic meals delivered to their home every day. The meals were specially designed for diabetes patients and consisted of 1,200 to 1,800 kcal, 16% protein, 60–64% carbohydrates, 20–25% fat, and approximately 9 g of salt. The meals were cooked and chilled immediately to 3°C prior to delivery. If a patient agreed to participate in the study, the subject had three sessions with dietitians at the start of intervention and after 4 months, and 12 months. The sessions were interactive and included a focus on type 2 diabetes, its symptoms, treatment (especially diet), and associated complications.
The individual diet counseling group (group D) involved patients having face-to-face consultation sessions (20 to 30 min) with dietitians every month (12 sessions over 12 months). Although the sessions had pre-planned topics such as diabetes risk factors, diet, physical activity and problem solving, the discussions were individualized and focused on specific individual problems, mainly diet. The goal was to equip the subjects with the necessary knowledge and skills to initiate behavioral changes. The subjects were encouraged to make intermediate goals for themselves by thinking about practical things they could use to change in their diet and daily exercise habits. For example, instead of an abstract goal such as “increase fiber intake and daily exercise”, a practical goal would be “to eat brown rice and walk 10,000 steps everyday”. The dietitians also addressed coping skills for improving psychosocial functions. The participants were also encouraged to measure and record their weight at home on a regular basis, but were not required to record their diet. The family members responsible for shopping and cooking were invited to join the sessions, with the importance of family participation and the support structures provided being emphasized.
The conventional dietary education group (group C) involved the patients receiving their usual outpatients management every month. At baseline, the control group was given general information about lifestyle and diabetes risk. This briefly information was provided individually by either a doctor or nurse at every visit with some printed material also being distributed. The message was to reduce weight, increase physical activity and make qualitative changes in dietary habits.
Laboratory analysis
Fasting blood samples were collected from all participants every month and examined by auto analyzer. HbA1c levels were determined by a latex cohesion method (JCA-BM2250, KYOWA MEDEX, Co., Ltd., Tokyo, Japan). Fasting blood glucose (FBG) levels were examined by the hexokinase method (JCA-BM12, Shino Test, Co., Ltd., Tokyo, Japan). Total cholesterol (T-Ch) and triglyceride (TG) levels were determined by enzyme assay. HDL cholesterol (HDL-Ch) levels by a direct method (Labospect 008K, Bio Majesty JCA-BM 8060, JEOL, Ltd., Tokyo, Japan) and LDL cholesterol (LDL-Ch) levels by an enzymatic method (Bio Majesty JCA-BM 8060, JEOL, Ltd., Tokyo, Japan).
Statistical analysis
Data were expressed as means ± SD. Statistical analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL). Differences between the three groups at baseline were determined by one-way analysis of variance (ANOVA). The Bonferroni/Dunnett post hoc test was used to determine the significant of continuous data and the χ2 test for significance of the categorical variables. Repeated-measures ANOVA was used to determined the difference between the groups and over times. When a significant difference was found by repeated-measures ANOVA, paired t tests with Bonferroni correction were applied to identify specific differences. A p value of less than 0.05 was considered statistically significant.
Results
The clinical characteristics and baseline laboratory data of the three intervention groups are shown in Table 1. There were no significant differences between the three groups with respect to age, body mass index (BMI), diabetes duration and glucose control methods or in terms of laboratory data, including baseline FBG, HbA1c, T-Ch, HDL-Ch, LDL-Ch and TG.
Table 1.
