Abstract
The ideal water-soluble dietary fiber for the fiber-enrichment of foods must be very low in viscosity, tasteless, odorless, and should produce clear solutions in beverages. Partially hydrolyzed guar gum (PHGG) produced from guar gum by enzymatic process has the same chemical structure with intact guar gum but less than one-tenth the original molecular length of guar gum, which make available to be used as film former, foam stabilizer and swelling agent. The viscosity of PHGG is about 10 mPa·s in 5% aqueous solution, whereas 1% solution of guar gum shows range from 2,000 to 3,000 mPa·s. In addition, PHGG is greatly stable against low pH, heat, acid and digestive enzyme. For these reasons, PHGG seems to be one of the most beneficial dietary fiber materials. It also showed that interesting physiological functions still fully exert the nutritional function of a dietary fiber. PHGG has, therefore, been used primarily for a nutritional purpose and became fully integrated food material without altering the rheology, taste, texture and color of final products. PHGG named as Benefiber® in USA has self-affirmation on GRAS status of standard grade PHGG. PHGG named as Sunfiber® is now being used in various beverages, food products and medicinal foods as a safe, natural and functional dietary fiber in all over the world.
Keywords: Cyamoposis tetragonolobus, partially hydrolyzed guar gum (PGHH), dietary fiber
Introduction
Dietary fibers are considered as important nutritive component for human health, and especially, water-soluble dietary fibers have received much attention due to its various physiological function. The dietary fibers can be defined as a wide variety of substances that belong to the family of carbohydrate and that resist to be digested by the endogenous secretions of the human digestive tract, which would include gums, pectin, lignin, cellulose and hemicellulose. It also includes resistant starch as a minor component remaining after the hydrolysis of starch by the addition of starch metabolizing enzymes in the assay procedure of the AOAC (Association of Official Agriculture Chemists) method. Basically, dietary fibers are natural polysaccharides and the origin of that is natural component.
Guar Gum as Dietary Fiber
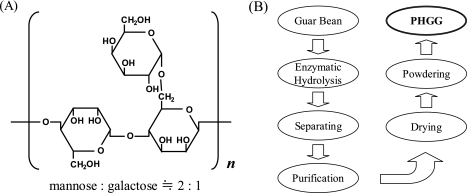
Guar gum, one of the most promising dietary fibers, is a gel-forming galactomannan obtained by grinding the endosperm portion of Cyamoposis tetragonolobus L., a leguminous plant grown for centuries mainly in India and Pakistan to produce the seed for human and animal food [1]. It was reported that guar gum was first used for food products in the U. S. in 1949 [2]. Since 1953, the seeds of the guar plant have been processed into guar gum, and widely used in modern food industry as thickener and emulsion stabilizer for both food and industrial purposes in all over the world with increasing amounts. Guar gum is structurally comprises long, straight chains of α-D-mannopyranosyl units linked together by β-D-(1-4)-glycosidic linkage. The hexose linked along this chain is α-D-galactopyranose and these side groups appear on both side of the mannose chain at about every other mannose link in the main chain [3] (Fig. 1A). Therefore, the ratio of mannose to galactose in galactomannan of guar gum has been known approximately 2:1 [4, 5]. Although a single molecular weight cannot be given due to difficulty for determination, it is estimated to be in the range of 200,000 to 300,000 daltons [6]. Galactomannan is presents in various natural sources such as coffee beans, soy beans, alfalfa seeds, pineapple, sugar beets, and locust beans [7]. There are certainly benefits when guar gum are included in human diets. However, guar gum is extremely viscous which results in the liquid products with high viscosity when it is added to enteral formulas at a physiologically effective concentration, thereby its utilization may be restricted for dietary fiber supplementation in actual food products, especially for liquid products. High viscosity of guar gum also decreases the protein efficacy [8] and lipid utilization [9] by interfering with the digestion and absorption of nutrient [10] when it dissolved into water, results in a slower gastric emptying [11].
Fig. 1.
