Abstract
Immune cell migration into and through mucosal barrier sites in general and airway sites in particular is a critical feature of immune and inflammatory responses, but the determinants of transepithelial (unlike transendothelial) immune cell traffic are poorly defined. Accordingly, we used primary culture airway epithelial cells and peripheral blood mononuclear cells to develop a cell monolayer system that allows for apical-to-basal and basal-to-apical T cell transmigration that can be monitored with quantitative immunofluorescence flow cytometry. In this system, T cell adhesion and subsequent transmigration were blocked in both directions by monoclonal antibodies (mAbs) against lymphocyte function-associated antigen 1 (LFA-1) or intercellular adhesion molecule 1 (ICAM-1) (induced by interferon γ [IFN-γ] treatment of epithelial cells). The total number of adherent plus transmigrated T cells was also similar in both directions, and this pattern fit with uniform presentation of ICAM-1 along the apical and basolateral cell surfaces. However, the relative number of transmigrated to adherent T cells (i.e., the efficiency of transmigration) was increased in the basal-to-apical relative to the apical-to-basal direction, so an additional mechanism was needed to mediate directional movement towards the apical surface. Screening for epithelial-derived β-chemokines indicated that IFN-γ treatment caused selective expression of RANTES (regulated upon activation, normal T cell expressed and secreted), and the functional significance of this finding was demonstrated by inhibition of epithelial–T cell adhesion and transepithelial migration by anti-RANTES mAbs. In addition, we found that epithelial (but not endothelial) cells preferentially secreted RANTES through the apical cell surface thereby establishing a chemical gradient for chemotaxis across the epithelium to a site where they may be retained by high levels of RANTES and apical ICAM-1. These patterns for epithelial presentation of ICAM-1 and secretion of RANTES appear preserved in airway epithelial tissue studied either ex vivo with expression induced by IFN-γ treatment or in vivo with endogenous expression induced by inflammatory disease (i.e., asthma). Taken together, the results define how the patterns for uniform presentation of ICAM-1 along the cell surface and specific apical sorting of RANTES may serve to mediate the level and directionality of T cell traffic through epithelium (distinct from endothelium) and provide a basis for how this process is precisely coordinated to route immune cells to the mucosal surface and maintain them there under normal and stimulated conditions.
Keywords: RANTES, intercellular adhesion molecule 1, airway epithelial cell, endothelial cell, asthma
Tcell trafficking into epithelial barriers (including gut, skin, and airway) may be critical for mucosal immunity and epithelial barrier function, but the basis for T cell movement into and through the epithelium is poorly defined. Schemes for transendothelial movement of T cells (extravasation) have been defined that depend on the coordinated expression of cell adhesion molecules and β-chemokines that interact with corresponding receptors on the lymphocyte surface (1). Thus, it might appear reasonable to propose that similar molecular mechanisms may regulate transepithelial movement of T cells. However, there are critical differences between epithelial and endothelial cell adhesion and transmigration, the most obvious of which may be that immune cell recruitment through endothelium and epithelium generally occur in opposite directions with respect to the cell's luminal surface and the bloodstream. For endothelium, the abluminal direction is apical-to-basal, whereas for epithelium, it is basal-to-apical. In the case of the endothelium, it appears that this directional process is coordinated by the actions of selectins, cell adhesion molecules, and chemokines, but the driving force for T cell movement across the epithelial barrier (if it exists at all) is uncertain.
Accordingly, we developed a system for monitoring T cell adhesion and transmigration through an epithelial model in apical-to-basal and basal-to-apical directions. T cell behavior was monitored by quantitative flow cytometry to avoid a need for extensive leukocyte purification, culture, and/or labeling—steps that may alter leukocyte function and, by their nature, may eliminate heterotypic cell–cell interactions that may be important in vivo. Monolayers were established with primary-culture human tracheobronchial epithelial cells (hTBECs)1 that exhibit differentiated structural and functional features of polarized epithelial barriers found in situ (2). In particular, monolayers of hTBECs emulate in vivo behavior with low basal levels of intercellular adhesion molecule (ICAM)-1 and cytokine-dependent increases in ICAM-1 expression (3, 4) governed chiefly by selective responsiveness of a specific IFN-γ–driven signal transduction pathway leading from the cell surface to the ICAM-1 gene promoter region in the nucleus (5–7). Expression of epithelial ICAM-1 and consequent interaction with T cell lymphocyte function-associated antigen 1 (LFA-1) appears to be the major determinant of T cell adhesion to the apical surface of the hTBEC monolayers (8, 9), but, as noted above, the extent to which ICAM-1– LFA-1 interaction or other molecular interactions might regulate T cell traffic through the epithelium and the directionality of movement was uncertain for airway and other epithelia. These findings obtained ex vivo now provide a molecular basis for T cell traffic into and through mucosal barriers based on the polarized secretion of the β-chemokine RANTES (regulated upon activation, normal T cell expressed and secreted) in the setting of uniform presentation of ICAM-1 along the cell surface. In addition, we present immunohistochemical and in situ hybridization data from normal and inflamed airway tissue to support the relevance of these patterns of expression for RANTES and ICAM-1 to mediate immune cell traffic in human subjects. Taken together with comparative data for endothelial cells, the findings offer a means for progressive movement from endothelium to environment through distinct cell-specific mechanisms for cell adhesion molecule expression and chemokine secretion.
Materials and Methods
Materials.
Recombinant human IFN-γ and TNF-α were gifts from Genentech Corp. (South San Francisco, CA); Ficoll-Hypaque was from Pharmacia (Piscataway, NJ); fluorospheres (Coulter Standard-Brite) that emit from 525 to 700 nm were from Coulter Cytometry (Miami, FL); streptavidin–Red 670 was from GIBCO BRL (Gaithersburg, MD); collagen (vitrogen 100) was from Celtrix Laboratories (Santa Clara, CA); Citra solution was from Biogenex (San Ramon, CA); 4-β-phorbol-12,13-dibutyrate, purified mouse IgG1, and other chemicals were from Sigma Chemical Co. (St. Louis, MO). Laboratory of Human Carcinogenesis (LHC) basal medium was obtained from Biofluids (Rockville, MD) and was supplemented with bovine pituitary extract, insulin, hydrocortisone, epidermal growth factor, transferrin, epinephrine, triiodothyronine, l-glutamine, calcium chloride, trace elements, penicillin, and streptomycin (LHC-8e) as described previously (8, 10). The cDNA for RANTES in pBluescript was a gift from T. Schall (DNAX, Palo Alto, CA; reference 11).
Antibodies.
Antibodies were obtained as follows: anti–ICAM-1 (CD54) mAb R6.5 (as an F[ab] fragment; reference 12) was a gift from R. Rothlein (Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT); anti-ICAM mAb 84H10 was obtained from Immunotech, Inc. (Westbrook, ME); anti-β2-integrin (LFA-1β, CD18) mAb producing hybridoma cell line TS1/18.1.2.11 (13) was obtained from the American Type Culture Collection (Rockville, MD) and intraperitoneal injection into BALB/c mice was used to produced ascites fluid enriched for mAb (14); one anti-RANTES IgG1 mAb and two affinity-purified goat anti-RANTES IgGs were gifts from T. Schall (DNAX, Palo Alto, CA); another anti-RANTES mAb and an affinity-purified goat anti-RANTES IgG were obtained from R&D Systems (Minneapolis, MN); anti-CD3 mAb Leu-4 conjugated with FITC or biotin (for T cell detection), anti–TCR-α/β mAb TCR-α/β-1 conjugated with FITC, anti–TCR-γ/δ mAb TCR-γ/δ-1 conjugated with PE, goat anti–mouse IgG1 conjugated with FITC, and negative control mAbs consisting of mouse IgG1 conjugated with FITC or PE were obtained from Becton Dickinson (Mountain View, CA); anti-Na,K-ATPase (α1 subunit) Ab was a gift from R. Mercer (Washington University, St. Louis, MO; reference 15).
Epithelial Cell Isolation and Culture.
Human tracheal tissue was obtained from lung transplant donors and routine autopsies (2–24 h postmortem). Subjects with lung disease were excluded from study. hTBECs were isolated from tracheal mucosal strips by enzymatic dissociation and then were cultured in LHC-8e medium on flasks coated with collagen/albumin as described previously (8). Cells were passaged by treatment with 0.05% trypsin/0.02% EDTA and studied up to passage 7.
To prepare standard (upright) epithelial cell monolayers, hTBECs were cultured to confluence on collagen-coated polycarbonate membrane inserts (8-μm pore size, 6.5-mm diam) in Transwell cell culture chambers (Costar, Cambridge, MA). Preliminary experiments confirmed that T cells do not adhere to inserts and pass through freely if no cell monolayer is present. To create inverted monolayers, membrane inserts were inverted and the bottom side was fitted with a section of a 1.7-ml microcentrifuge tube similar to methods described previously (16, 17). Cells were cultured to confluence on this bottom side (5–6 d), and then the microcentrifuge tube was removed and the inserts were placed upright into 24-well culture plates for an additional day before assay. Monolayer confluence was verified by Diff-Quik stain (Baxter, McGaw Park, IL), and monolayer integrity was monitored using transepithelial electrical resistance (17, 18). Electrical potential difference (PD) after passage of a defined current pulse was determined using a two-electrode volt–ohm meter (World Precision Instruments, Inc., Sarasota, FL); only monolayers showing stable resistance of >120 ohm × cm2 and 100% confluency were used for assay. Cell monolayers grew only on the plated side of the filter, and under these culture conditions, maintained epithelial-specific characteristics of differentiated structure and function (19).
Conditions for Adhesion/Transmigration Assay.
