Abstract
This report investigates the response of CD8+ T cells to antigens presented by B cells. When C57BL/6 mice were injected with syngeneic B cells coated with the Kb-restricted ovalbumin (OVA) determinant OVA257–264, OVA-specific cytotoxic T lymphocyte (CTL) tolerance was observed. To investigate the mechanism of tolerance induction, in vitro–activated CD8+ T cells from the Kb-restricted, OVA-specific T cell receptor transgenic line OT-I (OT-I cells) were cultured for 15 h with antigen-bearing B cells, and their survival was determined. Antigen recognition led to the killing of the B cells and, surprisingly, to the death of a large proportion of the OT-I CTLs. T cell death involved Fas (CD95), since OT-I cells deficient in CD95 molecules showed preferential survival after recognition of antigen on B cells. To investigate the tolerance mechanism in vivo, naive OT-I T cells were adoptively transferred into normal mice, and these mice were coinjected with antigen-bearing B cells. In this case, OT-I cells proliferated transiently and were then lost from the secondary lymphoid compartment. These data provide the first demonstration that B cells can directly tolerize CD8+ T cells, and suggest that this occurs via CD95-mediated, activation-induced deletion.
Keywords: CD8+ T lymphocytes, cytotoxic T lymphocytes, antigen presentation, B cells, ovalbumin
Bcells express relatively high levels of class I MHC molecules and therefore potentially play a role as APCs for CD8+ T cells. In vitro evidence suggests that B cells can stimulate IL-2 production and CTL activity by either primed CD8+ T cell clones or hybridomas (1–3). However, studies in B cell–deficient mice suggest that B cells are not required as APCs during the inductive phase of naive CD8+ T cell responses (4). In fact, it has been reported that B cells fail to induce naive CD8+ T cells to generate primary CTL activity in vitro (5) and can induce secondary in vitro unresponsiveness in CD8+ T cell clones (6). In adult mice, B cells have been shown to induce in vivo CTL tolerance to the minor antigen H-Y (7), but whether this was due to direct tolerance of the CD8+ T cell compartment was not addressed. CTL responses to H-Y are known to be CD4+ T cell dependent (8, 9), and there is a great deal of evidence that B cell presentation of antigen to mature CD4+ T cells is tolerogenic (10–16). Thus, it was unclear whether H-Y–specific CTL tolerance was due to the direct tolerance of CD8+ T cells, or occurred because H-Y–specific CD4+ helper T cells were tolerized. In this latter case, the CTLs themselves might have been completely unaffected by antigen-bearing B cells, but failed to be primed in the absence of CD4+ T cell help. In this report, we demonstrate that CD8+ T cells can be directly tolerized after encounter with antigen on B cells. The basis of this tolerance is examined.
Materials and Methods
Mice.
Mice were bred and maintained at the Walter and Eliza Hall Institute for Medical Research. OT-I mice have been described previously (17), and were maintained on the recombination-activating gene (RAG)1-1–deficient C57BL/6 (B6) background. Some experiments used OT-I mice expressing the lpr mutation (OT-I.lpr) or back-crossed to bm1; both of these strains were RAG-1 sufficient. For all experiments, mice between 8 and 16 wk of age were used.
B Cell Purification.
B cells were purified as described previously (16). In brief, spleen cells were depleted of red blood cells, and passed over a 30–35-ml Sephadex G-10 column (Amersham Pharmacia Biotech, Uppsala, Sweden) to remove adherent cells. T cells were then removed by treatment with a mixture of anti-Thy1.2 (J1j), anti-CD8 (3.168), and anti-CD4 (RL172) antibody supernatants at 4°C for 30 min, followed by two successive treatments with rabbit C (C-Six Diagnostics Inc., Mequon, WI) at 37°C for 20 min. After passage over a second Sephadex G-10 column, the cells were analyzed by flow cytometry using anti-B220– FITC and found to be >95% B220+. To separate small, resting B cells, the B cell suspension was centrifuged at 3,000 rpm for 30 min over a discontinuous Percoll (Amersham Pharmacia Biotech) density gradient containing ρ = 1.05–1.09 layers as described previously (16). Cells from the ρ = 1.07–1.08 interface were used as resting B cells. B cells were coated at 5 × 107/ml with 0, 0.1, 1.0, or 10 μg/ml OVA257–264 peptide in Hepes Eagle's Medium (HEM) containing 2.5% FCS for 60 min at 37°C and washed three times before counting. Mice were immunized intravenously with 10–15 × 106 B cells in HEM.
Dendritic Cell Generation.
Bone marrow–derived dendritic cells were prepared as described previously (18) with the following modifications. In brief, bone marrow cells from B6 mice were cultured in 94-mm tissue culture dishes (Greiner Labortechnik, Frickenhausen, Germany) at a density of 5 × 105/ml in complete DMEM with 10% FCS as well as 5% supernatant of X63-Ag8 myeloma cells transfected with murine GM-CSF (provided by Dr. David Gray, Hammersmith Hospital, London, UK). On day 3, nonadherent granulocytes were removed and the media replaced. Loosely adherent cells were harvested on day 6 and recultured overnight in fresh GM-CSF–containing media. Nonadherent dendritic cells were harvested the next day and used for immunization.
