Abstract
Recruitment of leukocytes from blood to tissue in inflammation requires the function of specific cell surface adhesion molecules. The objective of this study was to identify adhesion molecules that are involved in polymorphonuclear leukocyte (PMN) locomotion in extravascular tissue in vivo. Extravasation and interstitial tissue migration of PMNs was induced in the rat mesentery by chemotactic stimulation with platelet-activating factor (PAF; 10−7 M). Intravital time-lapse videomicroscopy was used to analyze migration velocity of the activated PMNs, and the modulatory influence on locomotion of locally administered antibodies or peptides recognizing various integrin molecules was examined. Immunofluorescence flow cytometry revealed increased expression of α4, β1, and β2 integrins on extravasated PMNs compared with blood PMNs. Median migration velocity in response to PAF stimulation was 15.5 ± 4.5 μm/min (mean ± SD). Marked reduction (67 ± 7%) in motility was observed after treatment with mAb blocking β1 integrin function (VLA integrins), whereas there was little, although significant, reduction (22 ± 13%) with β2 integrin mAb. Antibodies or integrin-binding peptides recognizing α4β1, α5β1, or αvβ3 were ineffective in modulating migration velocity.
Our data demonstrate that cell surface expression of β1 integrins, although limited on blood PMNs, is induced in extravasated PMNs, and that members of the β1 integrin family other than α4β1 and α5β1 are critically involved in the chemokinetic movement of PMNs in rat extravascular tissue in vivo.
Keywords: inflammation, leukocyte recruitment, adhesion molecules, integrins, polymorphonuclear leukocytes
Tissue recruitment of circulating leukocytes is a central event in the host defense against infectious and noxious agents. The extravasation process comprises a multistep reaction accomplished through a sequential interaction with vascular endothelium and extravascular matrix components. The initial steps in this process, i.e., rolling along the endothelium and firm adhesion, have been extensively studied both in vitro and in vivo, revealing the function of specific cell adhesion molecules of the selectin and integrin families (1). In contrast, the subsequent event of leukocyte migration in the extravascular tissue in response to a chemotactic stimulus is not well characterized, and the receptor interactions involved in this process are largely unknown.
Several cell surface receptors found on leukocytes recognize and bind extracellular matrix (ECM) components. For example, all known members of the β1 integrin (VLA, very late activation antigen) family bind to ECM proteins with varying affinity for specific ECM components, e.g., fibronectin, collagen, and laminin (2–5). Although β1 integrins have a widespread distribution, the expression on leukocytes has repeatedly been shown to be largely restricted to eosinophils, monocytes, and certain lymphocyte subsets, whereas expression on PMNs is limited (2). However, this view has been reconsidered in recent years because of data showing that activated or extravasated neutrophils may indeed express certain β1 integrins that potentially can mediate binding to ECM proteins (6–8).
The β2 integrins (CD11a–c/CD18) are expressed exclusively on leukocytes and mediate firm adhesion to vascular endothelium (9). Specific ligand binding has been shown for fibrinogen and factor X of the coagulation cascade, complement factor C3bi, and intercellular adhesion molecule (ICAM) 1 (1). Moreover, binding of PMNs to a variety of biological substrates (e.g., fibronectin, collagen) and nonbiological surfaces (such as plastic) has been reported to be β2 dependent, inasmuch as adhesion can be abrogated by CD11/CD18-blocking antibodies. Yet another member of the integrin family that has been demonstrated to bind ECM components and that is expressed in PMNs is the αvβ3 integrin, which binds to vitronectin and fibronectin (10).
The aim of this study was to investigate the role of major integrin receptors in PMN interstitial migration in vivo. The potential involvement of the fibronectin-binding integrin receptors in this process was of particular interest because of their previously documented roles in migration of various cell types (11–13). An intravital microscopy model was used for analyzing leukocyte locomotion in response to local chemotactic stimulation in extravascular tissue of the rat mesentery. Immunofluorescence flow cytometry revealed increased expression of integrins in extravasated PMNs. This included β1 integrins, which were shown by antibody-blocking experiments to be critically involved in the extravascular PMN migration. However, this process does not seem to engage the fibronectin-binding receptors α4β1 and α5β1.
Materials and Methods
Surgical Preparation.