These conditions were modified from those described previously for determining epithelial–T cell adhesion (8, 9). The mononuclear leukocyte fraction was isolated from peripheral blood of healthy donors by a one-step gradient through Ficoll-Hypaque. The PBMC fraction was resuspended in LHC-8e, and aliquots of 3 × 105 leukocytes/ 100 μl were added to the upper compartment of each Transwell chamber. Leukocytes were pretreated with or without phorbol dibutyrate (50 ng/ml for 15 min) and epithelial monolayers were pretreated with or without IFN-γ (100 units/ml for 24 h); each was then washed with LHC-8e. A phorbol dibutyrate concentration was chosen that causes maximal lymphocyte adherence (20), and an IFN-γ concentration was chosen that causes maximal adherence and ICAM-1 and RANTES expression (3, 9, 21). To some tissue culture wells, leukocytes were added that had been pretreated with anti–LFA-1β mAb (30 μg/ml) for 30 min at 37°C. In other wells, the cell monolayer was pretreated with anti–ICAM-1 mAb R6.5 F(ab) fragment (50 μg/ml) or anti-RANTES mAb (10 μg/ml) for 30 min at 37°C. Antibody concentrations were used which caused maximal inhibition of leukocyte adherence (8, 9), and antibodies were not removed during the subsequent adherence/transmigration period. Leukocyte adherence/transmigration was then allowed to occur for 1–4 h at 37°C in 5% CO2. Nonadherent leukocytes were removed by gently washing each well three times, and then adherent and transmigrated leukocytes were recovered as separate populations. Adherent leukocytes were removed by treatment with 0.05% trypsin/0.02% EDTA for 10 min at 37°C followed by neutralization with PBS containing 10% fetal bovine serum. Preliminary experiments established that these dissociation conditions did not alter flow cytometry determinants and resulted in maximal recovery of adherent leukocytes with no significant decrease in cell viability and no residual leukocytes adherent to the monolayer. For inverted monolayers, T cells freely cross the insert, and so were observed to adhere only to the monolayer on the underside of the filter. Microscopy of monolayers and complete dissociation of cells with trypsin each indicated that <0.1% of leukocytes were retained within the monolayer. Transmigrated leukocytes were recovered from the lower chamber by aspiration of medium.
Flow Cytometry Analysis of Adherent and Transmigrated Leukocytes.
Using an approach we have described previously (8, 9), aliquots from the starting mononuclear leukocyte preparation added to the monolayer and from the adherent and transmigrated leukocytes from each monolayer well were analyzed by fluorescence-activated flow cytometry in order to determine the number and percentage of adherent and transmigrated T cells for each condition. Each sample was washed and resuspended in PBS containing 1% FBS, incubated with 5 μl of anti-CD3 mAb (to distinguish T cells from other types of leukocytes or epithelial cells) for 1 h at 4°C followed by FITC-conjugated goat anti–mouse IgG for 1 h at 4°C, and then washed again and resuspended in 200 μl PBS containing 0.2% BSA. Next, 104 fluorospheres (to serve as an internal standard for quantitation) were added to each sample, and the samples were analyzed using an Epics Elite flow cytometer (Coulter Cytometry, Hialeah, FL). Gating conditions were established for the clearest separation of T cells and resulted in the use of a multivariate analysis of cell side scatter versus forward scatter as described previously (8, 9). Data was acquired (Coulter Elite Version 3 software; Coulter Cytometry) using 1,024-channel resolution over a four decade log gain setting. The software was set up to calculate the percentages of T cells and fluorospheres in each sample based on 30,000 events, and the number of fluorospheres added to the sample was calculated based on the manufacturer's specifications. The number of adherent or transmigrated T cells and percent adherence or transmigration in each sample was determined by the following equations: number of adherent or transmigrated T cells = (percent T cells)/(percent fluorospheres) × (number of fluorospheres added to the sample); percent adherence or transmigration = (number of adherent or transmigrated T cells/number of T cells added to the monolayer) × 100. Preliminary experiments indicated a high correlation (r = 0.99) between measurements of leukocytes by flow cytometry versus the values obtained for manual cell counting. For experiments analyzing α/β– versus γ/δ–T cell behavior, the same protocol was followed except that samples were incubated with 5 μl biotin-conjugated anti-CD3 mAb followed by streptavidin–Red 670, 5 μl FITC-conjugated anti–TCR-α/β mAb, and 5 μl PE-conjugated anti–TCR-γ/δ mAb for 1 h at 4°C, and then resuspended in 200 μl PBS containing 0.2% BSA. Exclusive gating conditions for separation of α/β- and γ/δ-bearing T cells was accomplished as noted previously (8).
Immunocytochemistry for ICAM-1.
Epithelial cell monolayers were cultured in Transwell chambers and treated with or without IFN-γ as described above. After treatment, the cell monolayers were fixed in methanol for 6 min at −20°C, pretreated with 2% gelatin in PBS for 1 h at 25°C to block nonspecific Ig binding, and then incubated with anti–ICAM-1 mAb 84H10 (1 μg/ml) and 2% gelatin in PBS for 1 h at 25°C. Next, the cells were rinsed with PBS, and then treated with FITC-conjugated goat anti– mouse IgG1 (1:20, vol/vol in PBS with 2% gelatin) for 1 h at 25°C. In addition, cells were incubated with anti-Na,K-ATPase Ab (5 μg/ml) followed by Cy-3–conjugated goat anti–mouse IgG to define the basolateral cell membrane (15, 22). Immunofluorescent cells were then viewed using Zeiss Axioplan microscope equipped with a laser confocal imaging system (MRC-500; BioRad Labs., Hercules, CA) and scanned along the z-axis in 2-μm steps. Images were generated at ×600 via a computer interface.
Immunoassay for RANTES.
Epithelial cell monolayers were cultured in Transwell chambers and treated with or without IFN-γ as described above. After treatment, monolayers were washed and then incubated for a 4-h adherence/transmigration period without or with PBMCs (untreated or treated with phorbol dibutyrate), and media from the upper and lower chambers was collected for RANTES levels using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). For comparison, apical and basal secretion of RANTES was also monitored in epithelial (hTBEC) versus human umbilical vein endothelial cell (HUVEC) monolayers treated with or without IFN-γ (100 units/ml) as well as IFN-γ plus TNF-α (100 units/ml) to maximize RANTES generation and release (21, 23).
Epithelial Tissue Procurement.
Endobronchial biopsy tissue was obtained by fiberoptic bronchoscopy using a University-approved protocol for healthy nonasthmatic control subjects (six male and four female aged 20–54 yr) and stable asthmatic subjects (three male and eight female aged 22–60 yr). Nonasthmatic subjects had no clinical history of asthma, normal spirometry (forced expiratory volume in 1s; FEV1 = 108 ± 14% predicted; range = 81–129% predicted), and normal airway reactivity to inhaled methacholine (provocative concentration for 20% decrease in FEV1; FEV1 PC20 > 16 mg/ml). Asthmatic subjects met clinical diagnostic criteria for asthma (24) and had more variable spirometry (FEV1 = 90 ± 12% predicted; range 66–111% predicted) and hyperreactivity to inhaled methacholine (FEV1 PC20 = 1.40 ± 1.20 mg/ml; range, 0.12–2.80 mg/ml). Positive prick skin test reactivity to a panel of allergens (house dust, trees, grasses, fungi, and dog and cat dander) was present in nine of the asthmatic subjects and three of the control subjects. Asthmatic subjects were treated with inhaled triamcinolone (1,600 μg/d) or fluticasone (1,760 μg/d) for 30 d before the first endobronchial biopsy. Glucocorticoid treatment was then discontinued, and subjects were monitored for an additional 6 wk or until peak expiratory flow had decreased by 20% at which time a second endobronchial biopsy was obtained.
Immunohistochemistry for ICAM-1 and RANTES.
For ICAM-1 immunostaining, endobronchial biopsies were frozen in OCT/ TBS (optimum cutting temperative/Triangular Biomedical Science) and cut into 6-μm thick sections that were washed in Tris-buffered saline, blocked by treatment with 2% gelatin, and incubated with anti–ICAM-1 mAb (1 μg/ml) for 18 h at 4°C. Primary Ab binding was detected with an FITC-conjugated donkey anti– mouse IgG secondary Ab and viewed by laser confocal microscopy as described above. In addition, some endobronchial biopsies were incubated in 8-well LabTek chambers containing LHC-8e media without and with IFN-γ (100 units/ml) for 24 h at 37°C. These biopsies (explants) were then frozen, sectioned, and immunostained for ICAM-1 in the same manner. Immunofluorescent sections were then viewed by laser confocal microscopy as described above.
For RANTES immunostaining, biopsies were fixed in 10% formalin, embedded in paraffin, and cut into 6-μm sections. Tissue sections were deparaffinized with xylene, rehydrated with graded ethanol solutions, and endogenous peroxidase activity was blocked by treatment with 3% hydrogen peroxide for 30 min at 25°C. Antigen exposure was facilitated by an antigen-retrieval method in which tissue specimens were placed in 10 mM Citra solution for 10 min at 98°C. After tissue specimens cooled to room temperature, nonspecific antigens were blocked by exposure to 5% nonimmune rabbit serum for 30 min at 25°C. Slides were then incubated with goat anti–human RANTES Ab (5 μg/ml) or nonimmune IgG for 24 h at 4°C. Excess free primary antibody was removed by washing with Tris-buffered saline, and bound antibody was detected by sequential incubation with a biotinylated rabbit anti–goat IgG secondary antibody (7.5 μg/ml) for 30 min at 25°C and then a preformed streptavidin–horseradish peroxidase complex (1:400 vol/vol) for 30 min at 25°C. The peroxidase reaction was carried out by exposure to 0.6 mM 3,3′-diaminobenzidine-HCl, 3 mM hydrogen peroxide, and 50 mM Tris-HCl (pH 7.6) for 30 min. Sections were then washed with water, lightly counterstained with hematoxylin, and dehydrated with graded ethanol solutions. Sections of tonsillar tissue processed with the same protocol served as a positive control for RANTES immunostaining. All sections were then viewed using a photomicrography system (model D-7082; Carl Zeiss, Inc., Thornwood, NY).
In Situ Hybridization for RANTES.
For RANTES riboprobe synthesis, a 0.41-kb human cDNA fragment (nt 1–410) was positionally cloned into the EcoRI and HindIII sites of pBluescript (Promega Corp., Madison, WI) in order to generate pBluescript-RANTES (1–410). Radiolabeled 35S-UTP sense and antisense cRNA transcripts were transcribed in vitro by T3 and T7 RNA polymerases, respectively, using the Gemini Riboprobe system (Promega Corp.). Riboprobes were subsequently precipitated with ethanol/acetate, washed, and counted, and an average of 3.0 × 106 cpm/μl were generated for each probe. In situ hybridization was performed as described previously (25). In brief, formalin-fixed tissue sections were deparaffinized, digested with proteinase K, and washed in 0.1 M triethanolamine buffer containing 0.25% acetic anhydride. Sections were incubated in 50 μl of hybridization solution containing 35S-labeled riboprobe (2 × 104 cpm/μl) at 60°C for 18 h in a humidified chamber and then washed under stringent conditions and processed for autoradiography for 15 d. Formalin-fixed preparations of IFN-γ–treated hTBECs were used as a positive control for RANTES mRNA expression.