CTL Challenge.
1 wk after immunization with B cells, B6 mice were challenged with an intravenous injection of 20–25 × 106 irradiated spleen cells loaded intracytoplasmically with OVA by osmotic shock, or with a subcutaneous injection of 10–20 μg OVA257–264 in CFA as described previously (19).
In Vitro Generation of OVA-specific CTLs.
CTLs were generated as described previously (19). LU/spleen were calculated as the total number of CTL effectors present after the 6 d of in vitro stimulation, divided by the number of effectors required to give 20% OVA-specific lysis (1 LU).
In Vitro Cultures of OVA-presenting B Cells with Activated OT-I Cells.
Activated OVA-specific CD8+ T cells were generated using LN cells from OT-I mice on (a) a B6 RAG-1–deficient background, (b) an lpr background, or (c) from OT-I.bm1 bone marrow into B6 chimeras. Cells were cultured at 2.5 × 105 cells/ ml for 6 d in 30 ml RPMI containing 10% FCS, 50 μM 2-ME, 2 mM l-glutamine, and 2.5 × 106 cells/ml irradiated syngeneic spleen cells coated with OVA257–264 at 0.1 μg/ml. On day 3, cultures were diluted twofold with RPMI containing 10% FCS, 50 μM 2-ME, 2 mM l-glutamine, and 20 U/ml rIL-2. To remove any contaminating B cells or CD4+ T cells, 6 d–activated OT-I cells were exposed to a mixture containing anti-HSA (J11d) and anti-CD4 (RL172) mAb supernatants for 30 min at 4°C and then treated with rabbit C for 20 min at 37°C. Cells were washed three times in HEM with 2.5% FCS and filtered through nylon mesh, and in some experiments, activated OT-I cells were purified further by passage over nylon wool. Finally, 5 × 105 activated OT-I cells were cultured together with a total of 5 × 105 cells consisting of a 1:1 mixture of B6 to bm1 B cells in 200 μl RPMI containing 10% FCS, 50 μM 2-ME, and 2 mM l-glutamine. Before in vitro culture, the mixture of B6 plus bm1 B cells was coated with OVA257–264 at 0.1 μg/ml or left uncoated. Up to six replicate cultures were set up for each T cell–B cell combination. 15 h later, the cells from each well were recovered and analyzed by flow cytometry using anti-B220–FITC (6B2) and anti-Kb (5-F-1)–biotin followed by Streptavidin-PE (Caltag Laboratories, Inc., San Francisco, CA).
Adoptive Transfer Experiments.
OT-I T cells were prepared as described previously (20). 4–7 × 106 OT-I cells were injected intravenously into (B6 × B6.Kathy)F1 host mice 1 d before immunization with 10–15 × 106 B cells. The LNs and spleen of each mouse were analyzed by flow cytometry on day 3, 7, or 14 after the initial B cell immunization using anti-Thy1.1–FITC (OX-7; PharMingen, San Diego, CA), anti-CD8–PE (CT-CD8a; Caltag Laboratories, Inc.), and anti-Vα2 (B20.1)–biotin followed by Streptavidin–Tri-color (Caltag Laboratories, Inc.). The number of OT-I cells was determined for each organ by multiplying the cell number by the percentage of Thy1.1−CD8+Vα2+ cells. For individual litters, the background number of Thy1.1−CD8+Vα2+ cells of a control littermate that did not receive an injection of OT-I cells was subtracted from the number of CD8 + Thy1.1−Vα2+ cells found in the same organ of each test host mouse. To measure the CTL function of adoptively transferred OT-I cells, 5, 2.5, 1.25, 0.63, 0.31, or 0.16 × 105 LN cells from the (B6 × B6.Kathy)F1 host mice were cultured in triplicate with 5 × 105 irradiated, OVA-loaded spleen cells in 200 μl RPMI containing 10% FCS, 50 μM 2-ME, and 2mM l-glutamine. The number of OT-I responders per well was calculated by multiplying the number of LN cells by the percentage of Thy1.1− CD8+Vα2+ cells as determined by flow cytometry. After 5 d, the cytotoxicity of each well was assessed on 104 51Cr-labeled EL4 cells alone or EL4 cells coated with 1 μg/ml OVA257–264 during 51Cr labeling as described (19).
Results
B Cell Presentation of Antigen Causes Direct Tolerization of CD8+ T Cells.