Wistar rats of either sex, weighing 200–250 g, were used in the experiments. Anesthesia was induced with equal parts of fluanison/fentanyl (10/0.2 mg/ml; Hypnorm; Janssen-Cilag Ltd., Saunderton, UK) and midazolam (5 mg/ml; Dormicum; Hoffman-La Roche, Basel, Switzerland) diluted 1:1 with sterile water (2 mg/kg intramuscularly). Body temperature was maintained at 37°C by a heating pad connected to a rectal thermistor. Laparotomy was performed, and a segment of the ileum was pulled outside the peritoneal cavity and placed on a heated transparent pedestal to allow microscopic observation of the mesenteric microvasculature. The exposed tissue was superfused with a warmed (37°C) bicarbonate buffer solution equilibrated with 5% CO2 in N2 to maintain physiological pH. The experiments were approved by the regional ethical committee for animal experimentation.
Intravital Microscopy.
The exposed rat mesentery was observed through a microscope (Orthoplan; Leitz, Wetzler, Germany) equipped with a water immersion lens (SW × 25, NA 0.60; Leitz). The microscopic image was televised (WV 1050 E/C; Panasonic, Osaka, Japan) and recorded on time lapse video (AG-6010; Panasonic) connected to a time/date generator (WJ-810; Panasonic). Recordings were made at one-seventh normal speed. Analysis of leukocyte migration in the mesenteric tissue was made off-line from the recorded video scenes during playback at normal speed. The migration path of individual leukocytes was drawn on a transparent film placed in front of the monitor for subsequent analysis with a digital image analyzer.
Experimental Procedure.
After positioning under the microscope, the exposed mesentery was soaked with 5 ml of buffer solution (37°C) containing platelet-activating factor (PAF; Sigma Chemical Co., St. Louis, MO) at a concentration of 10−7 M. The tissue was then covered with a transparent plastic film to provide continuous chemotactic stimulation by PAF. The top of the plastic film was continuously bathed with buffer to maintain temperature at 37°C. After 40 min of chemotactic stimulation, when numerous leukocytes had extravasated, time-lapse recording of leukocyte migration was undertaken, first for 20 min to assess basal migration rates in response to PAF stimulation and then for an additional 40 min in the presence of PAF and antibodies or peptides administered to the tissue at a concentration of 100 μg/ ml and 500 mM, respectively. A total of five different fields were analyzed from each animal. In each field, a minimum of five cells were selected at random and followed as long as they remained within the field of observation (at least 2 min). Cells that did not move during the observation time were not included in the analysis.
Antibody Diffusion in the Mesentery.
In separate experiments, the diffusion capability of the Ig molecules in the mesentery was confirmed. Pieces of intact mesenteric tissue (thickness: 20–40 μm) were mounted on plastic rings and placed on top of HBSS-filled wells (400 μl/well) of a 96-well tissue culture plate. 40 μl of FITC-conjugated murine IgG (100 μg/ml) were placed on the surface of the mesentery. The plate was incubated at 37°C for 10 min, and the fluorescence intensity of the IgG content in the upper and lower fluid compartments was measured in a fluorometer (Fluoroscan II; Labsystems Oy, Helsinki, Finland). Over a period of 10 min, an average of 7.5 ± 1.6% of applied antibody diffused through the mesentery per minute and cm2 (n = 3), indicating that there is no significant restriction for diffusion of the antibodies into the mesenteric tissue when being topically administered.
Staining of Leukocytes.
Representative samples of the mesentery stimulated with PAF 10−7 M for 1.5 h were stained with Wright/Giemsa (Sigma Chemical Co.) for 10 min in stock concentration and in dilution 1:1 with water for another 10 min at room temperature. Leukocytes in peripheral blood and in peritoneal fluid collected after 1.5 h PAF stimulation were similarly stained. After a thorough rinse of the specimens in HBSS, a differential leukocyte count was made with an ×100 oil-immersion objective. In addition to the Wright/Giemsa stain, leukocytes in the mesenteric tissue were stained by brief exposure to acridine orange (2.5 mg/ml; Sigma Chemical Co.) and viewed under fluorescent light to improve discrimination of nuclear morphology. Percentage of leukocyte count based on analysis of at least 50 leukocytes in each of 5–7 microscopic fields in each preparation is given in Table 1 (mean values of data from 3 animals). In another set of experiments, PAF-stimulated mesenteric tissue was incubated with antibodies against the β1, α4, or β2 integrin subunit for 30 min at 37°C. After washing, the tissue was incubated with FITC- or TRITC-conjugated F(ab)2 fragments of anti–mouse or –hamster IgG (diluted 1:50) at room temperature for 30 min. The tissue was again washed and fixed for 15 min in 4% paraformaldehyde. The tissue specimens were observed in normal transmitted light and with fluorescent epiillumination (filter block I2 and M2; Leitz Ploemopak). Specificity of antibody binding was confirmed by comparing the immunofluorescence obtained with specific primary mAb with that of isotype-matched irrelevant mAb at the same concentration and incubation time.