Statistical Analysis.
Values for leukocyte adherence were analyzed for statistical significance using a one-way analysis of variance (ANOVA) for a factorial experimental design. The multicomparison significance level for the one factor analysis of variance was 0.05. If significance was achieved by one-way analysis, post-ANOVA comparison of means was performed using Scheffe F tests.
Results
Polarity of T Cell Adhesion and Transmigration.
Epithelial cell monolayers (hTBECs) cultured on the underside of a Transwell insert (to measure basal-to-apical transmigration) exhibited consistent confluency (by microscopy) and constant transepithelial resistance (by potential difference) that was identical to that observed for standard upright culture conditions (for measuring apical-to-basal transmigration). Initial experiments with inverted (and upright) monolayers each indicated that maximal T cell adhesion occurred within 1 h and remained at a stable level for 4–8 h, whereas T cell transmigration exhibited a progressive and relatively linear increase with time (Fig. 1). The time-dependent nature of T cell transmigration was observed during unstimulated control conditions and under conditions where adhesion and transmigration were augmented by phorbol dibutyrate treatment of T cells, IFN-γ treatment of epithelial cells, or a combination of the two treatments.
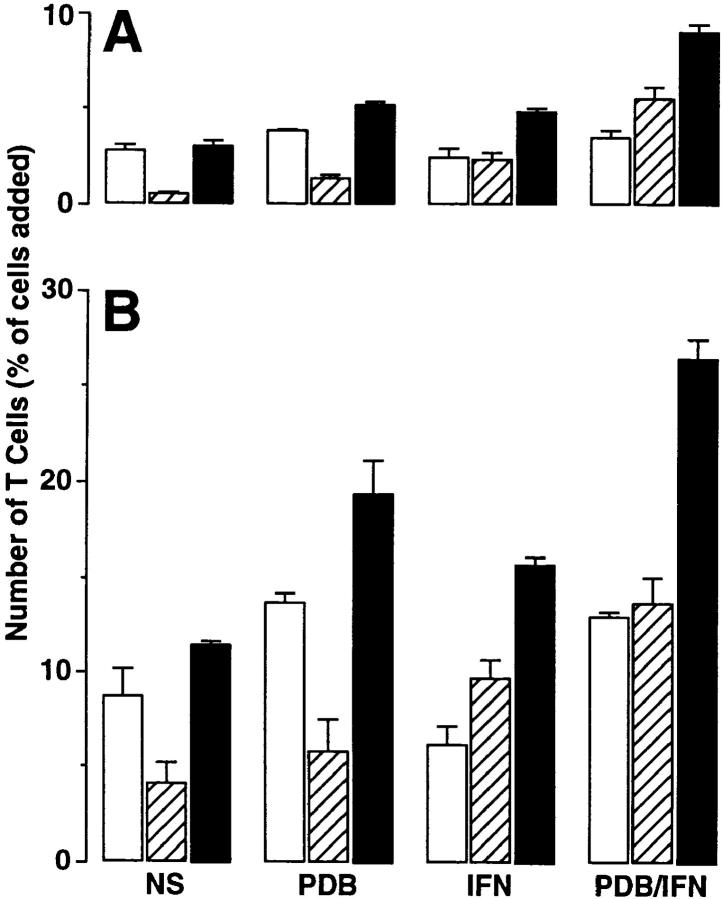
Figure 1.
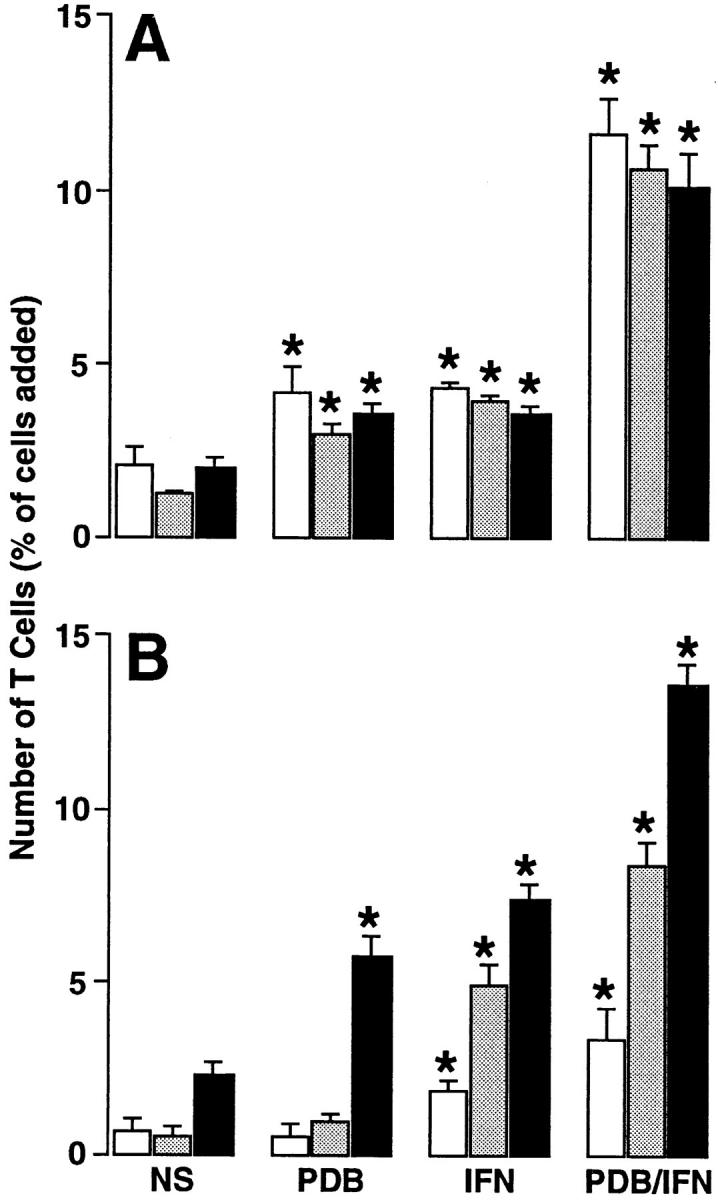
Time dependence of T cell adhesion (A) and transmigration (B) in the basal-to-apical direction using epithelial cell monolayers under the following conditions: no stimulation of monolayer or T cells (NS), T cell stimulation with phorbol dibutyrate (PDB; 50 ng/ml for 15 min), monolayer stimulation with IFN-γ (IFN; 100 units/ml for 24 h), and stimulation of both T cells and monolayers (PDB/IFN). After these treatments, 3 × 105 PBMCs were added to each Transwell for 1 (white bars), 2 (gray bars), or 4 (black bars) h, and adherent and transmigrated T cells were quantified using anti-CD3 mAb and immunofluorescence flow cytometry as described in Materials and Methods. Results are expressed as the percentage of T cells initially added to the monolayer system. Each value is the mean ± SEM (n = 5 samples) and is representative of three experiments. A significant increase from unstimulated level is indicated by *.
In comparing epithelial–T cell adhesion and transmigration in apical-to-basal versus basal-to-apical directions, we observed that the efficiency of transmigration (i.e., the relative number of transmigrated to adherent cells) was increased in the basal-to-apical direction (Fig. 2). Since the total number of adherent plus transmigrated T cells was similar in both directions, this efficiency reflected increased retention of T cells adherent to the apical compared with the basal epithelial cell surface, but increased transmigration of T cells adherent to the basal compared with the apical cell surface. The same patterns for preferential apical adhesion and basal-to-apical transmigration was observed for α/β and γ/δ T cell subsets (Fig. 3). However, the level of adhesion and transmigration was augmented in γ/δ compared with α/β T cells. These findings are in accord with the requirement of LFA-1 activation for epithelial–T cell adhesion and the higher level of activatable LFA-1 on γ/δ compared with α/β T cells (8).
Figure 2.
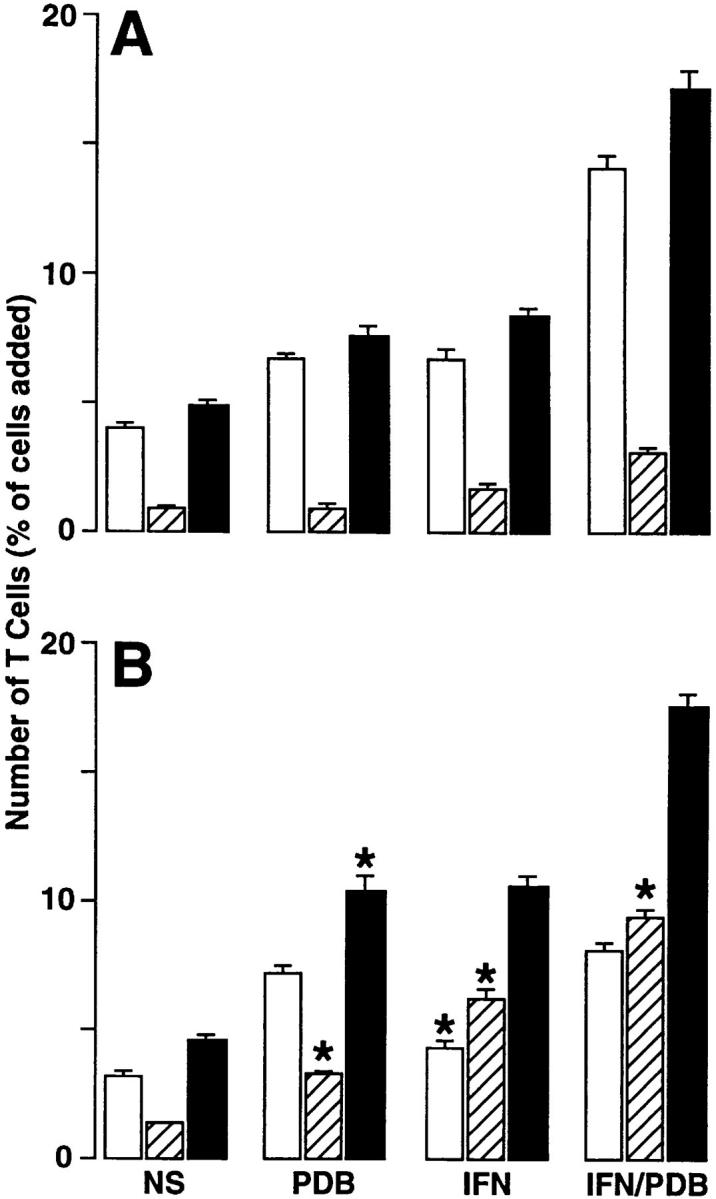
T cell adhesion and transmigration in the apical-to-basal (A) and basal-to-apical (B) directions. Aliquots of 3 × 105 PBMCs were added to Transwells containing epithelial (hTBEC) cell monolayers under the same activation conditions as described in Fig. 1. After a 4-h adherence/transmigration period, the adherent and transmigrated T cells were quantified using anti-CD3 mAb and flow cytometry. Results are expressed as the percentage of T cells added to the monolayer for adherent (white bars), transmigrated (striped bars), and adherent plus transmigrated cells (black bars). Each value is the mean ± SEM (n = 5 samples) and is representative of three experiments. A significant difference between apical-to-basal versus basal-to-apical values is indicated by *.