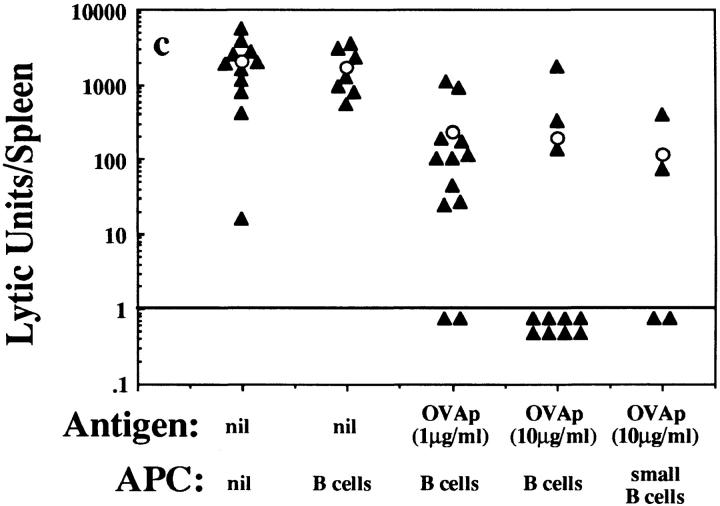
Presentation of antigen by B cells has been shown to cause CTL tolerance for the male-specific anti–H-Y response (7). However, this response is dependent on CD4+ T cell help (8, 9), raising the possibility that CTL tolerance occurred indirectly by the induction of CD4+ T cell tolerance. To investigate whether antigen-bearing B cells can induce tolerance by directly affecting the CD8+ T cell population, B cells coated with the Kb- restricted OVA peptide OVA257–264 were tested for their ability to tolerize OVA-specific CTL responses generated by intravenous injection of irradiated spleen cells loaded intracytoplasmically with OVA protein (OVA-loaded spleen cells [21]). Injection of B6 mice with 107 OVA257–264-coated B cells reduced the subsequent OVA-specific CTL response upon challenge with OVA-loaded spleen cells (Fig. 1 a). On average, the ability to generate OVA-specific CTLs was diminished 10-fold, but many mice showed >100-fold weaker responses (Fig. 1 c). Tolerance induction by B cells was long-lasting, as thymectomized mice injected with OVA257–264-coated B cells were unable to generate OVA-specific CTLs 12 wk after immunization (data not shown). The level of antigen expression also affected tolerance induction, which was slightly more efficient when B cells were coated with a higher concentration (10 vs. 1 μg/ml) of peptide (Fig. 1 c). Although CD4+ T cell tolerance induction by B cells has been shown to be more effective using resting B cells (11, 12), we found that for CD8+ T cells, small resting B cells were no more tolerogenic than unfractionated B cells (Fig. 1 c). Under similar conditions, peptide-pulsed dendritic cells were not tolerogenic, but instead primed hosts for stronger responses to OVA-loaded spleen cell challenge (data not shown).
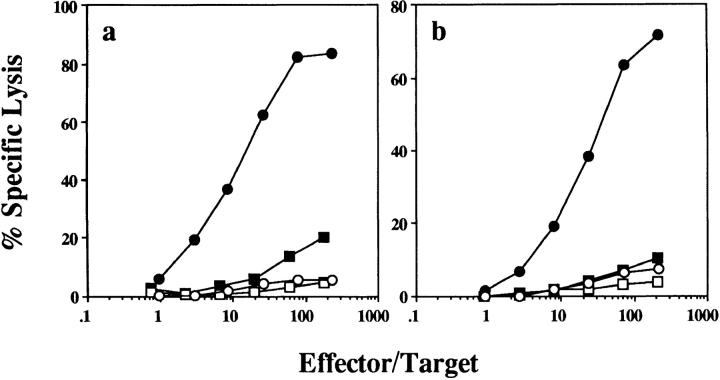
Figure 1.
OVA257–264-coated B cells induce OVA-specific CTL tolerance in B6 mice. B6 mice were injected intravenously with 107 OVA257–264-coated B6 B cells, unpulsed B6 B cells, or medium alone, and rechallenged 7 d later with (a and c) 25 × 106 OVA-loaded spleen cells injected intravenously or (b) 10 μg OVA257–264 in CFA injected subcutaneously. After an additional 7 d, their spleens were removed and stimulated in vitro for 6 d before a 4-h chromium release assay was performed. (a and b) Percent specific lysis of 51Cr-labeled OVA257–264-coated EL4 (filled symbols) and EL4 (open symbols) in mice injected with B cells coated with OVA257–264 at 10 μg/ml (squares) or with medium alone (circles). (c) The number of OVA-specific LU/spleen for individual mice (filled triangles) treated as in a, and calculated as described in Materials and Methods. OVAp, OVA257–264 coated. Open circles, average LU/spleen for each group.
We have found that the priming protocol used to measure CD8+ T cell tolerance induction by B cells, i.e., OVA-loaded spleen cell challenge, is CD4+ T cell dependent (19). Therefore, it was possible (though unlikely given that tolerization only involved the class I–restricted determinant) that CTL tolerance in this model may have also occurred indirectly through the induction of CD4+ T cell tolerance. To determine whether tolerance resulted from a direct effect on CD8+ T cells, we examined B cell tolerance induction under conditions where CTL generation was not CD4+ T cell dependent, i.e., by priming subcutaneously with OVA257–264 in CFA (19). OVA257–264-coated B cell–primed mice challenged subcutaneously with OVA257–264 in CFA also failed to generate OVA-specific CTLs (Fig. 1 b), confirming that the CD8+ T cells were directly tolerized.
Activated CD8+ T Cells Die after Recognizing Antigen on B Cells In Vitro.