Table 1.
Differential Leukocyte Count (Wright/Giemsa Stain)
| Percentage of total leukocytes counted | ||||||
|---|---|---|---|---|---|---|
| Blood | Peritoneal fluid | Mesentery tissue section | ||||
| Neutrophils | 20.4 ± 4.8 | 69.0 ± 12.5 | 85.7 ± 11.8 | |||
| Eosinophils | 1.4 ± 0.6 | 2.8 ± 1.2 | 5.9 ± 1.8 | |||
| Lymphocytes | 77.7 ± 9.8 | 1.2 ± 0.8 | – | |||
| Monocytes/ | 0.5 ± 0.4 | 27.0 ± 7.3 | 8.4 ± 5.8 | |||
| Macrophages | ||||||
Mean ± SD of leukocyte fractions after 1.5 h intraperitoneal stimulation with PAF 10−7 M (n = 3).
Immunofluorescence Flow Cytometric Analysis.
Leukocytes collected from rats of the same strain and weight as used in the in vivo experiments were used for analysis of integrin receptor expression. Leukocyte extravasation was induced by intraperitoneal injection of either 3% proteose peptone (Sigma Chemical Co.) or PAF 10−7 M in 10 ml HBSS. After 2 h, the animals were killed with methyl-ether and peritoneal leukocytes were harvested by washing the peritoneal cavity with 10 ml ice-cold HBSS. EDTA-anticoagulated blood was collected from the same animal, and leukocyte-rich plasma was obtained through dextran sedimentation. Blood and peritoneal leukocytes were washed twice at 150 g for 7 min at 4°C and resuspended in HBSS at a final concentration of 106 cells/ml. The leukocyte suspension was incubated with primary antibodies (10 μg/ml) or isotype-matched control antibodies for 20 min at 4°C and washed twice. FITC-conjugated F(ab)2 fragments of rabbit anti–mouse IgG (Dako, Glostrup, Denmark), donkey anti–rabbit IgG (Jackson Immunoresearch, West Grove, PA), and goat anti– hamster IgG (Jackson Immunoresearch) diluted 1:20 were used as second antibodies. After staining (20 min, 4°C), the cells were fixed (1% formaldehyde, FACS® Lysing Solution [Becton Dickinson, Mountain View, CA]), washed twice, and analyzed on a FACSort® flow cytometer (Becton Dickinson). The fluorescence intensity of 104 PMNs (>95% neutrophils; see Table 1) was analyzed by selective gating based on forward and side scatter parameters.
Antibodies and Peptides.
The following antibodies were used: mAb HMβ1-1 (PharMingen, San Diego, CA) and purified IgG from a rabbit antiserum (gift of K. Rubin, Uppsala, Sweden) against the rat β1 chain (CD29); mAbs CL26 (Upjohn, Kalamazoo, MI) and WT.3 (PharMingen), recognizing the rat β2 chain (CD18); mAbs MRα4 (PharMingen) and TA-2 (Serotec, Oxford, UK), which react with the rat integrin α4 subunit (CD49d); mAb HMα5-1 against the rat α5 (CD49e) (PharMingen); and a rabbit antiserum against human αvβ3 (CD51/CD61) (14). Function-blocking activity in rat systems has been documented for all antibodies used in this study. All antibody solutions were free of preservatives except MRα4 and HMα5-1, which contained 0.1% sodium azide. Control experiments showed that sodium azide in corresponding concentration did not influence cell locomotion. The integrin-binding peptides SLIDIP and ACRGDGWMCG (RGDGW), capable of functionally blocking α4β1 and α5β1 (15), were also used.
Statistical Analysis.
Statistical analysis was performed using the Wilcoxon signed rank test for paired observations. The results are presented as mean ± SD for the animals included in each experimental group (n ≥ 5 unless otherwise stated).
Results
β1-Integrin Cell Surface Expression Is Associated with PMN Extravasation.