Baseline clinical characteristics of the three study groups
| Meal delivery | Dietitian counseling | Conventional advise | p | |
|---|---|---|---|---|
| Number | 18 | 29 | 30 | |
| Sex (males/females) | 8/10 | 13/16 | 14/16 | 0.641 |
| Age (yrs) | 65.1 ± 10.9 | 62.0 ± 10.9 | 64.3 ± 10.7 | 0.488 |
| Body mass index (kg/m2) | 25.2 ± 5.4 | 23.8 ± 4.1 | 23.6 ± 2.9 | 0.398 |
| Systolic blood pressure (mmHg) | 126 ± 17 | 128 ± 18 | 127 ± 16 | 0.862 |
| Diastolic blood pressure (mmHg) | 77 ± 11 | 77 ± 13 | 76 ± 12 | 0.901 |
| Duration of diabetes (yrs) | 9.3 ± 9.4 | 11.1 ± 10.4 | 12.9 ± 6.2 | 0.374 |
| Fasting blood glucose (mg/dl) | 167 ± 30 | 181 ± 70 | 176 ± 49 | 0.777 |
| HbA1c (%) | 8.2 ± 1.2 | 8.5 ± 1.7 | 8.1 ± 1.2 | 0.529 |
| Total cholesterol (mg/dl) | 219 ± 29 | 214 ± 36 | 218 ± 38 | 0.890 |
| HDL-cholesterol (mg/dl) | 56 ± 17 | 59 ± 15 | 61 ± 16 | 0.557 |
| LDL-cholesterol (mg/dl) | 135 ± 28 | 122 ± 34 | 130 ± 34 | 0.410 |
| Triglycerides (mg/dl) | 154 ± 105 | 160 ± 118 | 142 ± 88 | 0.807 |
| Diabetes treatment | ||||
| Diet only | 4 | 11 | 10 | 0.531 |
| Oral hypoglycemic agents | 11 | 14 | 15 | 0.668 |
| Oral hypoglycemic agents + Insulin | 3 | 4 | 5 | 0.963 |
| Energy goals (kcal) | 1,455 ± 204 | 1,479 ± 190 | 1,490 ± 184 | 0.832 |
Data are means ± SD or n.
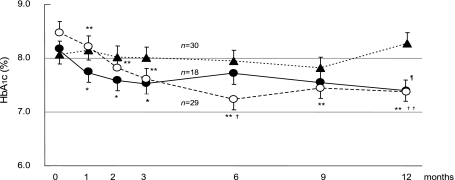
Our primary objective was glycemic control, as indicated by HbA1c levels. Fig. 1 shows HbA1c results in the three groups during the intervention period. In group M, HbA1c levels decreased from 8.2 ± 1.2% to 7.7 ± 1.1% after one month (p<0.05) and continued to decrease significantly after two and three months (p<0.05), with a reduction to 7.4 ± 0.8% after 12 month. In group D, HbA1c also decreased significantly overall throughout the 12 month period (p<0.01), from 8.5 ± 1.7% to 7.4 ± 1.1%. In contrast, in group C HbA1c increased slightly from 8.1 ± 1.2% at baseline to 8.3 ± 1.1% at the end of 12 month period. HbA1c levels in groups M and D were significantly lower than in group C at the end of the study (p<0.05 and p<0.01, respectively).
Fig. 1.
HbA1c levels after intervention in the group M (closed circle), D (open circle) and C (closed triangle). The data are expressed as means ± SE. *Significant difference from the baseline (p<0.05). **Significant difference from the baseline (p<0.01). ¶M group vs C group (p<0.05). †D group vs C group (p<0.05). ††D group vs C group (p<0.01).
Table 2 shows the changes in BMI and laboratory data from baseline to 12 months in the three dietary intervention groups. BMI tended to decrease in groups M and D, but remained unchanged in group C. FBG levels decreased significantly from 167 ± 30 to 128 ± 26 mg/dl in group M (p<0.05) and from 181 ± 70 to 145 ± 45 mg/dl in group D (p<0.01). No significant change was observed in group C. There were no significant changes in serum lipids levels in the three intervention groups, although serum TG levels were tended to decrease in groups M and D.
Table 2.