To investigate the mechanism of CTL tolerance induction by antigen-bearing B cells, we examined the in vitro response of activated CD8+ T cells to antigen-bearing B cells. For these experiments, in vitro–activated CD8+ T cells from the OVA-specific TCR transgenic line OT-I were used. These mice are of a B6 genotype. OT-I cells plus syngeneic B6 B cells (unpulsed or pulsed with OVA257–264) were cultured together for 15 h and then analyzed by flow cytometry to determine the survival of each cell population relative to an internal control population of bm1 B cells. These latter cells bear the Kbm1 MHC molecule and therefore cannot present OVA257–264 to OT-I cells (20). The survival of the OT-I cells (B220−, Kb+) and B6 B cells (B220+, Kb+) was then examined relative to the bm1 control B cells (B220+, Kb−) (Fig. 2, a and b). As expected, the activated OT-I cells killed a large proportion of B6 B cells, as seen by the reduction in B6 B cells relative to bm1 B cells in the presence of antigen. Surprisingly, there was also a dramatic loss of OT-I cells relative to the bm1 B cell control population. Six separate experiments revealed a consistent loss of the CD8+ CTLs during their 15-h culture with antigen-bearing B6 B cells. This result was the same whether the bm1 B cells were cocultured as described above, or whether they were simply added at the end of the culture period, just before flow cytometric analysis. In an example of the latter experiment, there were 2.1 × 105 OT-I cells and 1.7 × 105 B6 B cells present after 15 h of culture in the absence of peptide. When the B6 B cells were first coated with peptide, only 0.31 × 105 OT-I cells and 0.83 × 105 B6 B cells remained. This represents an 85% loss of OT-I cells and a 51% loss of B6 B cells.
Figure 2.
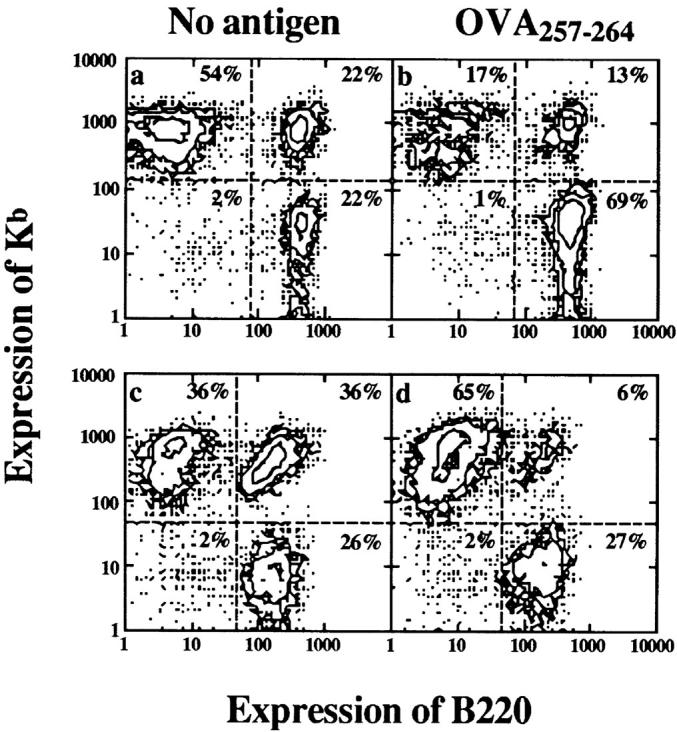
Activated OT-I cells kill peptide-coated B cells, but are themselves killed by a CD95-dependent mechanism during this recognition process. 5 × 105 previously stimulated (a and b) OT-I cells or (c and d) OT-I.lpr cells were cultured for 15 h with 5 × 105 B6 plus bm1 B cells coated without (a and c) or with (b and d) 0.1 μg/ml OVA257–264 peptide. The wells were then analyzed by flow cytometry using anti-B220–FITC and anti-Kb (5-F-1)–biotin followed by Streptavidin-PE.
The antigen-specific loss of OT-I cells was not due to fratricide as a result of re-presentation of OVA peptide by CTLs to each other, since OT-I cells bearing the nonpresenting Kbm1 molecule, instead of Kb, were also killed (data not shown). To investigate whether Fas (CD95)-mediated signaling (for a review, see reference 22) was involved in the death of activated CD8+ T cells, the OT-I mice were crossed to lpr mice, which express a genetic defect in CD95 (23). When activated OT-I.lpr cells were cultured for 15 h with antigen-bearing B cells, survival was greatly improved (Fig. 2, c and d). This suggested that activated cells were killed by a CD95-dependent mechanism. Unlike activated OT-I cells, naive OT-I cells did not kill antigen-bearing B cells and were not killed during this 15-h culture period (data not shown).
Expansion and Loss of Naive OT-I T Cells in Response to Antigen-bearing B Cells In Vivo.