The repeating unit of guar gum and partially hydrolyzed guar gum (PGHH) (A), and manufacturing scheme of PHGG from guar beans (B).
Guar Gum and Emzymatic Hydrolysis
Partially hydrolyzed guar gum (PHGG) is a natural water soluble dietary fiber. The guar plant, Cyamoposis tetragonolobus L. has been grown in India and Pakistan since ancient times and used for both human foods and animal feed stuffs. In 1950s, the seeds of the guar plant have been processed into guar gum in ever increasing amounts to meet the demand of the modern food industry. However, the viscosity of guar gum limits its applications in various foods especially liquid products. PHGG is produced by controlled partial enzymatic hydrolysis of guar gum seeds. As the metabolic, nutritional and analytical properties of the low-viscosity PHGG correspond to those of guar gum, this product is used primarily for a nutritional purpose, i.e. for the fiber-enrichment of processed foods. Its safety was confirmed by subchronic feeding study and mutagenecity test. Its specific chemical and physical properties could make it possible to improve the quality of food item world-widely.
Chemical Structure of PHGG
To solve the problems of guar gum as mentioned in above, PHGG was produced by controlled enzymatic hydrolysis using β-endo-mannanase, followed by sterilization and spray dry, and finally produced as shown in manufacturing scheme [12] (Fig. 1 (B)). The product was named as Sunfiber®, and of which repeating unit of structure is depicted in Fig. 1 (A). Endo-β-D-mannanase can hydrolyze galactomannan and thereby PHGG by selectively cutting the main mannan backbone-chain [13, 14], leaving the pendant galactosyl groups intact. The resulting product obtained through by the further processes, separating, purifying, drying and powdering, would have the same natural role with smaller galactomannan molecules in human diets. The molecular size of PHGG, was one-tenth the original length of guar gum, ranged from 1,000 to 100,000 daltons, and average molecular weight was 20,000 daltons when it was analyzed by gel filtration HPLC.
Physical Properties of PHGG
Guar gum formed a viscous colloidal solution when hydrated in cold water, while PHGG showed clear solution with lower viscosity than non-hydrolyzed guar gum. The viscosity of 1% aqueous solution of commercial food-grade guar gum ranged from 2,000 to 3,000 mPa·s, whereas that of a 5% solution of PHGG was less than 10 mPa·s, ideal viscosity for increment the dietary fiber content in beverages [15], which indicated that the β1→4 mannosidic linkages available for the enzyme attack strongly contribute to maintaining viscosity of guar gum [16, 17]. Low viscosity of PHGG provides a distinct advantage for the use of fiber in enteral feeding products that need to be administered through feeding tubes as the method of feeding access dictated by the clinical setting. PHGG is white power, soluble in water, colorless and transparent in water solution, and almost tasteless. When exposed to high temperature over long periods of time, the chemical structure of PHGG underwent no change and its viscosity was decreased consistently with increasing the temperature, which make it easy to application in manufacturing process with high temperature. PHGG is stable and soluble at various pH levels commonly found in foods as well as resistant to heat, acid, salt, high pressure and digestive enzyme [18, 19]. It appeared to have little or no interaction between PHGG and other common food ingredients. PHGG does not destabilize emulsions, change the viscosity of protein solution, affect to flavor and color of the products, or cause soluble materials to precipitate. In addition, PHGG prolongs the self-life of high starch foods such as bread by decreasing the turbidity of dextrin when it added to dextrin solution at low temperatures. The dietary fiber content of PHGG was very high, more than 75%, when measured by the AOAC method. The characteristics and properties of PHGG are summarized in Table 1.
Table 1.
