Abstract
The leukocyte adhesion molecule, L-selectin, mediates the recruitment of lymphocytes to secondary lymphoid organs via interactions with specific ligands presented on high endothelial venules (HEV). Although the HEV-derived ligands for L-selectin are still incompletely defined, they share a common sialomucin-like structure which is thought to present clustered oligosaccharides to the lectin domain of L-selectin. Podocalyxin-like protein (PCLP) is a transmembrane sialomucin that is similar in structure to the well-characterized L-selectin ligand CD34. PCLP has been shown previously to be expressed on the foot processes of podocytes in the kidney glomerulus as well as on vascular endothelium at some sites. We have determined that PCLP is present on HEV, where it binds to both recombinant L-selectin and the HEV-specific monoclonal antibody MECA-79. Furthermore, purified HEV-derived PCLP is able to support the tethering and rolling of lymphocytes under physiological flow conditions in vitro. These results suggest a novel function for PCLP as an adhesion molecule and allow the definition of conserved structural features in PCLP and CD34, which may be important for L-selectin ligand function.
Keywords: podocalyxin, L-selectin, CD34, high endothelial venule, homing
Maintenance of immune surveillance depends on the constant recirculation of lymphocytes from the blood through the vascular wall into the tissues and eventually back into the blood (1). Lymphocyte recruitment from the blood into all secondary lymphoid organs (except the spleen) as well as into many sites of chronic inflammation is mediated by specialized postcapillary venules called high endothelial venules (HEV)1 (2). These vessels are defined by the distinct, cuboidal morphology of their endothelial cells (3) and their luminal presentation of ligands for the leukocyte adhesion molecule, L-selectin (4). This lectin-like adhesion molecule is responsible for the initial tethering and rolling of lymphocytes on the endothelium before their integrin-mediated firm arrest and transmigration (5, 6). Studies using antibody blockade as well as gene inactivation have demonstrated the absolute requirement for L-selectin in the recruitment of lymphocytes to peripheral lymph nodes as well as a major role in recruitment into Peyer's patches and certain sites of inflammation (7–10).
The NH2-terminal C-type lectin domain of L-selectin mediates adhesion by binding to specific carbohydrate structures presented by glycoprotein ligands on the vascular endothelium (11). The HEV-derived ligands for L-selectin consist of a group of mucin-like glycoproteins which are able to present dense clusters of sulfated and sialylated O-linked oligosaccharides to L-selectin (12). Collectively, these glycoproteins are termed the peripheral node addressin (PNAd) (13). These proteins have been identified biochemically using either a recombinant L-selectin/IgG chimera (14) or the mAb MECA-79, which recognizes a sulfation-dependent posttranslational modification shared by L-selectin ligands (13, 15, 16). Both reagents bind specifically to HEV in histologic sections, are able to block the L-selectin–dependent adhesion of lymphocytes to HEV in vitro and in vivo (15, 17, 18), and both can be used to isolate the same set of glycoproteins from a detergent lysate of lymph node (16).
To date, four distinct ligands for L-selectin have been identified in mouse HEV. These are CD34, glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1), mucosal addressin cell adhesion molecule-1 (MAdCAM-1), and Sgp200 (16, 19–21). CD34 is a transmembrane mucin-like glycoprotein that is widely expressed on vascular endothelium (22). The specific glycoform expressed by HEV carries the MECA-79 epitope, binds L-selectin, and is capable of mediating L-selectin–dependent tethering and rolling of leukocytes under flow (16, 19, 23). GlyCAM-1 is a soluble, secreted molecule (4) which has been shown to be able to increase the avidity of β1 and β2 integrins on naive lymphocytes via ligation of L-selectin (24, 25). MAdCAM-1 is the predominant ligand for the α4β7 integrin in the HEV of Peyer's patch and mesenteric lymph node (26). In addition to its integrin-binding Ig-like domains, this molecule also contains a mucin-like domain. A subset of MAdCAM-1 is decorated with the MECA-79 epitope (20) and can serve as a ligand for L-selectin (6). Sgp200 is a 200-kD sulfated glycoprotein which has not yet been molecularly identified (16). In addition to these HEV ligands, P-selectin glycoprotein ligand-1 on leukocytes (27–29) can also function as a ligand for L-selectin.
In humans, the HEV-associated ligands for L-selectin are still poorly defined. MECA-79 has been shown to react with glycoproteins of 65, 105, 160, and 200-kD in human tonsil lysates (13). CD34 represents at least part of the 105-kD component, and it has been shown to represent 30% of the total MECA-79–reactive protein as well as 50% of the total adhesive activity of PNAd (23). The identities of the remaining glycoproteins have not yet been determined. Based on their diverse structures and expression patterns, the three defined ligands for L-selectin (i.e., CD34, GlyCAM-1, and MAdCAM-1) are likely to serve distinct roles in lymphocyte recruitment. The identification of the remaining ligands should allow the dissection of their unique functions as well as those that may be redundant.
Podocalyxin, originally identified in rat, is a sialoprotein present at high levels on the foot processes of podocytes in the kidney glomerulus, where it is thought to maintain the integrity of the filtration slits by contributing to the anionic glycocalyx of this structure (30). Although this protein remains to be identified molecularly in rat, a homolog called podocalyxin-like protein (PCLP) has been cloned from rabbit (31) and humans (32), and a more distant chicken homolog, known as thrombomucin, has been described recently (33). Interestingly, PCLP is similar in structure to CD34 in that both consist of a large NH2-terminal mucin-like domain followed by a disulfide-containing (and presumably globular) domain, a transmembrane domain, and a cytoplasmic tail. Additionally, CD34 (34–36) as well as podocalyxin and its homologs (31–33, 37) are expressed on the luminal surface of vascular endothelium in a variety of tissues. In this study, we demonstrate that PCLP is expressed in the HEV of secondary lymphoid organs, where it carries the MECA-79 epitope. Furthermore, MECA-79–reactive PCLP binds to recombinant L-selectin and is able to support the L-selectin–dependent tethering and rolling of lymphocytes under flow. These findings support a novel proadhesive function for PCLP in lymphocyte recruitment, and suggest that common structural features of CD34 and PCLP are important for their function as ligands for L-selectin.
Materials and Methods
Antibodies, Carbohydrates, and Ig Chimeras.
Mouse anti-PCLP mAbs 3D3 (IgG), 2A4 (IgM), and 4F10 (IgM) were generated as described (32) and produced as hybridoma culture supernatants. Additionally, the 3D3 antibody was produced as ascites. The MECA-79 (rat IgM) hybridoma was obtained from the American Type Culture Collection (Rockville, MD), produced as ascites, and purified on Sepharose-coupled anti–rat Igκ (Zymed Laboratories, Inc., South San Francisco, CA) as recommended by the manufacturer. Antibodies were biotinylated with NHS-biotin (Sigma Chemical Co., St. Louis, MO) as described (38). Purified L-selectin mAb LAM1-3 was obtained from Dr. Thomas Tedder (Duke University, Durham, NC). Fucoidin was purchased from Sigma Chemical Co. L-selectin/IgG and CD4/IgG chimeras consist of the entire extracellular domain of murine L-selectin or human CD4 fused to the Fc domain of human IgG1. These chimeric proteins were collected from the supernatants of transfected 293 cells and were protein A–purified as described previously (17). Arthrobacter ureafaciens sialidase was purchased from Oxford GlycoSystems Plc. (Abingdon, Oxford, UK). O-sialoglycoprotein endopeptidase (OSGE) was prepared by Cedarlane Labs. Ltd. (Hornby, Ontario, Canada) and purchased from Accurate Chemical and Science Corp. (Westbury, NY).