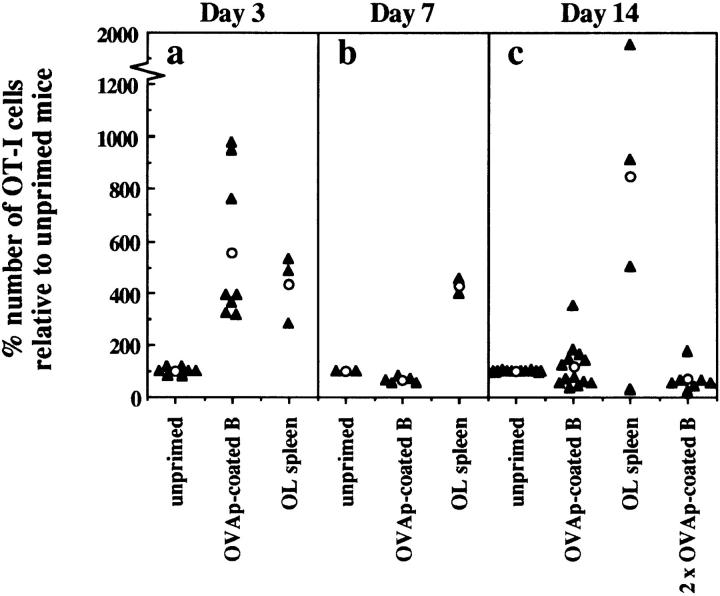
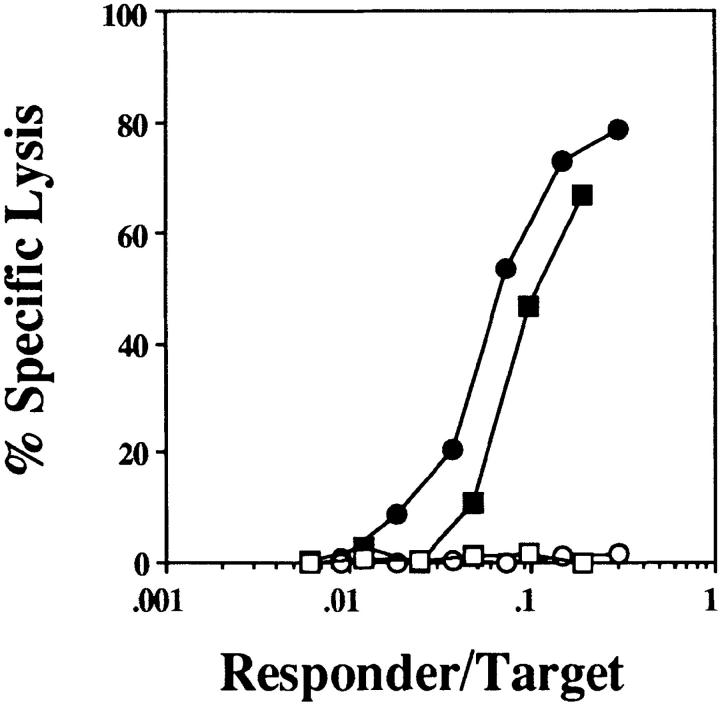
As shown earlier, CTL tolerance induction in B6 mice involved recognition of antigen on B cells by naive CD8+ T cells. To further characterize this response in vivo, naive OT-I cells were injected into Thy1 congenic (B6 × B6.Kathy)F1 mice. After injection of OVA257–264-coated B cells, the fate of the adoptively transferred OT-I cells was followed by flow cytometry. The number of OT-I cells recovered from the LNs and spleen of individual mice was then calculated as a percentage of the number of OT-I cells in unprimed control mice (Fig. 3). Exposure to antigen-bearing B cells resulted in a large expansion of OT-I cells by day 3, as seen by an increase in the relative number of OT-I cells in primed versus untreated controls (Fig. 3 a), and, in separate experiments, by the observed proliferation of carboxyfluorescein succinimidyl ester–labeled OT-I cells under similar conditions (data not shown). The extent of OT-I cell expansion was similar to that induced by OVA-loaded spleen cells (Fig. 3 a), which are known to induce strong OVA-specific CTL responses (21). However, by day 7 most of the OT-I cells generated in response to OVA257–264-coated B cells had disappeared (Fig. 3 b), and the number of OT-I cells remained similar to or below that of unprimed mice to day 14 (Fig. 3 c). In contrast, mice immunized with OVA-loaded spleen cells showed further expansion of OT-I cells up to day 14. The OT-I cells remaining in B cell–treated mice were not anergic, as they were able to lyse OVA- expressing targets as efficiently as naive OT-I cells, after 5 d restimulation in vitro (Fig. 4). Therefore, although an immunogenic form of OVA (OVA-loaded spleen cells) was able to induce sustained proliferation of OT-I cells, tolerance induction by B cells was characterized by proliferation and then rapid deletion of OVA-specific CD8+ T cells.
Figure 3.
Naive OT-I cells rapidly expand and disappear in response to antigen-bearing B cells in vivo. Naive OT-I cells were adoptively transferred into (B6 × B6.Kathy)F1 hosts 1 d before intravenous immunization with medium alone (unprimed), 107 OVA257–264-coated B cells (OVAp-coated B), or 25 × 106 OVA-loaded spleen cells (OL spleen). Mice given two injections of OVA257–264-coated B cells received their second injection 5 d after the first. On day 3 (a), 7 (b), and 14 (c) after the first immunization, the total number of OT-I cells in the spleen and LNs of individual mice was determined using flow cytometry and expressed as a percentage of the total number of OT-I cells from one or more unprimed control animals in each experiment (filled triangles). Open circles, average percentage of OT-I cells relative to unprimed controls for each group.