Figure 3.
Adhesion and transmigration for α/β (A) versus γ/δ (B) T cell subsets. Aliquots of 3 × 105 PBMCs were added to Transwells containing epithelial cell monolayers under the same activation conditions as described in Fig. 1. After a 4-h adherence/ transmigration period, the adherent and transmigrated T cells for each TCR-bearing subset were quantified using anti-CD3, anti– TCR-α/β, and anti–TCR-γ/δ mAbs and flow cytometry as described in Materials and Methods. Results are expressed as the percentage of that subset for adhered (white bars) or transmigrated (striped bars), as well as adherent plus transmigrated cells (black bars). The percentages of T cell subsets in the initial preparation bearing TCR-α/β- or -γ/δ was 95 and 5%, respectively. Each value is the mean ± SEM (n = 5 samples) and is representative of three experiments. A significant difference between γ/δ versus α/β values was present for all conditions.
Pattern of ICAM-1 Expression in Monolayers.
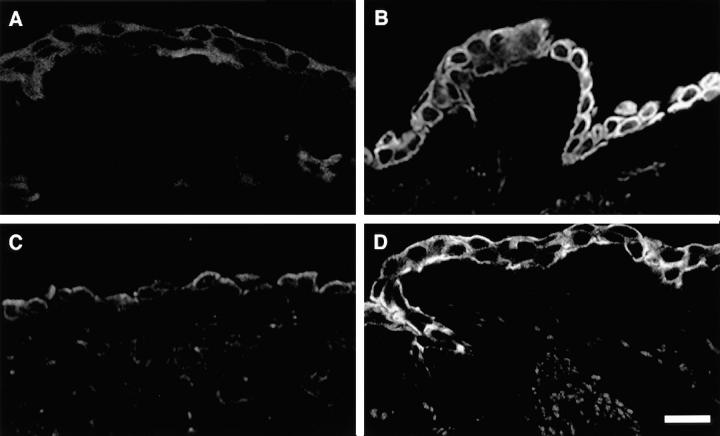
To determine the mechanism(s) for preferential T cell retention at the apical cell surface and transmigration via the basal cell surface, we first examined the distribution of ICAM-1 over the epithelial cell surface. To localize ICAM-1 expression on the apical and basolateral cell surfaces with respect to the tight junction, we used a marker of the basolateral cell surface (Na,K-ATPase) and visualized the z-axis of the monolayer by scanning laser confocal microscopy (Fig. 4). This technique (in contrast to monolayer embedding and mechanical transection) preserved epitopes on the basal cell surface and allowed us to document IFN-γ–dependent expression of ICAM-1 in a circumferential pattern with uniform distribution along both the apical and basolateral cell surfaces. Concomitant evidence for the functional significance of apical and basolateral expression of ICAM-1 came from measurements of ICAM-1–dependent adhesion and transmigration in the presence of blocking mAbs directed against ICAM-1 and LFA-1. Thus, IFN-γ/phorbol dibutyrate–stimulated levels of T cell adhesion and transmigration on apical (8, 9) or basal (Fig. 5) cell surfaces are returned completely to control levels by anti–ICAM-1 F(ab) or anti–LFA-1 mAb.
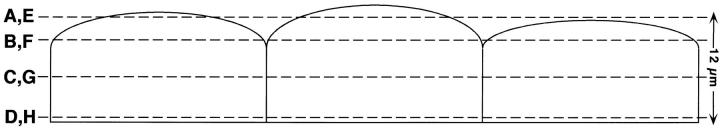
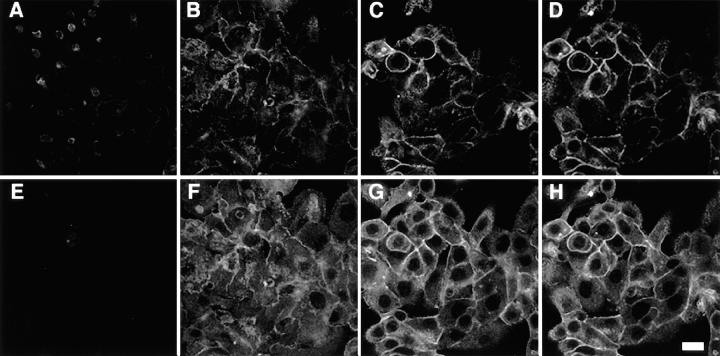
Figure 4.
Distribution of ICAM-1 over the apical and basolateral epithelial cell surfaces detected by scanning laser confocal microscopy. Cell monolayers were treated with IFN-γ (100 units/ml for 24 h), fixed in methanol, pretreated with 2% gelatin, and then incubated with anti–ICAM-1 mAb 84H10 (1 μg/ml) followed by FITC-conjugated goat anti–mouse IgG1 and with anti-Na,K-ATPase Ab followed by Cy-3–conjugated donkey anti–mouse IgG. Photomicrographs of four scans at indicated intervals depict the pattern of immunofluorescence extending from the apical to basal cell surface for ICAM-1 (A–D) and Na,K-ATPase (E–H). No fluorescence was detected for monolayers stained with nonimmune IgG or with only anti–mouse IgG (data not shown). Bar: 20 μm.
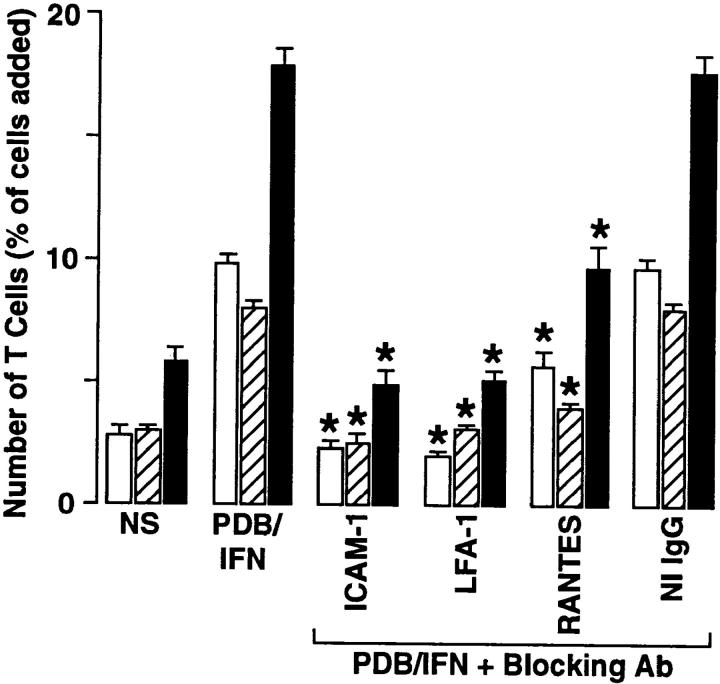
Figure 5.
Effect of mAb on T cell adhesion and transmigration. The epithelial–T cell system was unstimulated (NS) or stimulated with phorbol dibutyrate and IFN-γ (PDB/IFN) as described in the legend to Fig. 1. In addition, the PDB/IFN-stimulated system was treated with anti–ICAM-1 mAb R6.5 (50 μg/ml), anti–LFA-1β mAb (30 μg/ml), anti-RANTES mAb (10 μg/ml), or nonimmune IgG (50 μg/ml). The mAbs were added to both Transwell chambers 30 min before the addition of PBMCs, or in the case of anti–LFA-1β mAb, the PBMCs were pretreated for 30 min and then added to the chamber. After a 4-h adherence/transmigration period, the adherent and transmigrated T cells were quantified using anti-CD3 mAb and flow cytometry. Results are expressed as the percentage of T cells added to the monolayer for adherent (white bars), transmigrated (striped bars), and adherent plus transmigrated cells (black bars). Each value is the mean ± SEM (n = 5 samples) and is representative of four experiments. A significant decrease from the stimulated level is indicated by *.
Contribution of RANTES to T Cell Adhesion and Transmigration.
In view of the observations that ICAM-1–LFA-1 interaction was unlikely to account for preferential movement of T cells in the basal-to-apical direction, we performed concomitant studies of chemokine function in the airway epithelial cell model. Initial experiments indicated that IFN-γ stimulation of airway epithelial cells caused selective expression of RANTES (but not macrophage inflammatory protein [MIP]-1α, MIP-1β, monocyte chemoattractant protein [MCP]-1, MCP-3, or eotaxin) (21). Accordingly, we assessed the role of RANTES in mediating epithelial–T cell adhesion and transmigration. As in the case of ICAM-1 and LFA-1, we assessed the contribution of RANTES for increases in epithelial–T cell adhesion and transmigration by treatment with a specific blocking mAb under stimulation conditions that caused maximal adhesion and transmigration. For hTBEC monolayers (using IFN-γ stimulation of monolayers and phorbol dibutyrate stimulation of T cells to achieve maximal adherence and transmigration), mean stimulated T cell adhesion and transmigration were inhibited 58 and 80%, respectively, by anti-RANTES mAb (Fig. 5). The low level of transmigration remaining despite a maximally effective concentration of anti-RANTES mAb may indicate the contribution of other (as yet unidentified) chemoattractants derived from epithelial cells and/or T cells. Nonetheless, taken together, the results indicate that IFN-γ–stimulated airway epithelial–T cell transmigration is entirely dependent on ICAM-1–LFA-1 interaction and, among chemokines, most significantly influenced by RANTES interaction with its corresponding chemokine receptor (CCR1, 4, or 5) on the T cell (26).
Pattern of RANTES Secretion.