Cells.
Jurkat T cells were obtained from the laboratory of Dr. Art Weiss (University of California, San Francisco) and were maintained in RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and 5% heat-inactivated FCS (Hyclone, Logan, UT). Human T lymphocytes were isolated from venous blood samples using standard gradient techniques (25). In brief, blood was collected from healthy donors, and EDTA was added to a final concentration of 5 mM to prevent clotting and cell activation. The blood samples were diluted with 1/2 vol cation-free HBSS and layered over Histopaque 1077 (Sigma Chemical Co.). After centrifugation at 500 g for 20 min at room temperature, the mononuclear cell fraction was collected, diluted in cold cation-free HBSS supplemented with 0.2% BSA, and centrifuged to recover the isolated cells. T lymphocytes were obtained by depletion of B cells and monocytes. Peripheral blood mononuclear cells were treated with saturating amounts of mouse anti-CD14 (monocyte marker) and mouse anti-CD19 (B cell marker) followed by sheep anti–mouse IgG–coupled paramagnetic particles (Dynal Inc., Lake Success, NY). Labeled cells were then removed by immunomagnetic selection.
Immunohistochemistry.
Surgical specimens of human tonsil (obtained from the University of California, San Francisco) were either embedded in OCT medium (Miles Inc., Elkhart, IN) and frozen, or fixed in neutral-buffered formalin and paraffin embedded. Sections of paraffin-embedded human lymph node or appendix were obtained from archived specimens determined to be devoid of specific pathology. 12-μm frozen sections were fixed in 1% paraformaldehyde in 100 mM cacodylate, pH 7.3, for 20 min. For horseradish peroxidase detection, endogenous peroxidase was quenched with 0.3% H2O2, 0.2 M NaN3 in Dulbecco's PBS (PBS) for 20 min. Sections were blocked with 5% goat serum and stained with undiluted hybridoma culture supernatant overnight at 4°C. The sections were then incubated sequentially with 1:250 diluted biotinylated goat anti–mouse IgG (heavy and light chain– specific; Vector Laboratories, Inc., Burlingame, CA) in 5% goat serum and 2.5% human serum, then with ABC elite reagent (Vector Laboratories, Inc.) in PBS, and finally with AEC chromogen (Biomeda, Foster City, CA). After chromogen development, the sections were counterstained with aqueous hematoxylin and mounted in Permafluor (Shandon Lipshaw, Pittsburgh, PA). PBS was used for washing after each incubation. For fluorescence detection, frozen sections were fixed, blocked, and incubated with PCLP antibodies as above. Sections were then incubated sequentially with FITC-conjugated goat anti–mouse IgG (Zymed Laboratories, Inc.) in 5% goat serum and 2.5% human serum; 1 μg/ml biotinylated MECA-79 in 5% goat serum, 2.5% human serum, and 2.5% rat serum; and Texas red–conjugated streptavidin (Vector Laboratories, Inc.) in 5% goat serum, 2.5% human serum, and 2.5% rat serum. The sections were washed in PBS after each step and mounted in Vectashield (Vector Laboratories, Inc.).
PNAd Isolation.
Frozen surgical specimens of human tonsils were obtained from the Cooperative Human Tissue Network, Western Division (Case Western University, Cleveland, OH), which is funded by the National Cancer Institute. The tissue was homogenized in PBS containing 2% Triton X-100, 1 μg/ml leupeptin, 10 μg/ml aprotinin, 1 μM pepstatin, 10 μg/ml Pefablock, and 5 mM EDTA in a glass/Teflon homogenizer. This lysate was rocked at 4°C for 30 min followed by centrifugation at 20,000 g for 20 min at 4°C. The lysate was then diluted twofold with PBS and passed through a 0.2-μm filter. The clarified lysate was passed over a 1-ml Sepharose 4B column (CNBr-activated; Pharmacia Biotech, Piscataway, NJ) coupled to 2.4 mg affinity-purified MECA-79. The column was washed with PBS containing 0.1% Triton X-100 (PBS-TX) followed by PBS containing 25 mM n-octylglucoside. PNAd was then eluted with 100 mM triethylamine containing 25 mM n-octylglucoside and neutralized with 1/10 vol 3 M Tris, pH 6.8. This material was concentrated using a microconcentrator (Centricon-30; Amicon, Inc., Beverly, MA), diluted with PBS containing 25 mM n-octylglucoside, and reconcentrated. The purified protein was quantified using the BCA assay (Pierce Chemical Co., Rockford, IL), and purity was assessed by SDS-PAGE followed by silver-enhanced alcian blue staining (39). 7.5% gels were fixed in 25% ethanol, 10% acetic acid overnight. They were then stained with 0.125% alcian blue in fix solution for 1 h followed by three 30-min destaining steps with fix solution. Gels were subsequently incubated in 5% glutaraldehyde in H2O for 1 h, followed by four 30-min washes with 10% ethanol, 5% acetic acid. This was followed by three washes with H2O for 10 min each and a 30-min incubation in 0.4% silver nitrate. After rinsing briefly in water, gels were developed with 0.013% formaldehyde in 2.5% sodium carbonate. The development was stopped with 1% acetic acid.
Immunoprecipitation and Western Blotting.
Protein A–Sepharose Fast Flow beads (Pharmacia Biotech) were loaded with 200 μl of 3D3 hybridoma culture supernatant or 6 μg purified mouse IgG (Caltag Laboratories, Inc., San Francisco, CA) and washed with PBS-TX. 100 μl of PBS-TX containing ∼50 ng PNAd and 0.1 μl goat serum was then added to the beads and rocked overnight at 4°C. The supernatant was collected and pooled with two 100-μl PBS-TX washes of the beads. This unbound fraction was then precipitated with 4 vol acetone at −20°C for 1 h, collected by centrifugation, dissolved in SDS-PAGE sample buffer, and boiled. The beads were washed extensively with PBS-TX and eluted by boiling in sample buffer. After SDS-PAGE (7.5%), the gels were transferred in a Hoefer TE series electroblotter to Problott (Applied Biosystems, Inc., Foster City, CA) in 10 mM 3-[cyclohexylamino]-1-propane-sulfonic acid (CAPS), 10% methanol at 22 V for 16 h. The blot was then blocked in PBS containing 0.1% Tween 20 (PBST) and 3% nonfat dry milk for 1 h. Blots were then incubated sequentially in either 1 μg/ml MECA-79 or 1:50 diluted 3D3 supernatant in blocking solution, 1:1,000 diluted biotinylated mouse anti–rat Igμ (Caltag Laboratories, Inc.), or 1:1,000 diluted biotinylated goat anti–mouse IgG (Vector Laboratories, Inc.) in blocking solution, and finally 1:2,000 diluted horseradish peroxidase–conjugated streptavidin (Caltag Laboratories, Inc.) in PBST. Blots were washed with PBST after each incubation and developed using an ECL substrate (Amersham Corp., Arlington Heights, IL). For detection with the 3D3 mAb, 5–10 times more PNAd was used than for MECA-79 detection. Quantification was performed using Sigmagel software (Sigma Chemical Co.) after converting the data to a digital image using a transilluminating scanner.
Detection of PCLP mRNA.