Figure 4.
OT-I cells remaining at day 14 in B cell–treated mice possess full CTL effector function. 14 d after immunization with OVA257–264-coated B cells (squares) or medium alone (circles), the LN cells from adoptively transferred (B6 × B6. Kathy)F1 host mice were titrated into 200 μl microcultures and stimulated for 5 d with irradiated OVA-loaded spleen cells. The number of responder OT-I cells per well was determined by flow cytometry. The cytotoxicity was then measured in a 4-h chromium release assay using 51Cr-labeled OVA257–264-coated EL4 (filled symbols) and uncoated EL4 (open symbols) targets. This experiment was performed four times with two to four mice per group.
To test whether the incomplete deletion of OT-I cells was the result of insufficient antigen exposure, OT-I cell survival was examined after two injections of B cells, 5 d apart. Under these circumstances, deletion of OT-I cells was slightly more efficient (Fig. 3 c).
Discussion
Most studies examining tolerance induction by B cells have focussed on the response of CD4+ T cells (10–16). This report represents the first demonstration that naive CD8+ T cells can be directly tolerized by recognition of antigen on B cells. One other study has addressed the role of B cells as tolerogenic APCs in vivo for CD8+ T cell responses by showing that injection of male B cells into female mice resulted in H-Y–specific CTL tolerance (7). However, generation of H-Y–specific CTLs has been reported to require CD4+ T cell help (8, 9). Thus, failure to induce H-Y–specific CTLs might have simply reflected tolerant CD4+ T helper cells. We showed that OVA-specific CTLs were directly tolerized by OVA257–264-coated B cells, since mice primed with OVA257–264 peptide–coated B cells responded weakly to both OVA-loaded spleen cells (Fig. 1, a and c) and OVA peptide in CFA (Fig. 1 b). This latter response is CD4+ T cell independent, indicating that CTLs exposed to B cells bearing class I–restricted determinants are directly tolerized.
Since CTL tolerance has been reported to be the default response when CD4 help is unavailable (24, 25), it is possible that B cells induced CTL tolerance simply because they failed to provide determinants for stimulation of CD4+ T cell help. Even if class II–restricted determinants had been available, naive CD4+ T cells are reported to be tolerized by recognition of antigen on B cells, which should also lead to a lack of help and, consequently, CTL tolerance. It remains to be addressed whether provision of primed CD4+ T cell help, which cannot be tolerized by B cells, will allow B cells to stimulate naive CTL responses.
Our in vitro studies showed that peptide-coated B cells could be lysed by activated OT-I cells, confirming that CD8+ T cells could recognize antigen on B cells (Fig. 2). More importantly, however, these experiments revealed that activated, but not naive, OT-I cells died shortly after interacting with antigen-bearing B cells. The loss of activated OT-I cells after recognition of antigen on B cells suggested that the pathway to B cell–mediated tolerance induction may require the interaction of activated CD8+ T cells with B cells. Protection of activated OT-I.lpr CD8+ T cells from death implied that CD95-mediated signaling played an important role in this death pathway.
A great deal of evidence suggests that CD95-mediated signaling is the predominant mediator of CD4+ T cell death in vitro via a mechanism termed activation-induced cell death (AICD [26–30]). This is mediated through the interaction of CD95 with its ligand (CD95L [31, 32]), expressed on the same (26, 27) or neighboring (33, 34) antigen-activated T cells, and occurs within 24 h for previously activated cells (35). Although there is some evidence that CD95 is involved in the death of activated CD8+ T cells (33, 36, 37), TNFR/TNF-mediated apoptosis has also been suggested to be important (35, 38, 39). This latter mechanism takes 40–48 h to induce apoptosis of activated T cells (35). Our observation that up to 85% of activated CD8+ T cells were killed within 15 h argues against a role for TNFR signaling for B cell–induced deletion of CD8+ T cells. The fact that activated CD8+ T cells expressing the mutant CD95 gene were largely protected from deletion suggests that CD95/CD95L interactions are more important in this case.
It was at first surprising that naive OT-I cells, in contrast to activated OT-I cells, were not killed when cultured for 15 h in vitro with antigen-bearing B cells. Interestingly, cultures containing naive OT-I cells showed extensive proliferation on day 2 (data not shown). This is consistent with the idea that to be deleted, OT-I cells must first be activated and, as a consequence, may proliferate before being killed. However, the combined effects of proliferation, nutrient utilization, and activation-induced cell death in vitro made it very difficult to analyze the response of naive OT-I cells under these conditions; therefore, we concentrated our efforts on examining the response of naive OT-I cells in vivo.