The characterisitics and properties of partially hydrolyzed guar gum (PHGG, product name: Sunfiber®).
| Physical property | Structure type | Galactomannan |
|---|---|---|
| Molecular weight (average) | 20,000 daltons | |
| pH value | 6.7–7.0 | |
| Viscosity (5% solution) | ≤10 mPa·s | |
| Appearance | White powder | |
| Solubility in water | High | |
| Appearance on water | Colorless, Tansparent | |
| Sweetness |
≤10* |
|
| Bacteriological | Total plate count | ≤3,000/g |
| Coli forms |
Negative |
|
| Viscosity stability | Heat | ≤130°C |
| Acid | pH 3.0–7.0 | |
| Salt (NaCl) | ≤10% | |
| Digestive enzyme |
Resistance to amylase, pectinase, esterase, etc. |
|
| Composition | Dietary fiber | ≥75% |
| Moisture | ≤7% | |
| Ash | ≤2% | |
| Protein | ≤1.0% | |
| Heavy metal | ≤20 ppm |
*: The number was estimated compare to the sweetness of standard sugar, in which the sweetness of standard sugar, 3% sucrose, was considered as 100.
Safety Aspects of Guar Gum and PHGG
The safety data on guar gum may be largely available to establish the safety of its partially hydrolyzed analogue, PHGG. The safety of guar gum has been assessed by JECFA (Joint Expert Committee on Food Additives) in 1975 [20] and by the EEC’s SCF (European Economic Community, Scientific Committee on Foods) in 1978 [21]. In USA, guar gum has been considered GRAS (Generally Recognized As Safe) since 1974 in numerous food applications [22]. Guar gum did not elicit measurable mutagenic responses in the host-mediated assay using Salmonella [23], and was not carcinogenic in either species or sex [24].
When glycosidic linkage in mannose main chain of guar gum is partially broken and thereby shorten in molecular size, the question, however, can be arisen whether this product plays an equal action in human nutrition as the natural form or not. Therefore, it should be confirmed that the human body handles partially hydrolyzed guar gum, in the same manner as intact guar gum, and thereby partially hydrolyzed guar gum is safe because structural change might influence of its fate when ingested. Studies indicated that PHGG had not only lower molecular weight and lower viscosity than guar gum but also same physiological effect as the larger molecular weight soluble fiber in vivo tests [25, 26]. There is a report that the process of partial hydrolysis by enzyme breaks the guar gum down in the same manner as the human intestinal tract in which guar gum is metabolized by the colonic microflora as is intact guar gum, with the products of metabolism being the same, since in both cases metabolism is based on the fermentation of mannose and galactose [27]. Therefore, the partial hydrolysis is merely a predigestion step occurring in the normal digestion of the guar gum by the body. Because the structural change in guar gum is simply shortening the mannose chain, structural relationships between the mannan chain and the galactosyl side group in PHGG remains the same with unhydrolyzed guar gum. There is no evidence to assume that size or viscosity would have any influence on the safety of the hydrolyzed galactomannan molecules. The Life Sciences Research Organization of the Federation of American Societies for Experimental Biology commissioned a panel of 6 experts to assess the use of PHGG in consumer foods in 1993 [28], and the FDA accepted for filling a GRAS affirmation petition of PHGG in May 1995 [29]. A history of safety use has been established in Japan where the product is used as a dietary fiber in various foods since 1987. Toxicity of PHGG was tested with male and female Sprague-Dawley rats at dose levels of 0, 0.5 and 2.5 g/kg body weight/day for 28 days [26]. The results demonstrated that PHGG was well tolerated, and food consumption and body weight gain were not influenced by the treatment. Urinalysis, hematological examination and blood chemical analysis did not reveal any abnormalities that could be attributed to the treatment as well as no deaths and no changes were observed in the general condition of the rats. In another test, the dietary levels up to 10% PHGG were tolerated without any signs of toxicity in a subchronic (13-week) feeding study in rats [30]. The mutagenecity of PHGG was examined in a microbial reverse mutation assay with Salmonella typhimurium TA100 and TA98 strains, in which concentrations of up to 5 mg/plate did not have any effect on reverse mutation rates. From all these evidences, a low-viscous galactomannan (PHGG) can be, therefore, considered as safe. Administration of PHGG for 4 weeks to adult men at the dose of 36 g/day has resulted in no side effects [31], and a daily intake of 20–40 g/day PHGG has well tolerated and showed no side effects [32]. For the desired intake value, 20–30 g/day of dietary fiber has been recommended based on 2,000–2,500 kcal/day for an average in Japan. On the base of all of experiment data and published scientific evidence, PHGG is considered as safe and appropriate to use as a ingredient of enteral nutrition products and liquid oral supplement products for purpose of providing dietary fiber.