High endothelial cells (HEC) were purified from surgical specimens of human tonsils by immunomagnetic selection with MECA-79 by a modification of a previously described procedure (40). After collagenase digestion of the tonsils, stromal cells were resuspended at 2 × 108 cells/ml in staining buffer (PBS containing 1% BSA and 5 mM EDTA) and incubated with 0.3 μg of MECA-79 per 107 cells at 4°C for 15 min. Cells were collected by centrifugation and incubated with 30 μl of biotinylated mouse anti–rat Igμ (Caltag Laboratories, Inc.) for 15 min at 4°C. Cells were washed with staining buffer, 300 μl of streptavidin-coupled paramagnetic beads (Miltenyi Biotec Inc., Auburn, CA) was added, and cells were incubated at 4°C for 15 min. MECA-79–positive cells were isolated using an MS+ column and a mini-MACS magnet (Miltenyi Biotec Inc.) as recommended by the manufacturer. Purity was assessed microscopically by morphologic criteria as well as MECA-79 expression by immunofluorescence using cyanine-3–conjugated streptavidin (Caltag Laboratories, Inc.).
Human umbilical vein endothelial cells (HUVEC) were purchased from Clonetics (San Diego, CA) and were grown as recommended. Tonsillar lymphocytes were prepared by mincing surgical specimens of human tonsil and flushing the loose lymphocytes through a fine screen with cold RPMI 1640 medium.
Total RNA was isolated from HEC, HUVEC, and lymphocytes using RNAzol B (Tel-Test, Inc., Friendswood, TX). First-strand cDNA was made from 2 μg total RNA primed with random hexamers using AMV reverse transcriptase (GIBCO BRL, Gaithersburg, MD) according to the manufacturer's recommendations. Serial dilutions of cDNA were amplified using Klentaq polymerase mix (Clontech, Palo Alto, CA) and the following primers: for PCLP, 5′-TTTGGATCCCAGATGCCAGCCAGCTCTACG-3′ and 5′-TTTGAATTCCTTCATGTCACTGACCCCTGC-3′ were used, and for hypoxanthine phosphoribosyltransferase (HPRT), 5′-CCTGCTGGATTACATCAAAGCACTG-3′ and 5′-TCCAACACTTCGTGGGGTCCT-3′ were used. One half of the resulting amplified DNA was electrophoresed on 1% agarose gels and visualized by ethidium bromide staining. Quantification was performed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA) on digital images of gels obtained by optical scanning of photographs.
Purification of MECA-79–reactive PCLP.
A detergent lysate was prepared from 10 g of human tonsils as described for PNAd isolation. This material was passed twice over a 2-ml column of protein A–coupled Sepharose. The unbound material was then passed over a 0.5-ml protein A column to which 2 ml of 3D3 ascites had been bound and covalently coupled with dimethyl pimelimidate (Pierce Chemical Co.) as described (38). After washing with PBS-TX, the bound material was eluted with 100 mM triethylamine containing 0.1% Triton X-100 and neutralized with 1/10 vol 3 M Tris, pH 6.8. This sample was then purified on MECA-79–coupled Sepharose as described for PNAd isolation. 1/60 of the resulting material was analyzed by SDS-PAGE followed by MECA-79 Western blotting.
Parallel Plate Flow Chamber Analysis.
Purified PCLP was diluted 1:10 or 1:20 in Tris-buffered saline, pH 8.5, and coated onto bacteriological Petri dishes (Corning, Corning, NY) at 4°C overnight. For blocking, the plates were treated with 3% BSA for 1 h at room temperature. The substrate-coated slides were incorporated as the lower wall in a parallel plate flow chamber and mounted on the stage of an inverted phase–contrast microscope (Diaphot 200; Nikon, Inc., Melville, NY). All flow experiments were performed at room temperature. The wall shear stress was calculated as described previously (41). Cells were stored at a concentration of 107 cells/ml in cation-free HBSS containing 0.2% BSA, and were diluted 1:10 in HBSS containing 0.2% BSA immediately before perfusion into the flow chamber. Jurkat cells were infused into the chamber at a shear stress of 0.8 dynes/cm2 and T lymphocytes at 1.25 dynes/cm2. After three min, the cell tethering rate (number of cells that tethered per minute per field) was determined by analysis of two to four fields of view. Tethering was defined as the initial adhesive event, which results in the capture of a flowing cell by the substrate-coated surface. Cell tethering was followed by either continuous rolling or detachment from the substrate. Both types of interactions were included in the calculation of tethering rate. For inhibition studies, the cells (107/ml) were preincubated for 20 min in cation-free HBSS containing 0.2% BSA with 150 μg/ml LAM1-3 (anti–L-selectin) or 100 μg/ml fucoidin (Sigma Chemical Co.). The cell suspension was diluted 10-fold with HBSS containing 0.2% BSA before infusion into the flow chamber. The substrate was incubated with MECA-79 (ascites diluted 1:20) or 3D3 (anti-PCLP, ascites diluted 1:20) for 1 h at room temperature. Immobilized PCLP was treated with 50 mU/ml of sialidase in 0.1 M sodium acetate, pH 5.0, for 1 h at room temperature, or with 0.1 M sodium acetate, pH 5.0, alone as a control. OSGE was diluted 10-fold in PBS to a concentration of 0.24 mg/ml total protein, and was used to treat immobilized PCLP for 1 h at room temperature.
Results
HEC Express PCLP.
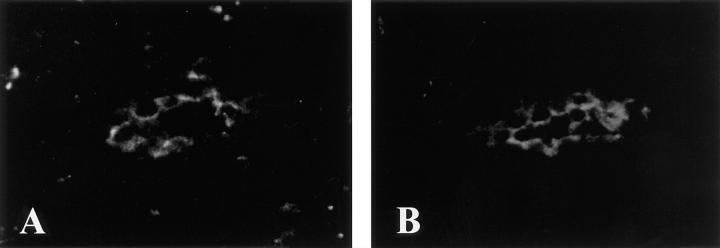
Since PCLP and its homologs are expressed by vascular endothelium in a number of sites (see above), we sought to determine if PCLP was present in HEV. Three different mAbs specific for human PCLP were used for immunohistochemical staining of sections of human secondary lymphoid organs. Two-color immunofluorescence experiments were performed by simultaneously staining frozen sections of human tonsil with a PCLP mAb (4F10) and MECA-79. As shown in Fig. 1, HEV were observed that expressed both PCLP and the MECA-79 epitope. PCLP was concentrated at the surface of the HEC on the luminal aspect of the vessels. Similar staining patterns were obtained with two other PCLP mAbs (3D3 and 2A4, not shown). Localization to the luminal face of vascular endothelium has also been reported for podocalyxin in kidney (30) and for thrombomucin in multiple tissues (33). HEV of paraffin-embedded samples of lymph node and appendix were also stained by the PCLP mAb 2A4 using immunoperoxidase techniques (Fig. 2, A–C), demonstrating that PCLP expression was present in HEV of both mucosal and nonmucosal lymphoid organs.
Figure 1.
Coexpression of MECA-79 and PCLP in HEV of tonsil. Frozen sections were stained simultaneously with the PCLP mAb 4F10 (A) and MECA-79 (B). A and B show the same HEV viewed with an FITC filter (A) or a Texas red filter (B).
Figure 2.

Expression of PCLP in secondary lymphoid organs. Frozen sections of tonsil and associated connective tissue (A and D) and paraffin-embedded sections of tonsil (E and F), appendix (B), and lymph node (C) were stained with the anti-PCLP mAb 2A4. Arrows, HEV. Arrowheads, PCLP-expressing capillaries. Bars, 200 μm (A), 100 μm (B, D, E, and F), and 50 μm (C). Data are representative of two different samples of tonsil and lymph node, and three samples of appendix.
PCLP expression was not restricted to HEV, as staining was associated with endothelial cells of many classes of blood vessels in these lymphoid organs. Fig. 2 shows PCLP staining in muscular arteries (Fig. 2 D), arterioles (Fig. 2 E), and capillaries (arrowheads, Fig. 2 B). These findings extend those of previous studies which demonstrated the expression of podocalyxin and its homologs on vascular endothelium in nonlymphoid tissues (30, 32, 33).