Deletional tolerance after antigen-specific activation in vivo has been reported for both CD4+ and CD8+ T cells responding to a variety of antigens. These include conventional peptide or protein antigens (40–42), superantigens (43–47), minor antigens (48), viral antigens (49, 50), and tissue-specific antigens (51–53). These studies were characterized by an early, transient period of antigen-specific T cell proliferation followed by rapid deletion of most responding T cells. OT-I cells displayed similar response kinetics upon exposure to peptide-coated B cells in vivo (Fig. 3). At day 3, there was marked proliferation of OT-I cells, but by day 7 the number of OT-I cells had declined to below prestimulation levels, where it remained. Altogether, our data suggest that B cells tolerize CD8+ T cells via activation of naive cells followed by CD95-mediated deletion of their activated progeny.
Interestingly, not all OT-I cells were deleted in response to OVA-bearing B cells. Unlike other models where those T cells that remain become hyporesponsive to further antigen challenge (40, 41, 44, 48, 52), the OT-I cells that were not deleted showed full CTL function (Fig. 4). Given that deletion is reported to be antigen dose dependent (42, 43), these cells may represent OT-I cells that were inadequately stimulated by the tolerogenic B cells. Perhaps all of the B cells were killed before all activated CD8+ T cells could reencounter antigen for induction of CD95-mediated death. We attempted to address this issue by introducing a second dose of OVA-coated B cells 5 d after the first immunization, but this only slightly enhanced OT-I deletion at day 14. However, in TCR transgenic models, changes to the extent and nature of tolerance induction may require very large variations in antigen dose due to the relatively high number of responsive T cells (52, 54).
It is important to note that we have not addressed whether B cells are unique in their ability to induce deletion of CD8+ T cells. It may be that any cell type lacking the appropriate accessory signals will cause such deletion. The aim of this report was to specifically examine the effect of antigen presentation by B cells to CD8+ T cells. To this end, we have shown that B cells are directly tolerogenic for CD8+ T cells. This tolerance appears to be preceded by activation and proliferation of antigen-specific CD8+ T cells. Once activated, CD8+ T cells appear to be susceptible to CD95-mediated killing by reencounter with antigen on B cells, at least in vitro. Taken together, our data suggest that B cell presentation of antigen to CD8+ T cells leads to activation followed by deletion of the antigen-specific population.
Acknowledgments
We thank Freda Karamalis, Paula Nathan, Jenny Falso, and Tatiana Banjanin for technical assistance.
This work was supported by the Cooperative Research Centre for Vaccine Technology, the National Institutes of Health (grant AI-29385), and grants from the National Health and Medical Research Council and the Australian Research Council.
Abbreviations used in this paper
- B6
C57BL/6
- HEM
Hepes Eagle's Medium
- RAG
recombination-activating gene
References
- 1.Ke Y, Kapp JA. Exogenous antigens gain access to the major histocompatibility complex class I processing pathway in B cells by receptor-mediated uptake. J Exp Med. 1996;184:1179–1184. doi: 10.1084/jem.184.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yefenof E, Zehavi-Feferman R, Guy R. Control of primary and secondary antibody responses by cytotoxic T lymphocytes specific for a soluble antigen. Eur J Immunol. 1990;20:1849–1853. doi: 10.1002/eji.1830200833. [DOI] [PubMed] [Google Scholar]
- 3.Barnaba V, Franco A, Alberti A, Benvenuto R, Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature. 1990;345:258–260. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- 4.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell–deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bruijn ML, Nieland JD, Schumacher TN, Ploegh HL, Kast WM, Melief CJ. Mechanisms of induction of primary virus-specific cytotoxic T lymphocyte responses. Eur J Immunol. 1992;22:3013–3020. doi: 10.1002/eji.1830221137. [DOI] [PubMed] [Google Scholar]
- 6.Hollsberg P, Batra V, Dressel A, Hafler D. Induction of anergy in CD8 T cells by B cell presentation of antigen. J Immunol. 1996;157:5269–5276. [PubMed] [Google Scholar]
- 7.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 8.Simpson E, Gordon RD. Responsiveness to HY antigen Ir gene complementation and target cell specificity. Immunol Rev. 1977;35:59–75. doi: 10.1111/j.1600-065x.1977.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 9.Husmann LA, Bevan MJ. Cooperation between helper T cells and cytotoxic T lymphocyte precursors. Ann NY Acad Sci. 1988;532:158–169. doi: 10.1111/j.1749-6632.1988.tb36335.x. [DOI] [PubMed] [Google Scholar]
- 10.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eynon EE, Parker DC. Parameters of tolerance induction by antigen targeted to B lymphocytes. J Immunol. 1993;151:2958–2964. [PubMed] [Google Scholar]
- 12.Morris SC, Lees A, Finkelman FD. In vivoactivation of naive T cells by antigen-presenting B cells. J Immunol. 1994;152:3777–3785. [PubMed] [Google Scholar]
- 13.Morris SC, Lees A, Holmes JM, Jeffries RD, Finkelman FD. Induction of B cell and T cell tolerance in vivoby anti-CD23 mAb. J Immunol. 1994;152:3768–3776. [PubMed] [Google Scholar]
- 14.Gilbert KM, Weigle WO. Tolerogenicity of resting and activated B cells. J Exp Med. 1994;179:249–258. doi: 10.1084/jem.179.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert KM, Weigle WO. B cell presentation of a tolerogenic signal to Th clones. Cell Immunol. 1992;139:58–71. doi: 10.1016/0008-8749(92)90099-b. [DOI] [PubMed] [Google Scholar]