Applications of Guar Gum and PHGG
In addition to the usage of guar gum in food reviewed in book written by Whistler and Hymowits [33], usage for pharmaceutical preparation was also suggested, in which a mixture of guar gum, a bovine gelatin hydrolysate, sodium glycinate, and an appropriate amount of flavoring agents was blended and heated to yield product which could be administered orally for the treatment of digestive tract disorders [34]. Because partially hydrolyzed guar gum (PHGG) is soluble in water, very low in viscosity, and almost free of color and taste, which correspond to those of guar gum, it can easily be used with sufficient amounts to give dietary fiber effect in food, and thereby it has widely been used in Japan as a new fiber resources since 1987 for a nutritional purpose. These specific chemical and physical properties could make it possible to improve the quality of food item. For example, addition of PHGG can improve in processing of cereals by increasing flow ability, provide body and mellow flavor in most beverages, stabilize the colloid system of dry and liquid meal replacements, mellow tartness and firm texture in yogurt, stabilize the foam system of shakes, improve suspension of particulate in soups and dressings and give good eating qualities in baked goods [12]. Also, PHGG has been widely used in food formulation such as beverages and breads [35], food binder [36], paper coating [37], and petroleum industry [38]. On the basis of those characteristics, the PHGG, partially hydrolyzed guar gum, is now being used in many different ways in a various fields of beverage and food as bulking agent (i.e. in Yogurt beverages, carbonated beverages, ice cream and starch syrup), alternative of wheat flour (i.e. in cookies), food stabilizer (i.e. in whipped cream), source of dietary fiber (i.e. in Bavarian desserts and custard pudding), substitute of sugar (i.e. in whipped cream and steamed bread), supplement for preparation of powder from liquid sugars, coating materials (i.e. in meringue cake with high-fat nuts and dried fish with luster), and inhibitor of starch dispersion from the surface of boiled rice without any change in taste and texture of final product.
Recommended Daily Intake of Dietary Fiber
The most thorough recent analysis of fiber intake in the United States was conducted by FASEB (Federation of American Societies for Experimental Biology) [39] as part of their “Physiological Effects and Health Consequences of Dietary Fiber” monograph prepared for FDA. FASEB summarized a number of intake studies and calculated ranges of fiber intake, whose data showed a daily per capita consumption of 11.1–23.3 g/day or 5.8–11.8 g/1,000 kcal [40]. The estimated total dietary fiber intake varies between about 10–25 g/day in most developed countries. A WHO study group has recommended a daily intake of about 37 g total dietary fiber. The FASEB expert panel has recommended a daily intake of 20–35 g/day total dietary fiber from foods for the healthy, adult population of the USA [37]. The minimum value was based on the effects of dietary fiber on intestinal function, while the maximum value was chosen with an eye towards the possibly deleterious effects of large amounts of dietary fiber on mineral balance. This recommendation calculates out to be 10 to 13 g dietary fiber/1,000 kcal. The American Diabetes Association has recommended a fiber intake of 40–50 g/day [41]. This level is significantly higher than the level recommended by FASEB. When Fredstrom et al. analyzed the soluble, insoluble, and total dietary fiber content of a number of currently marketed enteral products, the fiber content of these products varied widely, ranging from 5–20 g/1,000 kcal [42]. The insoluble fiber, soluble fiber, and total dietary fiber in one product were 5.4 g/1,000 kcal, 4.6 g/1,000 kcal, and 9.9 g/1,000 kcal, respectively; approximately 45% of the total dietary fiber in those products were soluble fiber. The desired intake value of 20–30 g/day of dietary fiber has been recommended in Japan based on 2,000–2,500 kcal/day.