It was also apparent from these experiments that although the majority of HEV in tonsils were positive for PCLP expression, a minority were weak or negative (Fig. 2 A). This observation was also noted in the two-color immunofluorescence experiments, where some MECA-79–positive vessels expressed little or no PCLP. In contrast, CD34 can be detected on virtually all HEV in sections of the same tonsil (not shown). However, these PCLP-negative HEV were not noted in lymph node or appendix, suggesting that the heterogeneity might have been the result of the inflamed state of the tonsil specimens.
A reverse transcriptase PCR assay was used to demonstrate the presence of PCLP mRNA in purified HEC. MECA-79–expressing cells were purified from collagenase-digested tonsillar stroma by immunomagnetic selection. This procedure produced 99% pure HEC with a slight contamination of lymphocytes, as determined by morphology and MECA-79 expression. Total RNA was extracted from HEC, tonsillar lymphocytes, and primary cultured endothelial cells (HUVEC). Serial dilutions of reverse-transcribed cDNA were then amplified with primers specific for PCLP or HPRT. The level of the constitutively expressed HPRT mRNA was used to normalize the amount of total cDNA in each sample. PCLP mRNA was readily detected in both HEC and HUVEC cDNA, but was only barely detectable in the lymphocyte sample (Fig. 3). The identity of the product that was amplified using PCLP-specific primers was confirmed by the demonstration of appropriately sized fragments after digestion with the restriction endonucleases Sac1 and Sty1 (data not shown). When the data were quantified and normalized for HPRT expression, PCLP mRNA levels were found to be approximately twofold greater in HUVEC than in HEC. Importantly, the PCLP expression level in HEC was at least 32-fold higher than in the lymphocyte sample, thus showing that the PCLP mRNA detected in the HEC preparation was not a result of lymphocyte contamination. Additionally, when reverse transcriptase was omitted from the cDNA reactions, no PCR product was observed (Fig. 3, (−)RT lanes), establishing that all products are the result of amplified cDNA and not contaminating genomic DNA.
Figure 3.
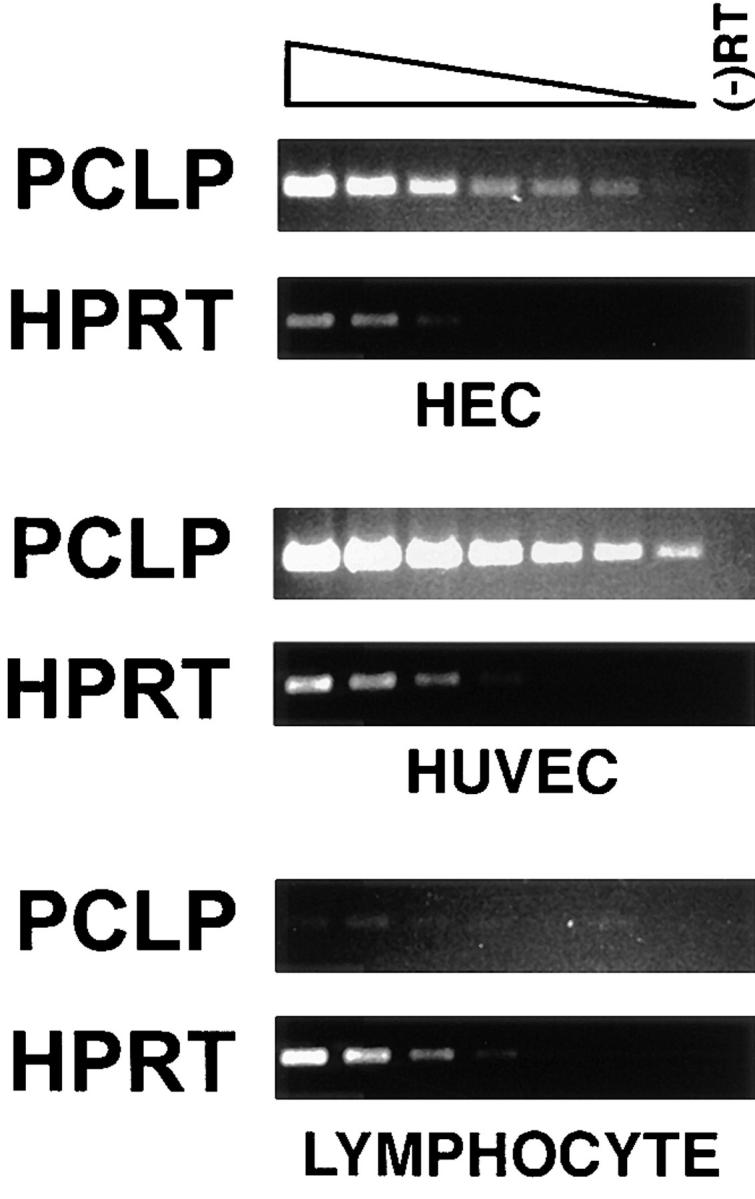
Detection of PCLP mRNA by PCR. Fragments of the PCLP and HPRT sequences were amplified by PCR from serial dilutions of cDNA from purified HEC, tonsillar lymphocytes, and HUVEC. (-)RT, PCR reactions using samples from which the reverse transcriptase was omitted. Note that a faint PCLP band is barely visible in the lowest dilution of lymphocyte cDNA.
Tonsillar PCLP Displays the MECA-79 Epitope and Binds L-selectin.
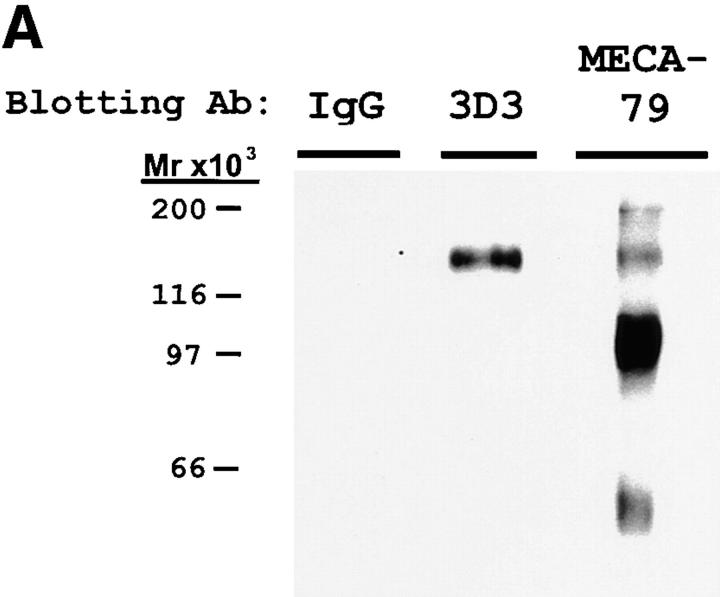
To determine whether HEV-derived PCLP carries the MECA-79 epitope and is therefore a potential ligand for L-selectin, we subjected MECA-79–purified tonsil lysate (i.e., PNAd) to Western blotting with the PCLP mAb 3D3 as well as MECA-79 (Fig. 4 A). MECA-79 detected major bands of 210, 160, 115, and 60 kD, in agreement with previous characterizations (13). The 3D3 antibody specifically recognized a 160-kD component in PNAd, which is consistent with the molecular weight of PCLP.
Figure 4.