- 16.Webb SR, Li JH, Wilson DB, Sprent J. Capacity of small B cell-enriched populations to stimulate mixed lymphocyte reactions: marked differences between irradiated vs. mitomycin C-treated stimulators. Eur J Immunol. 1985;15:92–96. doi: 10.1002/eji.1830150118. [DOI] [PubMed] [Google Scholar]
- 17.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett SR, Carbone FR, Karamalis F, Miller JFAP, Heath WR. Induction of a CD8 cytotoxic T lymphocyte response by cross-priming requires cognate CD4 help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurts C, Heath WR, Carbone FR, Allison J, Miller JFAP, Kosaka H. Constitutive class I–restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbone FR, Bevan MJ. Class I–restricted processing and presentation of exogenous cell–associated antigen in vivo. J Exp Med. 1990;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 24.Guerder S, Matzinger P. Activation versus tolerance: a decision made by T helper cells. Cold Spring Harbor Symp Quant Biol. 1989;2:799–805. doi: 10.1101/sqb.1989.054.01.093. [DOI] [PubMed] [Google Scholar]
- 25.Guerder S, Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J Exp Med. 1992;176:553–564. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 27.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 28.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 29.Owen-Schaub LB, Yonehara S, Crump WL, III, Grimm EA. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992;140:197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- 30.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 32.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillette FI, Sidman CL. A specific intercellular pathway of apoptotic cell death is defective in the mature peripheral T cells of autoimmune lpr and gldmice. Eur J Immunol. 1994;24:1181–1185. doi: 10.1002/eji.1830240526. [DOI] [PubMed] [Google Scholar]
- 34.Vignaux F, Golstein P. Fas-based lymphocyte-mediated cytotoxicity against syngeneic activated lymphocytes: a regulatory pathway? . Eur J Immunol. 1994;24:923–927. doi: 10.1002/eji.1830240421. [DOI] [PubMed] [Google Scholar]
- 35.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 36.Russell JH, Rush B, Weaver C, Wang R. Mature T cells of autoimmune lpr/lprmice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA. 1993;90:4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurts C, Heath WR, Kosaka H, Miller JFAP, Carbone FR. The peripheral deletion of autoreactive CD8+T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1) J Exp Med. 1998;188:415–420. doi: 10.1084/jem.188.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J Exp Med. 1996;184:485–492. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Speiser DE, Sebzda E, Ohteki T, Bachmann MF, Pfeffer K, Mak TW, Ohashi PS. Tumor necrosis factor receptor p55 mediates deletion of peripheral cytotoxic T lymphocytes in vivo. . Eur J Immunol. 1996;26:3055–3060. doi: 10.1002/eji.1830261235. [DOI] [PubMed] [Google Scholar]
- 40.Kyburz D, Aichele P, Speiser DE, Hengartner H, Zinkernagel RM, Pircher H. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur J Immunol. 1993;23:1956–1962. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- 41.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. . Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 42.Liblau RS, Tisch R, Shokat K, Yang X, Dumont N, Goodnow CC, McDevitt HO. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci USA. 1996;93:3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 44.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureusenterotoxin B. Nature. 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 45.MacDonald HR, Baschieri S, Lees RK. Clonal expansion precedes anergy and death of Vβ8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. . Eur J Immunol. 1991;21:1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- 46.McCormack JE, Callahan JE, Kappler J, Marrack PC. Profound deletion of mature T cells in vivoby chronic exposure to exogenous superantigen. J Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- 47.Gonzalo JA, Moreno de Alboran I, Ales-Martinez JE, Martinez C, Kroemer G. Expansion and clonal deletion of peripheral T cells induced by bacterial superantigen is independent of the interleukin-2 pathway. Eur J Immunol. 1992;22:1007–1011. doi: 10.1002/eji.1830220420. [DOI] [PubMed] [Google Scholar]
- 48.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 49.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 50.Zinkernagel RM, Moskophidis D, Kundig T, Oehen S, Pircher H, Hengartner H. Effector T-cell induction and T-cell memory versus peripheral deletion of T cells. Immunol Rev. 1993;133:199–223. doi: 10.1111/j.1600-065x.1993.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 51.Kurts C, Kosaka H, Carbone FR, Miller JFAP, Heath WR. Class I–restricted cross-presentation of exogenous self antigens leads to deletion of autoreactive CD8+T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanoue A, Bona C, von Boehmer H, Sarukhan A. Conditions that induce tolerance in mature CD4+T cells. J Exp Med. 1997;185:405–414. doi: 10.1084/jem.185.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertolino P, Heath WR, Hardy CL, Morahan G, Miller JFAP. Peripheral deletion of autoreactive CD8+ T cells in transgenic mice expressing H-2Kb in the liver. Eur J Immunol. 1995;25:1932–1942. doi: 10.1002/eji.1830250721. [DOI] [PubMed] [Google Scholar]
- 54.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]