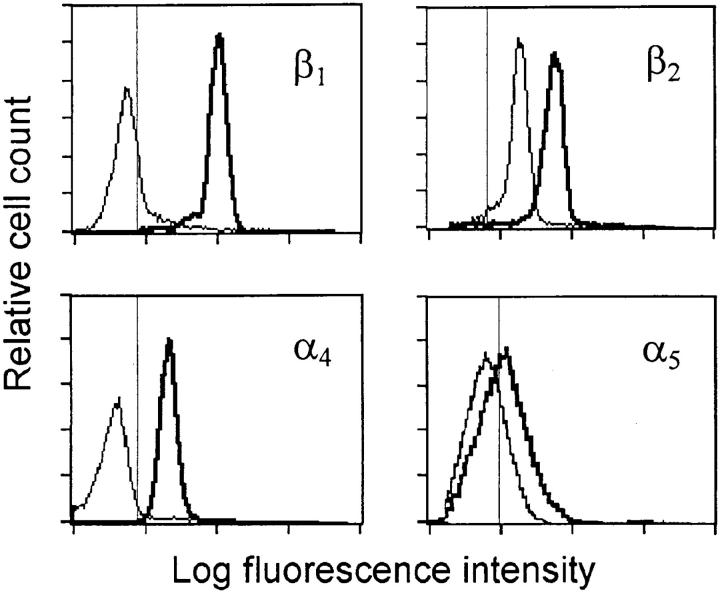
Flow cytometric assessment of cell surface molecule expression on neutrophils that had extravasated into the peritoneal cavity revealed positive staining for β1 (CD29) and α4 (CD49d) integrin molecules (Fig. 1 and Table 2). This pattern contrasted to that of blood PMNs where little or no staining for β1 and α4 was seen (Fig. 1), indicating that cell surface expression of β1 integrins is induced in conjunction with the extravasation process. Expression of α5 (CD49e) was limited in both cell populations. There was an increased expression of β2 integrins (CD18) on extravasated PMNs compared to their blood counterpart, whereas staining for αvβ3 (CD51/CD61) was similarly positive in both PMN populations (Table 2).
Figure 1.
Immunofluorescent staining of integrins on blood PMNs (thin line) and on extravasated PMNs collected from the peritoneal cavity (thick line). Thin vertical line indicates the 99th percentile of fluorescence events for cells stained with isotype matched control antibody. Histograms are representative tracings of three to five analyses for each antibody.
Table 2.
Integrin Expression on Blood and Extravasated rat PMNs
| Integrin | Median fluorescence intensity | |||
|---|---|---|---|---|
| Blood PMNs | Extravasated PMNs | |||
| β1 | 1.9 ± 0.6 | 47.5 ± 6.8 | ||
| β2 | 15.3 ± 3.2 | 52.5 ± 9.5 | ||
| α4 | 1.7 ± 0.3 | 16.5 ± 3.7 | ||
| α5 | 2.2 ± 0.9 | 4.4 ± 0.6 | ||
| αvβ3 | 10.0 ± 4.3 | 12.3 ± 3.7 | ||
Median fluorescence intensity is the staining intensity with specific antibody divided by that obtained with control antibody.
Preincubation of isolated blood PMNs with PAF (10−7 M) before antibody labeling did not result in β1 or α4 integrin expression dissimilar from that of untreated blood cells (data not shown).
PMN Migration In Vivo Is Dependent on β1 Integrins.
Topical stimulation of the rat mesentery with PAF (10−7 M) induced profound adhesion and extravasation of circulating leukocytes. At 30–40 min of chemotactic stimulation, numerous leukocytes (predominantly neutrophils, see Table 1) were migrating further in the extravascular tissue (Fig. 2 A). In accordance with the flow cytometric data, immunofluorescent staining of emigrated PMNs in the mesenteric tissue in situ showed surface expression of β1, α4, and β2 integrin molecules, as illustrated for α4 in Fig. 2 B.
Figure 2.