Western blotting and immunoprecipitation of PNAd with PCLP mAb. (A) PNAd was electrophoresed on 7.5% SDS gels and blotted with either MECA-79, the PCLP mAb 3D3, or control mouse IgG. (B) Samples of PNAd were immunoprecipitated (I.P.) with protein A–Sepharose loaded with either 3D3 (PCLP mAb) or control mouse IgG. The unbound supernatant (SUP) as well as the fraction bound to the antibody (PEL) were analyzed by MECA-79 Western blotting after 7.5% SDS-PAGE.
The identity of the 160-kD component of PNAd as PCLP was confirmed by immunoprecipitation with the 3D3 antibody (Fig. 4 B). When PNAd was immunoprecipitated with 3D3, the 160-kD band was specifically depleted from the unbound fraction, and only this component was detected in the bound fraction. This result demonstrates that the 160-kD band represented a MECA-79–reactive form of PCLP. In multiple experiments, the 3D3 antibody reproducibly depleted a maximum of 50% of the 160-kD band. Increasing the amount of antibody did not enhance the precipitation, suggesting that there were glycoforms of HEV-derived PCLP that were unreactive with the 3D3 antibody. Since this antibody was made against a nonglycosylated form of recombinant PCLP produced in bacteria, the existence of an unreactive native glycoform would not be surprising. However, it is formally possible that the unreactive material represented a distinct glycoprotein with the same apparent molecular weight.
To demonstrate a direct interaction between HEV-derived PCLP and L-selectin, the same preparation of PNAd was subjected to precipitation with a recombinant L-selectin/ IgG chimera (Fig. 5). Essentially all of the PCLP band (160 kD) and >90% of the total PNAd were precipitated with the L-selectin/IgG chimera, whereas no interaction was observed between any PNAd component and a control CD4/IgG chimera. Furthermore, the L-selectin– bound material could be eluted by the addition of EDTA. This demonstrates that PCLP and CD34, as well as the two unidentified PNAd components (210 and 65 kD), interacted with L-selectin in a calcium-dependent manner, a characteristic of selectin–carbohydrate interactions (12).
Figure 5.
Binding of PNAd to L-selectin. Samples of PNAd were precipitated with protein A beads loaded with either L-selectin–IgG (L-IgG) or with CD4/ IgG chimeras (CD4-IgG). The unbound supernatants (SUP) and EDTA elutions of bound material (EDTA) were electrophoresed on a 7.5% SDS gel and Western blotted with MECA-79. Arrowhead, The 160-kD band representing PCLP.
HEV-derived PCLP Is a Sialomucin.
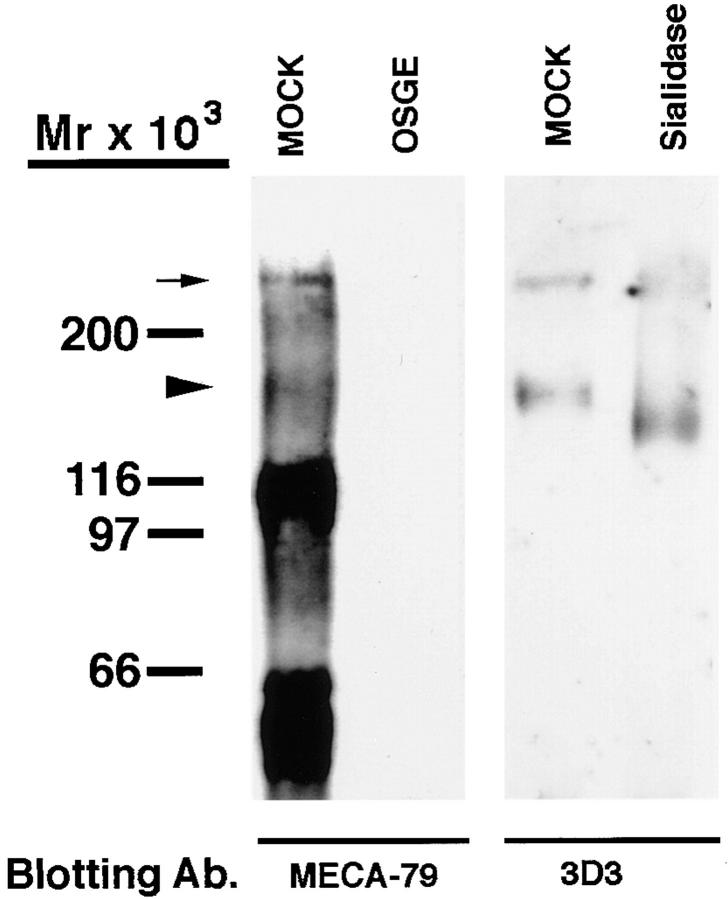
All HEV-derived L-selectin ligands described to date share a common sialomucin-like character (12, 23). Similarly, PCLP derived from the kidney glomerulus or microvascular endothelium is known to be modified extensively with sialylated oligosaccharides, a hallmark of sialomucins (31, 42). We sought to determine if the specific form of PCLP that is produced by HEV exhibits these structural features. Therefore, we subjected PNAd to digestion with OSGE, an endopeptidase that specifically hydrolyzes the peptide backbone of sialomucins (43). When PNAd was treated with OSGE, virtually all of the material detected by MECA-79 was eliminated, including the 160-kD PCLP band (Fig. 6). Interestingly, no low molecular weight cleavage products were detected, suggesting that either the resulting cleaved products were very small or that an intact peptide backbone is essential for the presentation of the MECA-79 epitope.
Figure 6.
OSGE and sialidase digestion of PCLP. PNAd was either treated with OSGE and Western blotted with MECA-79, or treated with sialidase and blotted with the PCLP mAb 3D3. Mock treatments were identical except the enzyme was omitted. Arrowhead, The 160-kD PCLP band. Small arrow, The presumed PCLP multimer.
To demonstrate directly the sialylation of HEV-derived PCLP, we subjected PNAd to sialidase digestion followed by Western blotting with the 3D3 antibody. This treatment resulted in a clear increase in the electrophoretic mobility of PCLP, thus confirming the presence of sialic acid (Fig. 6). In contrast, removal of sialic acid from many glycoproteins (35, 44), including glomerular PCLP (30, 45), results in a decrease in mobility due to a loss of charge. However, the behavior of HEV-derived PCLP was similar to that of the other PNAd components (data not shown), including GlyCAM-1 and CD34 (14). The sensitivity of the HEV-derived form of PCLP to both OSGE and sialidase demonstrates that it is sialomucin-like in structure, as is the case for all MECA-79–reactive ligands in HEV (3).
Western blotting of PNAd with the 3D3 antibody often revealed a high molecular weight band (>200 kD) in addition to the major 160-kD band (Fig. 6). We suspect that this high molecular weight species represented an SDS-stable multimerized form of PCLP, which has been described previously (45). The molecular weight of this band did not correspond to the major 210-kD component of PNAd. However, a minor band with a similar molecular weight to this putative multimer was observed occasionally in PNAd, and likely represented the same species (Fig. 6).
PCLP Mediates L-selectin–dependent Adhesion under Flow.