Conclusions
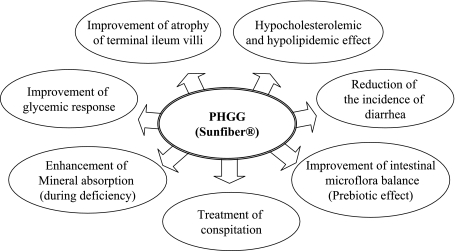
The ideal water-soluble dietary fiber for the fiber-enrichment of foods must be very low in viscosity, tasteless, odorless, and should produce clear solutions in beverages. Partially hydrolyzed guar gum (PHGG) produced from guar gum by enzymatic process has same chemical structure with intact guar gum but one-tenth the original molecular length of guar gum, which make available to be used as film former, foam stabilizer and swelling agent. The viscosity of PHGG is about 10 mPa·s in 5% aqueous solution, whereas 1% solution of guar gum shows range from 2,000 to 3,000 mPa·s. In addition, PHGG is greatly stable against low pH, heat, acid and digestive enzyme. PHGG seems to be one of the most beneficial dietary fiber materials. It showed that interesting physiological functions still fully exert the nutritional function of a dietary fiber. PHGG has, therefore, been used primarily for a nutritional purpose and became fully integrated food material without altering the rheology, taste texture and color of final products. PHGG named as Benefiber® in USA has self-affirmation on GRAS status of standard grade PHGG [29]. PHGG named as Sunfiber® is now being used in various beverages, food products and medicinal foods as a safe, natural and functional dietary fiber in all over the world (Fig. 2).
Fig. 2.
Physiological functions of partially hydrolyzed guar gum (PHGG).
References
- 1.Goldstein A.M., Alter E.N., Seaman J.K. In: Guar gum, in Industrial Gums, 2nd ed. Whistler R.L., BeMiller J.N., editors. Academic Press; New York: 1973. pp. 303–321. [Google Scholar]
- 2.FASEB (Federation of American Societies for Experimental Biology) Evaluation of the health aspects of guar gum as a food ingredient. Submitted under contract No. FDA 72-85. Bethesda: 1973. pp. 1–12. [Google Scholar]
- 3.Stephen A. M. In: Other plant polysaccharides, in The polysaccharides. Aspinall G.O., editor. Academic Press; New York: 1983. pp. 97–193. [Google Scholar]
- 4.Robinson G., Ross-Murphy S.B., Morris E.R. Viscosity-molecular weight relationships, intrinsic chains flexibility, and dynamic solution properties of guar galactomannan. Carbohyd. Res. 1982;107:17–32. [Google Scholar]
- 5.Englyst H.N., Cummings J.H. Improved method for measurement of dietary fiber as non-starch polysaccharides in plant foods. J. Assoc. Off. Anal. Chem. 1988;71:808–814. [PubMed] [Google Scholar]
- 6.Glicksman M. Gum technology in the Food Industry. Academic Press; New York: 1969. p. 590. [Google Scholar]
- 7.Dea I.C.M., Morrison A. In: Chemistry and interactions of seed galactomannans, in Advances in Carbohydrate Chemistry and Biochemistry, vol. 3. Tipson R.S., Horton D., editors. Academic Press; New York: 1975. pp. 241–312. [Google Scholar]
- 8.Poksay K.S., Schneeman B.O. Pancreatic and intestinal response to dietary guar gum in rats. J. Nutr. 1983;113:1544–1549. doi: 10.1093/jn/113.8.1544. [DOI] [PubMed] [Google Scholar]
- 9.Simons L.A., Gayst S., Balasubramaniam S., Ruys J. Long-term treatment of hypercholesterolaemia with a new palatable formulation of guar gum. Atherosclerosis. 1982;45:101–108. doi: 10.1016/0021-9150(82)90175-7. [DOI] [PubMed] [Google Scholar]