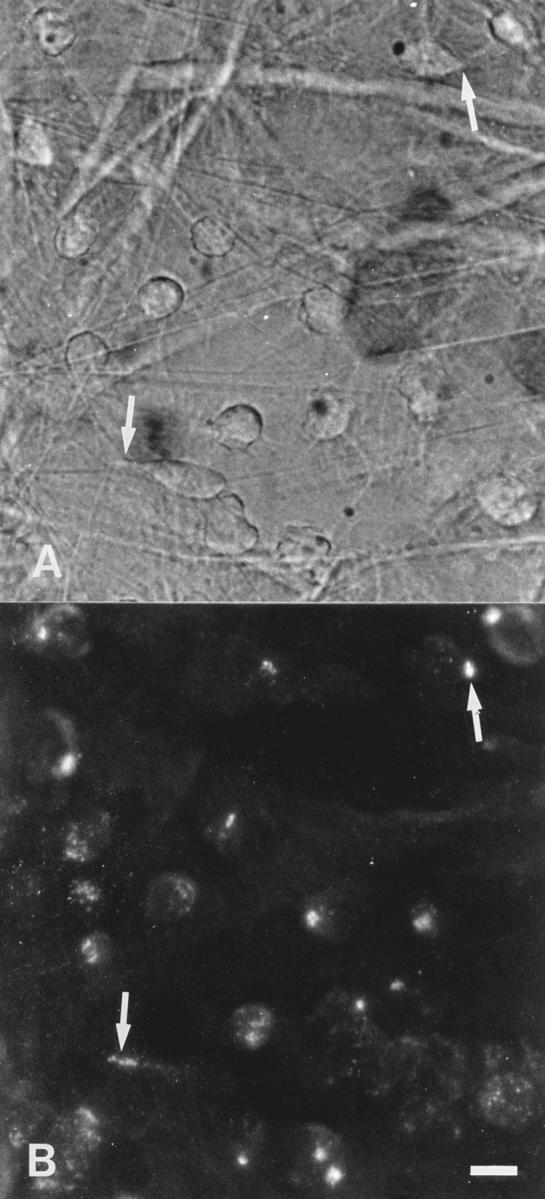
Micrographs showing migrating PMNs in a tissue section of the rat mesentery after stimulation with PAF (10−7 M) for 40 min (A), and immunofluorescent staining for α4 integrins in the same cells (B). The fluorescence is concentrated and localized to spots in most PMNs, indicating a polarized integrin expression (arrows), whereas in some cells a more scattered distribution is seen. Bar: 10 μm.
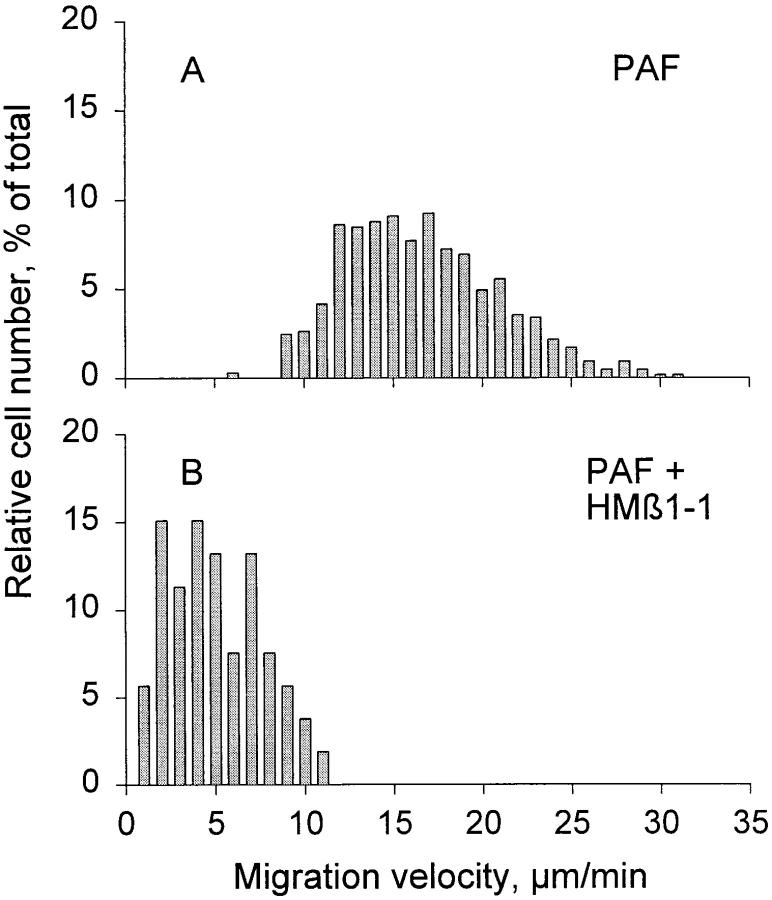
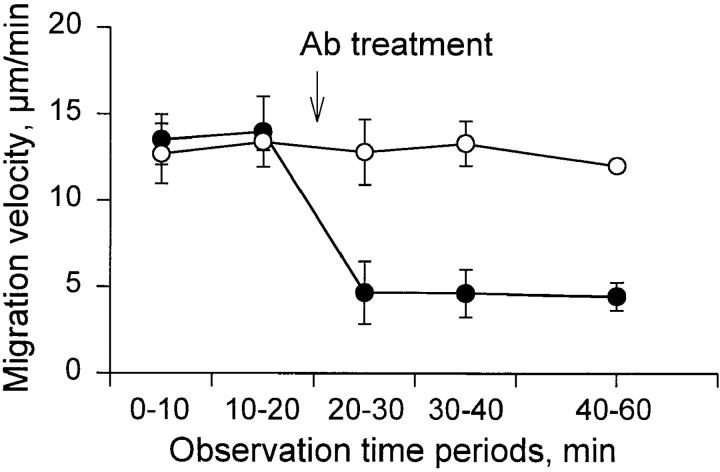
Fig. 3 A illustrates the frequency distribution of the migration velocity of individual PMNs in response to stimulation with PAF (649 cells in 30 animals total). Among these cells, the median migration velocity was 15.5 ± 4.5 μm/min (mean ± SD). The migration velocity was stable over a period of >1.5 h after induction of the chemotactic stimulus. The role of various integrins in PMN migration was evaluated by topical administration of antibodies to the tissue. Treatment with anti-β1 (mAb HMβ1-1) resulted in a pronounced inhibition of PMN locomotion. Migration velocity was reduced by 67 ± 7% (P <0.01; Fig. 4), yielding a median migration velocity of 4.6 ± 1.3 μm/min (Fig. 3 B). Notably, as evident from Fig. 3 B, the whole population of migrating cells, rather than a certain fraction, was affected by this antibody treatment. A less pronounced effect was observed with the polyclonal anti-β1 antibody, which reduced the migration velocity by 32 ± 15% (P <0.01; Fig. 4). No further inhibition was achieved when the antibody concentration was increased 10-fold. Treatment with two different antibodies against the β2 chain (CD18) also significantly reduced migration velocity, by 17 ± 14% (mAb CL26) and 22 ± 13% (mAb WT.3) (P <0.05; Fig. 4). An additive inhibitory effect was observed when anti-β2 mAb was administered together with the polyclonal anti-β1 serum. This combined treatment reduced migration velocity by 52 ± 18% (P <0.01; Fig. 4). On the other hand, coadministration of anti-β2 mAb with the anti-β1 mAb HMβ1-1 yielded no further inhibition of migration velocity above that seen with HMβ1-1 alone. The inhibitory effect of the various antibody treatments was observed within minutes after application and persisted throughout the observation period (>40 min) as shown for mAb HMβ1-1 (Fig. 5). Purified hamster, mouse, and rabbit IgG isotype standards did not influence migration velocity (103 ± 11, 95 ± 7, and 99 ± 8%, respectively).