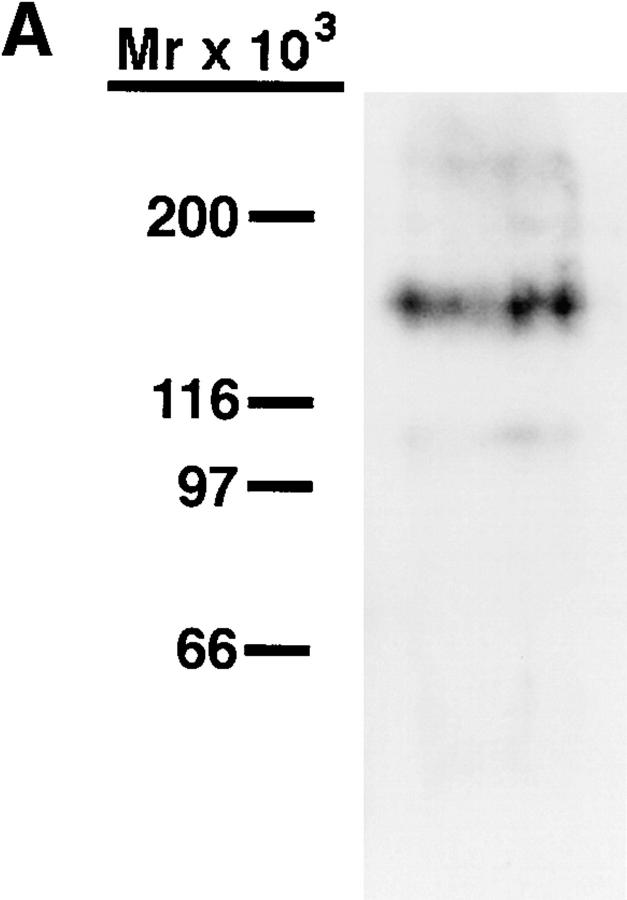
PNAd, as well as CD34 isolated from PNAd, have been shown to support the L-selectin–dependent tethering and rolling of lymphocytes under flow conditions (23). Therefore, we wished to examine whether HEV-derived PCLP isolated from human tonsils shared these properties. MECA-79–reactive PCLP was purified from detergent lysates of tonsil by sequential purification on 3D3-coupled Sepharose and MECA-79–coupled Sepharose. Western blotting of the purified material with MECA-79 showed a major band of 160 kD, as expected (Fig. 7 A). This material was coated onto one surface of a parallel plate laminar flow chamber, and either peripheral blood T lymphocytes or Jurkat T lymphoma cells were infused into the chamber at physiological flow rates. Under these conditions, both cell types tethered to the PCLP-coated surface (Fig. 7 B), but not to a surface coated with BSA. The rolling behavior of Jurkat cells was studied in detail. At 0.8 dynes/cm2, the velocity of Jurkat cells rolling on PCLP was 50.1 ± 14.1 μm/s, which was comparable to the velocity of these cells rolling on unfractionated PNAd (data not shown). This adhesion was dependent on L-selectin (Fig. 7 C), since it was inhibited completely by pretreatment of the Jurkat cells with either an L-selectin mAb or the L-selectin antagonist fucoidin (46). Furthermore, treatment of the coated PCLP with either OSGE or A. ureafaciens sialidase abrogated the interaction with Jurkat cells, consistent with the sialomucin-like structure of PCLP and the absolute requirement of sialic acid for L-selectin binding. Finally, treatment of the coated substrate with MECA-79 abrogated all interactions with Jurkat cells, establishing that lymphocyte L-selectin was binding to the immobilized PCLP and not to a MECA-79–unreactive contaminant. In contrast, treatment of the immobilized PCLP with the 3D3 antibody did not affect lymphocyte adhesion. This result was not surprising, since this antibody recognizes a protein determinant (32). Based on these data, we conclude that immobilized MECA-79– reactive PCLP was able to mediate the L-selectin–dependent tethering and rolling of lymphocytes under physiological flow conditions.
Figure 7.
Tethering of lymphocytes to purified PCLP under flow. (A) MECA-79 Western blotting of the purified PCLP, which was used in the flow chamber experiments, showing a major band at 160 kD. (B) Peripheral blood T lymphocytes or Jurkat T lymphoma cells were infused into the flow chamber at a constant wall shear stress, and the cell tethering rate to the PCLP-coated surface was determined. (C) Immobilized PCLP was treated with OSGE, sialidase, MECA-79, or PCLP mAb (3D3) before infusion of Jurkat cells. Jurkat cells were treated with L-selectin mAb (LAM1-3) or fucoidin before infusion. Error bars represent SD of measurements from at least three separate fields of view.
Discussion
Previously, it was speculated that PCLP on endothelial cells might serve as an antiadhesion molecule due to its strong negative charge, which in the kidney glomerulus appears to be involved in maintaining the filtration slits between podocyte foot processes via charge repulsion (31). This study demonstrates that PCLP is present on the luminal face of HEV, where it can serve as a proadhesive ligand for the leukocyte adhesion molecule, L-selectin. This HEV-derived PCLP was shown to bind to both recombinant L-selectin and the HEV-specific mAb MECA-79. Furthermore, MECA-79–reactive PCLP was capable of supporting the L-selectin–dependent tethering and rolling of lymphocytes under flow, demonstrating that PCLP has the ability to function as a ligand for L-selectin and therefore potentially participate in the recruitment of blood-borne lymphocytes to secondary lymphoid organs.
The ability of PCLP to function as both an antiadhesive molecule on the podocyte and a proadhesive molecule in HEV is likely imparted by tissue-specific posttranslational modifications. Evidence for this differential modification is provided by the distinct electrophoretic behavior of glomerulus- and HEV-derived PCLP after sialidase treatment, and the observation that HEV-derived PCLP binds to the MECA-79 antibody, which does not stain glomerular tissue (47). Both sulfation and fucosylation of HEV ligands are required for L-selectin binding (16, 48). Furthermore, HEV-derived GlyCAM-1 has been demonstrated to display both galactose-6-SO4 and N-acetyl-glucosamine-6-SO4 in the context of 6′- and 6-sulfo-sialyl Lewisx, respectively (49–51). Therefore, we suspect that the elaboration of these or related capping structures by HEC is essential for the function of PCLP as an adhesive ligand for L-selectin.
The expression of PCLP on both platelets and early hematopoietic progenitors (33) raises the possibility that this molecule may function as a selectin ligand at these sites as well as in HEV. Strong evidence has been presented for the existence of a ligand for P-selectin on platelets (52–54) and a ligand for L-selectin on the early hematopoietic progenitor cell line KG1a (55). Therefore, it is tempting to speculate that PCLP could be involved in a multitude of selectin-mediated adhesive interactions between endothelium, hematopoietic cells, and platelets.
Interestingly, PCLP shows striking similarities to CD34 in both general structure and expression pattern. In addition to a membrane-distal mucin-like domain, both proteins contain a membrane-proximal cysteine-rich domain, a transmembrane domain, and a sizable cytoplasmic tail. The mucin domains of PCLP and CD34 are characterized by a high content of serine, threonine, and proline, and in contrast to many other mucin-like proteins (56, 57), do not exhibit any obvious sequence repeats. As with CD34, dense O-glycosylation of the mucin domain is inferred because of the limited number of potential sites for N-glycosylation and the threefold discrepancy between the predicted molecular weight of the peptide and the apparent molecular weight of the glycoprotein by SDS-PAGE. The sensitivity of PCLP and CD34 (23) to OSGE and sialidase confirms that these proteins are sialomucin-like in structure. PCLP and CD34 differ in the length of their mucin domains, ∼290 and 130 amino acids long, respectively, and share no obvious sequence homology here. Despite these differences, the mucin domains of PCLP and CD34 are presumably responsible for direct interaction with L-selectin, since all of the HEV-derived L-selectin ligands that have been biochemically defined to date contain mucin-like domains (3, 23).