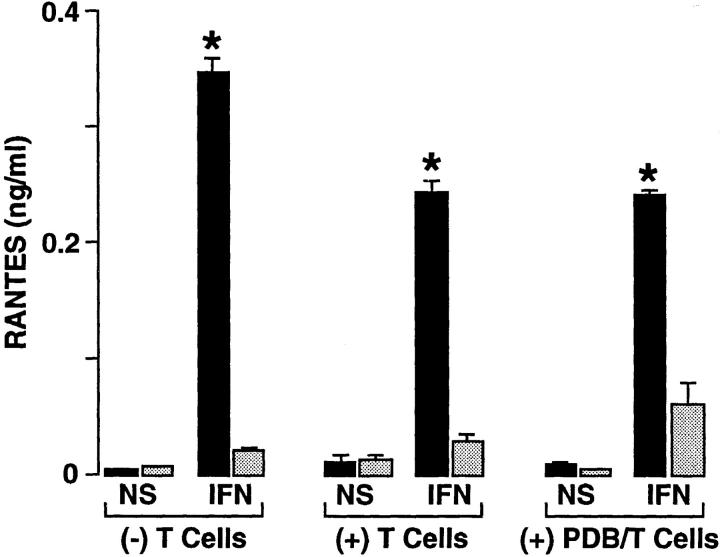
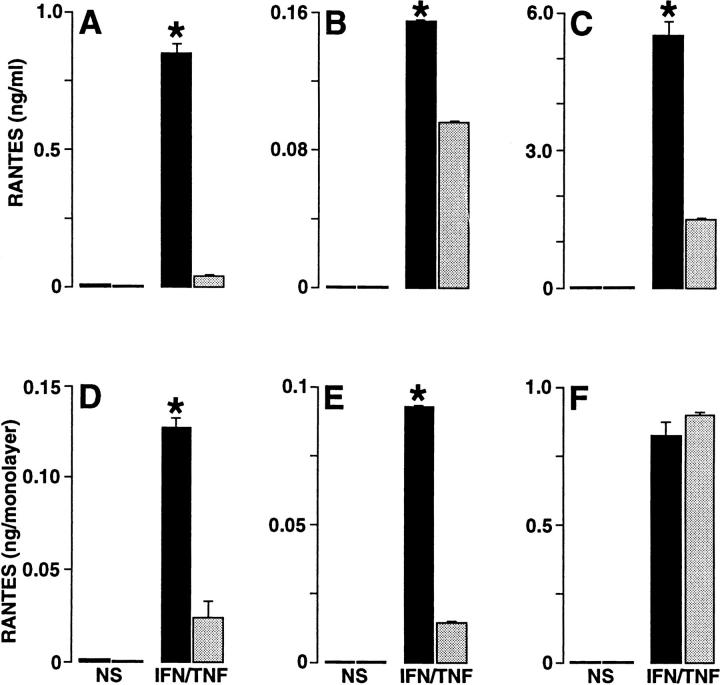
We next assessed the possible mechanism for RANTES to mediate a preferential directional movement of T cells across the epithelial cell monolayer in the basal-to-apical direction. Initial experiments indicated weak immunostaining of epithelial cells with five different anti-RANTES Ab preparations, suggesting that the secreted form of RANTES was responsible for its functional effect. In view of this possibility, we reasoned that transmigration might be mediated by a soluble chemical gradient for RANTES. Support for this possibility was obtained with measurements of RANTES in media collected from the apical versus basal aspect of the monolayers. Thus, apical levels of RANTES were ∼16-fold higher than basal levels under standard (upright) conditions for monolayer culture (Fig. 6). This differential level of RANTES persisted even after: (a) correcting for the smaller volume of media on the apical side of the cell monolayer (a fourfold difference), (b) adding unactivated or phorbol dibutyrate– activated T cells to the system, or (c) culturing cells in the upright or inverted manner with respect to the Transwell membrane (Figs. 6 and 7). The pattern for polarized secretion of RANTES combined with uniform expression of ICAM-1 was distinct for epithelial cells because endothelial (HUVEC) cell monolayers exhibited nonpolarized secretion of RANTES (Fig. 7) along with uniform expression of ICAM-1 (27). To achieve significant endothelial cell production (or maximal epithelial cell production) of RANTES, it was necessary to stimulate cells with TNF-α (in addition to IFN-γ; references 21, 23), but the apical distribution of RANTES secretion for epithelial cells was constant whether cells were stimulated with IFN-γ alone or with IFN-γ in combination with TNF-α (Fig. 7). Similarly, the distribution of RANTES across the cell could not be accounted for by diffusion of RANTES, because concentration gradients were maintained under these experimental conditions for epithelial and endothelial cell monolayers (Figs. 6 and 7).
Figure 6.
Concentration gradients of RANTES established by cultured epithelial cells in the apical (black bars) versus the basal (gray bars) side of the monolayer. Epithelial cell (hTBEC) monolayers were cultured upright in Transwell chambers and then were treated without (NS) or with IFN-γ (IFN, 100 units/ml) for 24 h at 37°C. After treatment, monolayers were incubated without or with PBMCs (untreated or treated with phorbol dibutyrate) for 4 h at 37°C (as was done for adherence/transmigration assays), and media from the upper (apical) and lower (basal) chambers was collected for RANTES immunoassay. Each value is the mean ± SEM (n = 3 samples assayed in triplicate). A significant difference for apical versus basal level of RANTES is indicated by *.
Figure 7.
Levels of RANTES secreted by monolayer cultures of upright epithelial cells (A and D), inverted epithelial cells (B and E), and upright endothelial cells (C and F). Epithelial (hTBEC) and endothelial (HUVEC) monolayers were cultured in Transwell chambers and then were treated without (NS) or with IFN-γ plus TNF-α (IFN/TNF; 100/ 100 units/ml) for 24 h at 37°C. After treatment, media from the upper chamber (150 μl) and lower chamber (600 μl) was collected for RANTES immunoassay. Each value is presented as the concentration (upper row, A–C) and the amount (lower row, D–F) of RANTES in the chamber. All values represent the mean ± SEM (n = 3 samples assayed in triplicate). A significant difference for apical versus basal level of RANTES is indicated by *.
Pattern of ICAM-1 Expression and RANTES Secretion in Airway Epithelium.
As discussed below, the relatively uniform distribution of ICAM-1 over the airway epithelial cell surface was distinct for polarized epithelial cells, which (at least in the case of colonic and alveolar epithelial cells) appear to limit expression of ICAM-1 to the apical surface (4, 28–30). To verify whether the pattern of ICAM-1 expression was representative of airway epithelium, we also determined the pattern of ICAM-1 presentation in endobronchial biopsies of airway epithelial tissue. The strategy was aimed at obtaining airway epithelial tissue from healthy control subjects that was stimulated ex vivo with IFN-γ (just as was done for epithelial cell monolayers) to express ICAM-1 or from asthmatic subjects that exhibited increased expression of endogenous ICAM-1 compared with nonasthmatic subjects even under baseline conditions (31). In both cases, confocal microscopy indicated that the pattern of ICAM-1 expression along the airway epithelial cell surface was identical to the one observed for epithelial cell monolayers. Thus, ICAM-1–expressing epithelial tissue either stimulated ex vivo to express ICAM-1 or obtained from asthmatic subjects that endogenously express ICAM-1 both exhibit expression uniformly along apical and basolateral surfaces (Fig. 8).
Figure 8.
Representative pattern of ICAM-1 expression in airway epithelium induced ex vivo by IFN-γ treatment or in situ by asthma. For ex vivo experiments (A and B), endobronchial biopsy tissue was obtained from a healthy control subject and treated without (A) or with (B) IFN-γ (100 units/ ml for 24 h). For in situ experiments (C and D), endobronchial biopsy tissue was obtained from normal control (C) or stable asthma (D) subjects. In all cases, tissues were frozen, sectioned, pretreated with 2% gelatin, and then incubated with anti–ICAM-1 mAb 84H10 (1 μg/ml). Primary Ab binding was detected with FITC-conjugated donkey anti–mouse IgG and was viewed using a scanning laser confocal photomicrography system. No fluorescence was detected for monolayers stained with nonimmune IgG or with only anti–mouse IgG (data not shown). Bar: 20 μm.
We next aimed to determine whether evidence of RANTES expression in isolated epithelial cells could also be extended to studies of epithelial tissue from endobronchial biopsies. Initial experiments with IFN-γ–stimulated epithelial cells (as noted above) indicated immunostaining was relatively weak (despite marked increases in corresponding mRNA levels by Northern blot analysis and in situ hybridization), and detection of RANTES signal above background in immune cells (using tonsillar tissue) required antigen-retrieval (data not shown). Accordingly, we used a combination of antigen-retrieval immunostaining and in situ hybridization to detect RANTES in endobronchial biopsy tissue, but even these approaches indicated only low levels of RANTES expression in epithelial tissue from healthy control or asthmatic subjects under stable baseline conditions (data not shown and Fig. 9). As discussed below, these findings are similar to ones reported by others (32–35). However, using a newly-developed protocol for controlled exacerbation of asthma during monitored glucocorticoid withdrawal, we observed a marked increase in expression of RANTES that was localized to airway epithelial cells (as well as an expanded population of immune cells) in asthmatic subjects (Fig. 9). As was the case for IFN-γ–stimulated epithelial cells, the asthma-associated increase in epithelial RANTES expression was more prominent by in situ hybridization (that detects cell-associated mRNA) than by immunostaining (that detects cell-associated protein). Taken together, these findings suggest that secretion of RANTES by airway epithelial cells is efficiently designed to achieve an appropriate extracellular site of action.
Figure 9.