Figure 3.
Frequency distribution of PMN migration velocity in extravascular tissue of the rat mesentery in response to chemotactic stimulation with PAF (10−7 M). (A) Migration velocities during control period (PAF alone). (B) Migration velocities after topical treatment with the anti-β1 mAb HMβ1-1.
Figure 4.
Effect of local treatment with various integrin antibodies and integrin-binding peptides on PMN migration velocity in the rat mesentery stimulated with PAF (10−7 M). Data are expressed as percent of migration velocity before treatment, and represent mean ± SD of five experiments for each reagent tested. Asterisk, denotes significant difference from control (P <0.05).
Figure 5.
Time course of inhibitory effect of the anti-β1 mAb HMβ1-1 on PMN migration velocity in the rat mesentery (•) compared with control antibody (○). Data are based on calculation of mean migration velocity during defined 10 or 20 min intervals, and presented as means ± SD of five separate experiments for each antibody. Note that the inhibitory effect on PMN locomotion persisted throughout the observation period.
Despite pronounced upregulation of the integrin α4 subunit (CD49d) on extravasated PMNs, no significant modulatory effect on the migration velocity was observed after treatment with either of the two anti-α4 mAbs. Antibodies against α5β1 or αvβ3 also did not influence PMN locomotion. Moreover, combined treatment with anti-β2 mAb together with either anti-α4 or anti-α4 plus anti-α5 mAb resulted in no further inhibition of migration velocity above that obtained with anti-β2 treatment alone (99 ± 4 and 95 ± 10%, respectively, n = 3). The integrin-binding peptides SLIDIP and RGDGW, which mimic natural ligand binding and block the function of α4β1 and α5β1, respectively, also had no effect on the migration velocity either in combination (data not shown) or alone (Fig. 4). There was no difference in the effect for any of the reagents tested when concentration was raised 10 times (data not shown).