Changes in BMI and laboratory data from baseline to endpoint in subjects in the intervention and control groups
| Meal delivery (n=18) | Dietitian counseling (n=29) | Conventional advice (n=30) | ||||
|---|---|---|---|---|---|---|
| before | endpoint | before | endpoint | before | endpoint | |
| BMI (kg/m2) | 25.2 ± 5.4 | 24.2 ± 5.8 | 23.8 ± 4.1 | 23.5 ± 3.6 | 23.6 ± 2.9 | 23.9 ± 3.1 |
| Fasting blood glucose (mg/dl) | 167 ± 30 | 128 ± 26* | 181 ± 70 | 145 ± 45** | 176 ± 49 | 167 ± 52 |
| HbA1c (%) †,†† | 8.2 ± 1.2 | 7.4 ± 0.8* | 8.5 ± 1.7 | 7.4 ± 1.1** | 8.1 ± 1.2 | 8.3 ± 1.1 |
| Total cholesterol (mg/dl) | 219 ± 29 | 202 ± 25 | 214 ± 36 | 202 ± 33 | 218 ± 38 | 203 ± 42 |
| HDL-cholesterol (mg/dl) | 56 ± 17 | 63 ± 13 | 59 ± 15 | 61 ± 18 | 61 ± 16 | 60 ± 12 |
| LDL-cholesterol (mg/dl) | 135 ± 28 | 128 ± 31 | 122 ± 34 | 116 ± 29 | 130 ± 34 | 119 ± 30 |
| Triglyceride (mg/dl) | 154 ± 105 | 110 ± 46 | 160 ± 118 | 130 ± 78* | 142 ± 88 | 135 ± 69 |
Data are means ± SD. Baseline vs endpoint: *p<0.05, **p<0.01.
Meal delivery vs conventional advice at the end of the study: †p<0.05.
Dietitian counseling vs conventional advice at the end of the study: ††p<0.01.
Discussion
To our knowledge, the present study is the first to evaluate the use of a diabetic meal delivery system. In this study, we showed that delivery of the diabetic meals led to improved glycemic control. Intervention with the diabetic meal delivery had a beneficial effect on HbA1c levels similar to that seen with individual dietary counseling.
It is well known that intensive blood glucose control decreases the risk of the micro- and macro-vascular complications [7, 8]. Our results therefore have important clinical implications, given that medical costs for diabetic patients are increasing and self-care for diabetes patients is considered to be of considerable benefit. For example, it costs approximately $2,100 a week to stay in hospital for diabetic education, whereas diabetic meal delivery costs only $740 for 6 dinners for 4 months. In addition, improving glucose control will reduce the economic costs associated with diabetic complications [9, 10]. For example, hemodialysis costs $50,000 a year for one diabetic patient with chronic renal failure.
There are several possible explanations for the improved glucose control in the diabetic meal delivery group. Firstly, the patients were able to learn and experience dietary changes as a result of receiving the diabetic meals at their home. In addition, the patients in the diabetic meal delivery group received medical data, with the majority showing improved glucose control soon after intervention, as a consequence of increased self-efficacy. These factors may have stimulated and motivated the patients to control their glucose levels more enthusiastically. After the period of diabetic meal delivery, the majority of patients gained dietary skills and continued to consume a proper diet during the 12 month study period. Our study provides evidence that diabetic meal delivery is as effective as individual counseling for the self-management of diabetes. The method was particularly practical for diabetic outpatients who were not able to receive either regular education and counseling by dietitians or stay in hospital for diabetes education. Secondly, delivery of meals was suitable for patients who were not able to depend on family participation or support. We often experience that it is difficult to motivate lifestyle change, especially diet, unless there is family understanding and support. In our study, all the participants were encouraged, but not required, to record their diet.
There were several limitations to our study. The first was the relatively small number of patients, especially in the diabetic meal delivery group. In this study, first we assigned 30 patients in each of the three groups. However, 12 patients in group M were not able to complete intervention with delivery of diabetic meals perfectly. The main reasons were particular food preferences and the cost of the meal delivery. It is therefore necessary to improve the quality, taste and cost of the diabetic meals in order that they are accepted by more patients. The second limitation was that the number of meals delivered each week and the period of meal delivery varied among the patients. Patients were free to choose both the frequency and the period of delivery. This difference may have affected the change in HbA1c levels over the 12 month period. However, we found there was no difference in metabolic levels or behavior modification between patients with different frequencies or periods of meal delivery. Further studies are required to examine how many meals or how frequently the diabetic meals need to be delivered in order to maintain optimal glycemic control and behavior modification in the future.