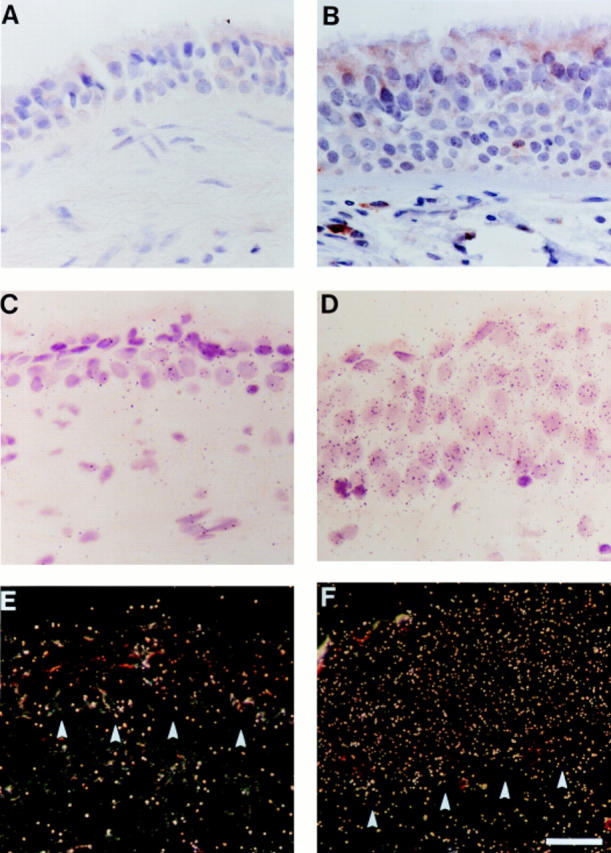
Representative pattern of RANTES expression in airway epithelium in asthma exacerbation provoked by glucocorticoid withdrawal. Endobronchial biopsy tissue was obtained from an asthmatic subject during treatment with inhaled glucocorticoid (A, C, and E) and after glucocorticoid withdrawal (B, D, and F), and then processed either for immunostaining (A and B) or in situ hybridization (C–F) for RANTES. For immunostaining, tissues were fixed in 10% formalin, embedded, sectioned, blocked with hydrogen peroxide, subjected to antigen retrieval, and then incubated with anti-RANTES Ab. Primary Ab binding was detected using a biotinylated secondary Ab and a streptavidin–peroxidase complex, and tissues were then counterstained with hematoxylin. Control staining with nonimmune IgG gave no detectable signal above background (not shown). Arrowheads, immunopositive epithelial cells and subepithelial immune cells. For in situ hybridization, formalin-fixed tissue sections were hybridized with 35S-labeled RANTES cRNA, subjected to autoradiography, and imaged by brightfield (C and D) and darkfield (E and F) microscopy. Hybridization with 35S-labeled RANTES RNA (sense probe) gave no detectable signal above background (data not shown). Arrowheads indicate junction of epithelial cell layer with the basement membrane. Bar: 30 μm.
Discussion
T cell traffic across epithelia is a critical feature of immune barrier function, but the signals for movement and their possible relationship to other polarized cell functions were uncertain. Our results establish a model for transepithelial T cell movement based on uniform and circumferential distribution of ICAM-1 along the cell surface and polarized secretion of RANTES through the apical cell surface. For ICAM-1, distribution on both apical and basolateral cell surfaces allows for efficient cell adhesion at the basal cell surface (to aid in transmigration) and at the apical cell surface (for retention along the airway). For RANTES, the pattern of preferential apical secretion provides for a soluble chemical gradient for T cell movement from the subepithelium (where levels are low) to the mucosal surface and maintenance there (where levels are higher). These patterns for localization of ICAM-1 expression and RANTES secretion found in isolated epithelial cells account appropriately for the levels of T cell adhesion and transmigration found in epithelial cell monolayers and with the available data for ICAM-1 and RANTES expression in airway epithelial tissue. Thus, ICAM-1 appears to be uniformly distributed around the mucosal epithelial cell surface under conditions for exogenous stimulation with IFN-γ or endogenous expression with asthma, and RANTES appears to be abundantly expressed and efficiently secreted by the same population of airway epithelial cells during asthmatic inflammation.
In attempting to understand the cellular mechanism for sorting membrane proteins (such as ICAM-1) or secretory proteins (such as RANTES), there is presently little information for airway epithelial cells. In other models of polarized epithelium (e.g., MDCK cells from kidney or CaCo-2 cells from intestine), it appears that membrane and secretory proteins are distributed to the polarized cell surface by exocytic and endocytic routes to varying degrees in different epithelial cell types, but in both routes, sorting to the apical domain is signal mediated and delivery to the basolateral domain represents the default pathway (36, 37). Thus, the apical pathway from the trans-Golgi network (for the exocytic route) or endosomal recycling (for the endocytic route) requires specific sorting signals, whereas the basolateral pathway represents the equivalent of the default pathway in nonpolarized cells. The definitive identification of sorting signals for transmembrane proteins is still uncertain, but most examples are targeted via extracytoplasmic domains, and in that regard, glycosyl phosphatidylinositol (GPI)-membrane anchoring may act as a targeting signal (38, 39). It is uncertain to what extent ICAM-1 might completely fit this paradigm, but analysis of the homologous neural cell adhesion molecule (NCAM) indicates that GPI-anchored forms are distributed to the apical surface, whereas transmembrane forms are targeted to the basolateral surface (40). For RANTES (or other β-chemokines), no polarized sorting has been previously documented, so no targeting signals have been defined (41).
The patterns for ICAM-1 expression and RANTES secretion in airway epithelial cells provide initial evidence of specialization and consequent cellular heterogeneity for directing immune cell traffic. For example, other types of epithelial cells (notably colonocytes and pneumocytes) express ICAM-1 only along the apical cell surface (28–30). Perhaps in this setting, ICAM-1 functions mostly in cell movement along that surface or acts in concert with other cell adhesion receptors to aid in host defense (e.g., as opsonins). Endothelial cells also appear to exhibit heterogeneity in the pattern of ICAM-1 expression. Pulmonary capillary and venular endothelial cells conserve this pattern of apical (luminal) expression under basal or endotoxin- and cytokine-stimulated conditions (4, 30). However, cytokine– (IL-1–) induced ICAM-1 exhibits uniform apical and basolateral expression over the surface of umbilical vein endothelial cells and so may contribute to bidirectional movement of immune cells across endothelium (27).
As was the case for ICAM-1, we found that RANTES was distributed to both sides of an (umbilical vein) endothelial cell monolayer. Our findings are consistent with a report that endothelial cells are unable to generate a significant gradient for other β-chemokines (MCP-1; reference 42). Endothelial cells remain polar in cell culture and exhibit polarized secretion of a number of biologically active molecules for directing immune cell traffic (43), suggesting that these cells lack the sorting signals that are present in some types of polarized epithelial cells for directing RANTES to the apical cell surface. Instead, endothelial cells appear to maintain proteoglycans capable of anchoring glycosaminoglycan-binding chemokines to either cell surface (44–46). Presumably, chemokine sequestration at the site of production maintains a high local concentration despite potential dilution by blood flow (47). After we submitted this report, a study of IL-8 and RANTES indicated that binding to the cell surface and transcytosis resulted in presentation of IL-8 and RANTES on both luminal and abluminal cell surfaces of venular endothelium (48). Binding to the endothelial cell depended on an intact COOH terminus (the immobilization domain), but the lack of polarity for this process (defined by administration of exogenous chemokine) indicates that it is distinct from what we have observed for endogenous RANTES production in epithelial cells. Furthermore, this nonpolar process might be expected to aid in haptotaxis, and perhaps this is useful for movement out of the circulation (often the predominant direction for immune cell flux) or in recirculation of immune cells. However, a polar process (such as the one we describe for RANTES) is required for effective chemotaxis and the consequent need to preferentially direct immune cell movement across the epithelium in a basal-to-apical direction. In fact, epithelial cells appear programmed to efficiently release β-chemokines either in culture or in vivo. Thus, endothelial cells may use nonpolarized expression of proteoglycans to permit chemokine-dependent haptotaxis of immune cells, whereas epithelial cells use polarized secretion of chemokine to generate a soluble chemokine-dependent gradient that mediates chemotaxis of immune cells across the epithelium. In all cases, it appears that a cell type–specific mechanism for directing RANTES secretion (and ICAM-1 expression) works in combination with specific determinants in the chemokine (or cell adhesion molecule) structure to achieve specialized direction of immune cell traffic.
We are unaware of other reports of transepithelial T cell traffic, but several groups have studied neutrophil traffic across epithelia. This work indicates that neutrophil transmigration across cultured airway epithelial and peritoneal mesothelial cells also occurs preferentially in the basal-to-apical direction and under some conditions may be mediated by IL-8 production (17, 49). In the case of mesothelial cells, IL-8 appears to be secreted at a slightly higher (onefold) rate towards the apical cell surface, but these determinations are influenced by back diffusion for this chemokine. Nonetheless, the findings suggest that directionality of chemokine secretion may also be used for parenchymal cells to direct neutrophils into inflammatory sites. In that regard, there are several reports describing the preferential migration of neutrophils across cultured enterocytes. Surface attachment of bacteria (Salmonella typhimurium) to intestinal T84 epithelial cells causes basolateral secretion of IL-8 (four- to fivefold), indicating that this α-chemokine is more likely responsible for recruiting neutrophils to the subepithelial space rather than directing the final movement of neutrophils across the epithelium in this cell system (50).
Intestinal T84 cells also respond to bacterial infection and cytokines (including IFN-γ) with expression of ICAM-1 on the apical cell surface, and this pattern of expression is preserved in vivo (28, 29). The investigators noted that apical expression of ICAM-1 by intestinal epithelial cells was a sign that ICAM-1 (like IL-8) was not involved in the transmigration of neutrophils (28, 29). This position was supported by the finding that ICAM-1 blockade did not influence basal-to-apical transmigration of neutrophils through intestinal epithelial monolayers in response to IFN-γ (29), presumably because neutrophils (unlike T cells) depend on the interaction of Mac-1 (instead of LFA-1) with an alternative Mac-1 ligand such as heparan sulfate glycans (instead of ICAM-1) (51). In this context, our results indicate that controls for transepithelial T cell movement are distinct from those controlling neutrophils and instead depend on coordinated interaction of epithelial ICAM-1 and RANTES with their corresponding immune cell receptors. The precise basis for the difference in T cell versus neutrophil transepithelial migration will require defining the nature of the chemokine stimulus for neutrophil transmigration. Similar uncertainty accompanies the nature of the soluble factor that regulates ICAM-1–dependent movement of monocytes across epithelium or endothelium (27).