Discussion
Extravasation and tissue accumulation of leukocytes is one of the key components in the host defense against invading pathogens. After their escape from the blood, the leukocytes need to migrate in the extravascular tissue, directed by a chemotactic stimulus, to reach the site of injury or infection. Studies of leukocyte migration in vitro have indicated the interaction of leukocytic cell surface receptors with different extracellular matrix components in this process (16). However, interactions with the multitudinous meshwork of biopolymers that characterizes native extracellular matrix and the function in vivo of leukocyte integrins in the locomotive process have yet to be defined. In this report, we demonstrate that β1 integrins, induced in PMNs in conjunction with their extravasation, are of critical functional importance for PMN locomotion in extravascular tissue. Our data on a physiological induction of β1 integrin expression in PMNs agree with recent in vitro and in vivo findings by Kubes and coworkers (7, 8), and contribute to a growing body of evidence that β1 integrin expression may reach significant levels also in neutrophils (6, 17, 18). Also, the upregulation of β2 integrins on extravasated PMNs is consistent with the activation-induced upregulation of β2 integrins on the leukocyte surface (19) and the critical role of this receptor complex in leukocyte adhesion to endothelium and diapedesis through the vessel wall in vivo (20).
A qualitatively similar pattern of PMN β1 integrin expression as obtained with flow cytometric analysis of extravasated PMNs isolated from the peritoneal cavity could be demonstrated by in situ immunostaining of PMNs migrating in the extravascular tissue of the mesentery. These findings are also of significance from a methodological point of view, since they illustrate that antibodies applied topically to the mesentery indeed do diffuse into the tissue, and by this route of administration will reach the migrating PMNs (see Materials and Methods). Thus, we may conclude that adequate antibody concentrations were achieved in the tissue at the level of the migrating cells (no additional effect was seen when antibody concentration was raised 10 times), and that restrictions in antibody transport could not account for the lack of effect of some of the reagents used.
Our quantitative measurements of PMN migration in the rat mesentery in vivo show that β1 and β2 integrins participate in extravascular PMN locomotion, and that they may cooperate in this process. Blockage of β1 and β2 integrin function impaired the ability of the leukocytes to migrate in the extravascular tissue as indicated by significant reductions in their migration velocity. Anti-β1 antibodies were clearly more effective in inhibiting PMN migration than were anti-β2, suggesting a predominant role of β1 integrins in the locomotive process in vivo. The additive inhibitory effect observed when anti-β2 mAb was coadministered with the polyclonal anti-β1 antiserum but not when combined with the anti-β1 mAb may suggest that a synergistic action of combined anti-β1 and anti-β2 treatment is detectable only when β1 integrins are insufficiently blocked (as was likely the case when the polyclonal anti-β1 antibody was used). In contrast to our findings with β2 integrin blockade, Bienvenu et al. (21), using a similar rat model, found no inhibition of the extravascular migration with anti-β2 treatment. Differences in the experimental protocol (e.g., the antibody concentration used) may explain the discrepant observations. Also, although statistically significant, the inhibition we found with anti-β2 was limited and may have been overlooked by these authors.
Although it has been shown for certain leukocyte subtypes that migration on various ECM matrices in vitro requires the function of specific integrin molecules (16, 22), this report is the first to demonstrate an in vivo role for β1 integrins in the extravascular locomotion of leukocytes. Even if it can not be deduced which β1 integrins are predominantly involved in the PMN locomotion, our data suggest, based on use of both monoclonal function-blocking antibodies and integrin-binding peptides, that the fibronectin binding receptors α4β1 and α5β1 do not participate in this process. This finding may seem surprising in light of the pronounced upregulation of α4 integrins on extravasated PMNs, and the central position being attributed to fibronectin in various aspects of cell migration (11, 12). Previous findings have suggested a role for α4β1 and α5β1 in migration of PMN from blood to tissue sites in vivo (17) or through fibroblast monolayers in vitro (18), seemingly in disparity with our direct observations in the rat mesentery. Possibly, mAb inhibition of integrin function in these studies interfered mainly with initial adhesion to endothelium or the fibroblast monolayer and less with the locomotive function. Interestingly, through direct observation of leukocyte migration in three-dimensional gels, an enhanced lymphocyte migration after anti-α4 mAb treatment (16), and reduced PMN locomotion after the gel being supplemented with fibronectin was demonstrated (23). These findings may suggest that fibronectin-binding integrins (e.g., α4β1 and α5β1) may support anchoring of the leukocytes to the substrate rather than promote their migratory movement.
Taken together, expression of β1 integrins, limited on blood PMNs, is induced in this cell population in conjunction with their emigration from blood to tissue. Our data demonstrate that molecules of this integrin family are critically involved in PMN locomotion in extravascular tissue in vivo. Hence, in addition to selectin and β2 integrin functions determining intravascular adhesive events, cell surface induction and engagement of β1 integrins is suggested to be yet another important physiological mechanism in the multistep process of PMN recruitment to sites of injury or infection.