COOH-terminal to their mucin-like domains, PCLP and CD34 contain regions with four and six cysteines, respectively, which likely form globular structures (see pp. 215–216 of reference 58). Following these domains and the predicted transmembrane regions are cytoplasmic tails, which are likely to serve essential functions, since the sequence of each has been highly conserved across species. Thus, the cytoplasmic tail of human CD34 shares >90% amino acid identity with its mouse and canine homologs (34, 59, 60), and this region of human PCLP shares 96% identity with its rabbit homolog and 84% identity with thrombomucin, its chicken homolog (31–33). Strikingly, it is also this region that is the most homologous between PCLP and CD34. These domains are nearly the same length, containing 75 and 74 amino acids in human PCLP and CD34, respectively, and share 25% identity overall. Moreover, there are three short segments which share >50% identity (Fig. 8). These observations not only support a possible evolutionary relationship of these two proteins, but also suggest the importance of the conserved regions in some common function. One possibility is a similar role in cell signaling. This possibility is supported by the presence of multiple potential serine/threonine kinase sites in both molecules (Fig. 8; references 32 and 61) and the observation that CD34 is phosphorylated after protein kinase C activation (62). This domain could also function in directing the specific cellular localization of these molecules.
Figure 8.
Alignment of PCLP and CD34 cytoplasmic tails. Regions depicted encompass residues 281–354 of human CD34, 309–382 of mouse CD34, 453–528 of human PCLP, and 477–551 of rabbit PCLP, and begin immediately after the predicted transmembrane domain of each protein. Regions of >50% homology are boxed. Potential protein kinase C (S/T-X-R/K) and casein kinase II (S/T-XX-D/E) phosphorylation sites are circled.
Given the similar structures and expression patterns of PCLP and CD34, it has been suggested that they could serve redundant roles under some circumstances. In particular, it was proposed that the lack of a drastic hematopoietic defect in the CD34 knockout mouse could be due to compensation by PCLP (33). Similarly, it is possible that the lack of a defect in lymphocyte recruitment to secondary lymphoid organs in these mice (63) might be due to compensation by PCLP or possibly other PNAd components. Although this interpretation implies some functional redundancy between these molecules, their coexpression in HEV suggests that they may possess distinct functions as well. Thus far, it has been demonstrated that both CD34 and PCLP are able to mediate the L-selectin–dependent tethering and rolling of lymphocytes under flow, but a direct comparison may be required to uncover distinct roles for these two glycoproteins in this process.
This study contributes to the definition of the endothelial ligands for L-selectin. In human tonsil, there are currently two MECA-79– and L-selectin–reactive glycoproteins, of 210 and 65 kD, which do not react with antibodies to either CD34 or PCLP. It remains to be determined whether the 65-kD component represents the human homolog of GlyCAM-1 or MAdCAM-1 (64). The 210-kD component is likely to represent the homolog of mouse Sgp200. The identification of CD34 and PCLP as HEV ligands for L-selectin, in conjunction with the identification of these additional ligands, will allow the dissection of both the distinct and redundant roles of each in the recruitment of lymphocytes from the blood via HEV.
Acknowledgments
This work was supported by grants to S.D. Rosen (R37 GM23547) and D.B. Kershaw (DK12264-01A1 and IP30HD28820) from the National Institutes of Health, a predoctoral fellowship to C. Sassetti (97-411) from the American Heart Association, and a postdoctoral fellowship to K. Tangemann (Ta 209/1-1) from Deutche Forschungsgemeinschaft.
Abbreviations used in this paper
- GlyCAM-1
glycosylation-dependent cell adhesion molecule-1
- HEC
high endothelial cell(s)
- HEV
high endothelial venule(s)
- HPRT
hypoxanthine phosphoribosyltransferase
- HUVEC
human umbilical vein endothelial cell(s)
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- OSGE
O-sialoglycoprotein endopeptidase
- PBS
Dulbecco's PBS
- PBST
PBS-Tween
- PBS-TX
PBS–Triton X-100
- PCLP
podocalyxin-like protein
- PNAd
peripheral node addressin
Footnotes
For their help in obtaining surgical specimens, we would like to thank the members of the Departments of Otolaryngology at the University of California, San Francisco (Drs. Murr, Lee, Lalwani, Dedo, and Schindler); Kaiser Permanente, San Francisco, CA (Dr. Dimeling); and Kaiser Permanente, Marin, CA (Drs. Mizano, Bauman, and Delfanti). In addition, we would especially like to thank the surgical support and scheduling personnel for their invaluable assistance.
References
- 1.Gowans JL, Knight EJ. The route of recirculation of lymphocytes in the rat. Proc R Soc Lond Ser B. 1964;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 2.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 3.Girard J-P, Springer TA. High endothelial venules (HEV): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 4.Kikuta A, Rosen SD. Localization of ligands for L-selectin in mouse peripheral lymph node high endothelial cells by colloidal gold conjugates. Blood. 1994;84:3766–3775. [PubMed] [Google Scholar]
- 5.Lawrence MB, Berg EL, Butcher EC, Springer TA. Rolling of lymphocytes and neutrophils on peripheral node addressin and subsequent arrest on ICAM-1 in shear flow. Eur J Immunol. 1995;25:1025–1031. doi: 10.1002/eji.1830250425. [DOI] [PubMed] [Google Scholar]
- 6.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 7.Gallatin W, Weissman I, Butcher E. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 8.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DC, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 9.Hamann A, Andrew DP, Joablonski-Westrich D, Holzmann B, Butcher EC. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. . J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 10.Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, Rahewsky K, Muller W. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 11.Lasky LA. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem. 1995;64:113–139. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 12.Rosen SD, Bertozzi CR. The selectins and their ligands. Curr Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 13.Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai Y, Singer MS, Fennie C, Lasky LA, Rosen SD. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991;113:1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmerich S, Butcher EC, Rosen SD. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA-79, an adhesion-blocking monoclonal antibody. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson SR, Imai Y, Fennie C, Geoffrey JS, Rosen SD, Lasky LA. A homing receptor IgG chimera as a probe for adhesive ligands of lymph node high endothelial venules. J Cell Biol. 1990;110:2221–2229. doi: 10.1083/jcb.110.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson SR, Fennie C, Lasky LA. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimera. Nature. 1991;349:164–167. doi: 10.1038/349164a0. [DOI] [PubMed] [Google Scholar]
- 19.Baumhueter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993;262:436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- 20.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 21.Lasky LA, Singer MS, Dowbenko D, Imai Y, Henzel WJ, Grimley C, Fennie C, Gillet N, Watson SR, Rosen SD. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992;69:927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- 22.Young PE, Baumhueter S, Lasky LA. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood. 1995;85:96–105. [PubMed] [Google Scholar]