- 10.Ikegami S., Tsuchihashi F., Harada H., Tsuchihashi N., Nishide E., Innami S. Effect of viscous indigestible polysaccharides on pancreatic-biliary secretion and digestive organs in rats. J. Nutr. 1990;120:353–360. doi: 10.1093/jn/120.4.353. [DOI] [PubMed] [Google Scholar]
- 11.Shah N., Mahoney R.R., Pellett P.L. Effect of guar gum, lignin and pectin on proteolytic enzyme levels in the gastrointestinal tract of the rat: a time-based study. J. Nutr. 1986;116:786–794. doi: 10.1093/jn/116.5.786. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg N.A., Sellman D. Partially hydrolyzed guar gum as a source of fiber. Cereal Foods World. 1998;43:703–707. [Google Scholar]
- 13.McCleary B.V. Modes of action of β-mannanase enzymes of diverse origin on legume seed galactomannans. Phytochemistry. 1979;18:757–763. [Google Scholar]
- 14.Balascio J.R., Pallmer J.K., Salyers A.A. Degradation of guar gum by enzymes produced by a bacterium from the human colon. J. Food Biochem. 1981;5:271–282. [Google Scholar]
- 15.Yamamoto T., Yamamoto S., Miyahara I., Matsumura Y., Hirata A., Kim M. Isolation or a β-Mannan Hydorlyzing Enzyme and Hydrolysis of Guar Gum by the Enzyme Isolated. Denpun Kagaku. 1990;37:99–105. [Google Scholar]
- 16.Dekker R.F., Richards G.N. Hemicellulases: their occurrence, purification, properties, and mode of action. Adv. Carbohydr. Chem. Biochem. 1976;32:277–352. doi: 10.1016/s0065-2318(08)60339-x. [DOI] [PubMed] [Google Scholar]
- 17.McCleary B.V., Neukom H. Effect of enzymic modification on the solution and interaction properties of galactomannans. Prog. Food Nutr. Sci. 1982;6:109–118. [Google Scholar]
- 18.Chudzikowski R.J. Guar gum and its applications. J. Soc. Cosmet. Chem. 1971;22:43–60. [Google Scholar]
- 19.Edwards C.A., Blackburn N.A., Craigen L., Davison P., Tomlin J., Sugden K., Johnson I.T., Read N.W. Viscosity of food gums determined in vitro related to their hypoglycemic actions. Am. J. Clin. Nutr. 1987;46:72–77. doi: 10.1093/ajcn/46.1.72. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Evaluation of certain food additives some food colors, thickening agents, and certain other substances. WHO Food Additives Series. 1975;8:38–43. [Google Scholar]
- 21.European Economic Community. Reports of the scientific committee for food on guar gum (E 412), in Reports of the scientific committee for food on emulsifiers, stabilizers, thickeners and gelling agents, 7th Series. Commission of the European Communities; Brussels-Luxenbourg: 1978. pp. 1–20. [Google Scholar]
- 22.Code of Federal Regulations. Guar gum, sec. 184.1339, in Food and Drug Administration, Department of Health and Human Services (Chap. 1), 21 C.F.R. U.S. Government Printing Office; Washington, DC: 1974. pp. 525–526. [Google Scholar]
- 23.Stanford Research Institute. Study of mutagenic effect of guar gum (FDA 71-16), in Report prepared under DHEW contract No. FDA 71-267. NTIS (National Technical Information Service), No. PB-221-815/4; Springfield, VA: 1972. [Google Scholar]
- 24.National Toxicological Program. Carcinogenesis bioassay of guar gum (CAS No. 9000-30-0) in F344 rats and B6C3F1 mice (Feed study). NIH Publ. Research Triangle Park; NC, USA: 1982. p. 127. [Google Scholar]