Acknowledgments
This study was supported by the Swedish Medical Research Council (grants 14X-4342 and 04P-10738); the Swedish Foundation for Health Care Sciences and Allergy Research (grant A98110); the IngaBritt and Arne Lundbergs Foundation; and the Karolinska Institutet. E. Ruoslahti was a Nobel Fellow at the Karolinska Institutet when this work was initiated.
References
- 1.Carlos TM, Harlan JM. Leukocyte–endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 2.Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 3.Ignatius MJ, Large TH, Houde M, Tawil JW, Barton A, Esch F, Carbonetto S, Reichardt LF. Molecular cloning of the rat integrin alpha 1–subunit: a receptor for laminin and collagen. J Cell Biol. 1990;111:709–720. doi: 10.1083/jcb.111.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987;105:1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gehlsen KR, Dillner L, Engvall E, Ruoslahti E. The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science. 1988;241:1228–1229. doi: 10.1126/science.2970671. [DOI] [PubMed] [Google Scholar]
- 6.Bohnsack JF, Zhou XN. Divalent cation substitution reveals CD18– and very late antigen–dependent pathways that mediate human neutrophil adherence to fibronectin. J Immunol. 1992;149:1340–1347. [PubMed] [Google Scholar]
- 7.Kubes P, Niu XF, Smith CW, Kehrli ME, Jr, Reinhardt PH, Woodman RC. A novel beta 1–dependent adhesion pathway on neutrophils: a mechanism invoked by dihydrocytochalasin B or endothelial transmigration. FASEB J. 1995;9:1103–1111. [PubMed] [Google Scholar]
- 8.Reinhardt PH, Ward CA, Giles WR, Kubes P. Emigrated rat neutrophils adhere to cardiac myocytes via α4integrin. Circ Res. 1997;81:196–201. doi: 10.1161/01.res.81.2.196. [DOI] [PubMed] [Google Scholar]
- 9.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 10.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 11.Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer JS, Schreiner CL, Giancotti FG, Ruoslahti E, Juliano RL. Motility of fibronectin receptor–deficient cells on fibronectin and vitronectin: collaborative interactions among integrins. J Cell Biol. 1992;116:477–487. doi: 10.1083/jcb.116.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aota, S., and K.M. Yamada. 1995. Fibronectin and cell adhesion: specificity of integrin-ligand interaction. In Advances in Enzymology and Related Areas of Molecular Biology. A. Meister, editor. Vol. 70. pp. 1–21. [DOI] [PubMed]
- 14.Suzuki S, Argraves WS, Pytela R, Arai H, Krusius T, Pierschbacher MD, Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci USA. 1986;83:8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koivunen E, Gay DA, Ruoslahti E. Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J Biol Chem. 1993;268:20205–20210. [PubMed] [Google Scholar]
- 16.Friedl P, Noble PB, Zänker KS. Lymphocyte locomotion in three-dimensional collagen gels. Comparison of three quantitative methods for analysing cell trajectories. J Immunol Methods. 1993;165:157–165. doi: 10.1016/0022-1759(93)90341-4. [DOI] [PubMed] [Google Scholar]
- 17.Issekutz TB, Miyasaka M, Issekutz AC. Rat blood neutrophils express very late antigen 4 and it mediates migration to arthritic joint and dermal inflammation. J Exp Med. 1996;183:2175–2184. doi: 10.1084/jem.183.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao JX, Issekutz AC. The beta 1 integrin, very late activation antigen–4 on human neutrophils can contribute to neutrophil migration through connective tissue fibroblast barriers. Immunology. 1997;90:448–454. doi: 10.1111/j.1365-2567.1997.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neeley SP, Hamann KJ, White SR, Baranowski SL, Burch RA, Leff AR. Selective regulation of expression of surface adhesion molecules Mac-1, L-selectin, and VLA-4 on human eosinophils and neutrophils. Am J Respir Cell Mol Biol. 1993;8:633–639. doi: 10.1165/ajrcmb/8.6.633. [DOI] [PubMed] [Google Scholar]
- 20.Arfors KE, Lundberg C, Lindbom L, Lundberg K, Beatty PG, Harlan JM. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987;69:338–340. [PubMed] [Google Scholar]
- 21.Bienvenu K, Harris N, Granger DN. Modulation of leukocyte migration in mesenteric interstitium. Am J Physiol. 1994;267:H1573–H1577. doi: 10.1152/ajpheart.1994.267.4.H1573. [DOI] [PubMed] [Google Scholar]
- 22.Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 23.Kuntz RM, Saltzman WM. Neutrophil motility in extracellular matrix gels: mesh size and adhesion affect speed of migration. Biophys J. 1997;72:1472–1480. doi: 10.1016/S0006-3495(97)78793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]