- 23.Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang ST, Singer MS, Giblin PA, Yednock TA, Bacon KB, Simon SI, Rosen SD. GlyCAM-1, a physiologic ligand for L-selectin, activates β2 integrins on naive peripheral blood lymphocytes. J Exp Med. 1996;184:1343–1348. doi: 10.1084/jem.184.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giblin PA, Hwang ST, Katsumoto TR, Rosen SD. Ligation of L-selectin on T lymphocytes activates β1 integrins and promotes adhesion to fibronectin. J Immunol. 1997;159:3498–3507. [PubMed] [Google Scholar]
- 26.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 27.Guyer DA, Moore KL, Lynam EB, Schammel CM, Rogelj S, McEver RP, Sklar LA. P-selectin glycoprotein ligand-1 (PSGL-1) is a ligand for L-selectin in neutrophil aggregation. Blood. 1996;88:2415–2421. [PubMed] [Google Scholar]
- 28.Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil–neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation on P-selectin in vitro. J Clin Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spertini O, Cordey A, Monai N, Giuffre L, Schapira M. P-selectin glycoprotein ligand 1 is a ligand for L-selectin on neutrophils, monocytes, and CD34+ hematopoietic progenitor cells. J Cell Biol. 1996;135:523–531. doi: 10.1083/jcb.135.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin—the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984;98:1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kershaw DB, Thomas PE, Wharram BL, Goyal M, Wiggins JE, Whiteside CI, Wiggins RC. Molecular cloning, expression and characterization of podocalyxin-like protein from rabbit as a transmembrane protein of glomerular podocytes and vascular endothelium. J Biol Chem. 1995;270:29439–29446. doi: 10.1074/jbc.270.49.29439. [DOI] [PubMed] [Google Scholar]
- 32.Kershaw DB, Beck SG, Wharram BL, Wiggins JE, Goyal M, Thomas PE, Wiggins RC. Molecular cloning and characterization of human podocalyxin-like protein. J Biol Chem. 1997;272:15708–15714. doi: 10.1074/jbc.272.25.15708. [DOI] [PubMed] [Google Scholar]
- 33.McMagny KM, Petterson I, Rossi F, Flamme I, Shevchenko A, Mann M, Graf T. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J Cell Biol. 1997;138:1395–1407. doi: 10.1083/jcb.138.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmons DL, Satterthwaite AB, Tenen DG, Seed B. Molecular cloning of a cDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol. 1992;148:267–271. [PubMed] [Google Scholar]
- 35.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland RD, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 36.Baumhueter S, Dybdal N, Kyle C, Lasky LA. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for L-selectin. Blood. 1994;84:2554–2565. [PubMed] [Google Scholar]
- 37.Kerjaschki D, Poczewski H, Dekan G, Horvat R, Balzar E, Kraft N, Atkins RC. Identification of a major sialoglycoprotein in the glycocalyx of human visceral glomerular epithelial cells. J Clin Invest. 1986;78:1142–1149. doi: 10.1172/JCI112694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harlow, E., and D. Lane. 1988. Antibodies: A Laboratory Manual. 726 pp. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 39.Moller HJ, Heinegare D, Poulson JH. Combined alcian blue and silver staining of subnanogram quantities of proteoglycans and glycosaminoglycans in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1993;209:169–175. doi: 10.1006/abio.1993.1098. [DOI] [PubMed] [Google Scholar]
- 40.Girard JP, Springer TA. Cloning from purified high endothelial cells of hevin, a close relative of the antiadhesive extracellular matrix protein SPARC. Immunity. 1995;2:113–123. doi: 10.1016/1074-7613(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 42.Schnitzer JE, Shen C-PJ, Palade GE. Lectin analysis of common glycoproteins detected on the surface of continuous microvascular endothelium in situand in culture: identification of sialoglycoproteins. Eur J Cell Biol. 1990;52:241–251. [PubMed] [Google Scholar]
- 43.Mellors A, Lo RY. O-sialoglycoprotease from Pasteurella haemolytica. . Methods Enzymol. 1995;248:728–740. doi: 10.1016/0076-6879(95)48049-8. [DOI] [PubMed] [Google Scholar]
- 44.Moore KL, Eaton SF, Lyons DE, Lichenstein HS, Cummings RD, McEver RP. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J Biol Chem. 1994;269:23318–23327. [PubMed] [Google Scholar]
- 45.Dekan G, Gabel C, Farquhar MG. Sulfate contributes to the negative charge of podocalyxin, the major sialoglycoprotein of the glomerular filtration slits. Proc Natl Acad Sci USA. 1991;88:5398–5402. doi: 10.1073/pnas.88.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imai Y, True DD, Singer MS, Rosen SD. Direct demonstration of the lectin activity of gp90MEL. J Cell Biol. 1990;111:1225–1232. doi: 10.1083/jcb.111.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segawa C, Wada T, Takaeda M, Furuichi K, Matsuda I, Hisada Y, Ohta S, Takasawa K, Takeda S, Kobayashi K, Yokoyama H. In situexpression and soluble form of P-selectin in human glomerulonephritis. Kidney Int. 1997;52:1054–1063. doi: 10.1038/ki.1997.428. [DOI] [PubMed] [Google Scholar]
- 48.Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 49.Hemmerich S, Bertozzi CR, Leffler H, Rosen SD. Identification of the sulfated monosaccharides of GlyCAM-1, an endothelial-derived ligand for L-selectin. Biochemistry. 1994;33:4820–4829. doi: 10.1021/bi00182a010. [DOI] [PubMed] [Google Scholar]
- 50.Hemmerich S, Rosen SD. 6′-sulfated sialyl Lewis x is a major capping group of GlyCAM-1. Biochemistry. 1994;33:4830–4835. doi: 10.1021/bi00182a011. [DOI] [PubMed] [Google Scholar]
- 51.Hemmerich S, Leffler H, Rosen SD. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- 52.Frenette PS, Johnson RC, Hynes RO, Wagner DD. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci USA. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boukerche H, Ruchaud-Sparagano M, Rouen C, Brochier J, Kaplan C, McGregor JL. A monoclonal antibody directed against a granule membrane glycoprotein (GMP-140/PADGEM, P-selectin, CD62P) inhibits ristocetin-induced platelet aggregation. Br J Haematol. 1996;92:442–451. doi: 10.1046/j.1365-2141.1996.d01-1485.x. [DOI] [PubMed] [Google Scholar]
- 54.Parmentier S, McGregor L, Catimel B, Leung LL, McGregor JL. Inhibition of platelet functions by a monoclonal antibody (LYP20) directed against a granule membrane glycoprotein (GMP-140/PADGEM) Blood. 1991;77:1734–1739. [PubMed] [Google Scholar]
- 55.Sackstein R, Fu L, Allen KL. A hematopoietic cell L-selectin ligand exhibits sulfate-independent binding activity. Blood. 1997;89:2773–2781. [PubMed] [Google Scholar]
- 56.Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 57.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 58.Barclay, A.N., M.H. Brown, S.K.A. Law, A.J. McKnight, M.G. Tomlinson, and P.A. van der Merwe. 1997. The Leukocyte Antigen Facts Book. Academic Press, London.
- 59.Brown J, Greaves MF, Molgaard HV. The gene encoding the stem cell antigen CD34 is conserved in mouse and expressed in haemopoietic progenitor cell lines, brain, and embryonic fibroblasts. Int Immunol. 1991;3:175–184. doi: 10.1093/intimm/3.2.175. [DOI] [PubMed] [Google Scholar]
- 60.McSweeney PA, Rouleau KA, Storb R, Bolles L, Wallace PM, Beauchamp M, Krizanac-Bengez L, Moore P, Sale G, Sandmaier B, et al. Canine CD34: cloning of the cDNA and evaluation of an antiserum to recombinant protein. Blood. 1996;88:1992–2003. [PubMed] [Google Scholar]
- 61.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 62.Fackler MJ, Civin CI, Sutherland DR, Baker MA, May WS. Activated protein kinase C directly phosphorylates the CD34 antigen on hematopoietic cells. J Biol Chem. 1990;265:1056–1061. [PubMed] [Google Scholar]
- 63.Suzuki A, Andrew DP, Gonzalo J-A, Fukumoto M, Spellberg J, Hashiyama M, Tadimoto H, Gerwin N, Webb I, Molineux G, et al. CD34-deficient mice have reduced eosinophil accumulation after allergen exposure and show a novel crossreactive 90-kD protein. Blood. 1996;87:3550–3562. [PubMed] [Google Scholar]
- 64.Shyjan AM, Bertagnolli M, Kenney CJ, Briskin MJ. Human mucosal addressin cell adhesion molecule-1 (MAdCAM-1) demonstrates structural and functional similarities to the α4β7-integrin binding domains of murine MAdCAM-1, but extreme divergence of mucin-like sequences. J Immunol. 1996;156:2851–2857. [PubMed] [Google Scholar]