Our experiments focus on the epithelial determinants (ICAM-1 and RANTES) of T cell transmigration, but present (and previous) results indicate that T cells also contain regulated determinants for adhesion and transmigration. Thus, a hierarchy for β1- and β2-integrin expression among T cell subsets is linked to TCR gene usage and emphasizes γ/δ T cell adhesion (8), and accordingly transmigration (our results). In particular, the higher levels of activatable LFA-1 on γ/δ versus α/β T cells result in higher rates of adhesion and consequent transmigration. The findings also confirm the importance of ICAM-1–LFA-1 interaction in regulating T cell transmigration as well as adhesion. Analogous to the variable level of integrin expression, the level of RANTES responsiveness also varies among T cell subsets (52, 53). Initial studies indicate a higher level of responsiveness in memory relative to naive T cells, although responsiveness appears preserved among unactivated and activated as well as CD4+ and CD8+ T cells (53). The lack of preferential responsiveness to RANTES may be a reflection of its capacity to use multiple β-chemokine receptors, but the expression levels of receptors in relation to T cells subsets (or TCR gene usage) is still only partly defined (26). After we submitted this work for publication, a report appeared indicating that RANTES may cause preferential chemotaxis of Th1 type (IFN-γ–producing) T cells in vitro (54). As discussed below, this data may be taken together with our findings for induction of RANTES expression during asthma exacerbation to explain the seemingly paradoxical enrichment of Th1 type T cells (detectable after pharmacologic stimulation) in this disease (55). The data also fit well with possible autoamplification of Th1 type T cell immune responses during conditions associated with IFN-γ production (such as respiratory viral infection; reference 56). In support of this possibility, we find that increases in ICAM-1 and RANTES expression localized to airway epithelial cells are consistently detected in a mouse model of viral bronchitis and hyperreactivity (57).
The relationship of RANTES responsiveness to transmigratory capacity may be further complicated by the capacity of RANTES to activate T cells (including activation of β1- and β2-integrins on the T cell surface) as well as cause chemotaxis (58–60). In fact, RANTES capacity for LFA-1 activation most likely accounts for the increased level of ICAM-1–dependent adhesion of unactivated T cells (Fig. 5). The relatively weak effect of RANTES on adhesion (compared with transmigration) is consistent with the relatively weak effect on integrin (including β2-integrin) activation in other systems (59, 61). Thus, in the case of the epithelium, the RANTES signal may confer some level of adherence (via basal secretion), but it is predominantly chemotactic (as a result of a more potent effect and a higher level of agonist at the apical surface). The requirement for a dual signal from ICAM-1 and RANTES is underscored by findings that blockade of either signal blocks T cell transmigration, and delivering either signal alone (e.g., by treatment with exogenous RANTES in the absence of IFN-γ–stimulated ICAM-1 expression) is insufficient to mediate transepithelial T cell traffic (Taguchi, M. and M.J. Holtzman, unpublished observation).
Our findings in isolated cells and in tissue now provide for a more complete picture of immune cell traffic during airway immunity and inflammation. In the setting of respiratory viral infection, the stimulatory effect of IFN-γ may complement direct effects of the virus on epithelial cells to augment ICAM-1 and RANTES expression and consequent immune cell traffic (57, 62). In the setting of asthma, we and others have also observed higher than normal expression of ICAM-1 in the airway epithelium (31, 63–66), although the present and distinct use of confocal laser microscopy and frozen tissue appears to provide a more precise image of ICAM-1 localization in epithelial tissue. Most reports indicate that RANTES is also expressed in the airway epithelium of normal and asthmatic subjects with mild disease, but only at low basal levels in each group (32–35). Accordingly, we sought an experimental protocol for endogenous exacerbation of asthma that might reflect natural flares of the disease. In that context, we developed a protocol for controlled glucocorticoid withdrawal that in some subjects results in asthma exacerbation characterized by increases in airway obstruction and immune cell infiltrate and that is associated with a marked increase in expression of RANTES localized to airway epithelial cells as well as immune cells. Thus, the epithelial system for cell adhesion and chemoattractant molecules appears engineered for host defense, but the same molecular systems appear abnormally activated in asthma depending on the severity of disease and treatment conditions.
The molecular basis for overexpression of epithelial ICAM-1 or RANTES in asthma is uncertain, since IFN-γ levels derived from Th1 type T cells are expected to be low in this condition compared with viral infection (67). Concomitant work has confirmed that there is little detectable IFN-γ production in asthmatic subjects under baseline or glucocorticoid-withdrawal conditions (Sampath, D., M. Castro, and M.J. Holtzman, unpublished observation). Even when airway tissue levels of IFN-γ appear to be low, however, there may be overactivation of IFN-γ signal transduction as evidenced by higher than normal levels of signal transducer and activator of transcription 1 (Stat1) activation (31). Thus, airway epithelial cells may be programmed for constitutive ICAM-1 expression (mediated by Stat1-dependent transcription; references 5–7) and inducible RANTES production (mediated by transcriptional and post-transcriptional events; references 68, 69) even in the apparent absence of viral infection or IFN-γ production. In the case of RANTES, inducible expression may reflect a coincidental and subliminal response to virus that does not result in IFN-γ production, or perhaps more likely may suggest an inherited or acquired propensity for overexpression of the RANTES gene in these subjects in response to a wider variety of inhaled stimuli. Defining these relationships of RANTES expression to exogenous and endogenous stimulation will require additional work on the molecular basis for RANTES expression as well as correlative protocols in human subjects. In the context of the present work, however, the pattern of epithelial ICAM-1 expression and RANTES production appears consistent in vitro and in vivo in healthy and diseased tissue, suggesting that this combination of cell adhesion and chemoattractant activities may efficiently regulate traffic of mucosal immune cells during normal host defense and abnormal inflammatory disease in the airway.
Abbreviations used in this paper
- HUVEC
human umbilical vein endothelial cell
- hTBEC
human tracheobronchial epithelial cell
- ICAM
intercellular adhesion molecule 1
- LFA
lymphocyte function-associated antigen
- LHC
Laboratory of Human Carcinogenesis
- MCP
monocyte chemoattractant protein
- RANTES
regulated upon activation, normal T cell expressed and secreted
Footnotes
The authors gratefully acknowledge valuable help and advice from Lisa Looper, Bill Parks, Jill Roby, and Theresa Tolley.
This research was supported by grants from the National Institutes of Health and the Alan A. and Edith L. Wolff Charitable Trust.
The first two authors contributed equally to this work.
References
- 1.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 2.Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures of human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 3.Look DC, Keller BT, Rapp SR, Holtzman MJ. Selective induction of intercellular adhesion molecule-1 by interferon-γ in human airway epithelial cells. Am J Physiol. 1992;263:L79–L87. doi: 10.1152/ajplung.1992.263.1.L79. [DOI] [PubMed] [Google Scholar]
- 4.Kang B-H, Manderschied BD, Huang Y-CT, Crapo JD, Chang L-Y. Contrasting response of lung parenchymal cells to instilled TNFα and IFNγ: the inducibility of specific cell ICAM-1 in vivo. . Am J Respir Cell Mol Biol. 1996;15:540–550. doi: 10.1165/ajrcmb.15.4.8879188. [DOI] [PubMed] [Google Scholar]
- 5.Look DC, Pelletier MR, Holtzman MJ. Selective interaction of a subset of interferon-γ response element binding proteins with the intercellular adhesion molecule-1 (ICAM-1) gene promoter controls the pattern of expression on epithelial cells. J Biol Chem. 1994;269:8952–8958. [PubMed] [Google Scholar]
- 6.Look DC, Pelletier MR, Tidwell RM, Roswit WT, Holtzman MJ. Stat1 depends on transcriptional synergy with Sp1. J Biol Chem. 1995;270:30264–30267. doi: 10.1074/jbc.270.51.30264. [DOI] [PubMed] [Google Scholar]
- 7.Walter MJ, Look DC, Tidwell RM, Roswit WT, Holtzman MJ. Targeted inhibition of interferon-γ– dependent ICAM-1 expression using dominant-negative Stat1. J Biol Chem. 1997;272:28582–28589. doi: 10.1074/jbc.272.45.28582. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima S, Roswit WT, Look DC, Holtzman MJ. A hierarchy for integrin expression and adhesiveness among T cell subsets that is linked to TCR gene usage and emphasizes Vδ1+γδ T cell adherence and tissue retention. J Immunol. 1995;155:1117–1131. [PubMed] [Google Scholar]
- 9.Nakajima S, Look DC, Roswit WT, Bragdon MJ, Holtzman MJ. Selective differences in vascular endothelial– vs. airway epithelial–T cell adhesion mechanisms. Am J Physiol. 1994;267:L422–L432. doi: 10.1152/ajplung.1994.267.4.L422. [DOI] [PubMed] [Google Scholar]
- 10.Lechner JF, LaVeck MA. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J Tissue Cult Methods. 1985;9:43–48. [Google Scholar]
- 11.Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, Krensky AM. A human T cell–specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 12.Smith CW, Rothlein R, Hughes BJ, Mariscalco MM, Rudloff HE, Schmalstieg FC, Anderson DC. Recognition of an endothelial determinant for CD18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988;82:1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Madrid F, Krensky AM, Ware CF, Robbins E, Strominger JL, Burakoff SJ, Springer TA. Three distinct antigens associated with human T-lymphocyte–mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci USA. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoyama, W.M. 1991. Monoclonal antibody supernatant and ascites fluid production. In Current Protocols in Immunology. J.E. Coligan, A.M. Kruisbeck, D.H. Margulies, E.M. Shevach, and W. Strober, editors. John Wiley & Sons, Inc., New York. 2.6.1–2.6.7. [DOI] [PubMed]