- 25.Favier M.L., Bost P.E., Guittard C., Demigne C., Remesy C. The cholesterol-lowering effect of guar gum is not the result of a simple diversion of bile acids toward fecal excretion. Lipids. 1997;32:953–959. doi: 10.1007/s11745-997-0123-z. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi H., Yang S.I., Fujiki M., Kim M., Yamamoto T., Greenberg N.A. Toxicity studies of partially hydrolyzed guar gum. J. Am. Coll. of Toxicol. 1994;13:273–278. [Google Scholar]
- 27.Nyman M., Asp N. Fermentation of dietary fibre components in the rat intestinal tract. Br. J. Nutr. 1982;47:357–357. doi: 10.1079/bjn19820047. [DOI] [PubMed] [Google Scholar]
- 28.Anderson S.A., Fisher K.D., Talbot J.M. Evaluation of the health aspects of using partially hydrolyzed guar gum as a food ingredient. Life Sciences Research Office, Federation of American Societies for Experimental Biology. Bethesda: 1993. [Google Scholar]
- 29.Angels R.M. Letter from Food and Drug Administration. May 23, 1995. [Google Scholar]
- 30.Takahashi H., Yang S.I., Kim M., Yamamoto T. Protein and energy utilization of growing rats fed on the diets containing intact or partially hydrolyzed guar gum. Comp. Biochem. Physiol. 1994;107A:255–260. doi: 10.1016/0300-9629(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi H., Yang S.I., Hayashi C., Kim M., Yamanaka J., Yamamoto T. Effect of partially hydrolyzed guar gum on fecal output in human volunteers. Nutr. Res. 1993;13:649–657. [Google Scholar]
- 32.Meier R., Beglinger C., Schneider H., Rowedder A., Gyr K. Effect of liquid diet with and without soluble fiber supplementation on intestinal transit and cholecystokinin release in volunteers. J. Parenteral Enteral Nutr. 1993;17:231–235. doi: 10.1177/0148607193017003231. [DOI] [PubMed] [Google Scholar]
- 33.Whistler R.L., Hymowits T. Guar; Agronomy, production, industrial use and nutrition. Purdue University Press, West Lafayette, Ind.; USA: 1979. p. 124. [Google Scholar]
- 34.Wellcome Foundation Ltd. Inhibition of gelation of polysaccharide gums in pharmaceuticals. Jap. Patent 55019290 A. 1980. p. 7. [Google Scholar]
- 35.Dainippon Pharmaceutical Co., Ltd. Dietary fibers and their manufacture by partial enzymatic digestion of plant polysaccharides. Jap. Patent 63269993 A. 1988. p. 7. [Google Scholar]
- 36.Toyo Boseki Co., Ltd. Method of producing guar gum of low viscosity. Jap. Patent 52038039 A. 1980. p. 8. [Google Scholar]
- 37.Hokuetsu Paper Mills Ltd. Water thinned antiseptic deodorant compositions for coating or impregnation. Jap. Patent 63035637 A. 1988. p. 7. [Google Scholar]
- 38.Baxter Laboratories Inc. (T Cayle, T., Morgaanville, N.J., and Schleich, H.) Mannan depolymerase enzyme combination. US Patent 3684710. 1972. p. 7. [Google Scholar]
- 39.Pilch S.M. Physiological effects and health consequences of dietary fiber. Submitted under FDA contract 223-84-2059. FASEB (Federation of American Societies for Experimental Biology); Bethesda: 1987. p. 256. [Google Scholar]
- 40.Marlett J.A., Chesters J.G. Measuring dietary fiber in human foods. J. Food Sci. 1985;50:410–414. [Google Scholar]
- 41.ADA (American Diabetes Association) Position statement; Nutritional recommendations and principles for individuals with diabetes mellitus. Diabetes Care. 1987;10:120–132. doi: 10.2337/diacare.10.1.126. [DOI] [PubMed] [Google Scholar]
- 42.Fredstrom S.B., Baglien K.S., Lampe J.W., Slavin J.L. Determination of the fiber content of enteral feedings. J. Parenteral Enteral Nur. 1991;15:450–453. doi: 10.1177/0148607191015004450. [DOI] [PubMed] [Google Scholar]