Methods of efficient self-management training are required in public health programs as from 2008 in Japan, all health insurance organizations will be responsible for educating people with metabolic problems or other health problems. In order to educate a larger group of people efficiently, previous studies have shown that a telephone- [11] or an internet-based blood glucose monitoring system are effective for diabetes management [12]. However, many elderly Japanese are not yet familiar with the use of the internet, while education over the telephone, although an effective psychological support may not be a suitable method for teaching facts about diet. The diabetic meal delivery service provides an opportunity for people with diabetes who are not able to receive individual counseling, or who live in rural and medically underserved areas, to have access to high-quality diabetes education. The observed difference in incidence between intervention groups and the usual care control group indicated that the intervention needs to be individualized and performed consistently in order to be effective in self-management, especially diet. During the study it proved necessary to explore methods to respond to increasing demands for individual self-management education. However, the findings presented in this paper need to be confirmed in a study on a larger number of patients.
In conclusion, this study provides evidence that intervention using delivery of diabetic meals in Japanese patients with type 2 diabetes is as effective as individual diet counseling for maintaining glycemic control. It is hoped that delivery of diabetic meals makes it easier to maintain an appropriate diet at home and also to motivate and empower underserved patients to start self-management and thereby avoid the serious complications of diabetes.
Abbreviations
- BMI
Body mass index
- FBG
Fasting blood glucose
- HbA1c
Glycated hemoglobin A1c
- HDL-Ch
High density lipoprotein cholesterol
- LDL-Ch
Low density lipoprotein cholesterol
- T-Ch
Total cholesterol
- TG
Triglyceride
References
- 1.Nathan D.M. Long-term complications of diabetes mellitus. N. Eng. J. Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 2.Ohkubo Y., Kishikawa H., Araki E., Miyata T., Isami S., Motoyoshi S., Kojima Y., Furuyoshi N., Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res. Clin. Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 4.Fritsche A., Stumvoll M., Renn W., Schmülling R.M. Diabetes teaching program improves glycemic control and preserves perception of hypoglycemia. Diabetes Res. Clin. Pract. 1998;40:129–135. doi: 10.1016/s0168-8227(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 5.Bloomgarden Z.T., Karmally W., Metzger M.J., Brothers M., Nechemias C., Bookman J., Faierman D., Ginsberg-Fellner F., Rayfield E., Brown W.V. Randomized controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Diabetes Care. 1987;10:263–275. doi: 10.2337/diacare.10.3.263. [DOI] [PubMed] [Google Scholar]
- 6.Norris S.L., Lau J., Smith S.J., Schmid C.H., Engelgau M.M. Self-management education for adults with type 2 diabetes. Diabetes Care. 2002;25:1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 8.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Eng. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Economic consequences of diabetes mellitus in the U.S. in 1997. Diabetes Care. 1998;21:296–309. doi: 10.2337/diacare.21.2.296. [DOI] [PubMed] [Google Scholar]
- 10.Caro J.J., Ward A.J., O’Brien J.A. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25:476–481. doi: 10.2337/diacare.25.3.476. [DOI] [PubMed] [Google Scholar]
- 11.Izquierdo R.E., Knudson P.E., Meyer S., Kearns J., Ploutz-Snyder R., Weinstock R.S. A comparison of diabetes education administered through telemedicine versus in person. Diabetes Care. 2003;6:1002–1007. doi: 10.2337/diacare.26.4.1002. [DOI] [PubMed] [Google Scholar]
- 12.Kwon H.S., Cho J.H., Kim H.S., Song B.R., Ko S.H., Lee J.M., Kim S.R., Chang S.A., Kim H.S., Cha B.Y., Lee K.W., Son H.Y., Lee J.H., Lee W.C., Yoon K.H. Establishment of blood glucose monitoring system using the internet. Diabetes Care. 2004;27:478–483. doi: 10.2337/diacare.27.2.478. [DOI] [PubMed] [Google Scholar]