- 15.Grindstaff KK, Blanco G, Mercer RW. Characterization of Na,K-ATPase isoform expression and activity in MDCK and Caco-2 epithelial cells. Epithelial Cell Biol. 1995;4:17–24. [PubMed] [Google Scholar]
- 16.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeillemaker AM, Mul FPJ, Hoynck van Papendrecht AAGM, Kuijpers TW, Roos D, Leguit P, Verbrugh HA. Polarized secretion of interleukin-8 by human mesothelial cells: a role in neutrophil migration. Immunology. 1995;84:227–232. [PMC free article] [PubMed] [Google Scholar]
- 18.Adams RB, Planchon SM, Roche JK. IFN-γ modulation of epithelial barrier function: time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- 19.Gruenert DC, Basbaum CB, Widdicombe JH. Long-term culture of normal and cystic fibrosis epithelial cells grown under serum-free conditions. In Vitro Cell Dev Biol. 1990;26:411–418. doi: 10.1007/BF02623833. [DOI] [PubMed] [Google Scholar]
- 20.Haskard D, Cavender D, Ziff M. Phorbol ester-stimulated T lymphocytes show enhanced adhesion to human endothelial cell monolayers. J Immunol. 1986;137:1429–1434. [PubMed] [Google Scholar]
- 21.Koga T, Look DC, Taguchi M, Holtzman MJ. Selective interferon-γ–dependent regulation of RANTES in airway epithelial cells. Am J Respir Crit Care Med. 1997;155:A751. . (Abstr.) [Google Scholar]
- 22.Boucher RC. Human airway ion transport. Am J Respir Crit Care Med. 1994;150:271–281. doi: 10.1164/ajrccm.150.1.8025763. [DOI] [PubMed] [Google Scholar]
- 23.Marfaing-Koka A, Devergne O, Gorgone G, Poprtier A, Schall TJ, Galanaud P, Emilie D. Regulation of the production of the RANTES chemokine by endothelial cells. J Immunol. 1995;154:1870–1878. [PubMed] [Google Scholar]
- 24.Meneely GR, Renzetti AD, Jr, Steele JD, Wyatt JP, Harris HW. Definitions and classification of chronic bronchitis, asthma, and pulmonary emphysema. Am Rev Respir Dis. 1962;85:762–768. [Google Scholar]
- 25.Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993;92:2858–2866. doi: 10.1172/JCI116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay CR. Chemokine receptors and T cell chemotaxis. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randolph GJ, Furie MB. Mononuclear phagocytes egress from an in vitro model of the vascular wall by migrating across endothelium in the basal to apical direction: role of intercellular adhesion molecule 1 and the CD11/ CD18 integrins. J Exp Med. 1996;183:451–462. doi: 10.1084/jem.183.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang GT-J, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule–1 (ICAM-1) expression and neutrophil adhesion. J Clin Invest. 1996;98:572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkos CA, Colgan SP, Diamond MS, Nusrat A, Liang TW, Springer TA, Madara JL. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol Med. 1996;2:489–505. [PMC free article] [PubMed] [Google Scholar]
- 30.Burns AR, Takei F, Doerschuk CM. Quantitation of ICAM-1 expression in mouse lung during pneumonia. J Immunol. 1994;153:3189–3198. [PubMed] [Google Scholar]
- 31.Sampath D, Castro M, Look DC, Holtzman MJ. Activation of the Stat1 transcription factor in asthma. J Allergy Clin Immunol. 1997;99:475. . (Abstr.) [Google Scholar]
- 32.Berkman N, Krishnan VL, Gilbey T, Newton R, O'Connor B, Barnes PJ, Chung KF. Expression of RANTES mRNA and protein in airways of patients with asthma. Am J Respir Crit Care Med. 1996;154:1804–1811. doi: 10.1164/ajrccm.154.6.8970374. [DOI] [PubMed] [Google Scholar]
- 33.Humbert M, Ying S, Corrigan C, Menz G, Barkans J, Pfister R, Meng Q, Van Damme J, Opdenakker G, Durham SR, Kay AB. Bronchial mucosal expression of the genes encoding chemokines RANTES and MCP-3 in symptomatic atopic and nonatopic asthmatics: relationship to the eosinophil-active cytokines interleukin (IL)-5, granulocyte macrophage-colony–stimulating factor, and IL-3. Am J Respir Cell Mol Biol. 1997;16:1–8. doi: 10.1165/ajrcmb.16.1.8998072. [DOI] [PubMed] [Google Scholar]
- 34.Fahy JV, Figueroa DJ, Wong HH, Liu JT, Abrams JS. Similar RANTES levels in healthy and asthmatic airways by immunoassay and in situ hybridization. Am J Respir Crit Care Med. 1997;155:1095–1100. doi: 10.1164/ajrccm.155.3.9116993. [DOI] [PubMed] [Google Scholar]
- 35.Wang JH, Devalia JL, Xia C, Sapsford RJ, Davies RJ. Expression of RANTES by human bronchial epithelial cells in vitro and in vivo and the effect of corticosteroids. Am J Respir Cell Mol Biol. 1996;14:27–35. doi: 10.1165/ajrcmb.14.1.8534483. [DOI] [PubMed] [Google Scholar]
- 36.Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- 37.Nelson WJ. Regulation of cell surface polarity from bacteria to mammals. Science. 1992;258:948–954. doi: 10.1126/science.1439806. [DOI] [PubMed] [Google Scholar]
- 38.Lisanti MP, Caras IW, Gilbert T, Hanzel D, Rodriguez-Boulan E. Vectorial apical delivery and slow endocytosis of a glycolipid-anchored fusion protein in transfected MDCK cells. Proc Natl Acad Sci USA. 1990;87:7419–7423. doi: 10.1073/pnas.87.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- 40.Powell SK, Cunningham BA, Edelman GM, Rodriguez-Boulan E. Targeting of transmembrane and GPI-anchored forms of N-CAM to opposite domains of a polarized epithelial cell. Nature. 1991;353:76–77. doi: 10.1038/353076a0. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–11706. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- 42.Randolph GJ, Furie MB. A soluble gradient of monocyte chemoattractant protein 1 promotes the transendothelial migration of monocytes in vivo. J Immunol. 1995;155:3610–3619. [PubMed] [Google Scholar]
- 43.Cottam DW, Corbitt RH, Gomez DE, Rees RC, Thorgeirsson UP. Alterations in endothelial cell proteinase and inhibitor polarized secretion following treatment with interleukin-1, phorbol ester, and human melanoma cell conditioned medium. J Cell Biochem. 1996;60:148–160. doi: 10.1002/(sici)1097-4644(19960101)60:1<148::aid-jcb17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 44.Rot A. Endothelial cell binding of NAP-1/IL-8: role of neutrophil emigration. Immunol Today. 1992;13:291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 46.Gilat D, Hershoviz R, Mekori YA, Vlodavsky I, Lider O. Regulation of adhesion of CD4+ T lymphocytes to intact or heparinase-treated subendothelial extracellular matrix by diffusible or anchored RANTES and MIP-1β. J Immunol. 1994;153:4899–4906. [PubMed] [Google Scholar]
- 47.Tanaka Y, Adams DH, Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today. 1993;14:111–114. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- 48.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 49.Liu L, Mul FPJ, Lutter R, Roos D, Knol EF. Transmigration of human neutrophils across airway epithelial cell monolayers is preferentially in the physiologic basolateral-to-apical direction. Am J Respir Cell Mol Biol. 1996;15:771–780. doi: 10.1165/ajrcmb.15.6.8969272. [DOI] [PubMed] [Google Scholar]
- 50.McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI. Surface attachment of Salmonella typhimuriumto intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diamond MS, Alon R, Parkos CA, Quinn MT, Springer TA. Heparin is an adhesive ligand for the leukocyte integrin Mac-1 (CD11b/CD18) J Cell Biol. 1995;130:1473–1482. doi: 10.1083/jcb.130.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 53.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+T cells in response to MIP-1α and MIP-1β. Science. 1994;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 54.Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- 55.Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, Holgate ST, Frew AJ, Howarth PH. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 56.Holtzman MJ, Look DC, Sampath D, Castro M, Koga T, Walter MJ. Control of epithelial immune-response genes and implications for airway immunity and inflammation. Proc Assoc Am Phys. 1998;110:1–11. [PubMed] [Google Scholar]
- 57.Walter MJ, Kajiwara N, Rucker J, Holtzman MJ. Induction of epithelial immune-response genes in a mouse model of viral bronchitis and hyperreactivity. FASEB J. 1998;12:A1453. . (Abstr.) [Google Scholar]
- 58.Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 59.Carr MW, Alon R, Springer TA. The C-C chemokine MCP-1 differentially modulates the avidity of β1 and β2 integrins on T lymphocytes. Immunity. 1996;4:179–187. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- 60.LLoyd AR, Oppenheim JJ, Kelvin DJ, Taub DD. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156:932–938. [PubMed] [Google Scholar]
- 61.Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. The biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holtzman, M.J., M. Castro, D.C. Look, M. O'Sullivan, and M.J. Walter. 1998. Regulation of epithelial-leukocyte interaction and epithelial immune-response genes. In Asthma and Rhinitis. W. Busse, and S. Holgate, editors. Blackwell Scientific, Cambridge, MA. In press.
- 63.Bentley AM, Durham SR, Robinson DS, Menz G, Storz C, Cromwell O, Kay AB, Wardlaw AJ. Expression of endothelial and leukocyte adhesion molecules intercellular adhesion molecule-1, E-selectin, and vascular cell adhesion molecule-1 in the bronchial mucosa in steady-state and allergen-induced asthma. J Allergy Clin Immunol. 1993;92:857–868. doi: 10.1016/0091-6749(93)90064-m. [DOI] [PubMed] [Google Scholar]
- 64.Vignola AM, Chanez P, Campbell AM, Pinel AM, Bousquet J, Michel F-B, Godard P. Quantification and localization of HLA-DR and intercellular adhesion molecule-1 (ICAM-1) molecules on bronchial epithelial cells of asthmatics using confocal microscopy. Clin Exp Immunol. 1994;96:104–109. doi: 10.1111/j.1365-2249.1994.tb06238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohkawara Y, Yamauchi K, Maruyama N, Hoshi H, Ohno I, Honma M, Tanno Y, Tamura G, Shirato K, Ohtani H. In situ expression of the cell adhesion molecules in bronchial tissues from asthmatics with air flow limitation: in vivoevidence of VCAM-1/VLA-4 interaction in selective eosinophil infiltration. Am J Respir Cell Mol Biol. 1995;12:4–12. doi: 10.1165/ajrcmb.12.1.7529029. [DOI] [PubMed] [Google Scholar]
- 66.Gosset P, Tillie-Leblond I, Janin A, Marquette C-H, Copin M-C, Wallaert B, Tonnel A-B. Expression of E-selectin, ICAM-1 and VCAM-1 on bronchial biopsies from allergic and non-allergic asthmatic patients. Int Arch Allergy Immunol. 1995;106:69–77. doi: 10.1159/000236892. [DOI] [PubMed] [Google Scholar]
- 67.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 68.Koga T, Look DC, Tidwell RM, Holtzman MJ. Mechanisms for cytokine regulation of β-chemokine expression in airway epithelial cells. Am J Respir Crit Care Med. 1998;157:A744. . (Abstr.) [Google Scholar]
- 69.Sardina E, Tidwell R, Koga T, Holtzman MJ. Regulation of RANTES gene expression by a 3′-UTR that lacks consensus sites for mRNA turnover. FASEB J. 1998;12:A1308. . (Abstr.) [Google Scholar]